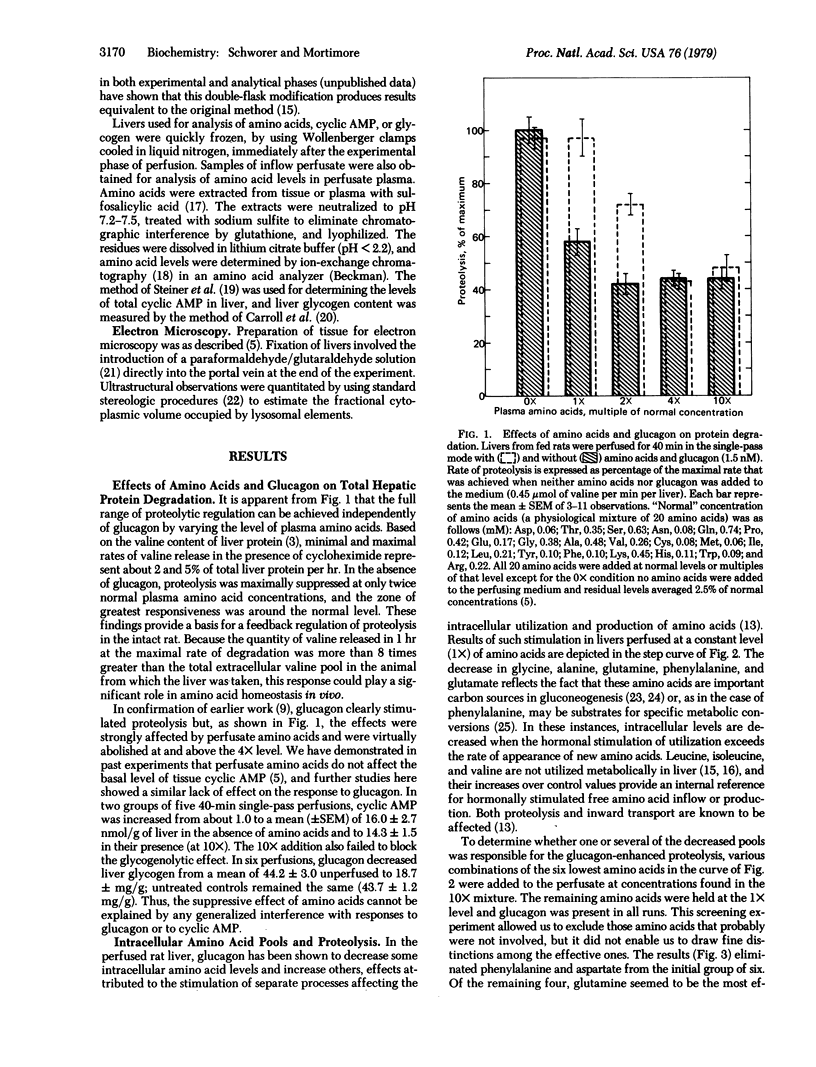
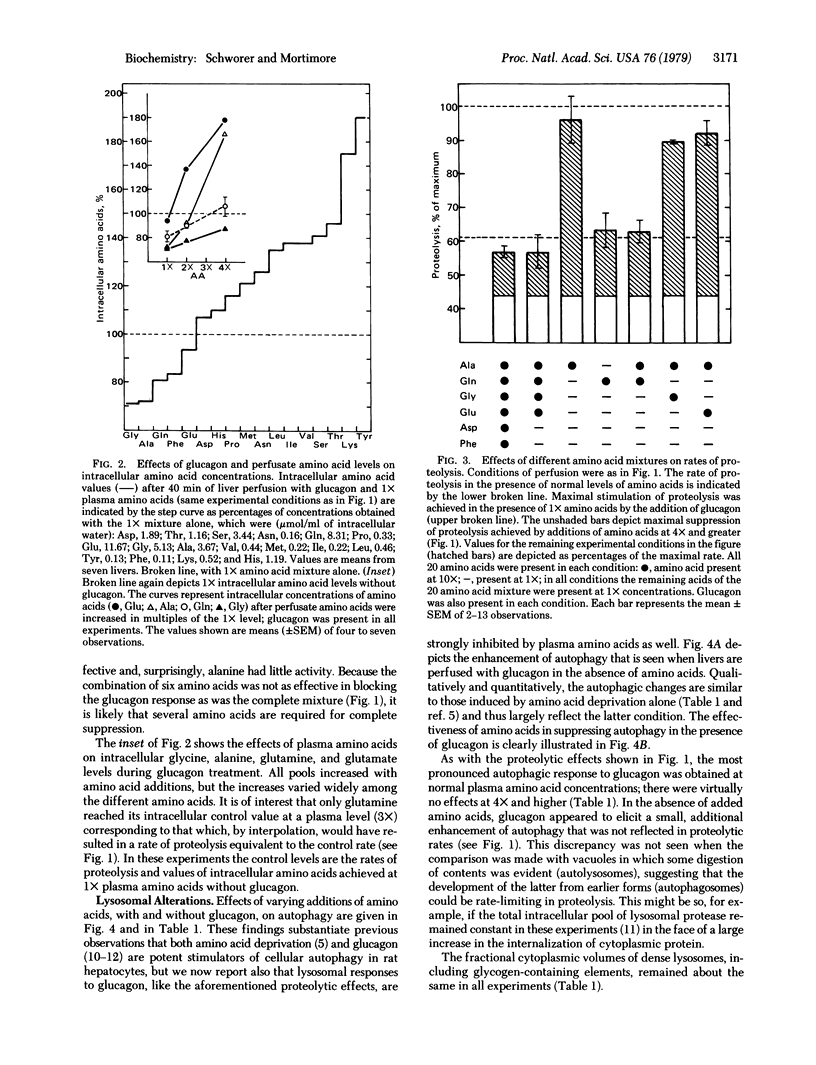
Abstract
Amino acid deprivation and glucagon are both potent inducers of autography and proteolysis in liver. Because glucagon enhanced the metabolic utilization of some amino acids, the catabolic response to both of these stimuli could be achieved by a lowering of intracellular amino acid pools. Alternatively, glucagon could act independently of amino acids. To clarify the mode of hormonal action and also the relationship between the two cellular responses, livers from fed rats were perfused, with and without glucagon, with plasma amino acids over a concentration range of 0 to 10 times normal. Individual amino acids constancy at each level was ensured by perfusion in the single-pass mode. Amino acids alone strongly regulated autophagy and proteolysis in a coordinated fashion; maximal suppression was achieved at twice normal concentration; both effects increased rapidly to maximum at less than normal concentration. Corresponding effects of glucagon, however, could be elicited only at intermediate amino acid levels. None was noted at 4 and 10 times normal; at 0, hormonal stimulation was minimal. The amino acid inhibition was selective because it did not block cyclic AMP production or glycogenolysis. Intracellular pool measurements and systematic alteration of perfusate amino acid composition indicated that the autophagic and proteolytic effects of glucagon are mediated by a hormonally induced depletion of glycine, alanine, glutamate, and glutamine; of these, glutamine alone is the most effective. We conclude that the stimulation of intracellular protein degradation in liver is a manifestation of deprivation-induced autophagy which results from a decrease in certain intracellular glucogenic amino acids, notably glutamine.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ASHFORD T. P., PORTER K. R. Cytoplasmic components in hepatic cell lysosomes. J Cell Biol. 1962 Jan;12:198–202. doi: 10.1083/jcb.12.1.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amenta J. S., Hlivko T. J., McBee A. G., Shinozuka H., Brocher S. Specific inhibition by NH4CL of autophagy-associated proteloysis in cultured fibroblasts. Exp Cell Res. 1978 Sep;115(2):357–366. doi: 10.1016/0014-4827(78)90289-6. [DOI] [PubMed] [Google Scholar]
