Abstract
Despite the important role of the antimalarial artesunate and its active metabolite dihydroartemisinin (DHA) in malaria treatment efforts, there are limited data on the pharmacokinetics of these agents in pediatric patients. This study evaluated the effects of body size and gender on the pharmacokinetics of artesunate-DHA using data from pediatric and adult malaria patients. Nonlinear mixed-effects modeling was used to obtain a base model consisting of first-order artesunate absorption and one-compartment models for artesunate and for DHA. Various methods of incorporating effects of body size descriptors on clearance and volume parameters were tested. An allometric scaling model for weight and a linear body surface area (BSA) model were deemed optimal. The apparent clearance and volume of distribution of DHA obtained with the allometric scaling model, normalized to a 38-kg patient, were 63.5 liters/h and 65.1 liters, respectively. Estimates for the linear BSA model were similar. The 95% confidence intervals for the estimated gender effects on clearance and volume parameters for artesunate fell outside the predefined no-relevant-clinical-effect interval of 0.75 to 1.25. However, the effect of gender on apparent DHA clearance was almost entirely contained within this interval, suggesting a lack of an influence of gender on this parameter. Overall, the pharmacokinetics of artesunate and DHA following oral artesunate administration can be described for pediatric patients using either an allometric scaling or linear BSA model. Both models predict that, for a given artesunate dose in mg/kg of body weight, younger children are expected to have lower DHA exposure than older children or adults.
INTRODUCTION
The World Health Organization (WHO) estimates that malaria infection was responsible for approximately 660,000 deaths in 2010. Young children bear a devastating extent of the global mortality burden associated with malaria, with approximately 86% of the deaths occurring among children <5 years of age (1). Malaria is caused by protozoa of the genus Plasmodium; the species P. falciparum and P. vivax are most commonly responsible for infections, with P. falciparum causing the vast majority of fatal infections. Derivatives of the endoperoxide antimalarial artemisinin efficiently effect profound reductions in parasite counts and are the cornerstone of the global treatment approach for acute malaria infection. Intravenous (i.v.) administration of artesunate (AS) is endorsed by the WHO for treatment of severe malaria, with oral artemisinin-based combination therapies (ACTs) recommended for treatment of uncomplicated malaria. ACTs couple artemisinin derivatives, which are rapidly eliminated from the body, with more slowly eliminated partner drugs. These partner drugs eradicate residual parasites and guard against the emergence of parasites with reduced artemisinin sensitivity (2).
Artesunate, a hemisuccinate ester of its active metabolite dihydroartemisinin (DHA), is the most water soluble of the artemisinin derivatives. Following absorption, artesunate is rapidly converted to DHA by hepatic and plasma esterases. DHA, in turn, undergoes glucuronide conjugation mediated by UGT2B7 and UGT1A9. DHA is considered the principal source of the antimalarial activity associated with oral artesunate treatment, largely due to the comparatively lower exposure to artesunate than to DHA observed following oral artesunate administration (3).
Given the therapeutic prominence of the artemisinin derivatives, and the particular vulnerability of the pediatric population to malaria-related mortality, any differences between children and adults in the disposition of artemisinins should be thoroughly characterized. However, as noted in two recent reviews, numerous gaps exist in our understanding of artemisinin derivative pharmacokinetics among pediatric patients (4, 5). The analysis that follows represents an attempt to describe the population pharmacokinetics of the artemisinin derivative artesunate and its active metabolite DHA in falciparum and vivax malaria patients participating in phase II and III trials for the novel ACT pyronaridine tetraphosphate-artesunate (PA); the primary focus of the analysis was the description of artesunate and DHA pharmacokinetics in pediatric patients.
As body size exerts a substantial influence on pediatric pharmacokinetics, a particular emphasis of this analysis was the evaluation of methods for describing the relationship between body size descriptors and clearance and volume parameters. The methods investigated included linear, estimated exponent, and allometric scaling models for body weight as well as linear and estimated exponent models for body surface area (BSA) and lean body mass. A further purpose of this analysis was to estimate the magnitude of covariate effects of potential clinical interest using a full-covariate-model approach. The final aim was to assess the sensitivity of the estimated parameter-body size relationships and covariate-parameter relationships to the inclusion or exclusion of adult data from the modeling data set.
MATERIALS AND METHODS
Data.
