Abstract
The study objective was to evaluate the pharmacokinetics (PK), antidrug antibody (ADA), and safety of motavizumab-YTE (motavizumab with amino acid substitutions M252Y/S254T/T256E [YTE]), an Fc-modified anti-respiratory syncytial virus (RSV) monoclonal antibody. Healthy adults (n = 31) were randomized to receive a single intravenous (i.v.) dose of motavizumab-YTE or motavizumab (0.3, 3, 15, or 30 mg/kg) and followed for 240 days. Clearance of motavizumab-YTE was significantly lower (71% to 86%) and the half-life (t1/2) was 2- to 4-fold longer than with motavizumab. However, similar peak concentrations and volume-of-distribution values, indicative of similar distribution properties, were seen at all dose levels. The sustained serum concentrations of motavizumab-YTE were fully functional, as shown by RSV neutralizing activity that persisted for 240 days with motavizumab-YTE versus 90 days postdose for motavizumab. Safety and incidence of ADA were comparable between groups. In this first study of an Fc-modified monoclonal antibody in humans, motavizumab-YTE was well tolerated and exhibited an extended half-life of up to 100 days. (This study has been registered at ClinicalTrials.gov under registration no. NCT00578682.)
INTRODUCTION
Respiratory syncytial virus (RSV) is a major cause of lower respiratory tract infections in infants and young children (1). No vaccine is currently approved for the prevention of RSV illness; however, palivizumab (MedImmune, Gaithersburg, MD) is available as prophylaxis against serious RSV disease in high-risk children. These high-risk children include infants born prematurely, children with chronic lung disease of prematurity (bronchopulmonary dysplasia), and children with congenital heart disease (2). RSV prophylaxis with palivizumab requires monthly intramuscular administration throughout the RSV season to maintain adequate serum concentrations. This monthly administration is necessary as immunoglobulin G (IgG) monoclonal antibodies (MAbs) are subject to proteolytic degradation and elimination, resulting in a serum half-life of approximately 20 days (3). An antibody with an extended half-life would reduce the required dosing frequency during the RSV season.
A growing body of evidence identifies the major histocompatibility complex class I-related neonatal Fc receptor (FcRn) as a critical receptor in maintaining IgG homeostasis and extending the serum half-life of IgG and albumin in humans (4, 5). FcRn was originally identified in the transport of maternal antibodies across the placenta and the fetal small intestines in rats (6). In adult tissues, FcRn localized on vascular endothelium is a key component in the antibody salvage pathway whereby serum IgG undergoes endocytosis and is sequestered in lysosomes (7).
A distinguishing characteristic of the IgG-FcRn pathway is obligate pH dependence. IgG-FcRn binding is driven by acidic pH (6.0) in the lysosome, whereas disassociation occurs at the neutral pH (7.4) of the extracellular environment (3). Acidification (pH 6.0 to 6.5) in the lysosomes enables the binding of FcRn to the Fc region of IgG with a low micromolar affinity and protects it from catabolism. The protected FcRn-bound IgG is subsequently shuttled to the cell surface and released into the extracellular environment. This process protects antibodies by decreasing their exposure to extracellular degradation (3). Selectively increasing the binding affinity of the Fc region of IgG to FcRn at pH 6.0 alone has been shown to increase the half-life in cynomolgus monkeys (8).
Motavizumab is an investigational recombinant humanized monoclonal antibody (MAb) against RSV that was derived by affinity maturation of the murine complementarity-determining regions of the heavy and light chains of palivizumab (9). Motavizumab and palivizumab function by blocking cell-to-cell fusion and inhibiting virus replication (10). Motavizumab has a serum half-life of approximately 24 days in children. Mutations in three amino acids (M252Y/S254T/T256E [YTE]) within the Fc region of motavizumab (referred to as mota-YTE) led to a 10-fold increase in in vitro FcRn binding at pH 6.0 for both humans and monkeys, consequently resulting in a 4-fold increase in in vivo serum half-life in monkeys (8). Antibody bioavailability in the lungs was also increased 4-fold due to sustained serum levels with mota-YTE. To date, the 4-fold increase in the serum half-life of mota-YTE represents the largest improvement in IgG serum longevity in a primate model. Additionally, in primates no antidrug antibodies (ADAs) were detected against mota-YTE, which suggests that the three novel Fc mutations were not more immunogenic than wild-type IgG1 in vivo (8).
With the promising finding of a prolonged serum half-life of the antibody and the absence of any safety findings in nonhuman primates, the present study in healthy adult volunteers was designed to evaluate the pharmacokinetics (PK) and safety profile of mota-YTE, the first Fc-modified monoclonal antibody to be studied in humans.
