Abstract
The Mycobacterium tuberculosis peptidoglycan is cross-linked mainly by l,d-transpeptidases (LDTs), which are efficiently inactivated by a single β-lactam class, the carbapenems. Development of carbapenems for tuberculosis treatment has recently raised considerable interest since these drugs, in association with the β-lactamase inhibitor clavulanic acid, are uniformly active against extensively drug-resistant M. tuberculosis and kill both exponentially growing and dormant forms of the bacilli. We have purified the five l,d-transpeptidase paralogues of M. tuberculosis (Mt1 to -5) and compared their activities with those of peptidoglycan fragments and carbapenems. The five LDTs were functional in vitro since they were active in assays of peptidoglycan cross-linking (Mt5), β-lactam acylation (Mt3), or both (Mt1, Mt2, and Mt4). Mt3 was the only LDT that was inactive in the cross-linking assay, suggesting that this enzyme might be involved in other cellular functions such as the anchoring of proteins to peptidoglycan, as shown in Escherichia coli. Inactivation of LDTs by carbapenems is a two-step reaction comprising reversible formation of a tetrahedral intermediate, the oxyanion, followed by irreversible rupture of the β-lactam ring that leads to formation of a stable acyl enzyme. Determination of the rate constants for these two steps revealed important differences (up to 460-fold) between carbapenems, which affected the velocity of oxyanion and acyl enzyme formation. Imipenem inactivated LDTs more rapidly than ertapenem, and both drugs were more efficient than meropenem and doripenem, indicating that modification of the carbapenem side chain could be used to optimize their antimycobacterial activity.
INTRODUCTION
Tuberculosis (TB) remains the second-leading infectious disease causing mortality, after AIDS, and it is estimated that one-third of the world's population is infected with Mycobacterium tuberculosis. According to the 2012 WHO report, there were 8.7 million new TB cases and 1.4 million deaths due to the disease in 2011 (1). TB treatment requires at least 6 months of chemotherapy with multiple drugs due to the poor efficacy of available antibiotics against particular forms of the M. tuberculosis bacilli that do not replicate (2, 3). Inappropriate use of the two first-line anti-TB drugs, isoniazid and rifampin, leads to the emergence of bacilli that are resistant to these drugs (multidrug-resistant M. tuberculosis [MDR-TB]), and widespread dissemination of these bacilli represents an obstacle to tuberculosis control (1). In 2011, the WHO estimated that there were 630,000 MDR-TB cases. The extensive use of second-line drugs has led to emergence of extensively drug-resistant M. tuberculosis (XDR-TB), which shows a very poor prognosis with an increasing mortality rate (4).
Except for bedaquiline, all drugs used to treat tuberculosis were approved more than 45 years ago, illustrating the complexity of TB drug development. β-Lactams are not used for TB treatment since M. tuberculosis produces a broad spectrum β-lactamase, BlaC, which inactivates all β-lactams with various efficiencies (5). However, BlaC is irreversibly inactivated by clavulanic acid, and the combination of this β-lactamase inhibitor with β-lactams of the carbapenem class has been reported to be bactericidal in vitro (6, 7). The combination is active against both exponentially growing M. tuberculosis and nonreplicating forms of the bacilli (7). Furthermore, the combination is uniformly active against XDR strains (7).
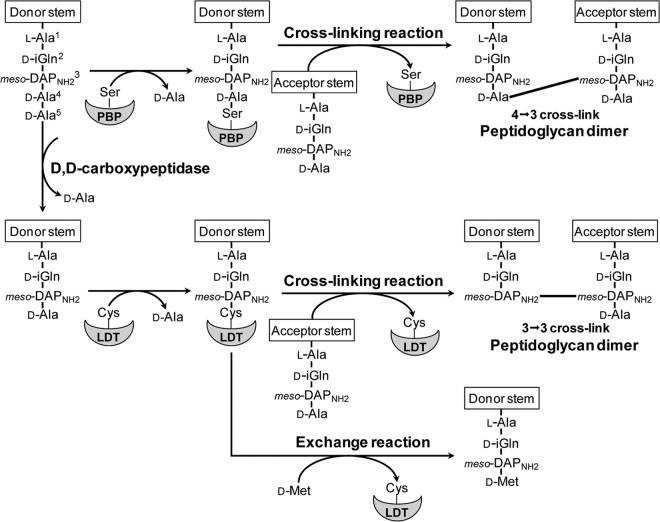
Antibiotics of the β-lactam family inhibit the last step of peptidoglycan polymerization. In most bacteria, the drugs inactivate the essential d,d-transpeptidase activity of classical penicillin-binding proteins (PBPs) (8, 9). These enzymes cross-link glycan chains by forming 4→3 peptide bonds connecting residues at the fourth and third positions of stem peptides (Fig. 1). Only 20% of the peptidoglycan cross-links are of the 4→3 type in mycobacteria, indicating that classical PBPs may only have a minor role in peptidoglycan polymerization (10). The majority of the cross-links are of the 3→3 type and are formed by l,d-transpeptidases (LDTs) (Fig. 1). PBPs and LDTs are structurally unrelated (11), contain active-site serine and cysteine residues (12), and use stem pentapeptide and tetrapeptide as the acyl donor substrate (12), respectively. Since pentapeptide is assembled in the cytoplasm, formation of the tetrapeptide donor substrate of LDTs requires a d,d-carboxypeptidase activity for cleaving the terminal d-Ala residue of peptidoglycan precursors (13, 14).