Two data sets were utilized for modeling. The first, termed the full data set, included data from both pediatric and adult participants in the five PA trials, which included one phase II and four phase III studies. The second data set, termed the pediatric data set, was a subset of the full data set; it contained data only for patients <12 years of age, the International Conference on Harmonisation (ICH) age category cutoff between children and adolescents (6).
A summary of patient demographics for the included clinical trials is given in Table 1. In all five trials, patients were administered PA once daily for 3 days without regard to food intake. In the phase II study (study 1), plasma samples were collected prior to the first dose of PA, and at 0.25, 0.5, 1, 1.5, 2.5, 4, 8, and 12 h following this dose; patients were administered 2, 3, or 4 mg/kg/day of artesunate (7). In the phase III studies, one sample was drawn during a 0.25- to 12-h window following either the first or second PA dose, and a second sample was drawn during that same time window following the third dose. During phase III studies, patients received artesunate doses ranging from 2.3 to 4.6 mg/kg/day. A granule formulation of PA was administered to approximately one-fourth of the patients in the phase II study and all of the patients in one of the phase III studies, while all other patients were given a tablet formulation (8). Written informed consent, in accordance with the Declaration of Helsinki, was obtained for participants in the studies, and approval for each study was granted by local ethics committees. For study 5, a pediatric study, written informed consent was provided by a parent or guardian, with patient assent sought where possible.
Table 1.
Summary of demographic and covariate data
| Parameter | Value for study |
|||||
|---|---|---|---|---|---|---|
| 1 (phase II) | 2 (phase III) | 3 (phase III) | 4 (phase III) | 5 (phase III) | All studies | |
| No. and description of subjects with pharmacokinetic data | 57 African children with falciparum malaria | 268 African and Asian children and adults with falciparum malaria | 196 African children and adults with falciparum malaria | 23 Asian children and adults with vivax malaria | 87 African children with falciparum malaria | 631 |
| Median artesunate dose (mg/kg) (interquartile range) | 3.4 (2.8, 3.9) | 3.3 (3.0, 3.6) | 3.3 (2.9, 3.9) | 3.4 (3.0, 3.7) | 3.0 (2.6, 3.3) | 3.3 (2.9, 3.6) |
| Median age of subjects (yr) (interquartile range) | 5 (4, 6) | 24 (19, 35) | 11 (8, 17) | 19 (14, 34) | 5 (3, 7) | 14 (7, 25) |
| Median wt of subjects (kg) (interquartile range) | 16 (14, 20) | 50 (44, 56) | 30 (25, 48) | 47 (35, 53) | 17 (14, 20) | 38 (22, 52) |
| % male subjects | 51 | 77 | 46 | 61 | 48 | 61 |
| Median parasite count (per μl) (interquartile range) | 6,304 (2,051, 14,926) | 12,838 (5,843, 31,168) | 12,607 (3,363, 29,408) | 10,275 (3,757, 15,692) | 10,074 (1,994, 44,068) | 11,462 (3,569, 29,060) |
| No. of patients administered granules | 15 | 0 | 0 | 0 | 87 | 102 |
| No. of patients <12 yr of age | 56 | 23 | 104 | 4 | 87 | 274 |
Sample handling.
Collected samples were processed as follows. Blood was collected into tubes containing potassium oxalate-sodium fluoride for the separation of plasma drawn at the times specified. The samples were centrifuged within 15 min of collection. Plasma was removed from cells and transferred into two approximately equal-volume aliquots in screw-cap cryovials immediately after centrifugation. The plasma samples were immediately frozen at or below −80°C in a laboratory freezer. The samples were later shipped separately via air express on dry ice to the Clinical Pharmacokinetics Laboratory at the College of Pharmacy, University of Iowa. All samples were stored at −80°C until drug analysis was performed.
Artesunate and dihydroartemisinin assay.
Plasma concentrations of artesunate and DHA were quantified according to a method described previously by Naik et al. (9). Briefly, plasma concentrations of artesunate and DHA were quantified by liquid chromatography-mass spectrometry (LC-MS). Chromatographic analysis was carried out on a Shimadzu model 2010 liquid chromatograph and mass spectrometer (Shimadzu, Columbia, MD, USA), using a LC-10AD solvent delivery system. The injection was made with a Shimadzu SIL-10AD automatic injector. The analysis was carried out by using a Synergi Max-RP 80A high-performance liquid chromatography (HPLC) column (75 mm by 4.6 mm by 4 μm; Phenomenex, Torrance, CA, USA), using a guard column (Phenomenex) with Phenomenex C-12 Max-RP cartridges. The lower limit of quantification was 1 ng/ml for both artesunate and DHA. The coefficients of variation for intraday precision and interday precision were <15% for AS and DHA.
