Abstract
We report the epidemiological impact of carbapenemase-producing Enterobacteriaceae (CPE) in Spain in 2012. Of the 237 carbapenemases detected, 163 were from the OXA-48 group, 60 were from VIM-1, 8 were from KPC-2, 5 were from IMP, and 1 was from NDM-1. Interhospital spread of carbapenemase-producing Klebsiella pneumoniae was due to a limited number of multilocus sequence types (MLST) and carbapenemase types, including ST15–VIM-1, ST11–OXA-48, ST405–OXA-48, ST101–KPC-2, and ST11–VIM-1. The number of CPE cases in Spain has increased sharply in recent years, due mainly to the emergence of OXA-48.
TEXT
In recent years, Enterobacteriaceae isolates, mainly Klebsiella pneumoniae, have increased their potential to become extensively drug resistant by acquiring resistance to carbapenems (1–3), due mainly to the production of carbapenemases.
In general, carbapenemases hydrolyze all β-lactam antibiotics (1–3). The most clinically important carbapenemases produced by Enterobacteriaceae are the class B metallo-β-lactamases (MBLs), represented by VIM, IMP, and NDM types, the class A enzymes of the KPC type, and the class D enzymes, represented by the OXA-48 type (3). In Spain, the number of reports on carbapenemase-producing Enterobacteriaceae (CPE) has increased in recent years (4–10). However, comprehensive assessment of the impact of CPE in Spain is still missing.
Our institute has run an active and unrestricted Antibiotic Resistance Surveillance Program at the national level since 2009. When this program was launched, all Spanish clinical microbiology laboratories and health-associated professionals were personally contacted and encouraged to submit their carbapenem-resistant Enterobacteriaceae isolates to our antibiotic reference lab for molecular and epidemiological characterization.
Enterobacteriaceae isolates were identified by standard microbiological methods and a MicroScan semiautomated system (MicroScan; Siemens Healthcare Diagnostics, Deerfield, IL, USA). If necessary, species identification was confirmed by 16S ribosomal DNA sequencing.
Antibiotic susceptibility testing was carried out by broth microdilution (panel type Neg MIC 31; MicroScan) and by the disc diffusion method according to the EUCAST guidelines (11). Isolates were considered nonsusceptible to carbapenems if they were either resistant or intermediate to at least one of the three carbapenem antibiotics tested (imipenem, meropenem, and ertapenem) according to EUCAST breakpoints (11). A modified Hodge test using an ertapenem disk was performed on all isolates. Inhibition of carbapenemase activity was carried out by comparing the inhibition zones obtained from ertapenem disks, with or without EDTA (10 μl 0.5 M solution) and phenyl-boronic acid (400 μg).
The presence of genes encoding carbapenemases, blaKPC, blaVIM, blaIMP, and blaNDM, was confirmed by PCR and DNA sequencing (5, 12, 13). Specific primers for PCR amplification and sequencing of blaOXA-48-like genes were designed according to GenBank (National Center for Biotechnology Information, National Institutes of Health, Bethesda, MD, USA) database entry AY236073 (OXA-48-TOT-F, 5′-TGCGTGTATTAGCCTTATCG-3′; OXA-48-TOT-R, 5′-TTTTTCCTGTTTGAGCACTTC-3′).
Multilocus sequence type (MLST) was determined in all carbapenemase-producing K. pneumoniae isolates according to the Institut Pasteur scheme (http://www.pasteur.fr/recherche/genopole/PF8/mlst/Kpneumoniae.html; data last accessed in May 2013). Escherichia coli isolates were typed by MLST according to the University College Cork (Cork, Ireland) scheme (http://mlst.ucc.ie/mlst/dbs/Ecoli; data last accessed in May 2013).
In 2012, 357 isolates of Enterobacteriaceae nonsusceptible to carbapenems were studied in detail, and only one isolate per patient was considered. They came from 49 Spanish hospitals (about 20% of all Spanish microbiology laboratories) located in 24 geographic areas. The estimated catchment population was about 21.5%, corresponding to approximately 10.5 million. Of these 357 isolates, 237 (66.4%) had a carbapenemase gene positively identified and distributed as follows: 203 (74.4%) carbapenemases were identified in 273 carbapenem-nonsusceptible K. pneumoniae isolates, 6 (75%) in 8 Klebsiella oxytoca isolates, 16 (36.4%) in 44 Enterobacter cloacae isolates, 2 (25%) in 8 Enterobacter aerogenes isolates, 4 (22.2%) in 18 Escherichia coli isolates, and 6 (100%) in 6 single isolates of Serratia marcescens, Morganella morganii, Citrobacter freundii, and Enterobacter spp. (Table 1).