Fig 1.
Reactions catalyzed by Mycobacterium tuberculosis d,d-transpeptidases (PBPs) and l,d-transpeptidases (LDTs).
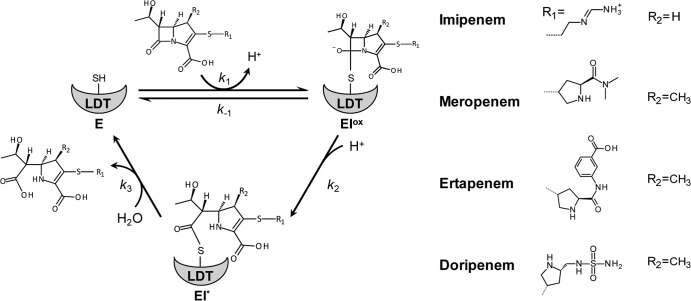
β-Lactam antibiotics inactivate peptidoglycan transpeptidases by forming a covalent adduct with the enzyme active-site residue. The reaction is analogous to the first step of the transpeptidation reaction that leads to enzyme acylation by the donor stem peptide (15). LDTs are efficiently inactivated by a single class of β-lactams, the carbapenems, such as imipenem, meropenem, ertapenem, and doripenem (16) (Fig. 2). LDTs are likely to be the essential targets of carbapenems in M. tuberculosis, since these enzymes are responsible for formation of a substantial majority of the cross-links in both the exponential and stationary phases of growth (10, 17). However, the d,d-carboxypeptidase activity of PBPs is an additional target, since tetrapeptide production is essential to generate the tetrapeptide donor substrate of LDTs (12, 14, 17). Classical d,d-transpeptidases may also be essential, although these enzymes have a minor contribution to peptidoglycan cross-linking.
Fig 2.
Reaction schemes for LDT inactivation by carbapenems. E, free enzyme form; EIox, oxyanion; EI*, acyl enzyme. SH, sulfhydryl of the catalytic cysteine.
The chromosome of M. tuberculosis strain H37Rv encodes five LDTs paralogues. LdtMt1 and LdtMt2 are functional in an in vitro peptidoglycan cross-linking assay and are thought to have distinct functions in vivo (10, 18). LdtMt2 is essential for virulence in a mouse model of acute infection (18), whereas LdtMt1 may have a role in adaptation to the nonreplicative state of the bacilli (10). The roles of the three remaining LDTs have not been investigated. To further characterize the targets of carbapenems in M. tuberculosis, we have purified the five l,d-transpeptidase paralogues and compared the in vitro activities of the enzymes with respect to peptidoglycan dimer formation and acylation by carbapenems.
MATERIALS AND METHODS
Production and purification of l,d-transpeptidases.
Recombinant plasmids for production of the soluble form of LdtMt1 (Rv0116c) containing an N-terminal hexahistidine tag have been previously described (19). For production of LdtMt2, LdtMt3, LdtMt4, andLdtMt5, portions of the genes from M. tuberculosis H37Rv were amplified by PCR using oligonucleotides described in Table 1. Heteroduplexes containing cohesive ends were ligated with vector pET-TEV digested with NdeI plus XhoI as previously described (19). Escherichia coli BL21(DE3) harboring recombinant plasmids was grown at 37°C with vigorous shaking in 2 liters of brain heart infusion broth (Difco) containing kanamycin (50 μg/ml) to an optical density at 600 nm of 0.9. Isopropyl-β-d-thiogalactopyranoside was added (0.5 mM), and incubation was continued for 18 h at 16°C. LDTs were purified from clarified lysates by affinity chromatography on Ni2+-nitrilotriacetate-agarose resin (Sigma) and by size exclusion chromatography (Superdex 75 HL26/60 column; GE HealthCare) in 100 mM sodium phosphate buffer (pH 6.4) containing 300 mM NaCl. l,d-Transpeptidases were concentrated by ultrafiltration (Amicon Ultra-4 centrifugal filter devices; Millipore) to a final concentration of ca. 1.5 mg/ml and stored at −65°C in the same buffer.