Base model.
Prior to model building, artesunate and DHA data were converted from ng/ml to nmol/liter values using the compounds' respective molecular weights. Visual exploratory data analysis was undertaken to examine the basic structure of the concentration-time data and to identify outliers. Due to the predominance of sparse sampling data, the more extensive full data set was primarily used for base model development; however, models which successfully converged with plausible estimates were implemented with the pediatric data set to assess for a reasonably similar model fit. Population pharmacokinetic modeling was performed by using NONMEM 7.2 (10), implemented on a Windows XP operating system with a G95 Fortran compiler. Model development and evaluation were facilitated through use of Perl Speaks NONMEM 3.5.3 (PsN) (11) and Pirana 2.6.0 (12).
For all models assessed in this analysis, interindividual variability (IIV) was modeled as following a log-normal distribution for all parameters, Pi = Ppop × eηi, where Pi is the estimated parameter value for individual i, Ppop represents the typical population estimate for the parameter, and ηi is the deviation of Pi from Ppop. The term η is assumed to be normally distributed with a mean of zero and a variance of ω2. For all models considered during base model development, both full and diagonal IIV variance-covariance matrices were evaluated. Models with some, but not all, covariance terms fixed to zero were assessed as deemed appropriate based on parameter estimates from full-matrix results.
Data were first modeled by using a simultaneously implemented parent metabolite model with first-order artesunate absorption and a one-compartment, first-order elimination model for both artesunate and DHA. The concentration data were natural log transformed, and an additive model for log-transformed data was applied, ln(Cij) = ln(Cpred,ij) + εij, where Cij and Cpred,ij represent the jth observed and model-predicted analyte concentrations, respectively, for individual i. The term εij denotes the residual random error for individual i and observation j, with ε assumed to be normally distributed with a mean of zero and a variance of σ2 in the population. Models with single and distinct ε distributions for the two analytes were assessed during early model building; for distinct distributions, the covariance term was either estimated or fixed to zero. Due to model stability problems encountered with alternative models, distinct ε values, with covariance fixed to zero, were utilized for all but early model building.
Estimation methods utilized during initial base model development included first-order conditional estimation (FOCE), importance sampling expectation maximization, and Laplacian estimation. Attempts were made to account for concentrations below the lower limit of quantification of the assay through implementation of the M2 and the M3 methods, as described previously by Beal (13).
Alternative models were tested to improve upon the initial base model, including the addition of a second DHA compartment as well as evaluation of multiple alternative absorption models including zero-order absorption, transit compartment absorption, mixed zero-order/first-order absorption, and parallel first-order absorption. Additionally, a model with η on artesunate bioavailability (and with a diagonal ω matrix and population bioavailability [F1] set to 1) was also assessed. Finally, based on research suggesting differences in artemisinin derivative pharmacokinetics between the most acute phase of infection and the convalescence phase (14), a model with F1 fixed to 1 for the first day of treatment, and estimated for days 2 and 3, was also evaluated.
Model selection was guided by the following factors: plausibility and precision of parameter estimates, goodness-of-fit plots, magnitude of residual variability, sensitivity of the model to initial estimates, minimum objective function value (MOFV), equal to minus twice the log likelihood function, and Akaike information criterion, equal to MOFV plus two times the number of parameters. Data for goodness-of-fit plots were stratified by age (<5 years, 5 through 11 years, 12 through 18 years, and older than 18 years) to identify if a given base model was associated with unique goodness-of-fit features in a particular age group. Finally, given that DHA is considered principally responsible for antimalarial activity following oral artesunate administration, concerns regarding appropriate fit of DHA data took precedence over parallel concerns regarding artesunate data.
Covariate modeling: body size descriptor.
