Abstract
We report the complete nucleotide sequence and analysis of pETBTY825, a Staphylococcus aureus TY825 plasmid encoding exfoliative toxin B (ETB). S. aureus TY825 is a clinical isolate obtained from an impetigo patient in 2002. The size of pETBTY825, 60.6 kbp, was unexpectedly larger than that of the archetype pETBTY4 (∼30 kbp). Genomic comparison of the plasmids shows that pETBTY825 has the archetype pETBTY4 as the backbone and has a single large extra DNA region of 22.4 kbp. The extra DNA region contains genes for resistance to aminoglycoside [aac(6′)/aph(2″)], macrolide (msrA), and penicillin (blaZ). A plasmid deletion experiment indicated that these three resistance elements were functionally active. We retrospectively examined the resistance profile of the clinical ETB-producing S. aureus strains isolated in 1977 to 2007 using a MIC determination with gentamicin (GM), arbekacin (ABK), and erythromycin (EM) and by PCR analyses for aac(6′)/aph(2″) and msrA using purified plasmid preparations. The ETB-producing S. aureus strains began to display high resistance to GM, which was parallel with the detection of aac(6′)/aph(2″) and mecA, after 1990. Conversely, there was no significant change in the ABK MIC during the testing period, although it had a tendency to slightly increase. After 2001, isolates resistant to EM significantly increased; however, msrA was hardly detected in ETB-producing S. aureus strains, and only five isolates were positive for both aac(6′)/aph(2″) and msrA. In this study, we report the emergence of a fusion plasmid carrying the toxin gene etb and drug resistance genes. Prevalence of the pETBTY825 carrier may further increase the clinical threat, since ETB-producing S. aureus is closely related to more severe impetigo or staphylococcal scalded-skin syndrome (SSSS), which requires a general antimicrobial treatment.
INTRODUCTION
Exfoliative toxin (ET) is an exotoxin produced by staphylococcal species, causing blisters on human and animal skin (1). ET-producing Staphylococcus aureus is involved in staphylococcal scalded-skin syndrome (SSSS) or Ritter disease and in bullous impetigo in neonates (1–3). Serologically, ETs causing diseases in human have been divided into three major serotypes: ETA, ETB, and ETD (4–6). All types cause intraepidermal cleavage in the granular layer, without epidermal necrolysis or inflammatory response in the skin (4, 5, 7). ETs are serine proteases that selectively cleave desmoglein 1, a desmosomal protein connecting epidermal cells present in the epidermis (8).
Virulence factors of staphylococci such as ET are accessory proteins, which are not essential for cell growth or division. Genetic determinants for these factors are often associated with mobile genetic elements, such as phages, plasmids, and pathogenicity islands (9–11). The eta gene is located on the genome of a temperate phage (ϕ ETA) (12), the etb gene is on a large plasmid (4, 13), and the etd gene is chromosomally located in a pathogenicity island (6).
We previously reported the complete nucleotide sequence of the ETB plasmid of strain S. aureus TY4, isolated from skin lesions of patients diagnosed with staphylococcal scalded-skin syndrome (SSSS) (13). The ETB plasmid (pETB) contains three copies of IS257, which divides the pETB genome into three regions: (i) a cadmium resistance operon-containing region, (ii) a lantibiotic gene-containing region, and (iii) the region where genes for plasmid replication and/or maintenance are dispersed. These genes include two virulence-related genes, the etb gene, and the ADP-ribosyltransferase ednC gene, which belongs to the C3 exoenzyme family. Further, we reported significant size variation of the ETB plasmid from various clinical strains.
During our genome project, we determined the nucleotide sequence of a new ETB plasmid from S. aureus strain TY825 from an impetigo patient. Comparative analysis of pETBTY4 and pETBTY825 showed that pETBTY825 carries three antibiotic resistance genes. Here we report a novel ETB plasmid contributing to the multidrug resistance of S. aureus. Additionally, we investigated the relevance of the pETBTY825 type and antimicrobial susceptibilities of ETB-producing S. aureus strains isolated between 1977 and 2007 in Japan.
MATERIALS AND METHODS
Bacterial strains.
S. aureus TY825 was isolated from the skin lesions of patients diagnosed with impetigo. Other S. aureus strains used in this study were from our laboratory collection of clinical isolates producing ETB.
Manipulation of DNA.
Routine DNA manipulations were performed using standard procedures (14). pETB was extracted from S. aureus TY825 and purified using a Qiagen midikit. The plasmid DNA was further purified by CsCl equilibration centrifugation, followed by isopropanol precipitation. Southern blotting of the DNA and hybridization were performed as described previously (15).
Shotgun sequencing, assembly, and annotation of pETBTY825.
The genome sequence of pETB DNA was determined using the random shotgun sequencing method as described previously (12). Collected sequences were assembled using SEQUENCHER DNA sequencing software (v3.0; Gene Codes). Gaps were closed by direct sequencing of the PCR products amplified with oligonucleotide primers designed to anneal to each end of the neighboring contigs. Initially, potential protein-encoding regions (open reading frames [ORFs]) that were ≥150 bp long were identified using MetaGeneAnnotator (16) and the InSilico molecular cloning software package, genomics edition (InSilico Biology Inc., Yokohama, Japan), and each ORF was reviewed manually for the presence of a ribosomal binding sequence. Functional annotation was assigned based on homology searches against the GenBank nonredundant protein sequence database using the program BLASTP (17). Protein and nucleotide sequences were compared with those in the sequence databases using the BLAST and FASTA programs implemented at the DDBJ (DNA Data Bank of Japan; http://www.ddbj.nig.ac.jp/).
Antimicrobial susceptibility testing.
The MIC determination was performed using the microdilution broth method (14) with the MicroScanWalkAway-96 system. The antibiotics tested were benzylpenicillin (PCG), ampicillin (ABPC), cefazolin (CEZ), cefotiam (CTM), cefozopran (CZOP), cefpirome (CPR), cefdinir (CFDN), cefditoren (CDTR), flomoxef (FMOX), imipenem (IPM), meropenem (MEPM), gentamicin (GM), arbekacin (ABK), erythromycin (EM), clindamycin (CLDM), minocycline (MINO), levofloxacin (LVFX), vancomycin (VCM), teicoplanin (TEIC), sulfamethoxazole-trimethoprim (ST), fosfomycin (FOM), and linezolid (LZD). Separately, the microdilution method was used to assess endpoints for the ABK, GM, and EM MICs according to the CLSI guidelines (18).
PCR scanning analysis.
Plasmid DNAs were isolated from ETB-producing S. aureus clinical strains in our laboratory stock and were used as templates for PCR scanning analysis (36). All primers were designed according to the nucleotide sequence of pETB (Table 1).
Table 1.
Oligonucleotides used for PCR amplification
| Purpose and gene or region | Primer | Oligonucleotide sequence (5′-3′) | Product size (bp) | Primer design reference or source |
|---|---|---|---|---|
| PCR | ||||
| etb | ET-3 | ATACACACATTACGGATAAT | 629 | 13 |
| ET-4 | CAAAGTGTCTCCAAAAGT | |||
| aac(6′)/aph(2′) | aac/aph-F | TACAGAGCCTTGGGAAGATG | 406 | 32 |
| aac/aph-R | CATTTGTGGCATTATCATCATATC | |||
| msrA | msr-F | TGCAAATGGCATACTATCGTC | 160 | 32 |
| msr-R | CAAGAACGCTCAAGTGCTTC | |||
| PCR scanning | ||||
| Region 1 | region_1-F | CCTAAAATTGTTTGAATAGTATC | 3,949 | This study |
| region_1-R | GGATTGAACTTCTGATAATCATT | |||
| Region 2 | region_2-F | CTTGTGTCTTTTTATGTGGATTG | 4,054 | This study |
| region_2-R | GACAATCTATTCATGATATAACT | |||
| Region 3 | region_3-F | TTTATCAAGATAATCCCTTATCG | 3,164 | This study |
| region_3-R | CACTTTTAAAATATGAACTAGGA | |||
| Region 4 | region_4-F | TGTAAAGTATCTCTATTTTTAGC | 3,150 | This study |
| region_4-R | CATTTAGGGGTATCTTATATATT | |||
| Region 5 | region_5-F | CTTAGACCTTATTTAAAATATCC | 2,019 | This study |
| region_5-R | CATAATTTTTGATAAAGTCCGTA | |||
| Region 6 | region_6-F | AAATTTCTTTTCTACCATTTTCG | 4,922 | This study |
| region_6-R | GTTAAAGATTTATTCCAACTACA | |||
| Region 7 | region_7-F | ATTTAGATAGAAAAGAAAGAGCG | 5,012 | This study |
| region_7-R | GATAAGCTTAAAGTAACTTCTTT |
Nucleotide sequence accession number.