- Arstila A. U., Trump B. F. Studies on cellular autophagocytosis. The formation of autophagic vacuoles in the liver after glucagon administration. Am J Pathol. 1968 Nov;53(5):687–733. [PMC free article] [PubMed] [Google Scholar]
- CARROLL N. V., LONGLEY R. W., ROE J. H. The determination of glycogen in liver and muscle by use of anthrone reagent. J Biol Chem. 1956 Jun;220(2):583–593. [PubMed] [Google Scholar]
- De Duve C., Wattiaux R. Functions of lysosomes. Annu Rev Physiol. 1966;28:435–492. doi: 10.1146/annurev.ph.28.030166.002251. [DOI] [PubMed] [Google Scholar]
- Dean R. T. Direct evidence of importance of lysosomes in degradation of intracellular proteins. Nature. 1975 Oct 2;257(5525):414–416. doi: 10.1038/257414a0. [DOI] [PubMed] [Google Scholar]
- Deter R. L., Baudhuin P., De Duve C. Participation of lysosomes in cellular autophagy induced in rat liver by glucagon. J Cell Biol. 1967 Nov;35(2):C11–C16. doi: 10.1083/jcb.35.2.c11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donlon J., Kaufman S. Glucagon stimulation of rat hepatic phenylalanine hydroxylase through phosphorylation in vivo. J Biol Chem. 1978 Oct 10;253(19):6657–6659. [PubMed] [Google Scholar]
- Elwyn D. H., Parikh H. C., Shoemaker W. C. Amino acid movements between gut, liver, and periphery in unanesthetized dogs. Am J Physiol. 1968 Nov;215(5):1260–1275. doi: 10.1152/ajplegacy.1968.215.5.1260. [DOI] [PubMed] [Google Scholar]
- Goldberg A. L., St John A. C. Intracellular protein degradation in mammalian and bacterial cells: Part 2. Annu Rev Biochem. 1976;45:747–803. doi: 10.1146/annurev.bi.45.070176.003531. [DOI] [PubMed] [Google Scholar]
- Hopgood M. F., Clark M. G., Ballard F. J. Inhibition of protein degradation in isolated rat hepatocytes. Biochem J. 1977 May 15;164(2):399–407. doi: 10.1042/bj1640399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IZZO J. L., GLASSER S. R. Comparative effects of glucagon, hydrocortisone and epinephrine on the protein metabolism of the fasting rat. Endocrinology. 1961 Feb;68:189–198. doi: 10.1210/endo-68-2-189. [DOI] [PubMed] [Google Scholar]
- Jefferson L. S., Schworer C. M., Tolman E. L. Growth hormone stimulation of amino acid transport and utilization by the perfused rat liver. J Biol Chem. 1975 Jan 10;250(1):197–204. [PubMed] [Google Scholar]
- Khairallah E. A., Mortimore G. E. Assessment of protein turnover in perfused rat liver. Evidence for amino acid compartmentation from differential labeling of free and tRNA-gound valine. J Biol Chem. 1976 Mar 10;251(5):1375–1384. [PubMed] [Google Scholar]
