Abstract
Invasive pulmonary aspergillosis (IPA) continues to rise in concert with increasing numbers of immune suppression techniques to treat other medical conditions and transplantation. Despite these advances, morbidity and mortality rates remain unacceptably high. One strategy used to optimize outcomes is antifungal pharmacodynamic (PD) examination. We explored the pharmacodynamics of a new triazole in development, isavuconazole, in a murine neutropenic IPA model. Ten A. fumigatus isolates were used, including four wild-type isolates and six cyp51 mutants. The MIC range was 0.125 to 8 mg/liter. Following infection, groups of mice were treated orally with the prodrug (BAL8557) at 40 to 640 mg/kg/12 h for 7 days. Efficacy was determined by quantitative PCR of lung homogenates. At the start of therapy, mice had 4.97 log10 conidial equivalents (CE)/ml of lung homogenate, and this increased to 6.82 log10 CE/ml of lung homogenate in untreated animals. The infection model was uniformly lethal in untreated control mice. The PD target endpoints examined included the static-dose AUC/MIC ratio and the 1-log10 killing AUC/MIC ratio. A stasis endpoint was achieved for all isolates with an MIC of ≤1 mg/liter and 1-log10 killing in all isolates with an MIC of ≤0.5 mg/liter, regardless of the presence or absence of the cyp51 mutation. The static-dose range was 65 to 617 mg/kg/12 h. The corresponding median free-drug AUC/MIC ratio was near 5. The 1-log10 killing dose range was 147 to 455 mg/kg/12 h, and the corresponding median free-drug AUC/MIC ratio was 11.1. These values are similar to those previously reported for other triazoles.
INTRODUCTION
Invasive pulmonary aspergillosis (IPA) is a common cause of illness and death in immunocompromised patients (1–7). The development of Aspergillus-active triazoles was a major step forward in therapy; however, morbidity and mortality rates remain unacceptably high. Additionally, the emergence of cyp51 mutant isolates with decreased susceptibility to triazoles is a threat to the efficacy of this drug class (8–13). Therefore, development of novel compounds and examination of the pharmacodynamic (PD) relationships of drug exposure, MIC, and outcome are necessary for the optimal use of these drugs.
PD studies integrate the pharmacokinetic (PK) properties, in vitro potency (MIC), and treatment efficacy of a drug. Common goals of these PD studies are to maximize clinical outcomes through dosing optimization and assist in susceptibility breakpoint determination. These investigations have been integral in the optimal use of antibiotics for bacterial infections and antifungal agents for mucosal and invasive candidiasis (14–18). However, only recently have PD investigations been used for filamentous fungal infections such as IPA (19–23).
Isavuconazonium sulfate (BAL8557) is the water-soluble prodrug of isavuconazole (BAL4815), a novel triazole compound with potent activity against numerous fungal pathogens, including Aspergillus species (24–28). After intravenous or oral administration, the prodrug is rapidly cleaved by plasma esterases to form the active drug isavuconazole (BAL4815) and the inactive cleavage product BAL8728 (25, 29, 30). The drug is currently in clinical development, including two phase III trials examining its efficacy for patients with IPA (http://clinicaltrials.gov, NCT00634049 and NCT00412893). The PD relationships of isavuconazole have been examined in experimental models of invasive candidiasis; however, for IPA, no PD evaluation has been done and targets are unknown. The objectives of the present study were to (i) examine the PD relationship of isavuconazole in a murine model of IPA and (ii) define the optimal isavuconazole exposure for infection due to both wild-type and cyp51 mutant isolates.
MATERIALS AND METHODS
Organisms.
Ten Aspergillus fumigatus isolates were chosen, including nine clinical isolates with and without cyp51 mutations and one laboratory isolate with an Fks1 mutation. Organisms were grown and subcultured on potato dextrose agar (PDA; Difco Laboratories, Detroit, MI). The organisms were chosen on the basis of similar fitness as determined by growth in lungs and mortality rates of untreated animals.
Drug.
Prodrug isavuconazonium sulfate (BAL8557) and isavuconazole (BAL4815) powders were provided by the sponsor (Astellas) for in vivo and in vitro studies, respectively. The prodrug was dissolved in sterile water and buffered to a pH of 4.0 prior to oral administration. Isavuconazole powder was dissolved in dimethyl sulfoxide in accordance with the sponsor's instructions prior to in vitro susceptibility testing. All of the dosages in used this study were administered by the oral route and based upon the oral prodrug dose. All concentration measurements (i.e., PK data and calculated pharmacokinetics) are based upon the active drug. A conversion factor is necessary to compare equivalent prodrug and active-drug amounts on a milligram-per-kilogram basis. This conversion factor was determined on the basis of a prodrug-to-drug equivalency rating of 1.863 (provided by the sponsor) and the 89% purity of the prodrug powder. Thus, the conversion factor for determining the equivalent isavuconazole dose from the prodrug dose was 0.48 (i.e., for every 1 mg/kg of prodrug administered orally, the equivalent in vivo isavuconazole dose would be 0.48 mg/kg). The purity of isavuconazole powder for in vitro susceptibility testing was >99%.