MATERIALS AND METHODS
Study design.
This was a first-in-human, phase 1, randomized, double-blind, single-dose, escalation study of the pharmacokinetics and safety of mota-YTE (registered at ClinicalTrials.gov under registration number NCT00578682). This study was conducted in accordance with the principles of the Declaration of Helsinki, any applicable laws and requirements, and any conditions required by a regulatory authority and/or IRB/Independent Ethics Committee. The protocol was approved by the Independent Investigational Review Board (Plantation, FL) before initiation. Written informed consent was obtained from each subject before conduct of any protocol-specific activity or study entry.
The study was conducted at a single site in the United States between 19 December 2007 and 5 February 2010. Thirty-one healthy subjects were randomly assigned in a 1:1 ratio to either mota-YTE or motavizumab based on one of four dose cohorts: cohort 1, 0.3 mg/kg dose group; cohort 2, 3 mg/kg dose group; cohort 3, 15 mg/kg dose group; and cohort 4, 30 mg/kg dose group. An interactive voice response system was used for randomization to a treatment arm (using a block size of 2) and assignment of blinded investigational product kits. Protocol-associated personnel, subjects, and clinical site staff were all blinded to treatment assignments. Each subject received a single intravenous infusion of either mota-YTE or motavizumab. The infusion time ranged from 15 min in the lowest-dose cohort to 140 min in the highest-dose cohort. All subjects were followed for safety up to 240 days from the infusion.
Participants.
Study subjects were required to be healthy, normotensive men or women, aged 18 to 45 years with a weight of ≤90 kg. Study subjects were excluded if they had acute illness or fever of ≥99.5°F at study entry, received immunoglobulin or blood products within 60 days before study entry, had received any investigational drug therapy or standard vaccine within 120 days before through 240 days after study drug administration, or had previously received palivizumab or motavizumab.
Study endpoints and assessments.
Endpoints included pharmacokinetic parameters, serum antidrug antibody (ADA) levels, and nasal wash drug levels. Durability of RSV neutralization activity of mota-YTE and motavizumab in serum was also assessed.
Blood was collected for PK parameters predose on day 0, immediately after infusion, at 0.5, 1, 4, 8, and 12 h after infusion, and at all visits through day 240. Serum samples for ADA analysis were collected predose on day 0 and on days 14, 28, 60, 90, 150, 210, and 240. Nasal wash samples were collected for PK assessment predose on day 0 and at every visit through day 240. Drug levels were measured using a validated enzyme-linked immunosorbent assay (ELISA) (serum lower limit of quantitation [LLOQ], 1.56 μg/ml; nasal wash LLOQ, 20 ng/ml). ADAs were detected using a validated electrochemiluminescent assay (positive titer, ≥1:30). Single-dose PK parameters were estimated by noncompartmental analyses using WinNonlin, version 5.2 (Pharsight Corporation, Mountain View, CA).
To assess RSV neutralizing activity, serum samples were selected randomly from higher-dose groups at two early (days 1 and 21), two intermediate (days 42 and 90), and two late (days 180 and 240) time points after drug administration. An RSV microneutralization assay was conducted as previously described (11, 12). Viral replication was measured using an F protein-specific ELISA. For calculations of 50% effective concentration (EC50) values for RSV neutralization, the amount of viral antigen present in wells at different dilutions of MAb was plotted as the A450 versus MAb concentration and analyzed by a four-parameter curve fitting of the sigmoid dose-response curves using Sigma Plot (Systat Software, Inc., San Jose, CA).
The safety and tolerability of mota-YTE were measured by adverse events (AEs) for the period immediately after receipt of investigational product through day 28, serious and targeted AEs (e.g., vasculitis, endocarditis, neuritis, glomerulonephritis, wheezing, abnormal pulmonary function tests, and hypersensitivity reactions) through day 240, and laboratory AEs and AEs of interest (i.e., liver function tests and reactions at the site of infusion) through day 90. In this first-in-human study, the investigators were instructed to report any laboratory and vital sign values that were outside the normal range as an AE, regardless of clinical significance. Thus, any minor variation from the normal reference range for laboratory values or vital signs was reported even though it would not be considered a clinically significant change.
Statistical analyses.
The phase 1 study of motavizumab in adults (study MI-CP101, 2004 [MedImmune, Gaithersburg, MD, data on file]) was used for sample size determination. The coefficient of variation for the area under the concentration-time curve (AUC) of motavizumab ranged from 10% to 22% across the dose range of 3 mg/kg to 30 mg/kg. Therefore, it was assumed that a 22% coefficient of variation for mota-YTE and a sample size of six subjects per arm in each dose cohort would be sufficient to detect a 2-fold increase in AUC values with ≥95% power. Mean AUC results for mota-YTE and motavizumab were compared using a Student t test.