The nucleotide sequence described here has been deposited in GenBank under accession number AP012467.
RESULTS
General overview and comparative analysis of the ETB plasmid.
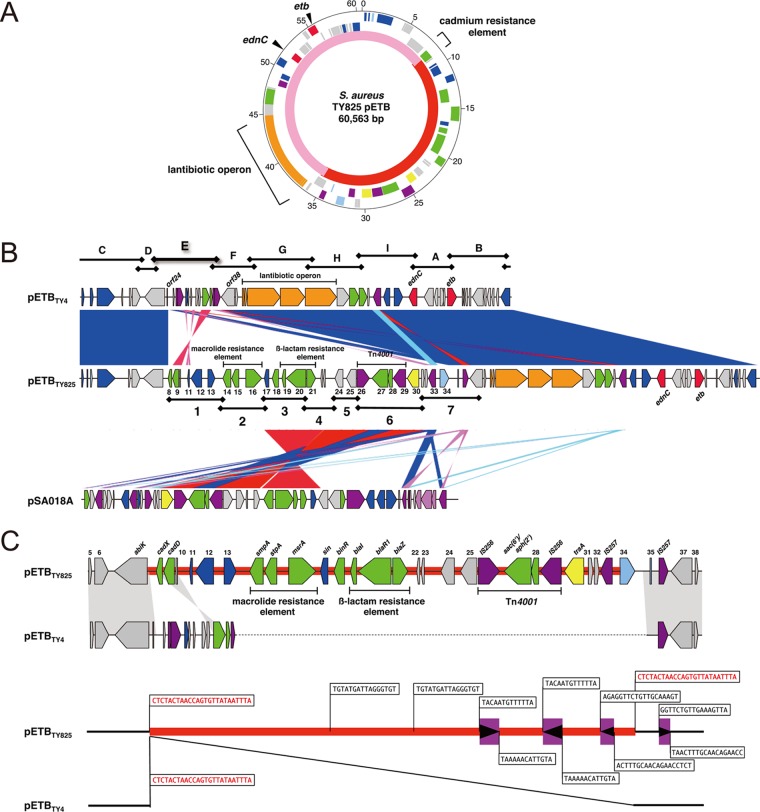
S. aureus TY825 was clinically isolated in 2002 from a lesion of an impetigo patient and is positive for the plasmid carrying etb (pETB). As a part of the genome project of clinically isolated S. aureus strains in Japan, the complete nucleotide sequence of pETBTY825 was determined using a shotgun approach. The fully assembled circular DNA sequence of pETBTY825 was 60,563 bp (Fig. 1A). The average GC content of pETBTY825 was 28.2%. We identified 63 potential protein-coding regions (Fig. 1A; Table 2). pETBTY825, which is 38,211 bp, is significantly larger than the archetype pETB (pETBTY4), which is ∼35 kb (13) (Fig. 1C). Comparison of pETBTY825 and pETBTY4 shows that pETBTY825 is a composite of pETBTY4 and a single large extra DNA region (22,352 bp) (Fig. 1; Table 2). Sequence alignment of both plasmids shows the extra DNA region was inserted between orf25 and orf37 in pETBTY4 (Fig. 1B). Examining the boundary nucleotide sequences of the extra DNA region, direct repeats of 25-bp sequences (5′-CTCTACTAACCAGTGTTATAATTTA-3′) were found (Fig. 1C). The genome organization of the backbone sequence of pETBTY825 corresponding to the pETBTY4 sequence was conserved (Fig. 1B). The genes etb and ednC, genetic elements for lantibiotic production, are present in the backbone sequence. Annotation of the extra DNA region identified a cadmium resistance element and three antibiotic resistance elements that confer resistance to aminoglycosides, macrolides, and β-lactams (Table 2; Fig. 1C) The aminoglycoside resistance gene, aac(6′)/aph(2″), encoding a bifunctional enzyme, is located between two IS256 elements, forming the 4.5-kb Tn4001, which is most frequently observed as the mobile element of aac(6′)/aph(2″) in Gram-positive bacteria (19, 20). AAC(6′)/APH(2″) primarily confers resistance to gentamicin, kanamycin, and tobramycin (21). The macrolide resistance element is composed of stpA, smpA, and msrA, whose products act as an ATP-dependent efflux pump conferring the so-called MS phenotype, i.e., inducible resistance to 14- and 15-membered ring macrolides and resistance to streptogramin type B (22, 23). The β-lactamase-dependent resistance element blaZ, two closely linked genes (blaI and blaR), and IS257 form Tn552. This transposon is frequently observed on a large plasmid as well as in the chromosome of staphylococci (24). However, the β-lactam resistance element of pETBTY825 and pSA018A lacks IS257 downstream of blaZ (Fig. 1A and B; Table 2). Identification of the sin recombinase gene immediately downstream of the element and the partial 12-bp resH sequence (5′-TGTATGATTAGG-3′) (25) on both sides of the element, a direct repeat, strongly suggests that the element was acquired as a block through Sin-dependent recombination. A cadmium resistance element is also present in pETBTY4 and was found at the extreme 5′ end of the extra DNA region with an inversion (Fig. 1B and C).
Fig 1.
(A) Circular genetic map of pETBTY825 from S. aureus TY825. From the outside in, the first circle shows the nucleotide sequence positions (in kb), the second and third circles show coding sequences transcribed clockwise and counterclockwise, respectively (red, pathogenic factor; green, antibiotic resistance gene; blue, DNA replication, recombination, and repair; light blue, transcription regulator; purple, transposase; yellow, conjugal transfer [tra]; orange, lantibiotic operon; and gray, conserved ORFs), and the fourth circle shows the backbone of pETBTY4 (pink) (GenBank accession no. AP003088) and the acquired region (red). (B) Structural comparison of pETBTY825 to pETBTY4 and the Staphylococcus plasmid pSA018A. Color shading indicates homologous regions. The approximately 16-kb extra DNA region of pETBTY825 was similarity matched with the Staphylococcus plasmid pSA018A (GenBank accession no. GQ900383). (C) IS elements are represented as purple boxes, and the directions of the transposase genes are indicated by arrowheads in the boxes. Sequences of the terminal inverted repeats of each IS elements are shown. Sequences of the terminal directed repeats of the acquired region (red) of pETBTY825 are shown.
Table 2.