Table 1.
Distribution of carbapenemase-producing Enterobacteriaceae in 2012 in Spain according to the national surveillance program of the Instituto de Salud Carlos III
| Species | No. of isolates (%) |
|||||
|---|---|---|---|---|---|---|
| With CBPa | OXA-48 group | VIM group | KPC group | IMP group | NDM group | |
| K. pneumoniae | 203 (85.6) | 153 | 40 | 6 | 3 | 1 |
| K. oxytoca | 6 (2.5) | 0 | 6 | 0 | 0 | 0 |
| E. cloacae | 16 (6.8) | 7 | 6 | 1 | 2 | 0 |
| E. aerogenes | 2 (0.8) | 2 | 0 | 0 | 0 | 0 |
| E. coli | 4 (1.7) | 1 | 3 | 0 | 0 | 0 |
| S. marcescens | 1 (0.4) | 0 | 0 | 1 | 0 | 0 |
| M. morganii | 1 (0.4) | 0 | 1 | 0 | 0 | 0 |
| C. freundii | 1 (0.4) | 0 | 1 | 0 | 0 | 0 |
| Enterobacter spp. | 3 (1.3) | 0 | 3 | 0 | 0 | 0 |
| Total | 237 | 163 | 60 | 8 | 5 | 1 |
CBP, carbapenemases.
A total of 149 (62.9%) isolates were from males and 76 (32.1%) were from patients ≥65 years old. Of the 237 CPE isolates, 162 (68.4%) produced clinical infections: 71 (43.8%) urinary tract infections (UTI), 37 (22.8%) blood infections, 28 (17.3%) respiratory tract infections, 13 (8%) wound infections, and 13 (8%) other infections. The remaining 75 isolates (31.6%) were obtained from carriers, mainly from rectal samples.
The carbapenemases detected were from the following groups: 163 from OXA-48 (84 from OXA-48 and 79 from OXA-245), 60 from VIM-1, 8 from KPC-2, 5 from IMP (2 from IMP-22 and 3 from IMP-8), and 1 from NDM-1 (Table 1). These CPE isolates came from 30 Spanish hospitals (average of 8.1 CPE isolates per hospital, range of 1 to 83) located in 14 geographic areas. Six hospitals had more than 10 CPE cases; the remaining 24 hospitals had between one and nine cases.
Susceptibility to carbapenem antibiotics is depicted in Table 2; all carbapenemase-producing isolates were ertapenem nonsusceptible, but of the OXA-48-like and VIM-1 producers, 66.3% and 15% were susceptible to imipenem, respectively.
Table 2.
Susceptibility to carbapenem antibiotics in carbapenemase-producing Enterobacteriaceae isolated in Spain (2012)
| Carbapenemase type (no. of isolates) | Carbapenem | MIC (μg/ml) |
%a |
||||
|---|---|---|---|---|---|---|---|
| Range | 50% | 90% | S | I | R | ||
| OXA-48 like (n = 163) | Ertapenem | 2–>4 | 4 | >4 | 0 | 0 | 100 |
| Imipenem | ≤1–>8 | 2 | >8 | 66.3 | 20.2 | 13.5 | |
| Meropenem | ≤1–>8 | 4 | >8 | 30 | 47.9 | 22.1 | |
| VIM-1 (n = 60) | Ertapenem | 1–>4 | 4 | >4 | 0 | 10 | 90 |
| Imipenem | ≤1–>8 | 4 | >8 | 15 | 55 | 30 | |
| Meropenem | ≤1–>8 | 8 | >8 | 18.3 | 40 | 41.7 | |
| IMP-like (n = 5) | Ertapenem | 4–>4 | >4 | >4 | 0 | 0 | 100 |
| Imipenem | ≤1–2 | 2 | 2 | 100 | 0 | 0 | |
| Meropenem | 2–>8 | 8 | >8 | 20 | 60 | 20 | |
| KPC-like (n = 8) | Ertapenem | >4–>4 | >4 | >4 | 0 | 0 | 100 |
| Imipenem | 4–>8 | 4 | >8 | 0 | 75 | 25 | |
| Meropenem | 2–>8 | 8 | >8 | 25 | 25 | 50 | |
S, susceptible isolates according to EUCAST breakpoints; I, intermediate isolates according to EUCAST breakpoints; R, resistant isolates according to EUCAST breakpoints.