Table 1.
Oligonucleotides used for amplification and cloning of l,d-transpeptidase genes
| l,d-Transpeptidase (strain; accession no.) and amplicona | Oligonucleotideb |
|---|---|
| Mt2 (Rv2518c; CAA16014.1) | |
| C | 5′AACATATGGCCGATCTGCTGGTGCC (for) |
| D | 5′TTCTCGAGTTACGCCTTGGCGTTACCGG (rev) |
| Mt3 (Rv1433; CAB09251.1) | |
| A | 5′TATGCAGTCTTACGGGTTCGCCGT (for) |
| 5′GTTATTCCTGCACAATGACCGGGT (rev) | |
| B | 5′TGCAGTCTTACGGGTTCGCCGT (for) |
| 5′TCGAGTTATTCCTGCACAATGACCGGGT (rev) | |
| Mt4 (Rv0192; CAB09732.1) | |
| C | 5′CATATGCCACACTGGGCTGAAGAACG (for) |
| D | 5′CTCGAGTTAGATCTGCCAGTCCTGGGCACC (rev) |
| Mt5 (Rv0483; CAB00944.1) | |
| A | 5′TATGGCCGGCAAAGTGACCAAGCT (for) |
| 5′GTTACCCACCCGGTCCGTTAGTAG (rev) | |
| B | 5′TGGCCGGCAAAGTGACCAAGCTGG (for) |
| 5′TCGAGTTACCCACCCGGTCCGTTAGTAG (rev) |
For cloning, amplicons A and B were mixed, denatured, and renatured to generate heteroduplexes with cohesive ends.
Initiation and stop codons are in italic. For Mt2 and Mt4, oligonucleotides C and D contained NdeI and XhoI sites (underlined). for, forward; rev, reverse.
Mass spectrometry analyses of l,d-transpeptidase acylation by carbapenems.
The formation of drug-enzyme adducts was tested by incubating l,d-transpeptidases (20 μM) with carbapenems (100 μM) at 20°C in 100 mM sodium phosphate buffer (pH 6.0). Five microliters of acetonitrile and 1 μl of 1% formic acid were added, and the reaction mixture was directly injected into the mass spectrometer (Qstar Pulsar I; Applied Biosystems) at a flow rate of 0.05 ml/min (acetonitrile, 50%; water, 49.5%; formic acid, 0.5% [by volume]). Spectra were acquired in the positive mode as previously described (16).
Kinetics of l,d-transpeptidase inactivation by carbapenems.
Fluorescence kinetic data were acquired with a stopped-flow apparatus (RX-2000; Applied Biophysics) coupled to a spectrofluorometer (Cary Eclipse; Varian) in 100 mM sodium phosphate (pH 6.0) at 10°C. Kinetic constants for LDT acylation by carbapenems were calculated as previously described (19, 20). The rates of hydrolysis of acyl enzymes were determined by spectrophotometry in sodium phosphate buffer (100 mM; pH 6.0) at 20°C in a Cary 100 spectrophotometer (Cary 100-Bio; Varian) as previously described (19).
Mass spectrometry analyses of transpeptidation reaction products.
The disaccharide tetrapeptide containing amidated meso-diaminopimelic acid (GlcNAc–MurNGlyc–l-Ala1–d-iGln2–meso-DapNH23–d-Ala4) was purified from Mycobacterium abscessus strain CIP104536T, and the concentration was determined by amino acid analysis after acid hydrolysis (21). In vitro formation of muropeptide dimers by LDTs (10 μM) was tested in 10 μl of 20 mM sodium phosphate buffer (pH 6.4) containing 60 mM NaCl and 4 mM disaccharide tetrapeptide. The reaction mixture was incubated for 2 h at 37°C and analyzed by electrospray mass spectrometry in the positive mode (Qstar Pulsar I; Applied Biosystems) as previously described (22). d-Met (1 mM) was added to the reaction mixture to assay the exchange of d-Ala4 by d-Met. The transpeptidases assayed were also tested with disaccharide pentapeptide GlcNAc–MurNGlyc–l-Ala1–d-iGln2–meso-DapNH23–d-Ala4–d-Ala5 (440 μM), which was purified from M. abscessus strain CIP104536T.