In vitro susceptibility testing.
All isolates were tested by broth microdilution in accordance with CLSI document M38-A2 (31). MICs were determined in duplicate three times. Median values are reported (see Table 1).
Table 1.
In vitro susceptibilities and in vivo fitness of selected A. fumigatus isolates
| A. fumigatus isolate | MIC (mg/liter) |
In vivo fitnessa | Comment | |
|---|---|---|---|---|
| Isavuconazole (BAL4815) | Posaconazole | |||
| AF41 | 0.25 | 0.25 | 1.85 | Wild type |
| AF293 | 1 | 0.5 | 2.20 | Wild type |
| DPL EC S 1 | 0.5 | 0.25 | 2.63 | Wild type |
| EMFR S678P | 0.25 | 0.25 | 2.42 | Fks1 S678P (echinocandin MIC, >16 mg/liter) |
| F11628 | 8 | 8 | 2.80 | cyp51 G138C |
| F14403 | 0.125 | 8 | 1.92 | cyp51 G54R |
| F16216 | 8 | 2 | 2.55 | cyp51 L98H + TR |
| AF72 | 2 | 2 | 2.32 | cyp51 G54E |
| F14532 | 1 | 1 | 2.52 | cyp51 M220T |
| F13737 | 4 | 2 | 2.45 | cyp51 G434C |
In vivo fitness is defined as the growth, measured in log10 CE/ml of lung homogenate, of the isolate in untreated animals until the time of death or sacrifice.
Animals.
Six-week-old Swiss/ICR specific-pathogen-free female mice weighing 23 to 27 g were used for all experiments (Harlan Sprague-Dawley, Indianapolis, IN). Animals were housed in groups of five and allowed access to food and water ad libitum. Animals were maintained in accordance with the American Association for Accreditation of Laboratory Care criteria (32). Moribund animals showing distress were sacrificed prior to the study endpoint (7 days) in accordance with the recommendations for the humane treatment of laboratory animals. The animal research committee of the William S. Middleton Memorial VA Hospital and the University of Wisconsin—Madison approved the animal studies.
Infection model.
Mice were rendered neutropenic (polymorphonuclear cell counts, <100/mm3) by the subcutaneous (s.c.) injection of 150 mg/kg cyclophosphamide on days −4 and −1. Prior studies have shown that this maintains neutropenia for 4 days; therefore, an additional injection (150 mg/kg) was administered on day +3 to ensure the maintenance of neutropenia throughout the 7-day experiment (20, 33, 34). Additionally, cortisone acetate was administered at 250 mg/kg s.c. on day −1 as previously described (20). Throughout the 7-day experiment, mice were also given ceftazidime at 50 mg/kg/day s.c. to prevent opportunistic bacterial infection. We have previously shown that 100% of the uninfected control animals given the above-described immune suppression and antibiotic prophylaxis survived to the study endpoint, whereas 100% of the infected controls that were left untreated died prior to the study endpoint (20).
Organisms were subcultured on PDA 5 days prior to infection and incubated at 37°C. On the day of infection, the inoculum was prepared by flooding the culture plate with 5 ml of normal saline and 0.05% Tween 20. Gentle agitation was used to release the conidia. The conidial suspension was collected and quantified by hemocytometer (Bright-Line, Hausser Scientific, Horsham, PA). The suspension was diluted to a final concentration of 1.2 × 107 conidia/ml. Viability was confirmed by plating the suspension and performing CFU counts.
An aspiration pneumonia model was used as previously described (20). Briefly, mice were anesthetized with a combination of ketamine and xylazine. Fifty microliters of the suspension of 1 × 107 to 2 × 107 conidia/ml was pipetted into the anterior nares with mice held upright to allow aspiration into the lungs. As previously shown and confirmed in the present study (data not shown), this results in invasive aspergillosis in over 90% of the animals and 100% mortality in untreated infected mice prior to the study endpoint (20). Drug treatment commenced 2 h after the initiation of infection.
Lung processing and organism quantitation.
Lung processing and quantitation of the Aspergillus burdens in the lungs of mice were performed as previously described (35, 36). Briefly, at the time of sacrifice of moribund animals or at the end of therapy (7 days), lungs were aseptically harvested and placed in a 2-oz sterile polyethylene Whirl-Pak bag (Nasco, Fort Atkinson, WI) containing 2 ml of sterile 0.85% NaCl. The lungs were manually homogenized by using direct pressure (37) to produce a primary homogenate. One milliliter of this primary homogenate was then placed in a sterile bead-beating tube (Sarstedt, Newton, NC) with 700 μl of 425- to 600-μm acid-washed glass beads (Sigma-Aldrich, St. Louis, MO). The primary homogenate was bead beaten in a BioSpec BeadBeater (BioSpec, Bartlesville, OK) for 90 s at 4,200 rpm to yield a secondary homogenate. One hundred microliters of this secondary homogenate was mixed with 100 μl Buffer ATL (Qiagen, Valencia, CA) and 20 μl proteinase K (Qiagen, Valencia, CA) and incubated overnight at 56°C with gentle agitation. DNA was then isolated in accordance with the DNeasy Blood and Tissue protocol (Qiagen, Valencia, CA). A final DNA elution step was performed with 100 μl elution buffer (Qiagen, Valencia, CA). The DNA was stored at −20°C until the day of PCR.