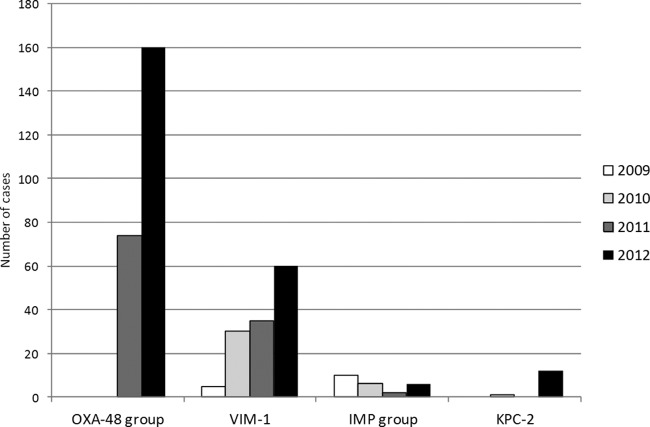
From 2009 to 2012, we observed an increase in the number of CPE isolates submitted to the surveillance program: 15 isolates in 2009, 38 in 2010, 112 in 2011, and 237 in 2012 (16-fold increase). The number of hospitals submitting cases increased from 6 in 2009 to 30 in 2012 (5-fold increase) (Fig. 1). Although VIM-1 was the first carbapenemase described in Spain (14), its frequency has been widely surpassed by the abrupt emergence of OXA-48 in the last 2 years (Fig. 1).
Fig 1.
Yearly evolution (2009–2012) of carbapenemase-producing Enterobacteriaceae in Spain and number of individual hospitals reporting cases to the national surveillance program of the Instituto de Salud Carlos III.
The frequency and distribution of carbapenemases are distinct in different countries. A rapid dissemination of KPC-producing K. pneumoniae was first noticed in the United States (3). Later, isolates producing KPC-2 and KPC-3 also emerged in Latin America, Israel, and Greece (1, 3). A recent study showed that KPC enzymes were the most common (89.5%) found in Italy (15). Outbreaks caused by OXA-48-producing K. pneumoniae have been described in several countries (1, 3, 16).
According to our data, OXA-48 is by far the most common carbapenemase type circulating in Spain in K. pneumoniae (75.4% in this study), followed by VIM (19.7%) (Table 1). The carbapenemase-producing K. pneumoniae isolates belonged to 12 different sequence types (STs) (Table 3), although most of them (88.7%) were carried by four major clones: ST11, ST15, ST16, and ST405. ST11, ST15, and ST16 have been described previously to be associated with different outbreaks due to extended-spectrum β-lactamases (ESBLs) or carbapenemase-producing K. pneumoniae (1, 7, 9, 17). ST405 was recently associated with OXA-48 production in Spain and Belgium (7, 8, 16) and was found in this study in eight hospitals from three geographic regions. These data may suggest that ST405 has been established in Spain and contributes to the dissemination of OXA-48.
Table 3.