RESULTS
Covalent inactivation of l,d-transpeptidases by carbapenems.
LDTs were independently incubated with four carbapenems (imipenem, meropenem, ertapenem, and doripenem), and formation of covalent adducts was assayed by electrospray mass spectrometry (Table 2). Mass increments corresponding to the masses of the antibiotics were detected, indicating that adducts were generated by formation of a thioester bond between the sulfhydryl group of the l,d-transpeptidase active-site cysteine and the carbonyl group of the carbapenem β-lactam ring (Fig. 2) (16). Formation of covalent adducts was not detected for LdtMt5, whereas the four remaining l,d-transpeptidases were acylated by each of the four carbapenems (Table 2). Thus, only four of the five M. tuberculosis l,d-transpeptidases are inactivated by carbapenems.
Table 2.
Masses of acyl enzymes
| l,d-Transpeptidase (residuesa) | Carbapenem (mass, Da) | Avg mass (Da)b |
|
|---|---|---|---|
| Calculated | Observed | ||
| Mt1 (32–251) | None | 26,092.3 | 26,092.6 |
| Imipenem (299.3) | 26,391.6 | 26,391.8 | |
| Meropenem (383.5) | 26,475.8 | 26,475.4 | |
| Ertapenem (475.5) | 26,567.8 | 26,567.5 | |
| Doripenem (420.1) | 26,512.4 | 26,512.6 | |
| Mt2 (55–408) | None | 40,236.8 | 40,236.7 |
| Imipenem | 40,536.1 | 40,538.0 | |
| Meropenem | 40,620.3 | 40,622.4 | |
| Ertapenem | 40,712.3 | 40,714.8 | |
| Doripenem | 40,656.9 | 40,657.3 | |
| Mt3 (32–271) | None | 28,160.6 | 28,161.1 |
| Imipenem | 28,459.9 | 28,460.9 | |
| Meropenem | 28,544.1 | 28,544.5 | |
| Ertapenem | 28,636.1 | 28,637.4 | |
| Doripenem | 28,580.7 | 28,581.5 | |
| Mt4 (1–366) | None | 41,178.2 | 41,178.9 |
| Imipenem | 41,477.5 | 41,478.0 | |
| Meropenem | 41,561.7 | 41,561.8 | |
| Ertapenem | 41,653.7 | 41,653.3 | |
| Doripenem | 41,598.3 | 41,597.7 | |
| Mt5 (50–451) | None | 45,140.3 | 45,140.2 |
| Imipenem | 45,439.6 | ND | |
| Meropenem | 45,523.8 | ND | |
| Ertapenem | 45,615.8 | ND | |
| Doripenem | 45,560.4 | ND | |
Portion of the M. tuberculosis H37Rv l,d-transpeptidase present in recombinant proteins produced in E. coli. Each l,d-transpeptidase contained an additional N-terminal 6× His tag (MHHHHHHENLYFQGHM). The N-terminal methionine was not present in the purified proteins.
Mass of enzyme or acyl enzyme. ND, not detected.
Kinetics of enzyme acylation.
We have previously shown that l,d-transpeptidase inactivation by carbapenems is a two-step reaction (Fig. 2) (20, 23). The first step is reversible and leads to formation of a tetrahedral intermediate, the oxyanion (EIox). The second step is irreversible and leads to formation of the acyl enzyme (EI*). Fluorescence kinetics indicated that LdtMt1, LdtMt2, LdtMt3, and LdtMt4, but not LdtMt5, were inactivated by carbapenems (Table 3), in agreement with mass spectrometry analyses (Table 2). The rate constants k1 and k2 for formation of the oxyanion and acyl enzyme, respectively, were determined by spectrofluorometry according to previously described procedures (20, 23). The k2/Kapp ratio provides an evaluation of the overall efficacy of the reaction based on estimates of the disappearance of free enzyme. Important variations in the velocity of the acylation reaction were detected between LDTs (Table 3). The dynamic range of k2/Kapp ratios reached 375 for inactivation of LdtMt1 and LdtMt2 by ertapenem. For imipenem, LdtMt3 was the most rapidly inactivated l,d-transpeptidase (k2/Kapp = 1.16 ± 0.03 μM−1 min−1), due to high rate constants for both oxyanion formation (k1 = 0.92 ± 0.03 μM−1 min−1) and acylation (k2 = 10.1 ± 0.2 min−1). LdtMt1 and LdtMt4 were also efficiently inactivated by imipenem (k2/Kapp = 0.61 ± 0.02 and 0.31 ± 0.01 μM−1 min−1, respectively). Inactivation of LdtMt2 by this antibiotic was less efficient (k2/Kapp = 0.081 ± 0.002 μM−1 min−1) due to slow formation of the oxyanion (k1 = 0.00067 ± 0.00002 μM−1 min−1). For the three remaining carbapenems, LdtMt1 was more efficiently inactivated than LdtMt2, LdtMt3, and LdtMt4. Among these three enzymes, LdtMt3 was acylated more efficiently by meropenem than LdtMt4, but similar values were observed for ertapenem and doripenem. For all carbapenems, the efficiency of acylation was the lowest for LdtMt2.