Quantitative PCR (qPCR) plates were prepared on the day of assay. Standard quantities of conidia were prepared by hemacytometer counting and used to generate standard curves. The results are reported as conidial equivalents (CE) per milliliter of lung homogenate. Samples were assayed in triplicate with a CFX96 real-time system (Bio-Rad, Hercules, CA). A single-copy gene, Fks1, was chosen for quantitation (38). The primer sequences used included forward primer 5′-GCCTGGTAGTGAAGCTGAGCGT-3′, reverse primer 5′-CGGTGAATGTAGGCATGTTGTCC-3′, and probe 5′–6-carboxyfluorescein–AGCCAGCGGCCCGCAAATG-MGB-3′ (Integrated DNA Technologies, Coralville, IA). The Fks1 mutation (EMFR S678P) was not located in the primer-probe area of the genome. All isolates have been previously studied in an experiment in which known quantities of conidia were used to spike lung homogenate to ensure that the primer-probe set performs similarly for all of the isolates over the dynamic range of interest (102 to 108) (20).
Pharmacokinetics.
The single-dose pharmacokinetics of isavuconazole (BAL4815) were determined in individual ICR/Swiss mice following the administration of the prodrug (BAL8557) at 10, 40, 160, and 640 mg/kg in a 0.2-ml volume by oral-gastric (OG) gavage. Plasma was collected from groups of three isoflurane-anesthetized mice at each of seven time points (0.5, 1, 2, 4, 8, 12, and 24 h). The plasma was stored at −80°C until the day of the drug assay. Drug concentration measurements were performed by the sponsor by liquid chromatography-mass spectrometry as previously described.
A noncompartmental model was used in the PK analysis. PK parameters, including the elimination half-life and the drug concentration at time zero, were calculated via nonlinear least-squares techniques. The area under the concentration-time curve (AUC) was calculated by the trapezoidal rule. For treatment doses in which kinetics were not directly determined, PK parameters were estimated by linear interpolation for those doses between two measured doses and by linear extrapolation for doses above or below the highest and lowest measured doses. Protein binding (99%) was based on previous studies of mice by the sponsor (personal communication).
PD index and magnitude.
The AUC/MIC ratio was used as the PD index for exploration of exposure-response relationships based upon previous PK/PD investigations with triazoles (19–22). Both total- and free (non-protein-bound)-drug concentrations were considered. Neutropenic mice were infected as described above. Treatment consisted of 2-fold increases in the prodrug (BAL8557) concentration (range, 40 to 640 mg/kg) administered every 12 h by OG gavage for 7 days. The doses were selected to vary the effect from maximal to no efficacy and included exposures expected to be achieved with regimens under study in clinical trials. Controls were used for each isolate and included 0-h and untreated controls. Four mice were included in each treatment and control group.
Data analysis.
The qPCR data were modeled according to the Hill-type dose-response equation log10 D = log10 (E/Emax − E)/N + log10 ED50, where D is the drug dose, E is the growth over the study period as measured by qPCR and represented as CE per milliliter of lung homogenate in untreated control mice, Emax is the maximal drug effect, N is the slope of the dose-response curve, and ED50 is the dose needed to achieve a 50% maximal effect. The AUC/MIC ratios of the total- and free-drug concentrations were determined for each isolate. The coefficient of determination (R2) was used to estimate the percent variance in the change in log10 CE per milliliter of lung homogenate over the treatment period for the different dosing regimens that could be attributed to the PD index (AUC/MIC ratio). The dose necessary for net stasis (static dose) and 1-log killing were determined when these endpoints were achieved. Additionally, the PD target total- and free-drug AUC/MIC ratios associated with these endpoints were calculated. The static dose and PD target total- and free-drug AUC/MIC ratios for wild-type and cyp51 mutant isolates were compared by t test for normally distributed data and by Mann-Whitney rank sum test for nonnormally distributed data.
RESULTS
Organism susceptibility and in vivo fitness.
The isavuconazole (BAL4815) susceptibility, genotype, and relative fitness in the in vivo murine model of each isolate are shown in Table 1. The MICs ranged from 0.25 to 1 mg/liter for wild-type isolates and from 0.125 to 8 mg/liter for cyp51 mutants. The organisms exhibited similar in vivo fitness levels on the basis of burden increases in untreated animals until the time of death or sacrifice. At the start of therapy, mice had 4.97 ± 0.33 log10 CE/ml of lung homogenate and the burden increased to 6.82 ± 0.51 log10 CE/ml of lung homogenate in untreated animals. Each isolate produced 100% mortality prior to the study endpoint in untreated animals, with death occurring between days 3 and 6 in all untreated animals.
Pharmacokinetics.