Features of pETBTY825 ORFs
| ORF | Position (bp) |
Strand | Gene | Length (aa)a | Translation signalb | Source | Description | Identity (%) | Overlapc (aa) | Accession no. | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Start | Stop | ||||||||||
| 1 | 231 | 455 | + | repA | 74 | GAGGTTTTTATTATG | S. aureus(pETB) | pETB_p18 (replication initiator protein A) | 100 | 74/74 | BAB78416 |
| 2 | 642 | 785 | + | rep | 47 | GAGAATAATGATATG | S. aureus TCH130 | Hypothetical protein (truncated replication protein) | 60.6 | 33/47 | ZP_04868980 |
| 3 | 1002 | 1217 | + | 71 | AGGGCTATGTAAAGAATTG | S. aureus(pETB) | pETB_p19 (transcriptional regulator protein) | 100 | 71/71 | NP_478362 | |
| 4 | 1590 | 3164 | + | repR | 524 | AGGAGGTGCAGACAATG | S. aureus(pETB) | pETB_p20 (plasmid replication protein RepR) | 100 | 524/524 | NP_478363 |
| 5 | 4397 | 4564 | + | 55 | S. aureus(pETB) | pETB_p22 (lipase) | 100 | 55/55 | NP_478365 | ||
| 6 | 4647 | 5378 | + | 243 | GAGGTATTCTTAATAAAATG | S. aureus(pETB) | pETB_p23 (cell wall-associated biofilm protein) | 92.4 | 243/243 | NP_478366 | |
| 7 | 5738 | 7510 | − | abiK | 590 | AGGAGAAAGGCTATG | S. aureus(pETB) | Abortive infection protein K | 100 | 590/590 | NP_478367 |
| 8 | 7914 | 8261 | − | cadX | 115 | AGGGTGCGATTTTATATG | S. lugdunensis(pLUG) | CadX | 100 | 115/115 | NP_054018 |
| 9 | 8280 | 8897 | − | cadD | 205 | GAGGTGTAATTATG | S. aureus(pETB) | Cadmium-binding protein | 99.5 | 205/205 | NP_478377 |
| 10 | 8966 | 9109 | − | 48 | S. epidernidis BCM-HMP0060 | Hypothetical protein | 97.9 | 48/48 | ZP_04824204 | ||
| 11 | 9670 | 9858 | − | 62 | AGGATTATATCGAAAACGTATG | S. epidernidis BCM-HMP0060 | Replication protein Rep | 93.4 | 62/62 | ZP_04824202 | |
| 12 | 9989 | 10960 | − | 323 | AGAGGTTTTTGTATG | S. saprophyticus ATCC 15305 | Replication initiator protein | 99.4 | 323/323 | YP_302585 | |
| 13 | 11475 | 12167 | + | 230 | GGAGGCCATTATATG | S. epidernidis BCM-HMP0060 | Partitioning protein | 80.9 | 230/230 | ZP_04824200 | |
| 14 | 12788 | 13558 | − | smpA | 256 | AGGAGGATCAATCGTAAAATG | S. epidernidis 968 | ABC transporter membrane protein | 100 | 256/256 | CAA83062 |
| 15 | 13560 | 14255 | − | stpA | 231 | AGGAGATAATTGTATG | S. epidernidis W23144 | ABC transporter ATP-binding protein | 100 | 231/231 | ZP_04796098 |
| 16 | 14792 | 16258 | + | msrA | 488 | AGGAGTGTATAAATATG | S. epidernidis W23144 | ABC transpoter permease protein (erythromycin resistance protein, MsrA) | 100 | 488/488 | ZP_04796097 |
| 17 | 16482 | 16910 | − | Sin | 142 | GGAGATCGATTCGTTGTG | S. aureus USA300_TCH959 | Recombinase Sin | 97.9 | 142/142 | YP_001569089 |
| 18 | 17217 | 17795 | − | binR | 192 | AGGAGGTTTGTATTTTG | S. aureus CF-Marseille | Tn552 DNA invertase BinR | 98.9 | 192/192 | ZP_04839235 |
| 19 | 18059 | 18439 | − | blaI | 126 | S. epidernidis ATCC 12228 | Beta-lactamase repressor BlaI | 100 | 126/126 | NP_863211 | |
| 20 | 18429 | 20228 | − | blaR1 | 599 | S. aureus JKD6008 | Beta-lactamase regulatory protein BlaR1 | 100 | 585/599 | ZP_03563212 | |
| 21 | 20293 | 21138 | + | blaZ | 281 | GGAGGGTTTATTTTG | S. aureus MRSA252 | Beta-lactamase | 99.6 | 281/281 | NP_878023 |
| 22 | 21500 | 21685 | − | 61 | AGGTTATGAAAGTAAATGTATG | S. epidernidis RP62A | Conserved hypothetical protein | 95.1 | 61/61 | YP_189789 | |
| 23 | 21757 | 21960 | − | 67 | AGGGGGAGTATCTTTG | S. epidernidis RP62A | Conserved hypothetical protein | 95.7 | 47/67 | YP_189789 | |
| 24 | 22751 | 23482 | − | 243 | GGAGGTAAGTTTTG | S. epidernidis RP62A | Conserved hypothetical protein | 97.1 | 243/243 | YP_189787 | |
| 25 | 23788 | 24666 | − | 292 | AGGACTGTTATATG | S. epidernidis ATCC 12228 | Hypothetical protein | 93.2 | 281/292 | NP_863227 | |
| 26 | 24729 | 25901 | + | IS256 | 390 | AGGAGGACTTTTACATG | E. faecalis V583 | IS256 transposase | 100 | 390/390 | NP_813928 |
| 27 | 26041 | 27480 | − | aac(6′)-aph(2′) | 479 | AGGTGATAAATAAATG | S. aureus Mu50 | Bifunctional AAC(6′)/APH(2″):6′-aminoglycoside N-acetyltransferase and 2″-aminoglycoside phosphotransferase | 100 | 479/479 | NP_115315 |
| 28 | 27481 | 27885 | − | 134 | AGGAGTCTGGACTTG | S. aureus(pLW043) | Acetyltransferase GNAT family protein | 100 | 134/134 | NP_878007 | |
| 29 | 27930 | 29102 | − | IS256 | 390 | AGGAGGACTTTTACATG | E. faecalis V583 | IS256 transposase | 100 | 390/390 | NP_813928 |
| 30 | 29203 | 30294 | − | traA | 363 | AGAGGAGGTAAAATCATG | S. epidernidis W23144 | Nickase TraA | 100 | 363/363 | ZP_03986061 |
| 31 | 30498 | 30767 | + | 89 | GGAGTTTTTTAATG | S. epidernidis W23144 | Conserved hypothetical protein | 100 | 89/89 | ZP_03986060 | |
| 32 | 30784 | 31023 | + | 79 | S. epidernidis W23144 | Conserved hypothetical protein | 100 | 79/79 | ZP_03986059 | ||
| 33 | 31131 | 31805 | − | IS257 | 224 | AGGAGTCTTCTGTATG | S. aureus(pV030-8) | IS257 transposase | 98.7 | 224/224 | YP_001653101 |
| 34 | 32145 | 32966 | + | 273 | AGGAGACCTAGTTAATG | S. aureus MRSA252 | LysR family regulatory protein | 96.3 | 273/273 | YP_040145 | |
| 35 | 33683 | 33823 | − | 46 | S. aureus(pEDINA) | pEDINA_p50 (transcriptional regulator) | 97.8 | 45/46 | YP_001573922 | ||
| 36 | 34209 | 34736 | + | IS257 | 175 | S. aureus(pETB) | pETB_p37 (IS257 transposase) | 100 | 175/175 | NP_478380 | |
| 37 | 34771 | 35913 | − | 380 | AGGAGAAACTATG | S. aureus(pETB) | pETB_p38 (putative ATP/GTP-binding protein) | 99.7 | 380/380 | NP_478381 | |