- Knowles S. E., Ballard F. J. Selective control of the degradation of normal and aberrant proteins in Reuber H35 hepatoma cells. Biochem J. 1976 Jun 15;156(3):609–617. doi: 10.1042/bj1560609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libby P., Goldberg A. L. Leupeptin, a protease inhibitor, decreases protein degradation in normal and diseased muscles. Science. 1978 Feb 3;199(4328):534–536. doi: 10.1126/science.622552. [DOI] [PubMed] [Google Scholar]
- MILLER L. L. DIRECT ACTIONS OF INSULIN, GLUCAGON, AND EPINEPHRINE ON THE ISOLATED PERFUSED RAT LIVER. Fed Proc. 1965 May-Jun;24:737–744. [PubMed] [Google Scholar]
- MORTIMORE G. E. Effect of insulin on potassium transfer in isolated rat liver. Am J Physiol. 1961 Jun;200:1315–1319. doi: 10.1152/ajplegacy.1961.200.6.1315. [DOI] [PubMed] [Google Scholar]
- Mallette L. E., Exton J. H., Park Effects of glucagon on amino acid transport and utilization in the perfused rat liver. J Biol Chem. 1969 Oct 25;244(20):5724–5728. [PubMed] [Google Scholar]
- Marty F. Cytochemical studies on GERL, provacuoles, and vacuoles in root meristematic cells of Euphorbia. Proc Natl Acad Sci U S A. 1978 Feb;75(2):852–856. doi: 10.1073/pnas.75.2.852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortimore G. E., Mondon C. E. Inhibition by insulin of valine turnover in liver. Evidence for a general control of proteolysis. J Biol Chem. 1970 May 10;245(9):2375–2383. [PubMed] [Google Scholar]
- Mortimore G. E., Neely A. N., Cox J. R., Guinivan R. A. Proteolysis in homogenates of perfused rat liver: responses to insulin, glucagon and amino acids. Biochem Biophys Res Commun. 1973 Sep 5;54(1):89–95. doi: 10.1016/0006-291x(73)90892-9. [DOI] [PubMed] [Google Scholar]
- Mortimore G. E., Schworer C. M. Induction of autophagy by amino-acid deprivation in perfused rat liver. Nature. 1977 Nov 10;270(5633):174–176. doi: 10.1038/270174a0. [DOI] [PubMed] [Google Scholar]
- Neely A. N., Cox J. R., Fortney J. A., Schworer C. M., Mortimore G. E. Alterations of lysosomal size and density during rat liver perfusion. Suppression by insulin and amino acids. J Biol Chem. 1977 Oct 10;252(19):6948–6954. [PubMed] [Google Scholar]