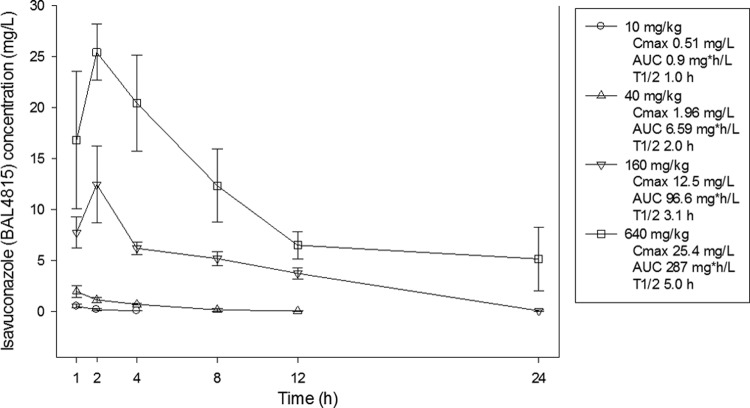
The time courses of isavuconazole (BAL4815) in the plasma of mice following OG doses of 640, 160, 40, and 10 mg/kg of the prodrug (BAL8557) are shown in Fig. 1. Peak levels were achieved within 2 h with each dosing regimen and ranged from 0.51 to 25.4 mg/liter. The elimination half-life in serum increased in a dose-dependent fashion from 1 to 5 h. The AUC from 0 h to infinity (AUC0-∞), as determined by the trapezoidal rule, ranged from 0.9 to 287 mg · h/liter. The AUC was relatively linear over the dose range (R2, 0.98).
Fig 1.
Plasma isavuconazole (BAL4815) concentrations after oral prodrug (BAL8557) administration at 640, 160, 40, and 10 mg/kg. Each symbol represents the geometric mean ± the standard deviation from three mice. The peak concentration (Cmax), 24-h AUC0-∞ (AUC), and elimination half-life (T1/2) are shown for each dose.
Dose-response curves.
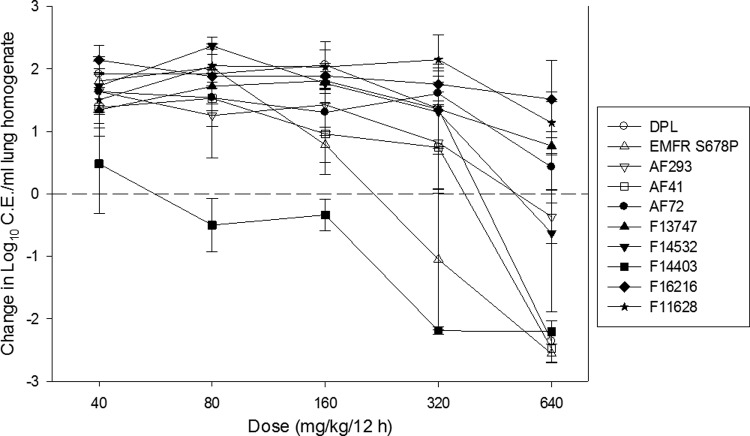
A dose-response relationship was observed for each isolate, with higher doses of isavuconazole achieving a larger microbiologic effect (Fig. 2). However, higher doses were necessary to achieve similar microbiologic effects against isolates with elevated isavuconazole MICs. A net static outcome and fungicidal activity were achieved for all wild-type isolates, as well as two of six cyp51 mutants. The maximal effect against wild-type isolates was an approximately 2-log10 reduction in the organism burden compared to that in the lungs at the start of therapy and a nearly 4-log10 reduction compared to untreated controls at the end of therapy.
Fig 2.
In vivo isavuconazole dose-response curves for multiple A. fumigatus isolates. The prodrug (BAL8557) was administered in 2-fold increasing concentrations of 40 to 640 mg/kg by the OG route every 12 h for 7 days. Open symbols represent wild-type isolates, and closed symbols represent cyp51 mutants. Each symbol represents the geometric mean organism burden ± the standard deviation as measured by qPCR.
PD index and target magnitude.
The doses needed to produce net growth suppression (i.e., the static dose) and to produce 1-log10 killing are shown in Table 2. Net stasis was achieved in all isolates that exhibited an MIC of ≤1 mg/liter, whereas 1-log10 killing was observed in all isolates with an MIC of ≤0.5 mg/liter. Therefore, we were able to estimate the static-dose AUC/MIC ratio target for all four wild-type isolates and two of six cyp51 mutants, with the latter two (F14403 and F14532) exhibiting lower MICs than the other four mutant isolates. The static doses for wild-type isolates ranged from 212 to 617 mg/kg/12 h. In comparison, the static doses for the two mutant isolates, F14403 and F14532, were 65 and 515 mg/kg/12 h, respectively. The 1-log10 killing PD target was achieved with three of four wild-type isolates and only a single mutant isolate. The 1-log10 killing doses for wild-type isolates ranged from 302 to 455 mg/kg/12 h, whereas the 1-log10 killing dose was 147 mg/kg/12 h for the single mutant isolate (F14403) with which this endpoint was achieved.
Table 2.