Table 3.
Kinetic constants for M. tuberculosis l,d-transpeptidase inactivation by carbapenems
| l,d-Transpeptidase and kinetic constant | Value (mean ±SD) for: |
|||
|---|---|---|---|---|
| Imipenem | Meropenem | Ertapenem | Doripenem | |
| Mt1 | ||||
| k1 × 103 (μM−1 min−1) | 460 ± 20 | 64 ± 2 | 930 ± 60 | 130 ± 10 |
| k2 × 103 (min−1) | 1,810 ± 20 | 3,600 ± 80 | 2,660 ± 50 | 470 ± 20 |
| k2/Kapp × 103 (μM−1 min−1) | 610 ± 20 | 75 ± 2 | 1,350 ± 50 | 89 ± 4 |
| k3 × 103 (min−1) | ≤0.12 | 1.04 ± 0.09 | 1.01 ± 0.12 | 0.58 ± 0.09 |
| Mt2 | ||||
| k1 × 103 (μM−1 min−1) | 67 ± 5 | 0.67 ± 0.02 | 4.0 ± 0.1 | 0.85 ± 0.04 |
| k2 × 103 (min−1) | 11,200 ± 700 | 660 ± 40 | 1,010 ± 30 | 580 ± 30 |
| k2/Kapp × 103 (μM−1 min−1) | 81 ± 2 | 0.48 ± 0.01 | 3.6 ± 0.1 | 0.56 ± 0.02 |
| k3 × 103 (min−1) | ≤0.06 | 0.72 ± 0.03 | 0.48 ± 0.08 | 0.34 ± 0.05 |
| Mt3 | ||||
| k1 × 103 (μM−1 min−1) | 920 ± 30 | 17 ± 1 | 87 ± 3 | 10.2 ± 0.5 |
| k2 × 103 (min−1) | 10,100 ± 200 | 6,800 ± 700 | 6,500 ± 600 | 4,500 ± 300 |
| k2/Kapp × 103 (μM−1 min−1) | 1,160 ± 30 | 21 ± 1 | 80 ± 2 | 12 ± 1 |
| k3 × 103 (min−1) | ≤0.12 | 1.42 ± 0.26 | 1.02 ± 0.06 | 0.38 ± 0.01 |
| Mt4 | ||||
| k1 × 103 (μM−1 min−1) | 250 ± 10 | 5.0 ± 0.3 | 31 ± 3 | NAa |
| k2 × 103 (min−1) | 9,900 ± 300 | 80 ± 20 | 18,000 ± 1,000 | NA |
| k2/Kapp × 103 (μM−1 min−1) | 310 ± 10 | 0.67 ± 0.024 | 37 ± 1 | 9.7 ± 1.7 |
| k3 × 103 (min−1) | ≤0.06 | 5.6 ± 0.6 | 3.9 ± 0.7 | 0.61 ± 0.27 |
NA, not applicable (constants k1 and k2 could not be determined since fluorescence kinetics were monophasic).
Important variations in the velocity of the acylation reaction were also detected between carbapenems. The dynamic range of k2/Kapp ratios reached 460 for inactivation of LdtMt4 by imipenem and meropenem. Based on the k2/Kapp ratios (Table 3), the overall efficacies of the four carbapenems could be ranked as imipenem > ertapenem > meropenem = doripenem.
In vitro cross-linking of purified peptidoglycan fragments.