RESULTS
Study subjects.
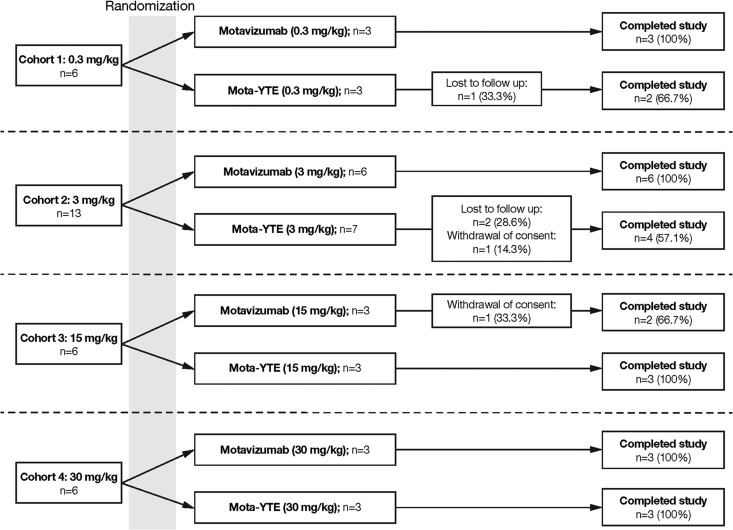
A total of 31 healthy adults were randomly assigned to receive mota-YTE (n = 16) or motavizumab (n = 15) at a single site in the United States between 19 December 2007 and 5 February 2010. Twenty-six (84%) of the 31 subjects completed the study: 12/16 (75%) were mota-YTE recipients, and 14/15 (93.3%) were motavizumab recipients (Fig. 1). Four subjects (25%) who received mota-YTE and one subject (6.7%) who received motavizumab did not complete the study. Reasons for not completing the study included failure to follow up (n = 3) and withdrawal of consent (n = 2).
Fig 1.
Disposition of subjects in the four treatment cohorts.
Overall, demographic characteristics were similar between the mota-YTE and motavizumab groups, with the exception of a higher proportion of men in the mota-YTE group (12/16 [75.0%]) than in the motavizumab group (8/15 [53.3%]) (Table 1). The majority of the subjects across the mota-YTE and motavizumab groups were white (14/16 [87.5%] and 11/15 [73.3%], respectively) and not of Hispanic or Latino ethnicity (15/16 [93.8%] and 13/15 [86.7%], respectively). The mean age overall was 30.5 years and 26.3 years in the mota-YTE and motavizumab groups, respectively. The mean body weight was 76.57 kg and 69.55 kg in the mota-YTE and motavizumab groups, respectively.
Table 1.
Demographics
| Parameter | Value for the parameter by group |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0.3 mg/kg |
3 mg/kg |
15 mg/kg |
30 mg/kg |
All |
||||||
| Mota-YTE (n = 3) | Mota (n = 3) | Mota-YTE (n = 7) | Mota (n = 6) | Mota-YTE (n = 3) | Mota (n = 3) | Mota-YTE (n = 3) | Mota (n = 3) | Mota-YTE (n = 16) | Mota (n = 15) | |
| Mean age (yr [SD]) | 37.0 (4.6) | 27.3 (6.0) | 29.0 (8.5) | 26.5 (7.7) | 30.3 (7.8) | 25.3 (5.7) | 27.7 (7.6) | 26.0 (2.0) | 30.5 (7.6) | 26.3 (5.7) |
| No. of male subjects (%) | 1 (33.3) | 2 (66.7) | 6 (85.7) | 3 (50.0) | 3 (100.0) | 1 (33.3) | 2 (66.7) | 2 (66.7) | 12 (75.0) | 8 (53.3) |
| No. of white subjects (%) | 3 (100.0) | 3 (100.0) | 5 (71.4) | 5 (83.3) | 3 (100.0) | 0 (0.0) | 3 (100.0) | 3 (100.0) | 14 (87.5) | 11 (73.3) |
| No. of subjects of non-Hispanic/Latino, ethnicity (%) | 3 (100.0) | 3 (100.0) | 6 (85.7) | 5 (83.3) | 3 (100.0) | 3 (100.0) | 3 (100.0) | 2 (66.7) | 15 (93.8) | 13 (86.7) |
| Mean wt (kg [SD]) | 75.30 (7.30) | 68.47 (4.70) | 78.03 (9.99) | 71.75 (12.15) | 79.77 (5.95) | 66.53 (4.52) | 71.27 (10.25) | 69.23 (13.95) | 76.57 (8.64) | 69.55 (9.53) |
Pharmacokinetics.