Distribution of Klebsiella pneumoniae MLST clones producing carbapenemases in Spain in 2012 according to the national surveillance program of the Instituto de Salud Carlos III
| ST | Carbapenemase | No. of cases | No. of hospitals | Geographic source(s) |
|---|---|---|---|---|
| 11 | OXA-245 | 76 | 1 | Málaga |
| VIM-1 | 14 | 6 | Madrid, Guadalajara | |
| OXA-48 | 12 | 7 | Madrid | |
| KPC-2 | 3 | 3 | Madrid, Ciudad Real | |
| NDM-1 | 1 | 1 | Alicante | |
| 15 | VIM-1 | 15 | 4 | Madrid, Barcelona, Ávila |
| OXA-48 | 4 | 2 | Madrid, Málaga | |
| 405 | OXA-48 | 38 | 8 | Madrid, Barcelona, Guadalajara |
| 16 | OXA-48 | 17 | 2 | Asturias |
| 147 | VIM-1 | 5 | 1 | Alicante |
| 340 | VIM-1 | 5 | 1 | Madrid |
| 437 | OXA-245 | 3 | 1 | Málaga |
| 101 | KPC-2 | 3 | 2 | Madrid |
| 464 | IMP-8 | 3 | 1 | Almeria |
| 846 | OXA-48 | 2 | 1 | Madrid |
| 13 | OXA-48 | 1 | 1 | Barcelona |
| 1235 | VIM-1 | 1 | 1 | Guadalajara |
The most common ST-carbapenemase associations found are detailed in Table 3. Only two STs carried more than one type of carbapenemase: ST11 (OXA-48, OXA-245, VIM-1, KPC-2, and NDM-1) and ST15 (VIM-1 and OXA-48). It should be emphasized the apparent capacity of ST11 to carry and disseminate different types of carbapenemases (1, 7, 17).
The four carbapenemase-producing E. coli isolates belonged to four different STs: ST10, ST226, and ST1152, with one case each producing VIM-1, and ST131 that produced OXA-48.
Our results are based on a large representative sample of Spanish CPE cases, but reporting of CPE is not mandatory in this country so far. Recent global data about the spread of CPE in Spain are not available; one multicenter study carried out in 2009 in Spain detected only 43 CPE cases, mainly VIM-1 and IMP-22 (5).
Only 13.5% of the isolates producing carbapenemases in this study were K. pneumoniae or E. coli isolated from blood, suggesting that EARS-Net may underestimate the occurrence of carbapenem-resistant Enterobacteriaceae. From 2011 to 2012, imipenem-nonsusceptible K. pneumonia has increased from <1% to 1.7% according to Spanish EARS-Net databases (unpublished data); similarly, according to Surveillance Program data depicted in Fig. 1, the number of carbapenemase-producing Enterobacteriaceae cases more than doubled between 2011 and 2012.
It is remarkable that, from 2009 to 2012, the number of hospitals reporting CPE increased by a factor of five. This fact may suggest that a recent epidemiological change may have occurred in this country, characterized by a rapid increase in the number of cases of CPE causing both nosocomial outbreaks and single infections (Table 3). A second significant factor explaining this trend may be that hospitals have increased awareness of CPE.
In summary, our data suggest that the impact of CPE in Spain has dramatically increased in the last years. Interhospital spread of several K. pneumoniae clone-carbapenemase combinations have been detected in this study, mainly ST15–VIM-1, ST11–OXA-48, ST405–OXA-48, ST101–KPC-2, and ST11–VIM-1. To address the emergence and spread of CPE, urgent measures are required, including early detection and the rapid implementation of control measures.
ACKNOWLEDGMENTS
This study was supported by the Antibiotic Resistance Surveillance Program of the Spanish Centro Nacional de Microbiología of the Instituto de Salud Carlos III. Verónica Bautista was supported by the Ministerio de Economía y Competitividad, Instituto de Salud Carlos III, cofinanced by the European Development Regional Fund “A way to achieve Europe” ERDF, Spanish Network for the Research in Infectious Diseases (REIPI RD12/0015).
We thank the Genomics Unit of the Centro Nacional de Microbiología for its support in DNA sequencing. We also thank the team of curators of the Institut Pasteur MLST system (Paris, France) for importing novel profiles and/or isolates at http://www.pasteur.fr/mlst.
The Spanish Microbiology National Center (CNM) of the Instituto de Salud Carlos III, a public-health and research Institution, runs an active Antibiotic Resistance Surveillance Program.