In vivo PD efficacy of isavuconazole in an immunocompromised murine pulmonary aspergillosis modela
| Isolate or parameter | MIC (mg/liter) | Static dose (mg/kg/12 h) | Static-dose 24-h AUC (mg · h/liter | Static-dose 24-h AUC/MIC ratio | Static-dose 24-h free-drug AUC/MIC ratio | 1-Log killing dose (mg/kg/12 h) | 1-Log killing 24-h AUC (mg · h/liter) | 1-Log killing 24-h AUC/MIC ratio | 1-Log killing 24-h free-drug AUC/MIC ratio |
|---|---|---|---|---|---|---|---|---|---|
| AF41 | 0.25 | 360 | 278 | 1,111 | 11.1 | 421 | 311 | 1,242 | 12.4 |
| AF293 | 1 | 617 | 415 | 415 | 4.15 | ||||
| DPL EC S 1 | 0.5 | 393 | 295 | 591 | 5.91 | 455 | 328 | 657 | 6.57 |
| EMFR S678P | 0.25 | 212 | 199 | 794 | 7.94 | 302 | 247 | 988 | 9.88 |
| F11628 | 8 | ||||||||
| F14403 | 0.125 | 65.0 | 45.9 | 367 | 3.67 | 147 | 154 | 1,235 | 12.4 |
| F16216 | 8 | ||||||||
| AF72 | 2 | ||||||||
| F14532 | 1 | 515 | 361 | 361 | 3.61 | ||||
| F13747 | 4 | ||||||||
| Median | 376 | 287 | 503 | 5.03 | 362 | 279 | 1,111 | 11.1 | |
| SD | 200 | 131 | 298 | 2.98 | 139 | 78.7 | 276 | 2.76 |
Blank cells represent strains for which the endpoint was not achieved.
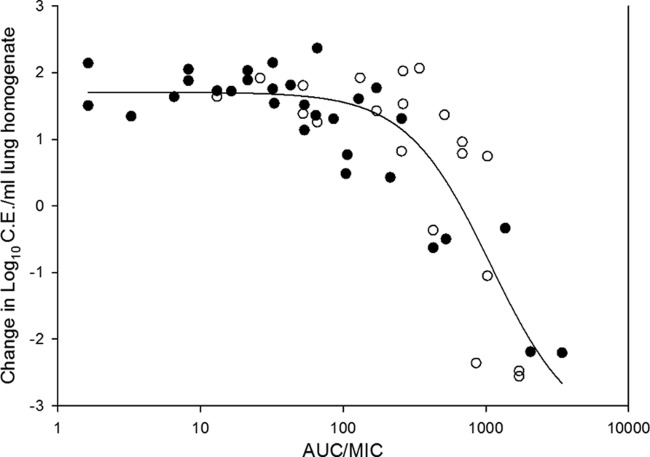
Total- and free-drug AUC/MIC ratio PD targets are shown in Table 2. The free-drug AUC/MIC ratios associated with net stasis in the wild-type group ranged from 4.15 to 11.1, whereas this endpoint was slightly lower for the two mutant isolates at 3.61 to 3.67. The difference between the two groups was not statistically significant (P = 0.18). For all of the isolates where net stasis was achieved, the median static-dose free-drug AUC/MIC ratio was 5.0. The 1-log10 killing free-drug AUC/MIC ratio was roughly 2-fold higher than the static-dose PD target, with a median value of 11.1. The AUC/MIC ratios and treatment outcome for all organisms were fitted to the Hill sigmoid dose-response model, and the relationship is shown in Fig. 3. The AUC/MIC ratio was a strong predictor of the observed outcome, with an R2 value of 0.75.
Fig 3.
Relationship between the PD index total-drug AUC/MIC ratio and the treatment efficacy of isavuconazole against 10 A. fumigatus isolates. Open symbols represent wild-type isolates, and closed symbols represent cyp51 mutants. Each data point represents the geometric mean organism burden of four mice. A best-fit line based on the Hill equation is included.
DISCUSSION
Aspergillus-active triazoles have become the cornerstone of the prevention and treatment of invasive aspergillosis (39). Preclinical animal model PK/PD investigation has proven useful for optimizing therapy for numerous pathogens but has been underused for filamentous fungal pathogens such as Aspergillus. These PD studies are important in the development of novel triazoles, such as isavuconazole, to provide a framework for predicting drug exposures that are expected to achieve a successful therapeutic outcome. Additionally, with the emergence of Aspergillus resistance to the triazole drug class, mediated by mutations in the cyp51 gene, these studies are integral in the examination of susceptibility breakpoints.