Substrates of the l,d-transpeptidases were prepared by isolation of M. abscessus sacculi, treatment with muramidases, and purification of the resulting disaccharide peptide fragments by reverse-phase high-pressure liquid chromatography (rpHPLC). Incubation of LdtMt1, LdtMt2, LdtMt4, and LdtMt5 with a disaccharide tetrapeptide resulted in formation of peptidoglycan dimers containing a 3→3 cross-link (Fig. 1 and Table 4). Formation of disaccharide tripeptide by cleavage of the meso-DAPNH23–d-Ala4 peptide bond was not detected, indicating that the four l,d-transpeptidases did not display l,d-carboxypeptidase activity (data not shown). The four enzymes are specific for a donor containing a stem tetrapeptide, since formation of dimers was not observed with disaccharide pentapeptide GlcNAc–MurNGlyc–l-Ala–d-iGln–mesoDAPNH2–d-Ala–d-Ala (data not shown). Incubation of the four enzymes with disaccharide tetrapeptide and d-methionine resulted in the exchange of d-Ala4 by d-Met (Fig. 1 and Table 4). Formation of peptidoglycan dimers and exchange of d-Ala4 by d-Met were not observed with LdtMt3.
Table 4.
Observed monoisotopic masses of l,d-transpeptidase reaction productsa
| l,d-Transpeptidase | Calculated mass (Da) of reaction product |
|
|---|---|---|
| Peptidoglycan dimer (1,817.66 Da) | Exchange of d-Ala by d-Met (1,013.35 Da) | |
| Mt1 | 1,817.81 | 1,013.44 |
| Mt2 | 1,817.81 | 1,013.43 |
| Mt3 | NDb | ND |
| Mt4 | 1,817.81 | 1,013.43 |
| Mt5 | 1,817.79 | 1,013.43 |
l,d-Transpeptidases were incubated with GlcNAc–MurNGlyc–l-Ala–d-iGln–mesoDAPNH2–d-Ala alone (peptidoglycan dimer) or with d-Met (exchange of d-Ala by d-Met).
ND, not detected.
DISCUSSION
The increasing incidence of MDR and XDR tuberculosis indicates that there is an urgent need for new anti-TB drugs (24). In this context, LDTs are attractive targets, since the M. tuberculosis peptidoglycan contains a majority of 3→3 cross-links formed by these enzymes (10, 17). To investigate peptidoglycan inhibition by β-lactams, we have characterized five M. tuberculosis l,d-transpeptidase paralogues with respect to peptidoglycan dimer formation and acylation by carbapenems. We have obtained functional forms of the five l,d-transpeptidases, since each enzyme was active in the cross-linking assay (Mt5), the β-lactam acylation assay (Mt3), or both (Mt1, Mt2, and Mt4).
LdtMt3 was not active in the peptidoglycan cross-linking assay. In E. coli, two l,d-transpeptidases catalyze peptidoglycan cross-linking, whereas three additional paralogues anchor the Braun lipoprotein to peptidoglycan (25, 26). This observation suggests that LdtMt3 may perform as-yet-unknown cross-linking reactions in M. tuberculosis.
LdtMt5 was not inactivated by carbapenems but was active in the peptidoglycan cross-linking assay. The antibacterial activity of carbapenems suggests that LdtMt5 cannot compensate for the activity of the other l,d-transpeptidases. In Streptococcus pneumoniae and E. coli, it has been established that multiple d,d-transpeptidases are essential for peptidoglycan polymerization (9).
Carbapenems also inactivate d,d-carboxypeptidase DacB and prevent formation of tetrapeptide stems in the M. tuberculosis peptidoglycan (17). Although d,d-carboxypeptidases are considered unessential in E. coli (27), these enzymes may be required for peptidoglycan polymerization in M. tuberculosis, since they generate the tetrapeptide donor stem for formation of 3→3 cross-links by LDTs (17). In agreement, we have shown here that the four LDTs that are active in the cross-linking assay are specific for donor substrates containing a stem tetrapeptide. Ldtfs from Enterococcus faecalis is the only known l,d-transpeptidase that uses both tetrapeptide and pentapeptide stems as acyl donors (22). In Enterococcus faecium, stem tetrapeptides are produced in the cytoplasm by metallo-d,d-carboxypeptidase DdcY, which cleaves the C-terminal d-Ala residue of UDP-MurNAc pentapeptide (14). The genome of M. tuberculosis does not code for a DdcY homologue, indicating that tetrapeptide stems are produced only by d,d-carboxypeptidases belonging to the PBP family in this species.