| 38 | 36042 | 36272 | + | 76 | TAAGCTGCTGCTGTATATTATG | S. aureus(pETB) | pETB_p39 (conserved hypothetical protein) | 100 | 76/76 | NP_478382 | |
| 39 | 36635 | 36823 | + | sacaA | 62 | TAAAGCGTGGTGATTCTTATG | S. aureus(pETB) | pETB_p40 (lantibiotic structural protein Sac-alpha-A) | 100 | 62/62 | NP_478383 |
| 40 | 36847 | 37050 | + | sacbA | 67 | TAAGGTGGTATTTTTATG | S. aureus(pETB) | pETB_p41 (lantibiotic structural protein Sac-beta-A) | 100 | 67/67 | NP_478384 |
| 41 | 37069 | 39966 | + | sacM1 | 965 | GGAGATAGTTCATAATG | S. aureus(pETB) | pETB_p42 (lantibiotic mersacidin modifying enzyme SacM1) | 100 | 965/965 | NP_478385 |
| 42 | 39968 | 42130 | + | sacT | 720 | GAGGTGTAATATG | S. aureus(pETB) | pETB_p43 (lantibiotic mersacidin ABC transporter system SacT) | 100 | 720/720 | NP_478386 |
| 43 | 42127 | 44880 | + | sacM2 | 917 | AAGGAGTGTGGAGTTTG | S. aureus(pETB) | pETB_p44 (lantibiotic mersacidin modifying enzyme SacM2) | 99.9 | 917/917 | NP_478387 |
| 44 | 44896 | 45996 | + | 366 | AGGAGCGTAAATATTTG | S. aureus(pETB) | pETB_p45 (conserved hypothetical protein) | 100 | 365/366 | NP_478388 | |
| 45 | 46011 | 46880 | + | 289 | AGGAGAATTCTGATG | S. aureus(pETB) | pETB_p46 (multidrug efflux ABC transporter ATP-binding protein) | 100 | 289/289 | NP_478389 | |
| 46 | 46864 | 47589 | + | 241 | GGAGGTTCTAAAATTG | S. aureus(pETB) | pETB_p47 (putative membrane protein) | 100 | 241/241 | NP_478390 | |
| 47 | 47618 | 47791 | + | 57 | GGAGGAATTTTAATG | S. aureus(pETB) | pETB_p48 (conserved hypothetical protein) | 100 | 57/57 | NP_478391 | |
| 48 | 48118 | 48792 | − | IS257 | 224 | GAGGTGCAGAGGATG | S. aureus(pETB) | pETB_p49 (IS257 transposase) | 100 | 224/224 | NP_478392 |
| 49 | 49007 | 49573 | − | res | 188 | GAGGTTATATTTGAATG | S. aureus(pETB) | pETB_p50 (recombinase Res) | 100 | 188/188 | NP_478393 |
| 50 | 50164 | 50955 | + | 263 | AGGTACCAATTTATG | S. aureus(pETB) | pETB_p01 (replication-associated protein) | 100 | 263/263 | NP_478344 | |
| 51 | 51488 | 52231 | − | ednC | 247 | AAGGAGTCTTTTATG | S. aureus(pETB) | epidermal cell differentiation inhibitor EDINC | 100 | 247/247 | NP_478345 |
| 52 | 52801 | 53631 | − | 276 | AAGGAGAATGAGGCATTG | S. aureus(pETB) | pETB_p03 (conserved hypothetical protein) | 99.6 | 276/276 | NP_478346 | |
| 53 | 53708 | 54022 | − | 104 | AAGGAGAGAAAATAATG | S. aureus(pETB) | pETB_p04 (conserved hypothetical protein) | 100 | 104/104 | NP_478347 | |
| 54 | 54175 | 54591 | + | 138 | GAGGTGTATTAAAATG | S. aureus(pETB) | pETB_p05 (conserved hypothetical protein) | 100 | 138/138 | NP_478348 | |
| 55 | 54833 | 55666 | + | etb | 277 | AAGGAGGTTTTATATATG | S. aureus(pETB) | exfoliative toxin B | 100 | 277/277 | NP_478350 |
| 56 | 55760 | 55921 | − | 53 | S. aureus MN8 | conserved hypothetical protein | 56.9 | 51/53 | ZP_03987549 | ||
| 57 | 56732 | 56881 | − | 49 | AGGAGGCATTTATTATG | S. aureus(pETB) | pETB_p11 (conserved hypothetical protein) | 100 | 49/49 | NP_478354 | |
| 58 | 57008 | 57958 | − | 316 | AAGGAGTAGTTAAGATG | S. aureus(pETB) | pETB_p12 (extracellular protein) | 100 | 316/316 | NP_478355 | |
| 59 | 58022 | 58234 | − | 70 | GGAGGTAACCTAAATATG | S. aureus(pETB) | pETB_p13 (conserved hypothetical protein) | 100 | 70/70 | NP_478356 | |
| 60 | 58309 | 58653 | − | mutS | 114 | GGAACAATTG | S. aureus(pWBG749) | putative DNA mismatch repair protein MutS | 83.1 | 98/118 | NP_478357 |
| 61 | 58713 | 58925 | − | 70 | GAGGGTTTTACAAATG | S. aureus(pETB) | pETB_p15 (conserved hypothetical protein) | 100 | 70/70 | NP_478358 | |
| 62 | 59090 | 59332 | − | 80 | AGGAGAGATACTATG | S. aureus(pETB) | pETB_p16 (conserved hypothetical protein) | 100 | 80/80 | NP_478359 | |
| 63 | 59501 | 60307 | − | parA | 268 | GGAGGTGGAAGCAATG | S. aureus(pETB) | pETB_p17 (plasmid partition protein ParA) | 100 | 268/268 | NP_478360 |
aa, amino acids.
Underlining indicates a putative ribosome binding site complementary to the 3′ end of the 16S rRNA; boldface indicates the start codon.
Overlap indicates the number of overlapping amino acids/total number of amino acids.
A homology search of the extra DNA region shows a ca. 16-kb extra DNA region in pETBTY825 containing the aminoglycoside resistance element (Tn4001) and β-lactam resistant element showed nearly a perfect match with the sequence of pSA018A from a clinical coagulase-negative Staphylococcus sp. strain CDC 25 isolated from a human (Fig. 1B).
Antimicrobial susceptibilities of S. aureus TY825 in the presence or absence of pETB.
To examine the functional activities of these resistance elements in pETBTY825, we constructed a pETB-defective strain of TY825 (26), and compared its antimicrobial susceptibility profile to that of the wild type. We determined the MICs of several clinically relevant antibiotics using the broth microdilution method (Table 3). As expected, the wild type was resistant to benzylpenicillin (MIC ≥ 8 μg/ml), ampicillin (MIC ≥ 8 μg/ml), gentamicin (MIC ≥ 8 μg/ml), and erythromycin (MIC ≥ 4 μg/ml). Conversely, the pETB-defective strain TY825 showed significantly decreased MICs of gentamicin (MIC ≤ 1 μg/ml), arbekacin (MIC ≤ 1 μg/ml), erythromycin (MIC ≤ 0.25 μg/ml), benzylpenicillin (MIC ≤ 2 μg/ml), and ampicillin (MIC ≤ 2 μg/ml). TY825 was also resistant to fosfomycin (MIC ≥ 16 μg/ml); however, the deletion of pETBTY825 did not alter the MIC of fosfomycin. These results clearly demonstrated that the resistance elements of pETBTY825 were functionally active and conferred resistance to these antibiotics.
Table 3.