- Novikoff A. B., Shin W. Y. Endoplasmic reticulum and autophagy in rat hepatocytes. Proc Natl Acad Sci U S A. 1978 Oct;75(10):5039–5042. doi: 10.1073/pnas.75.10.5039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross B. D., Hems R., Krebs H. A. The rate of gluconeogenesis from various precursors in the perfused rat liver. Biochem J. 1967 Mar;102(3):942–951. doi: 10.1042/bj1020942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seglen P. O. Protein degradation in isolated rat hepatocytes is inhibited by ammonia. Biochem Biophys Res Commun. 1975 Sep 2;66(1):44–52. doi: 10.1016/s0006-291x(75)80292-0. [DOI] [PubMed] [Google Scholar]
- Steiner A. L., Parker C. W., Kipnis D. M. Radioimmunoassay for cyclic nucleotides. I. Preparation of antibodies and iodinated cyclic nucleotides. J Biol Chem. 1972 Feb 25;247(4):1106–1113. [PubMed] [Google Scholar]
- Ward W. F., Chua B. L., Li J. B., Morgan H. E., Mortimore G. E. Inhibition of basal and deprivation-induced proteolysis by leupeptin and pepstatin in perfused rat liver and heart. Biochem Biophys Res Commun. 1979 Mar 15;87(1):92–98. doi: 10.1016/0006-291x(79)91651-6. [DOI] [PubMed] [Google Scholar]
- Ward W. F., Mortimore G. E. Compartmentation of intracellular amino acids in rat liver. Evidence for an intralysosomal pool derived from protein degradation. J Biol Chem. 1978 May 25;253(10):3581–3587. [PubMed] [Google Scholar]
- Weibel E. R. Stereological principles for morphometry in electron microscopic cytology. Int Rev Cytol. 1969;26:235–302. doi: 10.1016/s0074-7696(08)61637-x. [DOI] [PubMed] [Google Scholar]
- Williamson D. H., Lopes-Vieira O., Walker B. Concentrations of free glucogenic amino acids in livers of rats subjected to various metabolic stresses. Biochem J. 1967 Aug;104(2):497–502. doi: 10.1042/bj1040497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodside K. H., Mortimore G. E. Suppression of protein turnover by amino acids in the perfused rat liver. J Biol Chem. 1972 Oct 25;247(20):6474–6481. [PubMed] [Google Scholar]
- Woodside K. H., Ward W. F., Mortimore G. E. Effects of glucagon on general protein degradation and synthesis in perfused rat liver. J Biol Chem. 1974 Sep 10;249(17):5458–5463. [PubMed] [Google Scholar]
- Zahlten R. N., Hochberg A. A., Stratman F. W., Lardy H. A. Glucagon-stimulated phosphorylation of mitochondrial and lysosomal membranes of rat liver in vivo. Proc Natl Acad Sci U S A. 1972 Apr;69(4):800–804. doi: 10.1073/pnas.69.4.800. [DOI] [PMC free article] [PubMed] [Google Scholar]