The members of the Spanish Collaborating Group for the Antibiotic Resistance Surveillance Program are Alejandro Gonzalez Praetorius and Sonia Solís del Baño (H. Universitario de Guadalajara, Guadalajara, Mexico), Esteban Aznar and Carolina Campelo (Laboratorio BrSalud, San Sebastián de los Reyes, Madrid, Spain), Rocío Martínez-Ruiz and Isabel Sánchez-Romero (H. Puerta de Hierro, Majadahonda, Madrid, Spain), Mateu Espasa and Dionisia Fontanals (Corporació Sanitària Parc Taulí), Luisa García-Picazo (H. de El Escorial, Madrid, Spain), Ana Fleites (H. Universitario Central de Asturias, Oviedo, Spain), Adelina Gimeno (H. General Universitario de Alicante), Alberto Delgado-Iribarren (Hospital Universitario Fundación Alcorcón, Madrid, Spain), María Dolores Miguel Martínez (H. de Cabueñes, Gijón), José Luis Hernández (H. de Cruces, Barakaldo, Vizcaya), Gloria Trujillo (H. San Joan de Deu, Fundación Althaia, Manresa, Barcelona), Yolanda Gil and María Almagro (H. Universitario de Móstoles, Móstoles, Madrid, Spain), Emilia Cercenado (H. General Universitario Gregorio Marañón, Madrid, Spain), Fernando Buñuel (H. Universitario San Juan de Alicante), Teresa Cabezas (H. de Poniente, Almería, Spain), Ma Pilar Ortega (Complejo Asistencial Universitario de Burgos), Rafael Carranza (H. General La Mancha Centro, Ciudad Real, Spain), Ma del Mar López (H. de la Marina Baixa, Villajoyosa, Alicante, Spain), Ma Isabel Fernández-Natal (Complejo Asistencial Universitario de León), Carmen Gómez (HM Hospitales, Madrid, Spain), Isabel Wilhelmi (H. Severo Ochoa, Leganés, Madrid, Spain), Amparo San Pedro (H. Nuestra Señora de Sonsoles, Ávila, Spain), Concepción Baladón (Lab Echevarne).
Footnotes
Published ahead of print 16 September 2013
REFERENCES
- 1. Cantón R, Akóva M, Carmeli Y, Giske CG, Glupczynski Y, Gniadkowski M, Livermore DM, Miriagou V, Naas T, Rossolini GM, Samuelsen Ø, Seifer H, Woodford N, Nordmann P, European Network on Carbapenemases 2012. Rapid evolution and spread of carbapenemases among Enterobacteriaceae in Europe. Clin. Microb. Infect. 18:413–431 [DOI] [PubMed] [Google Scholar]
- 2. Nordmann P, Dortet L, Poirel L. 2012. Carbapenem resistance in Enterobacteriaceae: here is the storm! Trends Mol. Med. 18:263–272 [DOI] [PubMed] [Google Scholar]
- 3. Tzouvelekis LS, Markogiannakis A, Psichogiou M, Tasios PT, Daikos GL. 2012. Carbapenemases in Klebsiella pneumoniae and other Enterobacteriaceae: an evolving crisis of global dimensions. Clin. Microb. Rev. 25:682–707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gómez-Gil MR, Paño-Pardo JR, Romero-Gómez MP, Gasior M, Lorenzo M, Quiles I, Mingorance J. 2010. Detection of KPC-2-producing Citrobacter freundii isolates in Spain. J. Antimicrob. Chemother. 65:2695–2697 [DOI] [PubMed] [Google Scholar]
- 5. Miró E, Agüero J, Larrosa MN, Fernández A, Conejo MC, Bou G, González-López JJ, Lara N, Martínez-Martínez L, Oliver A, Aracil B, Oteo J, Pascual A, Rodríguez-Baño J, Zamorano L, Navarro F. 2013. Prevalence and molecular epidemiology of acquired AmpC β-lactamases and carbapenemases in Enterobacteriaceae isolates from 35 hospitals in Spain. Eur. J. Clin. Microbiol. Infect. Dis. 32:253–259 [DOI] [PubMed] [Google Scholar]
- 6. Oteo J, Hernández-Almaraz JL, Gil-Antón J, Vindel A, Fernández S, Bautista V, Campos J. 2010. Outbreak of VIM-1-carbapenemase-producing Enterobacter cloacae in a pediatric intensive care unit. 2010. Pediatr. Infect. Dis. J. 29:1144–1146 [DOI] [PubMed] [Google Scholar]