PK/PD studies of isavuconazole have been limited to two previous murine disseminated-candidiasis models (40; A. J. Lepak, K. Marchillo, J. VanHecker, and D. R. Andes, submitted for publication). Both showed a very strong relationship between the PD index AUC/MIC ratio and treatment outcome. Results from the present study also demonstrated a strong relationship between the total dose and effect in a murine IPA model. While this is the first PK/PD examination of the triazole isavuconazole for Aspergillus, previous studies using these models with the triazoles voriconazole and posaconazole provide the opportunity to compare results across the class. Employing the same model and diverse group of isolates as in the present study, we have previously shown that a posaconazole free-drug AUC/MIC ratio of approximately 1 was associated with net stasis (20). Similar studies by other investigators using pulmonary and intravascular models found that a posaconazole free-drug AUC/MIC ratio of 1.67 was associated with the ED50, and a value of 3.2 was associated with a 50% increase in survival, respectively (19, 21). A previous study with voriconazole in a murine pulmonary aspergillosis model observed a 50% maximal effect with a free-drug AUC/MIC ratio of 11 (22). The static-dose PD target free-drug AUC/MIC ratio identified in this study for isavuconazole at a median value of 5.0 is congruent with these other in vivo PD triazole studies. For comparison, the ED50, which is shown in Fig. 3, was equivalent to a free-drug AUC/MIC ratio of approximately 7.
In model used in the present study, we used qPCR, which has previously been shown to provide a large dynamic range between effective and ineffective therapy and reproducibility among biological replicates and correlates very well with mortality rates (20). The static dose and 1-log10 dose were the primary PD target endpoints used in this study. It is unclear which PD endpoint in the animal model correlates with the optimal treatment effect in patients. Further clinical PK/PD studies using large sets of patient data, organism susceptibility, and treatment outcomes to delineate the optimal clinical PK/PD target are urgently needed.
In summary, we have shown that the isavuconazole PD index AUC/MIC ratio correlates well with treatment outcome in a murine model of IPA. The MIC was a strong predictor of success or failure regardless of the presence or absence of a cyp51 mutation. Mutations that lead to elevated MICs of other triazoles did not universally correlate with elevated isavuconazole MICs. The median total- and free-drug 24-h AUC/MIC ratio PD targets for net stasis were 503 and 5, respectively. These targets correlate well with other triazole studies using this model. Further clinical study with isavuconazole for IPA is warranted, and it may be a useful addition to the triazole armamentarium for invasive aspergillosis.
ACKNOWLEDGMENTS
Astellas provided funding for the studies described here.
We kindly thank David Perlin for providing isolates DPL EC S 1 and EMFR S678P.
Footnotes
Published ahead of print 7 October 2013
REFERENCES
- 1. Baddley JW. 2011. Clinical risk factors for invasive aspergillosis. Med. Mycol. 49(Suppl. 1):S7–S12 [DOI] [PubMed] [Google Scholar]
- 2. Kontoyiannis DP, Marr KA, Park BJ, Alexander BD, Anaissie EJ, Walsh TJ, Ito J, Andes DR, Baddley JW, Brown JM, Brumble LM, Freifeld AG, Hadley S, Herwaldt LA, Kauffman CA, Knapp K, Lyon GM, Morrison VA, Papanicolaou G, Patterson TF, Perl TM, Schuster MG, Walker R, Wannemuehler KA, Wingard JR, Chiller TM, Pappas PG. 2010. Prospective surveillance for invasive fungal infections in hematopoietic stem cell transplant recipients, 2001-2006: overview of the Transplant-Associated Infection Surveillance Network (TRANSNET) Database. Clin. Infect. Dis. 50:1091–1100 [DOI] [PubMed] [Google Scholar]
- 3. Pappas PG, Alexander BD, Andes DR, Hadley S, Kauffman CA, Freifeld A, Anaissie EJ, Brumble LM, Herwaldt L, Ito J, Kontoyiannis DP, Lyon GM, Marr KA, Morrison VA, Park BJ, Patterson TF, Perl TM, Oster RA, Schuster MG, Walker R, Walsh TJ, Wannemuehler KA, Chiller TM. 2010. Invasive fungal infections among organ transplant recipients: results of the Transplant-Associated Infection Surveillance Network (TRANSNET). Clin. Infect. Dis. 50:1101–1111 [DOI] [PubMed] [Google Scholar]
- 4. Sherif R, Segal BH. 2010. Pulmonary aspergillosis: clinical presentation, diagnostic tests, management and complications. Curr. Opin. Pulm. Med. 16:242–250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Thompson GR, III, Patterson TF. 2011. Pulmonary aspergillosis: recent advances. Semin. Respir. Crit. Care Med. 32:673–681 [DOI] [PubMed] [Google Scholar]