Antimicrobial susceptibilities of S. aureus TY825 in the presence and absence of pETBa
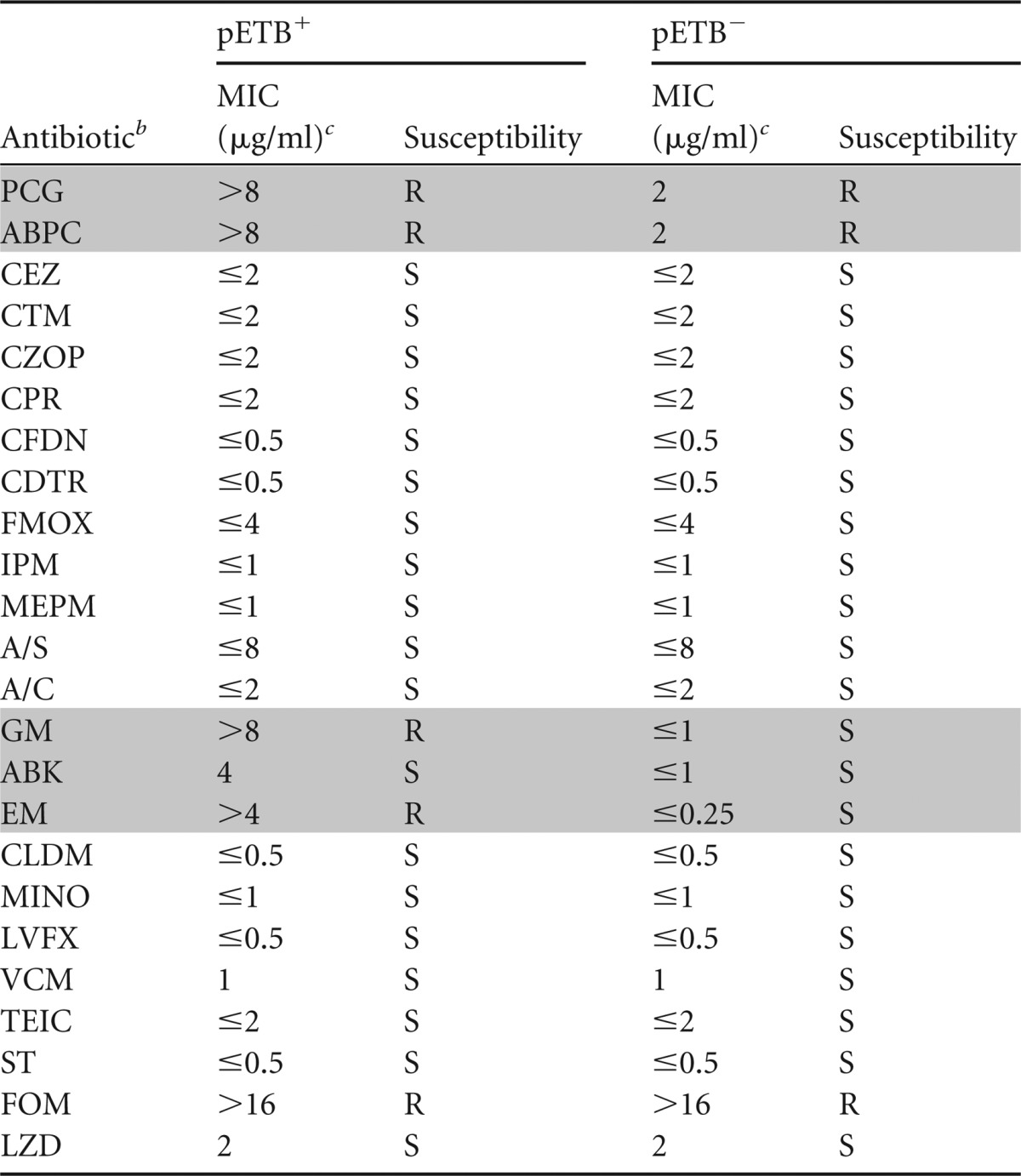
Shading indicates antimicrobial agents whose susceptibility was altered by the loss of pETB.
A/S, ampicillin-sulbactam; A/C, amoxicillin-clavulanic acid.
MICs were determined by using the Microscan system panel of antibiotics (Siemens Healthcare Diagnostics, Tokyo, Japan). S, susceptible; R, resistant.
Antimicrobial susceptibility to EM and GM in clinically isolated ETB-producing S. aureus strains.
For the treatment of impetigo/SSSS, GM is often used as an ointment, and a macrolide is one of the choices for empirical therapy. Additionally, ABK has frequently been used for the treatment of methicillin-resistant S. aureus (MRSA) in Japan since 1990, and aac(6′)/aph(2″) has been identified as one of the risk factors for ABK resistance in recent years (27, 28). Since the proportion of ETB-producing S. aureus causing impetigo/SSSS is significantly higher in Japan than in Western countries (29), we retrospectively examined the MICs of GM, ABK, and EM and genes for resistance to aminoglycosides [aac(6′)/aph(2′)] and macrolides (msrA) detected in pETBTY825 by PCR (Table 1), using the purified plasmid fractions of 86 randomly selected ETB-producing clinical isolates (1977 to 2007) stored in our laboratory (Table 4). Of note, an increase in MRSA strains causing impetigo/SSSS has been reported in recent years (30). Therefore, mecA was also examined in the MRSA strains by using PCR.
Table 4.
Antimicrobial susceptibility testing and PCR analysis of clinically isolated ETB-producing S. aureus strains
| Strain | Yr | Diagnosis | MIC (μg/ml) |
PCR result |
||||
|---|---|---|---|---|---|---|---|---|
| ABK | GM | EM | mecA | aac(6′)/aph(2″) | msrA | |||
| TY468 | 1977 | SSSS | 1 | 1 | 0.125 | − | − | − |
| TY469 | 1977 | SSSS | 1 | 1 | 0.125 | − | − | − |
| TY470 | 1977 | SSSS | 2 | 1 | 0.125 | − | − | − |
| TY471 | 1981 | SSSS | 1 | 1 | 0.125 | − | − | − |
| TY472 | 1981 | Impetigo | 1 | 1 | 0.125 | − | − | − |
| TY473 | 1982 | SSSS | 1 | 1 | 64 | − | − | − |
| TY474 | 1982 | SSSS | 1 | 1 | 0.125 | − | − | − |
| TY477 | 1978 | Impetigo | 0.5 | 1 | 2 | − | − | − |
| TY478 | 1979 | SSSS | 1 | 1 | 32 | − | − | − |
| TY479 | 1980 | SSSS | 1 | 1 | 1 | − | − | − |
| TY480 | 1980 | SSSS | 1 | 0.5 | >128 | − | − | − |
| TY481 | 1980 | SSSS | 2 | 1 | >128 | − | − | − |
| TY482 | 1980 | SSSS | 2 | 1 | 1 | − | − | − |
| TY484 | 1980 | SSSS | 1 | 2 | 0.125 | − | − | − |
| TY485 | 1981 | SSSS | 1 | 2 | 0.125 | − | − | − |
| TY487 | 1982 | Impetigo | 0.5 | 0.5 | 0.125 | − | − | − |
| TY488 | 1982 | Impetigo | >0.5 | 1 | 0.125 | − | − | − |
| TY489 | 1982 | SSSS | 1 | 1 | 128 | − | − | − |
| TY490 | 1982 | SSSS | 1 | 1 | 0.128 | − | − | − |
| TY491 | 1983 | SSSS | 1 | 2 | 128 | − | − | − |
| TY502 | 1983 | Impetigo | 1 | 2 | 2 | − | − | − |
| TY507 | 1983 | SSSS | 2 | 4 | 0.25 | − | − | − |
| TY519 | 1984 | Impetigo | 2 | 1 | 1 | − | − | − |
| TY520 | 1984 | Impetigo | 2 | 4 | 0.125 | − | − | − |
| TY522 | 1984 | Impetigo | 2 | 4 | 0.125 | − | − | − |
| TY561 | 1987 | SSSS | 4 | >128 | 0.125 | − | + | − |
| TY564 | 1988 | Impetigo | 2 | 1 | >128 | − | − | − |
| TY565 | 1988 | SSSS | 1 | 1 | >128 | − | − | − |
| TY573 | 1989 | Impetigo | 4 | 4 | 0.125 | − | − | − |
| TY576 | 1989 | SSSS | 4 | 16 | 0.125 | − | − | − |
| TY4 | 1990 | SSSS | 2 | 32 | >128 | + | + | |
| TY580 | 1992 | SSSS | 4 | >128 | 0.125 | + | + | − |
| TY36 | 1999 | Impetigo | 8 | >128 | >128 | + | + | − |
| TY49 | 1999 | Impetigo | 2 | >128 | 2 | + | + | − |
| TY54 | 1999 | Impetigo | 2 | >128 | 0.125 | + | + | − |
| TY56 | 1999 | Impetigo | 4 | >128 | 0.125 | + | + | − |
| TY64 | 1999 | Impetigo | 1 | 1 | 0.125 | − | − | − |
| TY69 | 1999 | Impetigo | 4 | >128 | >128 | + | + | − |
| TY93 | 1999 | Impetigo | 4 | >128 | >128 | − | + | − |
| TY97 | 1999 | Impetigo | 8 | >128 | 0.125 | − | + | − |
| TY110 | 1999 | Impetigo | 4 | >128 | >128 | + | + | − |
| TY119 | 2000 | ND | 32 | >128 | 0.5 | + | − | − |
| TY145 | 2000 | ND | 1 | 8 | 0.5 | − | + | − |
| TY146 | 2000 | ND | 1 | 8 | 0.5 | − | + | − |
| TY162 | 2000 | Atopy | 32 | >128 | 0.25 | − | + | − |
| TY174 | 2000 | Atopy | 8 | >128 | 0.5 | − | + | − |
| TY189 | 2001 | SSSS | >128 | >128 | >128 | + | + | − |
| TY213 | 2001 | SSSS | >128 | >128 | >128 | + | + | − |
| TY219 | 2001 | SSSS | 16 | >128 | 0.25 | + | + | − |