The maximum concentrations in serum (Cmax) of mota-YTE and motavizumab increased in a dose-proportional manner (Fig. 2). Mean Cmax values ranged from 9 μg/ml to 938 μg/ml across the dose range of 0.3 mg/kg to 30 mg/kg. The similar Cmax and similar volume-of-distribution values (volume of distribution at steady state [Vss], 4.6 to 8.3 liters for mota-YTE and 5.2 to 9.5 liters for motavizumab) (Table 2) indicate no difference in the distribution properties of the mota-YTE molecule compared with motavizumab.
Fig 2.
Dose proportionality of mota-YTE and motavizumab maximum concentration and area under the curve.
Table 2.
Motavizumab and mota-YTE mean pharmacokinetic parameter values
| Dose and treatment | Mean value for the parametera |
|||||
|---|---|---|---|---|---|---|
| Cmax (μg/ml) | Clearance (ml/day) | Half-life (days) | Vol of distribution (liters) | AUClast (μg · day/ml) | AUCinf (μg · day/ml) | |
| 0.3 mg/kg | ||||||
| Mota-YTE | 8.8 (16.2) | 58.5 (7.8) | 69.5 (2.4) | 5.8 (2.7) | 300.4 (3.8) | 342.8 (7.8) |
| Motavizumab | 8.9 (24.1) | 203.2 (12.4) | 18.9 (28.3) | 5.3 (27.3) | 90.4 (14.6) | 99.5 (13.1) |
| 3 mg/kg | ||||||
| Mota-YTE | 82.8 (31.8) | 44.2 (19.6) | 100.4 (10.5) | 6.3 (17.3) | 4,193.3* (6.4) | 5,224.0* (4.2) |
| Motavizumab | 59.3 (39.1) | 322.8 (46.7) | 22.3 (33.5) | 8.5 (39.7) | 784.6 (58.9) | 850.5 (56.8) |
| 15 mg/kg | ||||||
| Mota-YTE | 324.1 (15.0) | 67.5 (18.7) | 84.3 (18.6) | 8.3 (11.5) | 15,328.7† (11.4) | 18,022.3† (13.6) |
| Motavizumab | 232.9 (4.3) | 325.6 (34.2) | 20.4 (52.2) | 9.5 (12.7) | 2,903.8 (19.0) | 3,262.1 (26.8) |
| 30 mg/kg | ||||||
| Mota-YTE | 938.1 (3.6) | 43.3 (3.8) | 73.2 (4.0) | 4.6 (1.1) | 44,159.4† (14.9) | 49,276.6† (13.7) |
| Motavizumab | 898.2 (13.9) | 164.9 (33.7) | 34.4 (25.8) | 5.2 (13.5) | 13,180.6 (31.3) | 13,303.3 (31.3) |
The coefficient of variation (percent) is given in parentheses. AUC, area under the curve; Cmax, maximum concentration, infinity. Significant difference is indicated as follows: *, P < 0.001 for mota-YTE compared with motavizumab; †, P < 0.01 for mota-YTE compared with motavizumab.
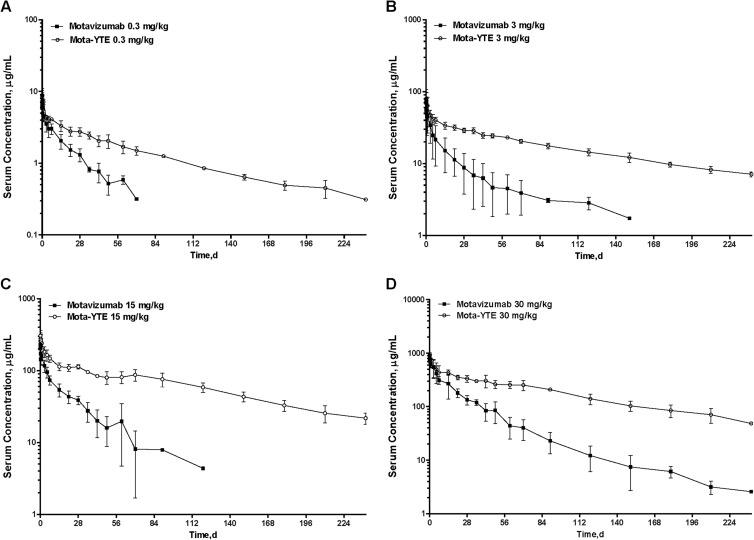
In contrast, serum clearance was decreased 71 to 86% (43 to 68 ml/day) with mota-YTE compared with motavizumab (165 to 326 ml/day). Correspondingly, the half-life of mota-YTE increased 2- to 4-fold, from 19 to 34 days for motavizumab to 70 to 100 days for mota-YTE. For example, at the lowest dose level of 0.3 mg/kg, motavizumab serum concentrations had declined to below detection limits by day 90, whereas mota-YTE was detectable in serum for 240 days.