- 6. Upton A, Kirby KA, Carpenter P, Boeckh M, Marr KA. 2007. Invasive aspergillosis following hematopoietic cell transplantation: outcomes and prognostic factors associated with mortality. Clin. Infect. Dis. 44:531–540 [DOI] [PubMed] [Google Scholar]
- 7. Baddley JW, Andes DR, Marr KA, Kontoyiannis DP, Alexander BD, Kauffman CA, Oster RA, Anaissie EJ, Walsh TJ, Schuster MG, Wingard JR, Patterson TF, Ito JI, Williams OD, Chiller T, Pappas PG. 2010. Factors associated with mortality in transplant patients with invasive aspergillosis. Clin. Infect. Dis. 50:1559–1567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chandrasekar PH. 2005. Antifungal resistance in Aspergillus. Med. Mycol. 43(Suppl. 1):S295–S298 [DOI] [PubMed] [Google Scholar]
- 9. Howard SJ, Arendrup MC. 2011. Acquired antifungal drug resistance in Aspergillus fumigatus: epidemiology and detection. Med. Mycol. 49(Suppl. 1):S90–95 [DOI] [PubMed] [Google Scholar]
- 10. Howard SJ, Cerar D, Anderson MJ, Albarrag A, Fisher MC, Pasqualotto AC, Laverdiere M, Arendrup MC, Perlin DS, Denning DW. 2009. Frequency and evolution of azole resistance in Aspergillus fumigatus associated with treatment failure. Emerg. Infect. Dis. 15:1068–1076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Snelders E, van der Lee HA, Kuijpers J, Rijs AJ, Varga J, Samson RA, Mellado E, Donders AR, Melchers WJ, Verweij PE. 2008. Emergence of azole resistance in Aspergillus fumigatus and spread of a single resistance mechanism. PLoS Med. 5:e219. 10.1371/journal.pmed.0050219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. van der Linden JW, Snelders E, Kampinga GA, Rijnders BJ, Mattsson E, Debets-Ossenkopp YJ, Kuijper EJ, Van Tiel FH, Melchers WJ, Verweij PE. 2011. Clinical implications of azole resistance in Aspergillus fumigatus, The Netherlands, 2007-2009. Emerg. Infect. Dis. 17:1846–1854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Verweij PE, Mellado E, Melchers WJ. 2007. Multiple-triazole-resistant aspergillosis. N. Engl. J. Med. 356:1481–1483 [DOI] [PubMed] [Google Scholar]
- 14. Ambrose PG, Bhavnani SM, Rubino CM, Louie A, Gumbo T, Forrest A, Drusano GL. 2007. Pharmacokinetics-pharmacodynamics of antimicrobial therapy: it's not just for mice anymore. Clin. Infect. Dis. 44:79–86 [DOI] [PubMed] [Google Scholar]
- 15. Andes D. 2006. Pharmacokinetics and pharmacodynamics of antifungals. Infect. Dis. Clin. North Am. 20:679–697 [DOI] [PubMed] [Google Scholar]
- 16. Craig WA. 1998. Pharmacokinetic/pharmacodynamic parameters: rationale for antibacterial dosing of mice and men. Clin. Infect. Dis. 26:1–10 [DOI] [PubMed] [Google Scholar]
- 17. Drusano GL. 2007. Pharmacokinetics and pharmacodynamics of antimicrobials. Clin. Infect. Dis. 45(Suppl. 1):S89–S95 [DOI] [PubMed] [Google Scholar]
- 18. Hope WW, Drusano GL. 2009. Antifungal pharmacokinetics and pharmacodynamics: bridging from the bench to bedside. Clin. Microbiol. Infect. 15:602–612 [DOI] [PubMed] [Google Scholar]
- 19. Howard SJ, Lestner JM, Sharp A, Gregson L, Goodwin J, Slater J, Majithiya JB, Warn PA, Hope WW. 2011. Pharmacokinetics and pharmacodynamics of posaconazole for invasive pulmonary aspergillosis: clinical implications for antifungal therapy. J. Infect. Dis. 203:1324–1332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lepak AJ, Marchillo K, Vanhecker J, Andes DR. 2013. Posaconazole pharmacodynamic target determination against wild-type and Cyp51 mutant isolates of Aspergillus fumigatus in an in vivo model of invasive pulmonary aspergillosis. Antimicrob. Agents Chemother. 57:579–585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mavridou E, Bruggemann RJ, Melchers WJ, Mouton JW, Verweij PE. 2010. Efficacy of posaconazole against three clinical Aspergillus fumigatus isolates with mutations in the cyp51A gene. Antimicrob. Agents Chemother. 54:860–865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mavridou E, Bruggemann RJ, Melchers WJ, Verweij PE, Mouton JW. 2010. Impact of cyp51A mutations on the pharmacokinetic and pharmacodynamic properties of voriconazole in a murine model of disseminated aspergillosis. Antimicrob. Agents Chemother. 54:4758–4764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Seyedmousavi S, Melchers WJ, Mouton JW, Verweij PE. 2013. Pharmacodynamics and dose-response relationships of liposomal amphotericin B against different azole-resistant Aspergillus fumigatus isolates in a murine model of disseminated aspergillosis. Antimicrob. Agents Chemother. 57:1866–1871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Guinea J, Pelaez T, Recio S, Torres-Narbona M, Bouza E. 2008. In vitro antifungal activities of isavuconazole (BAL4815), voriconazole, and fluconazole against 1,007 isolates of zygomycete, Candida, Aspergillus, Fusarium, and Scedosporium species. Antimicrob. Agents Chemother. 52:1396–1400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Odds FC. 2006. Drug evaluation: BAL-8557—a novel broad-spectrum triazole antifungal. Curr. Opin. Investig. Drugs 7:766–772 [PubMed] [Google Scholar]