| TY226 | 2001 | ND | 8 | 16 | >128 | − | + | − |
| TY228 | 2001 | Abscess | 1 | 8 | 0.25 | − | − | − |
| TY229 | 2001 | SSSS | 1 | 4 | >128 | − | − | − |
| TY632 | 2002 | Impetigo | 32 | >128 | 2 | − | + | + |
| TY825 | 2002 | Impetigo | 4 | >128 | 16 | − | + | + |
| TY1020 | 2002 | Impetigo | 4 | >128 | 16 | − | + | + |
| TY1603 | 2002 | Impetigo | 4 | >128 | 32 | − | + | + |
| TF2753 | 2005 | Impetigo | 16 | >128 | 0.125 | + | + | − |
| TF2754 | 2005 | Impetigo | 8 | >128 | >128 | + | + | − |
| TF2778 | 2005 | Impetigo | 16 | >128 | 1 | − | + | − |
| TF2780 | 2005 | Impetigo | 4 | >128 | >128 | − | + | − |
| TF2791 | 2005 | Impetigo | 8 | >128 | >128 | + | + | − |
| TF2799 | 2005 | Impetigo | 2 | >128 | 0.125 | + | + | − |
| TF2800 | 2005 | Impetigo | 8 | >128 | >128 | + | + | − |
| TF2802 | 2005 | Impetigo | 16 | >128 | >128 | + | + | − |
| TF2809 | 2005 | Impetigo | 8 | >128 | 0.125 | + | + | − |
| TF2815 | 2005 | Impetigo | 4 | >128 | >128 | − | + | − |
| TF2816 | 2005 | Impetigo | 4 | >128 | 2 | − | + | − |
| TF2817 | 2005 | Impetigo | 4 | >128 | 2 | − | + | − |
| TF2818 | 2005 | Impetigo | 2 | >128 | >128 | − | + | − |
| TF2825 | 2005 | ND | 2 | >128 | >128 | + | + | − |
| TF2829 | 2005 | Impetigo | 4 | >128 | 0.125 | + | + | − |
| TF2846 | 2005 | Impetigo | 2 | >128 | >128 | + | + | − |
| TF2848 | 2005 | Impetigo | 8 | >128 | 0.125 | − | + | − |
| TF2920 | 2005 | Impetigo | 64 | >128 | >128 | − | + | − |
| TF2932 | 2005 | Impetigo | >16 | >128 | 0.125 | − | + | − |
| TF2939 | 2005 | Impetigo | >16 | >128 | >128 | + | + | − |
| TF3056 | 2005 | Impetigo | 2 | >128 | 8 | − | + | + |
| TF3371 | 2006 | SSSS | 4 | >128 | 128 | + | + | − |
| TF3516 | 2007 | ND | 2 | 64 | 1 | − | + | − |
| TF3520 | 2007 | ND | 2 | 32 | 128 | − | + | − |
| TF3526 | 2007 | ND | 4 | >128 | 128 | + | + | − |
| TF3543 | 2007 | ND | 4 | >128 | 128 | + | + | − |
| TF3546 | 2007 | ND | 2 | 128 | 128 | + | + | − |
| TF3563 | 2007 | ND | 2 | >128 | >128 | + | + | − |
| TF3564 | 2007 | ND | 4 | >128 | >128 | + | + | − |
| TF3571 | 2007 | ND | 1 | >128 | >128 | + | + | − |
| TF3578 | 2007 | ND | 2 | >128 | >128 | + | + | − |
| TF3583 | 2007 | ND | 1 | >128 | >128 | + | + | − |
| TF3585 | 2007 | ND | 2 | >128 | >128 | + | + | − |
| TF3586 | 2007 | ND | 1 | 2 | 0.125 | − | − | − |
| TF3591 | 2007 | ND | 2 | 2 | 0.25 | − | − | − |
| TF3598 | 2007 | ND | 8 | >128 | >128 | + | + | − |
| TF3600 | 2007 | ND | 2 | 128 | 0.25 | − | + | − |
| TF3602 | 2007 | ND | 8 | >128 | 2 | + | + | − |
| TF3612 | 2007 | ND | 8 | >128 | >128 | + | + | − |
Boldface indicates strains that were selected for PCR scanning analysis. ND, no diagnosis data.
ETB-producing S. aureus strains isolated in the 1970s and 1980s were largely susceptible to ABK, GM, and EM (Table 4). However, MICs of GM sharply changed after 1992, and ETB-producing S. aureus strains began to display high resistance to GM. This high resistance almost perfectly matched the detection of aac(6′)/aph(2″). Further, the detection of aac(6′)/aph(2″) paralleled the detection of mecA. Conversely, there was no significant change in the ABK MICs during the test period, with only a slight increase from 1 to 2 to 8 μg/ml after 1989. There was no correlation between ABK MIC and the presence or absence of aac(6′)/aph(2″). Resistance to EM was sporadically found in strains from the 1970s and 1980s. After 2001, strains resistant to EM significantly increased. Notably, however, msrA was rarely detected in ETB-producing S. aureus strains, and only five strains were positive for both aac(6′)/aph(2″) and msrA by PCR.
PCR scanning of ETB-producing S. aureus strains positive for aac(6′)/aph(2″) and msrA.
Detection of both aac(6′)/aph(2″) and msrA suggests that these five strains (TY632, TY825, TY1020, TY1603, and TF3056) possess a TY825-type pETB. We therefore examined the genome organization of the 22-kb extra DNA region of the plasmids isolated from the four strains using the PCR scanning method. We generated seven pairs of primers whose PCR products cover all of the 22-kb extra DNA region. All pairs of primers yielded PCR products with the expected sizes in only one strain, TF3056, besides TY825 (Fig. 2). The other three strains were found to possess a DNA region containing macrolide and β-lactam resistance elements but lack the DNA region corresponding to the aminoglycoside resistance element.
Fig 2.
