Abstract
Use of amphotericin B-impregnated bone cement in combination with systemic antifungals for the treatment of coccidioidal osteomyelitis offers the potential for sustained local concentrations of drug at the site of the infection. Amphotericin B levels in bone of up to 5.1 μg/g have been demonstrated 4 months after placement of bone cement.
TEXT
Delivery of local antimicrobials has become an important tool in the treatment of bone and joint disease. While the incidence of fungal osteomyelitis is rare, it nevertheless presents numerous therapeutic challenges in the treatment and management of such cases (1). Fungal infections are often biofilm related and may be recalcitrant to care despite extensive surgical debridement and systemic antifungals. High concentrations of systemic antifungals can be associated with toxicity and often achieve inadequate concentrations at the site of infection (2). Use of amphotericin B-impregnated bone cement is not well characterized, and little is known about depot delivery of antifungal agents. Our report describes an immunocompetent patient with coccidioidal infection of the right ankle who was successfully treated with amphotericin B-loaded bone cement and systemic itraconazole.
A 68-year-old man presented to our institution for evaluation of a right ankle lesion. In 2004, he presented to an outside hospital with a soft mass on the medial ankle which had fluctuated in size over the prior 2 years. At that time, he had a magnetic resonance imaging (MRI) which revealed a mass and signal in the adjacent bone. He was told it was a ganglion cyst and declined further treatment. At the time of presentation to our institution, he noticed a nonhealing ulcer on the dorsolateral midfoot and weight loss of 20 pounds over the preceding 4 months. Aspiration of the hindfoot mass revealed Coccidioides spp. on culture, and the initial serum complement fixation (CF) titer for Coccidioides was 1:8. His past medical history included coronary artery disease, hypertension, and tobacco use, and HIV testing was negative. He was born and raised in San Francisco, CA, but lived in Coalinga, CA (within California's Central Valley).
On referral to the infectious disease service, the patient was noted to have a 3- by 3-cm rubbery, soft mass around the right lateral malleolus, draining thin fluid with surrounding erythema. MRI showed abnormal signal of the lateral calcaneus. His coccidioidal CF antibody titers remained at 1:8. He was started on fluconazole 2 weeks prior but was switched to 200 mg itraconazole twice daily, as it is the preferred agent for bony disease (3).
Three months later, the patient presented to the emergency department with worsening ankle pain. His physical exam was notable for right ankle cellulitis with a draining abscess on the dorsolateral foot. His serum itraconazole level was 0.5 μg/ml. MRI of the ankle showed extensive destructive changes within the distal fibula, tibia, and hindfoot, with enhancing soft tissue inflammation. The orthopedic service was consulted and planned for staged surgical management once the soft tissue envelope improved with antifungal treatment.
Eight weeks later, after taking itraconazole for 5 months, the patient was scheduled for a right ankle arthrotomy, talectomy, incision and drainage, and application of a multiplane external fixator with placement of antibiotic cement. Amphotericin B deoxycholate (100 mg; powder for injection) was prepared in hydroxyapatite beads and placed medially and superiorly into the bone. The carrier material created was a Palacos cement spacer (Heraeus Medical, Germany) that was molded into the shape of the defect. Amphotericin B is heat stable to 170°C, and the maximum temperature created by the cement measured intraoperatively was 60°C (4). Two antibiotic spacers were placed: one for the talar neck and one for the talar body, with vancomycin and tobramycin. Surgical pathology samples showed fibrocollagenous tissue with acute-on-chronic inflammation and necrosis consistent with osteomyelitis. No organisms were identified on histologic slides, and there was no growth of bacteria or fungi from the right ankle tissue samples after 4 weeks. Serum amphotericin B levels were drawn 48 h postprocedure and were undetectable, at <0.1 μg/ml. His postoperative course was unremarkable, and the patient was discharged within 72 h.