- 26. Perkhofer S, Lechner V, Lass-Florl C. 2009. In vitro activity of isavuconazole against Aspergillus species and zygomycetes according to the methodology of the European Committee on Antimicrobial Susceptibility Testing. Antimicrob. Agents Chemother. 53:1645–1647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Warn PA, Sharp A, Denning DW. 2006. In vitro activity of a new triazole BAL4815, the active component of BAL8557 (the water-soluble prodrug), against Aspergillus spp. J. Antimicrob. Chemother. 57:135–138 [DOI] [PubMed] [Google Scholar]
- 28. Rudramurthy SM, Chakrabarti A, Geertsen E, Mouton JW, Meis JF. 2011. In vitro activity of isavuconazole against 208 Aspergillus flavus isolates in comparison with 7 other antifungal agents: assessment according to the methodology of the European Committee on Antimicrobial Susceptibility Testing. Diagn. Microbiol. Infect. Dis. 71:370–377 [DOI] [PubMed] [Google Scholar]
- 29. Schmitt-Hoffmann A, Roos B, Heep M, Schleimer M, Weidekamm E, Brown T, Roehrle M, Beglinger C. 2006. Single-ascending-dose pharmacokinetics and safety of the novel broad-spectrum antifungal triazole BAL4815 after intravenous infusions (50, 100, and 200 milligrams) and oral administrations (100, 200, and 400 milligrams) of its prodrug, BAL8557, in healthy volunteers. Antimicrob. Agents Chemother. 50:279–285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Schmitt-Hoffmann A, Roos B, Maares J, Heep M, Spickerman J, Weidekamm E, Brown T, Roehrle M. 2006. Multiple-dose pharmacokinetics and safety of the new antifungal triazole BAL4815 after intravenous infusion and oral administration of its prodrug, BAL8557, in healthy volunteers. Antimicrob. Agents Chemother. 50:286–293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Clinical and Laboratory Standards Institute (CLSI) 2008. Reference method for broth dilution antifungal susceptibility testing of filamentous fungi; approved standard—second edition, CLSI document M38-A2. Clinical and Laboratory Standards Institute, Wayne, PA [Google Scholar]
- 32. National Research Council Committee on the Care and Use of Laboratory Animals Institute of Laboratory Animal Resources and Commission on Life Sciences 1996. Guide for the care and use of laboratory animals. National Academy Press, Washington, DC [Google Scholar]
- 33. Andes D, Craig WA. 1998. In vivo activities of amoxicillin and amoxicillin-clavulanate against Streptococcus pneumoniae: application to breakpoint determinations. Antimicrob. Agents Chemother. 42:2375–2379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lewis RE, Liao G, Hou J, Prince RA, Kontoyiannis DP. 2011. Comparative in vivo dose-dependent activity of caspofungin and anidulafungin against echinocandin-susceptible and -resistant Aspergillus fumigatus. J. Antimicrob. Chemother. 66:1324–1331 [DOI] [PubMed] [Google Scholar]
- 35. Bowman JC, Abruzzo GK, Anderson JW, Flattery AM, Gill CJ, Pikounis VB, Schmatz DM, Liberator PA, Douglas CM. 2001. Quantitative PCR assay to measure Aspergillus fumigatus burden in a murine model of disseminated aspergillosis: demonstration of efficacy of caspofungin acetate. Antimicrob. Agents Chemother. 45:3474–3481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Vallor AC, Kirkpatrick WR, Najvar LK, Bocanegra R, Kinney MC, Fothergill AW, Herrera ML, Wickes BL, Graybill JR, Patterson TF. 2008. Assessment of Aspergillus fumigatus burden in pulmonary tissue of guinea pigs by quantitative PCR, galactomannan enzyme immunoassay, and quantitative culture. Antimicrob. Agents Chemother. 52:2593–2598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Walsh TJ, McEntee C, Dixon DM. 1987. Tissue homogenization with sterile reinforced polyethylene bags for quantitative culture of Candida albicans. J. Clin. Microbiol. 25:931–932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Herrera ML, Vallor AC, Gelfond JA, Patterson TF, Wickes BL. 2009. Strain-dependent variation in 18S ribosomal DNA copy numbers in Aspergillus fumigatus. J. Clin. Microbiol. 47:1325–1332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Walsh TJ, Anaissie EJ, Denning DW, Herbrecht R, Kontoyiannis DP, Marr KA, Morrison VA, Segal BH, Steinbach WJ, Stevens DA, van Burik JA, Wingard JR, Patterson TF. 2008. Treatment of aspergillosis: clinical practice guidelines of the Infectious Diseases Society of America. Clin. Infect. Dis. 46:327–360 [DOI] [PubMed] [Google Scholar]
- 40. Warn PA, Sharp A, Mosquera J, Spickermann J, Schmitt-Hoffmann A, Heep M, Denning DW. 2006. Comparative in vivo activity of BAL4815, the active component of the prodrug BAL8557, in a neutropenic murine model of disseminated Aspergillus flavus. J. Antimicrob. Chemother. 58:1198–1207 [DOI] [PubMed] [Google Scholar]