We have previously shown that ertapenem and imipenem are the most efficient β-lactams for in vitro inactivation of LdtMt1, with high rate constants for formation of the oxyanion (k1) and acylation (k2) (19). The meropenem side chain moderately impaired drug binding (lower k1), whereas that of doripenem had an additional unfavorable impact on the acylation step (lower k2) (19). The analyses of LdtMt2, LdtMt3, and LdtMt4 reported in the present study revealed greater differences between carbapenems. For these enzymes, inactivation was more rapid with imipenem than with ertapenem, whereas acylation by meropenem and doripenem was very slow. These results indicate that modification of the side chains of carbapenems could be used to optimize the antibacterial activity of these drugs. Detection of multiple LDTs with highly diverse inactivation kinetics also suggests that combination therapy including two β-lactams may lead to synergism.
ACKNOWLEDGMENTS
This work was supported by the seventh Framework Program of the European Community, Project Open Collaborative Model for Tuberculosis Lead Optimization (ORCHID, 261378). M.C. was the recipient of a scholarship from Paris Descartes University, V.D. was supported by a Poste d'Accueil, Institut National de la Santé et de la Recherche Médicale, and S.T. was supported by ORCHID.
We thank D. Blanot for the determination of muropeptide concentrations by amino acid analysis.
Footnotes
Published ahead of print 16 September 2013
REFERENCES
- 1. Anonymous 2012. Tuberculosis 2011. WHO report. WHO, Geneva, Switzerland [Google Scholar]
- 2. Dick T. 2001. Dormant tubercle bacilli: the key to more effective TB chemotherapy? J. Antimicrob. Chemother. 47:117–118 [DOI] [PubMed] [Google Scholar]
- 3. Wayne LG, Sohaskey CD. 2001. Nonreplicating persistence of Mycobacterium tuberculosis. Annu. Rev. Microbiol. 55:139–163 [DOI] [PubMed] [Google Scholar]
- 4. Kliiman K, Altraja A. 2009. Predictors of extensively drug-resistant pulmonary tuberculosis. Ann. Intern. Med. 150:766–775 [DOI] [PubMed] [Google Scholar]
- 5. Wang F, Cassidy C, Sacchettini JC. 2006. Crystal structure and activity studies of the Mycobacterium tuberculosis beta-lactamase reveal its critical role in resistance to beta-lactam antibiotics. Antimicrob. Agents Chemother. 50:2762–2771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hugonnet JE, Blanchard JS. 2007. Irreversible inhibition of the Mycobacterium tuberculosis beta-lactamase by clavulanate. Biochemistry 46:11998–12004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hugonnet JE, Tremblay LW, Boshoff HI, Barry CE 3rd Blanchard JS 2009. Meropenem-clavulanate is effective against extensively drug-resistant Mycobacterium tuberculosis. Science 323:1215–1218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sauvage E, Kerff F, Terrak M, Ayala JA, Charlier P. 2008. The penicillin-binding proteins: structure and role in peptidoglycan biosynthesis. FEMS Microbiol. Rev. 32:234–258 [DOI] [PubMed] [Google Scholar]
- 9. Zapun A, Contreras-Martel C, Vernet T. 2008. Penicillin-binding proteins and beta-lactam resistance. FEMS Microbiol. Rev. 32:361–385 [DOI] [PubMed] [Google Scholar]
- 10. Lavollay M, Arthur M, Fourgeaud M, Dubost L, Marie A, Veziris N, Blanot D, Gutmann L, Mainardi JL. 2008. The peptidoglycan of stationary-phase Mycobacterium tuberculosis predominantly contains cross-links generated by l,d-transpeptidation. J. Bacteriol. 190:4360–4366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Biarrotte-Sorin S, Hugonnet JE, Delfosse V, Mainardi JL, Gutmann L, Arthur M, Mayer C. 2006. Crystal structure of a novel beta-lactam-insensitive peptidoglycan transpeptidase. J. Mol. Biol. 359:533–538 [DOI] [PubMed] [Google Scholar]
- 12. Mainardi JL, Fourgeaud M, Hugonnet JE, Dubost L, Brouard JP, Ouazzani J, Rice LB, Gutmann L, Arthur M. 2005. A novel peptidoglycan cross-linking enzyme for a beta-lactam-resistant transpeptidation pathway. J. Biol. Chem. 280:38146–38152 [DOI] [PubMed] [Google Scholar]