Mota-YTE had a significantly greater area under the curve to last measurable time point (AUClast) and AUC to infinity (AUCinf) than motavizumab at all dose levels: 0.3 mg/kg (P < 0.001 and P = 0.026, respectively), 3 mg/kg (P < 0.001 for both), 15 mg/kg (P = 0.004 and P = 0.005, respectively), and 30 mg/kg (P = 0.004 and P = 0.003, respectively). Across all doses, serum concentrations of mota-YTE persisted for a longer period than those of motavizumab (Fig. 3). Moreover, intersubject variability in serum concentrations was lower with mota-YTE than with motavizumab across all doses.
Fig 3.
Means (± standard deviations) of mota-YTE and motavizumab serum concentrations after a single dose. d, days.
Mota-YTE and motavizumab were not detectable in nasal wash aspirates at the 0.3 mg/kg dose level (detection limit of 20 ng/ml) but were detectable at the higher dose levels. In the 3 mg/kg, 15 mg/kg, and 30 mg/kg dose groups, respectively, mean Cmax values in nasal wash aspirates were 220.8 ng/ml, 198.0 ng/ml, and 231.4 ng/ml for mota-YTE and 86.4 ng/ml, 33.9 ng/ml, and 135.2 ng/ml for motavizumab. In the 30 mg/kg dose groups, mota-YTE and motavizumab were observed in nasal wash aspirates for up to 180 and 42 days, respectively.
RSV neutralizing activity.
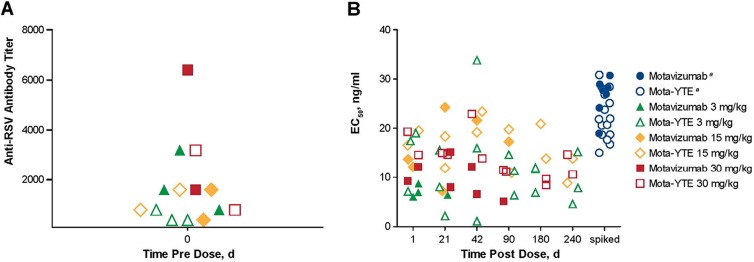
Of the 31 subjects participating in the study, 14 were randomly selected from higher-dose groups for RSV microneutralization testing (mota-YTE, n = 7; motavizumab, n = 7). All subjects demonstrated preexisting RSV antibodies before administration of mota-YTE or motavizumab. Predose anti-RSV neutralizing antibody titers were used to determine baseline RSV neutralizing activity. The baseline RSV neutralization titer was subtracted from measured values to account for preexisting antibody induced by natural RSV exposure. Microneutralization assays demonstrated RSV neutralizing activity in the serum of subjects up to day 240 postdose for mota-YTE and up to day 90 postdose for motavizumab (Fig. 3). Despite the decline in serum concentrations of mota-YTE and motavizumab over time, the EC50 values were comparable between day 1 and the later time points, suggesting that both antibodies remain fully functional for their RSV neutralizing activities (Fig. 4).
Fig 4.
(A) Predose baseline anti-RSV neutralizing antibody titers. (B) Postdose anti-RSV neutralizing activity of mota-YTE and motavizumab. EC50, half-maximal effective concentration. aSpiked concentrations were derived by protein quantification normalized to dilution.
Antidrug antibody response.
Antidrug antibody responses were detectable in 25% (4/16) of subjects in the mota-YTE groups and 20% (3/15) of subjects in the motavizumab groups. Individual ADA titers ranged from 1:30 to 1:240 for mota-YTE and from 1:30 to 1:120 for motavizumab (Table 3). The presence of ADA did not affect the pharmacokinetics of mota-YTE, whereas the presence of anti-motavizumab ADA increased clearance of motavizumab in two out the three ADA-positive subjects.
Table 3.
Antidrug antibodies and pharmacokinetics after mota-YTE and motavizumab treatment
| Treatment group (drug and dose) | Titera | Positive titer [study day(s)] | CL (liters/day)b | t1/2 (days)b |
|---|---|---|---|---|
| Mota-YTE | ||||
| 0.3 mg/kgc | 1:120 | 14 | NC | NC |
| 3 mg/kg | 1:60 | 150, 210, 240 | 0.0512 | 106 |
| ≤1:30d | 210 | 0.0322 | 111 | |
| 15 mg/kg | 1:120–1:240 | 150, 210, 240 | 0.0774 | 85.2 |
| Motavizumab | ||||
| 3 mg/kg | 1:30 | 150, 210 | 0.522 | 18.9 |
| 1:120 | 150, 210, 240 | 0.336 | 15.7 | |
| 15 mg/kg | 1:60–1:120 | 150, 210, 240 | 0.453 | 13.2 |
Each titer represents one subject.