At the 1-month follow-up in the clinic, the patient remained stable without pain. Laboratory findings were notable for a serum creatinine level of 1.74 mg/dl; inflammatory markers were significantly decreased. Serum levels of amphotericin B and itraconazole were <0.1 μg/ml and 0.6 μg/ml, respectively. He continued on itraconazole and was encouraged to take it with an acidic drink to improve absorption. After 4 months, the patient received a planned ankle pan-talar fusion with a femoral head allograft, revision of the talar space external fixator, and removal of antibiotic spacers. A pseudomembrane had developed, but there was no evidence of deep infection or purulence. Prior to the removal of the pseudomembrane, two bone tissue samples were sent for itraconazole and amphotericin B levels. The bone samples were homogenized and tested at The University of Texas at San Antonio Fungus Testing Laboratory (UTHSCSA). The two bone samples yielded amphotericin B levels of 2.3 μg/g and 5.1 μg/g by high-performance liquid chromatography (HPLC) (Table 1). The HPLC assay was validated in plasma by using the results of six replicates of spiked amphotericin B plasma samples at levels of 0.05, 0.25, 5.0, and 15 μg/ml over four different run days. Each control limit was within 15% of its theoretical value (UTHSCSA, personal correspondence). Itraconazole and its hydroxyl metabolite were tested via ultraperformance liquid chromatography-mass spectrometry and were both undetectable in bone. Concurrent serum itraconazole levels were 0.9 μg/ml.
Table 1.
Serum and tissue concentrations of antifungals throughout therapy
| Weeka | Itraconazole (serum; μg/ml) | Hydroxy-itraconazole (serum; μg/ml) | Itraconazole (bone; μg/g) | Amphotericin B (serum; μg/ml) | Amphotericin B (bone; μg/g) |
|---|---|---|---|---|---|
| 5 | 0.3 | 0.4 | |||
| 14 | 0.3 | 0.5 | |||
| 17 | 0.5 | 0.9 | |||
| 22 | 0.4 | 0.8 | <0.1 | ||
| 25 | 0.6 | 1.2 | <0.1 | ||
| 29 | 0.9 | 1.4 | |||
| 33 | Sample 1, undetectable; | Sample 1, 2.3; | |||
| sample 2, undetectable | sample 2, 5.1 | ||||
| 43 | 0.5 | 1.0 |
Week 0 is the first day the patient presented to our institution. At week 22, the patient received his first surgery with implantation of amphotericin B-impregnated bone cement spacer. At week 33, the patient was admitted for revision of his external fixator and removal of the antimicrobial spacer. Bone tissue samples were collected during this procedure.
Four months after the procedure, radiographs demonstrated hindfoot fusion at the site of the previous infection (Fig. 1). The multiplane external fixator frame was removed, and no purulence was noted intraoperatively. He remained on itraconazole, and during a 12-month follow-up visit, the patient was doing well with no significant problems or signs of active infection.
Fig 1.
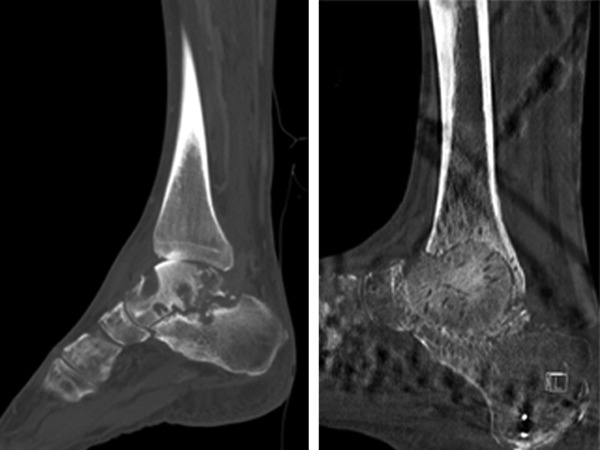
Left, preoperative sagittal computed tomographic (CT) scan of the right foot showing extensive osseous abnormalities within the talus and calcaneous; right, postfusion sagittal CT scan of the same area showing tibio-talar fusion with resolution of osteomyelitis.
Use of antimicrobial-impregnated beads or cement in addition to systemic therapy has been increasingly incorporated into clinical practice in the treatment of osteomyelitis (5–7). Since the majority of bone and joint infections are caused by bacterial pathogens, research and experience with antifungal agents have been relatively sparse. Primarily, data regarding efficacy and safety are available with antibiotics such as aminoglycosides and vancomycin (8). This report offers additional insight into treatment with amphotericin B bone cement for severe right ankle osteomyelitis caused by Coccidioides spp.