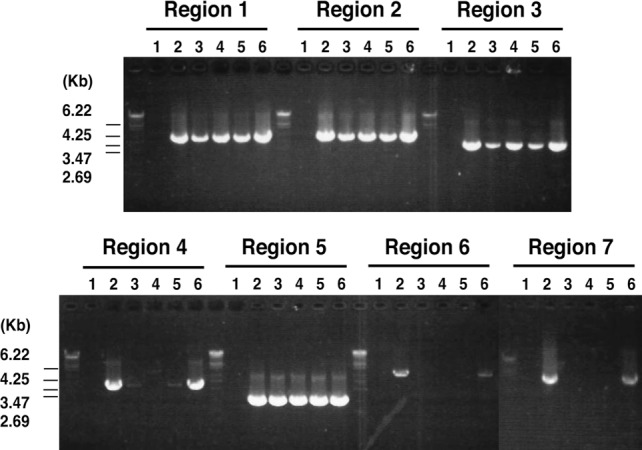
PCR scanning analysis of pETB plasmids. The gene organization of the acquired region in the pETBTY825 plasmid was examined using PCR scanning analysis. Various combinations of the 14 primers that target the selected seven genes were used. A schematic view is shown in Fig. 1B. The results of the PCR analysis of regions 1 to 7 are shown. By comparing the length of each amplified fragment with that from pETB, the regional heterogeneity was determined. Results with pETB from the following strains are shown in the indicated lanes: 1, TY4; 2, TY825; 3, TY632; 4, TY1020; 5, TY1603; and 6, TF3056.
DISCUSSION
In this study, we sequenced the pETB plasmid of the clinical isolate TY825, obtained in 2002 from a lesion of an impetigo patient. pETBTY825 is significantly larger than the archetype pETBTY4 and has a single extra DNA region (22,352 bp). Comparative analysis suggested that pETBTY825 was generated from pETBTY4 by acquiring a single 22-kb block of extra DNA. In a previous study, we reported that region D of pETBTY4 is highly heterogeneous in size, based on PCR scanning analysis of plasmids from clinical isolates (13). However, the extra DNA region of pETBTY825 was found to be inserted into the region corresponding to region E of pETBTY4. A nearly perfect match of ca. 16 kb in the extra DNA region of pETBTY825 with the partial sequence of a plasmid from a coagulase-negative staphylococcus (CNS) may imply that S. aureus acquired this region by horizontal transfer from resident CNS on the skin.
According to the PCR analysis for aac(6′)/aph(2″) and msrA and subsequent PCR scanning analysis of the pETB plasmid from the clinical isolates, the pETBTY825 type was rare and found in only two strains, TY825 and TF3056. It should be noted that the frequency of strains positive for both mecA and aac(6′)/aph(2″) markedly increased after 1990. In recent studies, community-associated MRSA with type IVc SCCmec was shown to possess Tn4001 in the J3 region (30–32). Tn4001 is composed of two IS256 elements flanking aac(6′)/aph(2″) and orf28. We therefore screened for SCCmec type IVc in the ETB-producing MRSA strains isolated after 1990. Only two strains (TF3371 and TF3571) among the all mecA-positive strains were typed as SCCmec type IVc, suggesting that SCCmec type IVc was rare among ETB-producing MRSA strains. Therefore, aac(6′)/aph(2″) in ETB-producing strains isolated after 1990 may be attributable to a plasmid other than pETB or a chromosome site other than SCCmec.
Antimicrobial susceptibility testing of TY825 and the pETB-defective strain indicated that aac(6′)/aph(2″) contributes to an increase in MICs of GM/ABK, but the effect on the MIC of ABK was slight. Earlier studies reported that AAC(6′)/APH(2″) modifies both gentamicin and arbekacin (19), but ABK was later found to be a poor substrate of AAC(6′)/APH(2″) (33). Barada et al. suggested that the presence of aph(3′)-III in addition to aac(6′)/aph(2″) is required for full resistance to ABK (27). This might explain the lack of correlation between ABK MIC and the presence or absence of aac(6′)/aph(2″) in clinical isolates.
The msrA and mef genes display inducible resistance to erythromycin by encoding an ATP-dependent efflux pump (23, 34). Our data, however, clearly indicated that msrA was not principally responsible for the macrolide resistance in ETB-producing S. aureus strains. Nakaminami et al. reported that the gene products of ermA, ermB, and ermC were major macrolide resistance traits in S. aureus strains causing impetigo/SSSS (32). These three genes (ermA, ermB, and ermC) display resistance to macrolides by methylation of the ribosomal target site (30, 35). Those authors also demonstrated the presence of msrA at a low frequency in S. aureus strains causing impetigo/SSSS (32). Our data support their observations.
A previous study suggested that there is an association between the ET serotype and the clinical severity of staphylococcal blistering diseases (29). ETB-producing S. aureus is more frequently isolated from SSSS or the severe form of impetigo than ETA-producing S. aureus. For the treatment of SSSS, β-lactams were a primary choice together with an ointment of GM. However, in recent years, it has become evident that ETB-producing S. aureus in Japan is almost 100% resistant to GM and the proportion of resistance to β-lactam and EM is significantly higher than those isolated before 1989 (Table 4). Our study suggests that the emergence of an ETB plasmid carrying multiple resistance genes partly contributes to an increase in multiple resistance of ETB-producing S. aureus. Most impetigo/SSSS patients are young children and neonates, and SSSS patients, especially newborns, require admission and general treatment. But quinolone and tetracycline are not first choices for treatment, and available antimicrobials are limited in the current situation. Thus, special caution may be necessary for the treatment of SSSS/severe impetigo caused by ETB-producing S. aureus strains in Japan.
ACKNOWLEDGMENTS
We thank M. Takeda for skillful assistance and R. Kuwahara for MIC measurement. We thank Jim Nelson and Larry Strand for editorial assistance.
The project was supported in part by Grant-in-Aid for Priority Areas “Applied Genomics” from the Ministry of Education, Culture, Sports, Science and Technology of Japan, Grant-in-Aid for Young Scientists (B) 22790408, from the Japan Society for the Promotion of Science, and by Health Labor Sciences Research Grants for Research on Allergic Diseases and Immunology from the Ministry of Health, Labor and Welfare.