- 13. Mainardi JL, Morel V, Fourgeaud M, Cremniter J, Blanot D, Legrand R, Fréhel C, Arthur M, van Heijenoort J, Gutmann L. 2002. Balance between two transpeptidation mechanisms determines the expression of beta-lactam resistance in Enterococcus faecium. J. Biol. Chem. 277:35801–35807 [DOI] [PubMed] [Google Scholar]
- 14. Sacco E, Hugonnet JE, Josseaume N, Cremniter J, Dubost L, Marie A, Patin D, Blanot D, Rice LB, Mainardi JL, Arthur M. 2010. Activation of the l,d-transpeptidation peptidoglycan cross-linking pathway by a metallo-d,d-carboxypeptidase in Enterococcus faecium. Mol. Microbiol. 75:874–885 [DOI] [PubMed] [Google Scholar]
- 15. Tipper DJ, Strominger JL. 1965. Mechanism of action of penicillins: a proposal based on their structural similarity to acyl-d-alanyl-d-alanine. Proc. Natl. Acad. Sci. U. S. A. 54:1133–1141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mainardi JL, Hugonnet JE, Rusconi F, Fourgeaud M, Dubost L, Nguekam Moumi A, Delfosse V, Mayer C, Gutmann L, Rice LB, Arthur M. 2007. Unexpected inhibition of peptidoglycan l,d-transpeptidase from Enterococcus faecium by the beta-lactam imipenem. J. Biol. Chem. 282:30414–30422 [DOI] [PubMed] [Google Scholar]
- 17. Kumar P, Arora K, Lloyd JR, Lee IY, Nair V, Fischer E, Boshoff HI, Barry CE., 3rd 2012. Meropenem inhibits d,d-carboxypeptidase activity in Mycobacterium tuberculosis. Mol. Microbiol. 86:367–381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gupta R, Lavollay M, Mainardi JL, Arthur M, Bishai WR, Lamichhane G. 2010. The Mycobacterium tuberculosis protein LdtMt2 is a nonclassical transpeptidase required for virulence and resistance to amoxicillin. Nat. Med. 16:466–469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Dubée V, Triboulet S, Mainardi J, Ethève-Quelquejeu M, Marie A, Dubost L, Hugonnet JE, Arthur M. 2012. Inactivation of Mycobacterium tuberculosis l,d-transpeptidase LdtMt1 by carbapenems and cephalosporins. Antimicrob. Agents Chemother. 56:4189–4195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Triboulet S, Arthur M, Mainardi JL, Veckerlé C, Dubée V, Nguekam Moumi A, Gutmann L, Rice LB, Hugonnet JE. 2011. Inactivation kinetics of a new target of beta-lactam antibiotics. J. Biol. Chem. 286:22777–22784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Auger G, van Heijenoort J, Mengin-Lecreulx D, Blanot D. 2003. A MurG assay which utilises a synthetic analogue of lipid I. FEMS Microbiol. Lett. 219:115–119 [DOI] [PubMed] [Google Scholar]
- 22. Magnet S, Arbeloa A, Mainardi JL, Hugonnet JE, Fourgeaud M, Dubost L, Marie A, Delfosse V, Mayer C, Rice LB, Arthur M. 2007. Specificity of l,d-transpeptidases from gram-positive bacteria producing different peptidoglycan chemotypes. J. Biol. Chem. 282:13151–13159 [DOI] [PubMed] [Google Scholar]
- 23. Triboulet S, Dubée V, Lecoq L, Bougault C, Mainardi JL, Rice LB, Ethève-Quelquejeu M, Gutmann L, Marie A, Dubost L, Hugonnet JE, Simorre JP, Arthur M. 2013. Kinetic features of l,d-transpeptidase inactivation critical for β-lactam antibacterial activity. PLoS One 8:e67831. 10.1371/journal.pone.0067831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mainardi JL, Hugonnet JE, Gutmann L, Arthur M. 2011. Fighting resistant tuberculosis with old compounds: the carbapenem paradigm. Clin. Microbiol. Infect. 17:1755–1756 [DOI] [PubMed] [Google Scholar]
- 25. Magnet S, Bellais S, Dubost L, Fourgeaud M, Mainardi JL, Petit-Frère S, Marie A, Mengin-Lecreulx D, Arthur M, Gutmann L. 2007. Identification of the l,d-transpeptidases responsible for attachment of the Braun lipoprotein to Escherichia coli peptidoglycan. J. Bacteriol. 189:3927–3931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Magnet S, Dubost L, Marie A, Arthur M, Gutmann L. 2008. Identification of the l,d-transpeptidases for peptidoglycan cross-linking in Escherichia coli. J. Bacteriol. 190:4782–4785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Denome SA, Elf PK, Henderson TA, Nelson DE, Young KD. 1999. Escherichia coli mutants lacking all possible combinations of eight penicillin binding proteins: viability, characteristics, and implications for peptidoglycan synthesis. J. Bacteriol. 181:3981–3993 [DOI] [PMC free article] [PubMed] [Google Scholar]