Among the antifungal class, amphotericin B enjoys a wide spectrum of activity, but its use is limited by systemic toxicities (2). Common adverse effects of systemic therapy include infusion-related reactions (pyrexia, thrombophlebitis, and hypotension), acute nephrotoxicity, and electrolyte disturbance. Many patients who require long-term therapy with amphotericin B are unable to maintain an adequate course because of these unacceptable side effects. Therefore, delivery of local antifungals is an attractive method to avoid systemic toxicity and achieve adequate concentrations of drug at the site of infection. The ideal drug should be heat stable, be water soluble, and exhibit adequate elution characteristics from the bone cement without compromising the mechanical properties of the cement (9).
There is no consensus regarding the ideal dose or formulation of amphotericin B to mix with bone cement for effective local treatment of joint infections. Marra et al. first described the use of amphotericin B-loaded polymethylmethacrylate (PMMA) bone cement to treat osteomyelitis caused by Candida albicans (4). In this study, 700 mg of amphotericin B sterile powder for injection mixed in bone cement achieved concentrations as high as 3.2 mg/liter in surgical wound fluid 50 h after implantation. Concentrations dropped to 0.71 mg/liter within 72 h, and no further samples were analyzed after 5 days. Goss et al. reported undetectable levels of amphotericin B after 7 days in an in vitro study, with the conclusion that the choice of bone cement may impact elution characteristics of the antifungal (10).
Another point of concern is whether the strength of bone cement decreases with the addition of high-dose poragen to allow greater delivery of amphotericin B. Cefazolin was chosen in one study as the poragen and mixed with 200 mg of amphotericin B, compared to the PMMA bone cement containing amphotericin B alone (11). While the cumulative mass of amphotericin B released over 15 h was greater with the addition of the poragen than with amphotericin B alone (12.76 μg/cylinder compared to 1.74 μg/cylinder, P < 0.001), the compressive strength was also statistically significantly decreased.
Few studies have sampled bone tissue concentrations of amphotericin B. Pharmacokinetic data from an oncology study where patients received liposomal amphotericin B for treatment of invasive fungal infections demonstrated widely variable bone marrow concentrations, with values between 0 to 3.2 mg/liter documented (12). Existing animal models that sampled bone marrow concentrations of amphotericin B after several doses of therapy showed higher peak concentrations with liposomal amphotericin B (mean concentration, 39.5 μg/g) and amphotericin B colloidal dispersion (mean concentration, 53.1 μg/g) than with amphotericin B deoxycholate (mean concentration, 8.0 μg/g) (13). The clinical significance of these concentrations for humans is uncertain, given that the vascularity of bone marrow may equate to large differences in drug levels compared to those of bone tissue itself.
To our knowledge, this is the first case report which obtained detectable amphotericin B levels at the site of infection 4 months after the initial placement of bone cement. Additionally, it is interesting to note that while itraconazole serum levels were detectable throughout the patient's clinical course, drug levels were undetectable in bone. The study which established itraconazole as the agent of choice in skeletal disease measured serum itraconazole concentrations but did not examine levels at the site of the infection (3). Other pharmacokinetic studies have shown as high as a 5-fold increase in bone tissue compared to in plasma following a single dose of 200 mg itraconazole (14). This report demonstrates that even with detectable serum itraconazole levels, bone concentrations may not be predictable, and alternative treatment strategies may be necessary in salvage situations. Other antifungals, such as voriconazole, have been used to treat bone and joint disease, although experience has largely been limited to Aspergillus and Candida osteomyelitis (15). While the elution properties and safety of amphotericin B-impregnated bone cement still warrant further study, this study demonstrates successful local delivery of amphotericin B while avoiding systemic adverse effects of the drug.