Footnotes
Published ahead of print 30 September 2013
REFERENCES
- 1. Ladhani S, Joannou CL, Lochrie DP, Evans RW, Poston SM. 1999. Clinical, microbial, and biochemical aspects of the exfoliative toxins causing staphylococcal scalded-skin syndrome. Clin. Microbiol. Rev. 12:224–242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Farrell AM. 1999. Staphylococcal scalded-skin syndrome. Lancet 354:880–881 [DOI] [PubMed] [Google Scholar]
- 3. Melish ME, Glasgow LA. 1971. Staphylococcal scalded skin syndrome: the expanded clinical syndrome. J. Pediatr. 78:958–967 [DOI] [PubMed] [Google Scholar]
- 4. Arbuthnott JP, Billcliffe B. 1976. Qualitative and quantitative methods for detecting staphylococcal epidermolytic toxin. J. Med. Microbiol. 9:191–201 [DOI] [PubMed] [Google Scholar]
- 5. Kondo I, Sakurai S, Sarai Y. 1973. Purification of exfoliatin produced by Staphylococcus aureus of bacteriophage group 2 and its physicochemical properties. Infect. Immun. 8:156–164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Yamaguchi T, Nishifuji K, Sasaki M, Fudaba Y, Aepfelbacher M, Takata T, Ohara M, Komatsuzawa H, Sugai M. 2002. Identification of the Staphylococcus etd pathogenicity island which encodes a novel exfoliative toxin, ETD, and EDIN-B. Infect. Immun. 70:5835–5845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Elias PM, Fritsch P, Epstein JEH. 1977. Staphylococcal scalded skin syndrome. Clinical features, pathogenesis, and recent microbiological and biochemical developments. Arch. Dermatol. 113:207–219 [DOI] [PubMed] [Google Scholar]
- 8. Amagai M, Matsuyoshi N, Wang ZH, Andi C, Stanley JR. 2000. Toxin in bullous impetigo and staphylococcal scalded-skin syndrome targets desmoglein 1. Nat. Med. 6:1275–1277 [DOI] [PubMed] [Google Scholar]
- 9. Betley MJ, Borst DW, Regassa LB. 1992. Staphylococcal enterotoxins, toxic shock syndrome toxin and streptococcal pyrogenic exotoxins: a comparative study of their molecular biology. Chem. Immunol. 55:1–35 [PubMed] [Google Scholar]
- 10. Lindsay JA, Ruzin A, Ross HF, Kurepina N, Novick RP. 1998. The gene for toxic shock toxin is carried by a family of mobile pathogenicity islands in Staphylococcus aureus. Mol. Microbiol. 29:527–543 [DOI] [PubMed] [Google Scholar]
- 11. Novick RP, Schlievert P, Ruzin A. 2001. Pathogenicity and resistance islands of staphylococci. Microbes Infect. 3:583–594 [DOI] [PubMed] [Google Scholar]
- 12. Yamaguchi T, Hayashi T, Takami H, Nakasone K, Ohnishi M, Nakayama K, Yamada S, Komatsuzawa H, Sugai M. 2000. Phage conversion of exfoliative toxin A production in Staphylococcus aureus. Mol. Microbiol. 38:694–705 [DOI] [PubMed] [Google Scholar]
- 13. Yamaguchi T, Hayashi T, Takami H, Ohnishi M, Murata T, Nakayama K, Asakawa K, Ohara M, Komatsuzawa H, Sugai M. 2001. Complete nucleotide sequence of a Staphylococcus aureus exfoliative toxin B plasmid and identification of a novel ADP-ribosyltransferase, EDIN-C. Infect. Immun. 69:7760–7771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Clinical and Laboratory Standards Institute 2008. Third approved standard M31–A3 Clinical and Laboratory Standards Institute, Wayne, PA [Google Scholar]
- 15. Sugai M, Fujiwara T, Akiyama T, Ohara M, Komatsuzawa H, Inoue S, Suginaka H. 1997. Purification and molecular characterization of glycylglycine endopeptidase produced by Staphylococcus capitis EPK1. J. Bacteriol. 179:1193–1202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Noguchi H, Taniguchi T, Itoh T. 2008. MetaGeneAnnotator: detecting species-specific patterns of ribosomal binding site for precise gene prediction in anonymous prokaryotic and phage genomes. DNA Res. 15:387–396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Altschul SF, Madden TL, Schaffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ. 1997. Gapped BLAST and PSI-BLAST: a new generation of protein database search program. Nucleic Acids Res. 25:3389–3402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Clinical and Laboratory Standards Institute 2005. Performance standards for antimicrobial susceptibility testing; fifteenth informational supplement M100–S15 Clinical and Laboratory Standards Institute, Wayne, PA [Google Scholar]
- 19. Lyon BR, Gillespie MT, Byrne ME, May JW, Skurray RA. 1987. Plasmid-mediated resistance to gentamicin in Staphylococcus aureus: the involvement of a transposon. J. Med. Microbiol. 23:101–110 [DOI] [PubMed] [Google Scholar]
- 20. Rouch DA, Byrne ME, Kong YC, Skurray RA. 1987. The aacA-aphD gentamicin and kanamycin resistance determinant of Tn4001 from Staphylococcus aureus: expression and nucleotide sequence analysis. J. Gen. Microbiol. 133:3039–3052 [DOI] [PubMed] [Google Scholar]
- 21. Ramirez MS, Tomasky ME. 2010. Aminoglycoside modifying enzymes. Drug Resist. Updat. 13:151–171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ross JI, Eady EA, Cove JH, Cunliffe WJ, Baumberg S, Wootton JC. 1990. Inducible erythromycin resistance in staphylococci is encoded by a member of ATP-binding transport super-gene family. Mol. Microbiol. 4:1207–1214 [DOI] [PubMed] [Google Scholar]
- 23. Ross JI, Eady EA, Cove JH, Baumberg S. 1995. Identification of a chromosomally encoded ABC-transport system with which the staphylococcal erythromycin exporter MsrA may interact. Gene 153:93–98 [DOI] [PubMed] [Google Scholar]
- 24. Rowland SJ, Dyke KG. 1990. Tn552, a novel transposable element from Staphylococcus aureus. Mol. Microbiol. 4:961–975 [DOI] [PubMed] [Google Scholar]
- 25. Rowland SJ, Stark WM, Boocock MR. 2002. Sin recombinase from Staphylococcus aureus: synaptic complex architecture and transposon targeting. Mol. Microbiol. 44:607–619 [DOI] [PubMed] [Google Scholar]
- 26. Kato F, Kadomoto N, Iwamoto Y, Bunai K, Komatsuzawa H, Sugai M. 2011. Regulatory mechanism for exfoliative toxin production in Staphylococcus aureus. Infect. Immun. 79:1660–1670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Barada K, Hanaki H, Ikeda S, Yamaguchi Y, Akama H, Nakae T, Inamatsu T, Sunakawa K. 2007. Trends in the gentamicin and arbekacin susceptibility of methicillin-resistant Staphylococcus aureus and the genes encoding aminoglycoside-modifying enzymes. J. Infect. Chemother. 13:74–78 [DOI] [PubMed] [Google Scholar]
- 28. Iwaki M, Noguchi N, Nakaminami H, Sasatsu M, Ito M. 2011. Antimicrobial activity and frequency of spontaneous gentamicin-resistant mutants in bacteria related skin infections. Yakugaku Zasshi 131:1653–1659 [DOI] [PubMed] [Google Scholar]
- 29. Yamasaki O, Yamaguchi T, Sugai M, Chapuis-Cellier C, Arnaud F, Vandenesch F, Etienne J, Lina G. 2005. Clinical manifestations of staphylococcal scalded-skin syndrome depend on serotypes of exfoliative toxins. J. Clin. Microbiol. 43:1890–1893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Noguchi N, Nakaminami H, Nishijima S, Kurokawa I, So H, Sasatsu M. 2006. Antimicrobial agent of susceptibilities and antiseptic resistance gene distribution among methicillin-resistant Staphylococcus aureus isolates from patients with impetigo and staphylococcal scalded skin syndrome. J. Clin. Microbiol. 44:2119–2125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ito T, Okuma K, Ma XX, Yuzawa H, Hiramatsu K. 2003. Insights on antibiotic resistance of Staphylococcus aureus from its whole genome: genomic island SCC. Drug Resist. Updat. 6:41–52 [DOI] [PubMed] [Google Scholar]
- 32. Nakaminami H, Noguchi N, Ikeda M, Hasui M, Yamamoto S, Asano T, Senoue M, Sasatsu M. 2008. Molecular epidemiology and antimicrobial susceptibilities of 273 exfoliative toxin-encoding-gene-positive Staphylococcus aureus isolates from patients with impetigo in Japan. J. Med. Microbiol. 57:1251–1258 [DOI] [PubMed] [Google Scholar]
- 33. Ishino K, Ishikawa J, Ikeda Y, Hotta K. 2004. Characterization of a bifunctional aminoglycoside-modifying enzyme with novel substrate specificity and its gene from a clinical isolate of methicillin-resistant Staphylococcus aureus with high arbekacin resistance. J. Antibiot. Tokyo 57:679–686 [DOI] [PubMed] [Google Scholar]
- 34. Leclercq R. 2002. Mechanisms of resistance to macrolides and lincosamides: nature of the resistance elements and their clinical implications. Clin. Infect. Dis. 34:482–492 [DOI] [PubMed] [Google Scholar]
- 35. Zmantar T, Kouidhi B, Miladi H, Bakhrouf A. 2011. Detection of macrolide and disinfectant resistance genes in clinical Staphylococcus aureus and coagulase-negative staphylococci. BMC Res. Notes 4:1–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Nakayama K, Takashima K, Ishihara H, Shinomiya T, Kageyama M, Kanaya S, Ohnishi M, Murata T, Mori H, Hayashi T. 2000. The R-type pyocin of Pseudomonas aeruginosa is related to P2 phage, and the F-type is related to lambda phage. Mol. Microbiol. 38:213–231 [DOI] [PubMed] [Google Scholar]