Data correspond to the study day with the first positive result. CL, serum clearance; NC, not calculated; t1/2, terminal phase half-life.
One subject in the 0.3 mg/kg mota-YTE group and one in the 30 mg/kg mota-YTE group with anti-mota-YTE ADA had detectible titers predose (suggesting that these are false positives), and they are not included in table.
Borderline positive.
Safety profile.
Adverse events (AEs) through day 28 were reported in 15/16 subjects (93.8%) who received mota-YTE and in 14/15 subjects (93.3%) who received motavizumab, for a total of 43 and 39 AEs, respectively. There were no deaths, serious AEs, targeted AEs, or AEs resulting in discontinuation of study drug in either treatment group.
The frequencies of AEs were generally similar in both treatment groups. The most common AEs for mota-YTE and motavizumab, respectively, were increased respiratory rate (50.0% versus 46.7%), headache (25.0% versus 33.3%), decreased hemoglobin (18.8% versus 20.0%), proteinuria (12.5% versus 26.7%), and upper respiratory tract infection (0% versus 20.0%). All AEs in the mota-YTE groups and all except one AE in the motavizumab treatment arms were mild or moderate in severity. The one exception occurred in a subject who received motavizumab at 3 mg/kg and experienced grade 3 proteinuria with normal blood urea nitrogen and creatinine levels. The proteinuria was noted on a scheduled follow-up visit 28 days after dosing, resolved 8 days after onset, and was deemed possibly related to the investigational drug by the site investigator. The rate or severity of AEs did not increase with dose escalation in either treatment group. In this small study, there were no reported adverse events consistent with a hypersensitivity reaction in either treatment group.
The frequencies of treatment-related AEs were similar in both treatment groups. Thirty treatment-related events (15 for each treatment) were reported in 7/16 (43.8%) mota-YTE recipients and in 9/15 (60.0%) motavizumab recipients. Having elevated hepatic transaminases was designated an AE of interest and was reported in two subjects (12.5%) in the mota-YTE 3 mg/kg treatment group and in one subject (6.7%) in the motavizumab 3 mg/kg treatment group. These events were mild in severity and not considered to be clinically significant.
DISCUSSION
This is the first study of a humanized monoclonal antibody engineered to have increased binding affinity to FcRn to extend the serum half-life in humans. In healthy adult subjects, inclusion of the YTE mutation in the Fc domain of motavizumab (mota-YTE) decreased clearance by 71% to 86% and increased serum half-life 2- to 4-fold (up to 100 days) compared with the parent antibody, motavizumab. The fold increase in serum half-life observed in this study is similar to increases observed for mota-YTE in preclinical studies conducted in cynomolgus monkeys (8). Overall, the pharmacokinetics of mota-YTE demonstrates increased systemic exposure compared with motavizumab. In addition to increased serum persistence of antibody levels, mota-YTE maintained RSV neutralizing activity up to 240 days, a 2.7-fold increase in duration of activity relative to motavizumab. The increased persistence of serum levels of mota-YTE was not associated with an increase in the percentage of subjects with detectable ADA relative to motavizumab. Moreover, the inclusion of the YTE substitution did not induce an appreciable increase in ADA titers among the healthy adults in this study. Overall, mota-YTE and motavizumab ADA titers were low (≤1:240) and did not impact PK.
A number of monoclonal antibodies with targeted Fc mutations are currently in development for a variety of diseases (13). These mutations include QL mutations on amino acid residues 250 and 428, an N434 mutation on a single amino acid, AAA mutations on amino acid residues 307, 380, and 434, and LS mutations on amino acid residues 428 and 434. The mutated antibodies increased FcRn binding, ranging from 3.4- to 500-fold compared with the parent antibody, and resulted in various magnitudes of half-life extension in preclinical studies in monkeys. IgG-FcRn interactions provide a control point for manipulating the pharmacokinetics of IgG monoclonal antibodies used in therapy, and strategies have been developed to both increase and decrease serum persistence of IgG based on this interaction (13). Although some therapeutic strategies have targeted endogenous FcRn, site-directed mutagenesis of the Fc region of IgG monoclonal antibody has been the preferred strategy for regulating antibody longevity. To date, IgG mutations that have increased IgG-FcRn affinity center around five groups of mutations targeting discrete amino acids in the Fc region (13). Fc mutations that increase binding affinity at pH 7.4 and pH 6.0 can accelerate elimination of IgG instead of extending its half-life (7). Fc mutations that increase FcRn binding at pH 6.0 with no binding at pH 7.4 ensure efficient recycling of IgG and extension of half-life (7, 14–16).