Footnotes
Published ahead of print 3 September 2013
REFERENCES
- 1. Azzam K, Parvizi J, Jungkind D, Hanssen A, Fehring T, Springer B, Bozic K, Della Valle C, Pulido L, Barrack R. 2009. Microbiological, clinical, and surgical features of fungal prosthetic joint infections: a multiinstitutional experience. J. Bone Joint Surg. Am. 91(Suppl 6):142–149 [DOI] [PubMed] [Google Scholar]
- 2. Thompson GR, III, Cadena J, Patterson TF. 2009. Overview of antifungal agents. Clin. Chest Med. 30:203–215 [DOI] [PubMed] [Google Scholar]
- 3. Galgiani JN, Catanzaro A, Cloud GA, Johnson RH, Williams PL, Mirels LF, Nassar F, Lutz JE, Stevens DA, Sharkey PK, Singh VR, Larsen RA, Delgado KL, Flanigan C, Rinaldi MG. 2000. Comparison of oral fluconazole and itraconazole for progressive, nonmeningeal coccidioidomycosis. A randomized, double-blind trial. Ann. Intern. Med. 133:676–686 [DOI] [PubMed] [Google Scholar]
- 4. Marra F, Robbins GM, Masri BA, Duncan C, Wasan KM, Kwong EH, Jewesson PJ. 2001. Amphotericin B-loaded bone cement to treat osteomyelitis caused by Candida albicans. Can. J. Surg. 44:383–386 [PMC free article] [PubMed] [Google Scholar]
- 5. Deelstra JJ, Neut D, Jutte PC. 2013. Successful treatment of Candida albicans-infected total hip prosthesis with staged procedure using an antifungal-loaded cement spacer. J. Arthroplasty 28:374.e5-e8. [DOI] [PubMed] [Google Scholar]
- 6. Wu MH, Hsu KY. 2011. Candidal arthritis in revision knee arthroplasty successfully treated with sequential parenteral-oral fluconazole and amphotericin B-loaded cement spacer. Knee Surg. Sports Traumatol. Arthrosc. 19:273–276 [DOI] [PubMed] [Google Scholar]
- 7. Cunningham B, McLaren AC, Pauken C, McLemore R. 2012. Liposomal formulation increases local delivery of amphotericin from bone cement: a pilot study. Clin. Orthop. Relat. Res. 470:2671–2676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Joseph TN, Chen AL, Di Cesare PE. 2003. Use of antibiotic-impregnated cement in total joint arthroplasty. J. Am. Acad. Orthop. Surg. 11:38–47 [DOI] [PubMed] [Google Scholar]
- 9. Sealy PI, Nguyen C, Tucci M, Benghuzzi H, Cleary JD. 2009. Delivery of antifungal agents using bioactive and nonbioactive bone cements. Ann. Pharmacother. 43:1606–1615 [DOI] [PubMed] [Google Scholar]
- 10. Goss B, Lutton C, Weinrauch P, Jabur M, Gillett G, Crawford R. 2007. Elution and mechanical properties of antifungal bone cement. J. Arthroplasty 22:902–908 [DOI] [PubMed] [Google Scholar]
- 11. Kweon C, McLaren AC, Leon C, McLemore R. 2011. Amphotericin B delivery from bone cement increases with porosity but strength decreases. Clin. Orthop. Relat. Res. 469:3002–3007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ellis M. 2008. New dosing strategies for liposomal amphotericin B in high-risk patients. Clin. Microbiol. Infect. 14(Suppl 4):55–64 [DOI] [PubMed] [Google Scholar]
- 13. Groll AH, Mickiene D, Piscitelli SC, Walsh TJ. 2000. Distribution of lipid formulations of amphotericin B into bone marrow and fat tissue in rabbits. Antimicrob. Agents Chemother. 44:408–410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Willems L, van der Geest R, de Beule K. 2001. Itraconazole oral solution and intravenous formulations: a review of pharmacokinetics and pharmacodynamics. J. Clin. Pharm. Ther. 26:159–169 [DOI] [PubMed] [Google Scholar]
- 15. Studemeister A, Stevens DA. 2011. Aspergillus vertebral osteomyelitis in immunocompetent hosts: role of triazole antifungal therapy. Clin. Infect. Dis. 52:966. [DOI] [PubMed] [Google Scholar]