- 7. Oteo J, Hernández JM, Espasa M, Fleites A, Sáez D, Bautista V, Pérez-Vázquez M, Fernández-García MD, Delgado-Iribarren A, Sánchez-Romero I, García-Picazo L, Miguel MD, Solís S, Aznar E, Trujillo G, Mediavilla C, Fontanals D, Rojo S, Vindel A, Campos J. 2013. Emergence of OXA-48-producing Klebsiella pneumoniae and the novel carbapenemases OXA-244 and OXA-245 in Spain. J. Antimicrob. Chemother. 68:317–321 [DOI] [PubMed] [Google Scholar]
- 8. Paño-Pardo JR, Ruiz-Carrascoso G, Navarro-San Francisco C, Gómez-Gil R, Mora-Rillo M, Romero-Gómez MP, Fernández-Romero N, García-Rodríguez J, Pérez-Blanco V, Moreno-Ramos F, Mingorance J. 2013. Infections caused by OXA-48-producing Klebsiella pneumoniae in a tertiary hospital in Spain in the setting of a prolonged, hospital-wide outbreak. J. Antimicrob. Chemother. 68:89–96 [DOI] [PubMed] [Google Scholar]
- 9. Sánchez-Romero I, Asensio A, Oteo J, Muñoz-Algarra M, Isidoro B, Vindel A, Alvarez-Avello J, Balandín-Moreno B, Cuevas O, Fernández-Romero S, Azañedo L, Sáez D, Campos J. 2012. Nosocomial outbreak of VIM-1-producing Klebsiella pneumoniae isolates of multilocus sequence type 15: molecular basis, clinical risk factors, and outcome. Antimicrob. Agents Chemother. 56:420–427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tato M, Coque TM, Ruíz-Garbajosa P, Pintado V, Cobo J, Sader HS, Jones RN, Baquero F, Cantón R. 2007. Complex clonal and plasmid epidemiology in the first outbreak of Enterobacteriaceae infection involving VIM-1 metallo-β-lactamase in Spain: toward endemicity? Clin. Infect. Dis. 45:1171–1178 [DOI] [PubMed] [Google Scholar]
- 11. The European Committee on Antimicrobial Susceptibility Testing 2013. Clinical breakpoints. http://www.eucast.org/clinical_breakpoints
- 12. Woodford N, Tierno PM, Jr, Young K, Tysall L, Palepou MF, Ward E, Painter RE, Suber DF, Shungu D, Silver LL, Inglima K, Kornblum J, Livermore DM. 2004. Outbreak of Klebsiella pneumoniae producing a new carbapenem-hydrolyzing class A β-lactamase, KPC-3, in a New York medical center. Antimicrob. Agents Chemother. 48:4793–4799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Oteo J, Domingo-García D, Fernández-Romero S, Saez D, Guiu A, Cuevas O, Lopez-Brea M, Campos J. 2012. Abdominal abscess due to NDM-1-producing Klebsiella pneumoniae in Spain. J. Med. Microbiol. 61:864–867 [DOI] [PubMed] [Google Scholar]
- 14. Tórtola MT, Lavilla S, Miró E, González JJ, Larrosa N, Sabaté M, Navarro F, Prats G. 2005. First detection of a carbapenem-hydrolyzing metalloenzyme in two enterobacteriaceae isolates in Spain. Antimicrob. Agents Chemother. 49:3492–3494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Giani T, Pini B, Arena F, Conte V, Bracco S, Migliavacca R, Survey Participants AMCLI-CRE. Pantosti A, Pagani L, Luzzaro F, Rossolini G. 2013. Epidemic diffusion of KPC carbapenemase-producing Klebsiella pneumoniae in Italy: results of the first countrywide survey, 15 May to 30 June 2011. Euro Surveill. 18(22):pii=20489 http://www.eurosurveillance.org/ViewArticle.aspx? ArticleId=20489 [PubMed] [Google Scholar]
- 16. Glupczynski Y, Huang TD, Bouchahrouf W, Rezende de Castro R, Bauraing C, Gérard M, Verbruggen AM, Deplano A, Denis O, Bogaerts P. 2012. Rapid emergence and spread of OXA-48-producing carbapenem-resistant Enterobacteriaceae isolates in Belgian hospitals. Int. J. Antimicrob. Agents 39:168–172 [DOI] [PubMed] [Google Scholar]
- 17. Woodford N, Turton JF, Livermore DM. 2011. Multiresistant Gram-negative bacteria: the role of high-risk clones in the dissemination of antibiotic resistance. FEMS Microbiol. Rev. 35:736–755 [DOI] [PubMed] [Google Scholar]