Whereas other antibodies have demonstrated interesting findings in monkeys (13), this current study is the first report of a YTE-mutated IgG1 monoclonal antibody in humans that clearly demonstrates an extended serum half-life while retaining functional activity of the antibody compared with motavizumab. The similar degrees of half-life extension seen over the wide dose range of 0.3 mg/kg to 30 mg/kg indicate nonsaturability of the FcRn system following conventional therapeutic doses of monoclonal antibodies. This confirms the high capacity of the FcRn salvage system in humans and is not surprising given the high levels (1,275 mg/dl) of circulating endogenous IgG (17).
With regard to safety, mota-YTE has a safety profile that is similar to that of the parent antibody, motavizumab. Mota-YTE was generally well tolerated in study subjects, with similar frequencies and types of self-limited AEs reported in both treatment arms. In addition, there was no appreciable difference in the percentages of subjects with detectable ADA for mota-YTE or motavizumab recipients.
Preclinical studies of other YTE-mutated monoclonal antibodies targeting interleukin-6 (IL-6) (18) and alpha toxin secreted by Staphylococcus aureus (19) have demonstrated significant half-life extensions. A similar magnitude of half-life extension with no loss of biological activity as that seen with mota-YTE in this study is expected for other monoclonal antibodies that follow linear pharmacokinetics. Thus, this technology can be used to reduce dosing frequency for patients treated with monoclonal antibodies, particularly in chronic disease settings.
Conclusion.
This is the first clinical study of an Fc-modified, humanized monoclonal antibody in human subjects demonstrating a significant increase in serum half-life of up to 100 days with mota-YTE. Mota-YTE was well tolerated and had a safety profile that was similar to that of the parent antibody. Although this was a study of an anti-RSV monoclonal antibody, the YTE triple mutation on the Fc region is likely to extend the serum half-life of any IgG1 monoclonal antibody. In this study, the YTE triple mutation did not impact the distribution properties of the monoclonal antibody or its functional activity. These results support the application of YTE technology to other antibodies as a means of potential reduction in dosing frequency for various disease settings.
ACKNOWLEDGMENTS
We thank Ruth Pereira of MedImmune for her critical review of the manuscript and her valuable comments.
This study was sponsored by MedImmune, the biologics arm of AstraZeneca. G.J.R., W.F.D., K.J., N.K.P., and M.P.G. are employees of MedImmune; all employees own stock/stock options of AstraZeneca. G.A.L. and R.C. were employees of MedImmune at the time of the analysis. MedImmune was involved in the study design, collection, analysis, and interpretation of data, the writing of this report, and the decision to submit this paper for publication.
We thank Tracy E. Bunting-Early, Susan E. DeRocco, John E. Fincke, and Gerard P. Johnson of Complete Healthcare Communications, Inc. (Chadds Ford, PA) for editorial assistance, which was funded by MedImmune.
Footnotes
Published ahead of print 30 September 2013
REFERENCES
- 1. Hall CB, Weinberg GA, Iwane MK, Blumkin AK, Edwards KM, Staat MA, Auinger P, Griffin MR, Poehling KA, Erdman D, Grijalva CG, Zhu Y, Szilagyi P. 2009. The burden of respiratory syncytial virus infection in young children. N. Engl. J. Med. 360:588–598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. American Academy of Pediatrics 2012. Respiratory syncytial virus, p 609–617 In Pickering LK, Baker CJ, Kimberlin DW, Long SS. (ed), Red book: 2012 report of the Committee on Infectious Diseases, 29th ed. American Academy of Pediatrics, Elk Grove Village, IL [Google Scholar]
- 3. Mould DR, Sweeney KR. 2007. The pharmacokinetics and pharmacodynamics of monoclonal antibodies—mechanistic modeling applied to drug development. Curr. Opin. Drug Discov. Devel. 10:84–96 [PubMed] [Google Scholar]
- 4. Roopenian DC, Akilesh S. 2007. FcRn: the neonatal Fc receptor comes of age. Nat. Rev. Immunol. 7:715–725 [DOI] [PubMed] [Google Scholar]
- 5. Simister NE, Mostov KE. 1989. An Fc receptor structurally related to MHC class I antigens. Nature 337:184–187 [DOI] [PubMed] [Google Scholar]
- 6. Simister NE, Rees AR. 1985. Isolation and characterization of an Fc receptor from neonatal rat small intestine. Eur. J. Immunol. 15:733–738 [DOI] [PubMed] [Google Scholar]
- 7. Dall'Acqua WF, Woods RM, Ward ES, Palaszynski SR, Patel NK, Brewah YA, Wu H, Kiener PA, Langermann S. 2002. Increasing the affinity of a human IgG1 for the neonatal Fc receptor: biological consequences. J. Immunol. 169:5171–5180 [DOI] [PubMed] [Google Scholar]
- 8. Dall'Acqua WF, Kiener PA, Wu H. 2006. Properties of human IgG1s engineered for enhanced binding to the neonatal Fc receptor (FcRn). J. Biol. Chem. 281:23514–23524 [DOI] [PubMed] [Google Scholar]
- 9. Abarca K, Jung E, Fernandez P, Zhao L, Harris B, Connor EM, Losonsky GA, Motavizumab Study Group 2009. Safety, tolerability, pharmacokinetics, and immunogenicity of motavizumab, a humanized, enhanced-potency monoclonal antibody for the prevention of respiratory syncytial virus infection in at-risk children. Pediatr. Infect. Dis. J. 28:267–272 [DOI] [PubMed] [Google Scholar]
- 10. Groothuis JR, Hoopes JM, Hemming VG. 2011. Prevention of serious respiratory syncytial virus-related illness. II: Immunoprophylaxis. Adv. Ther. 28:110–125 [DOI] [PubMed] [Google Scholar]
- 11. Anderson LJ, Hierholzer JC, Bingham PG, Stone YO. 1985. Microneutralization test for respiratory syncytial virus based on an enzyme immunoassay. J. Clin. Microbiol. 22:1050–1052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wu H, Pfarr DS, Johnson S, Brewah YA, Woods RM, Patel NK, White WI, Young JF, Kiener PA. 2007. Development of motavizumab, an ultra-potent antibody for the prevention of respiratory syncytial virus infection in the upper and lower respiratory tract. J. Mol. Biol. 368:652–665 [DOI] [PubMed] [Google Scholar]
- 13. Kuo TT, Aveson VG. 2011. Neonatal Fc receptor and IgG-based therapeutics. MAbs 3:422–430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Yeung YA, Leabman MK, Marvin JS, Qiu J, Adams CW, Lien S, Starovasnik MA, Lowman HB. 2009. Engineering human IgG1 affinity to human neonatal Fc receptor: impact of affinity improvement on pharmacokinetics in primates. J. Immunol. 182:7663–7671 [DOI] [PubMed] [Google Scholar]
- 15. Suzuki T, Ishii-Watabe A, Tada M, Kobayashi T, Kanayasu-Toyoda T, Kawanishi T, Yamaguchi T. 2010. Importance of neonatal FcR in regulating the serum half-life of therapeutic proteins containing the Fc domain of human IgG1: a comparative study of the affinity of monoclonal antibodies and Fc-fusion proteins to human neonatal FcR. J. Immunol. 184:1968–1976 [DOI] [PubMed] [Google Scholar]
- 16. Wang W, Lu P, Fang Y, Hamuro L, Pittman T, Carr B, Hochman J, Prueksaritanont T. 2011. Monoclonal antibodies with identical Fc sequences can bind to FcRn differentially with pharmacokinetic consequences. Drug Metab. Dispos. 39:1469–1477 [DOI] [PubMed] [Google Scholar]
- 17. Veys EM, van Leare M. 1973. Serum IgG, IgM, and IgA levels in ankylosing spondylitis. Ann. Rheum. Dis. 32:493–496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Faggioni R, Yao Z, Liang M, Schneider A, Vainshtein I, Lee R, Roskos L. 2010. Effect of the YTE mutation on the PK/PD of MEDI5117, abstr T2091. Abstr. Am. Assoc. Pharm. Sci. Natl. Biotechnol. Conf., San Francisco, CA, 17 to 19 May 2010. American Association of Pharmaceutical Scientists, Arlington, VA [Google Scholar]
- 19. Yu X, Iciek L, Criste R, Sellman B, Stover C, Jafri H, Esser M, Roskos L, Robbie G. 2013. Modeling of pharmacokinetics (PK) of a YTE-monoclonal antibody targeting Staphylococcus aureus alpha toxin in cynomolgus monkeys: human PK prediction, abstr T2123. Abstr. Am. Assoc. Pharm. Sci. Natl. Biotechnol. Conf., San Francisco, CA, 17 to 19 May 2010. American Association of Pharmaceutical Scientists, Arlington, VA [Google Scholar]