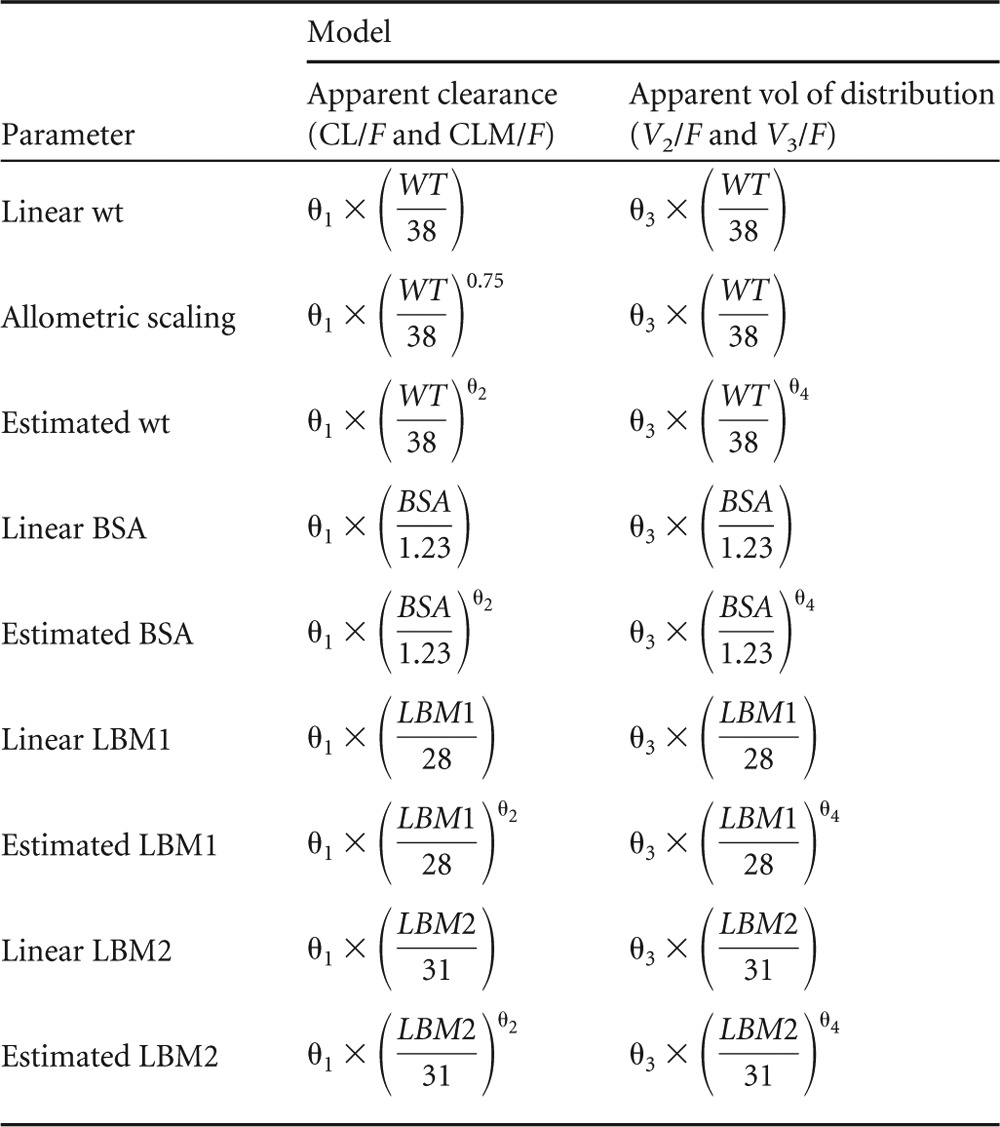
Following base model development, relationships between various body size descriptors and the clearance and volume parameters of artesunate and DHA were modeled. These descriptors included total body weight, body surface area (BSA), and two estimators of lean body mass (LBM1 and LBM2). The formulas for these descriptors are given in Table 2.
Table 2.
Formulas for body size descriptors used in modelinga
| Body size descriptor | Age or wt range | Formula |
|---|---|---|
| BSA (m2) | All ages | (wt0.5378) × (ht0.3964) × 0.024265 |
| LBM1 (kg) | Age ≤ 5 yr | Total body wt |
| 5 < age < 18 yr | Males, ln(LBM) = −2.8990 + 0.8064 × ln(ht) + 0.5674 × ln(wt) + 0.0000185 × wt2 − 0.0153 × (BMIz)2 + 0.0132 × age | |
| Females, ln(LBM) = −3.8345 + 0.954 × ln(ht) + 0.6515 × ln(wt) − 0.0102 × BMIz2 | ||
| Age ≥ 18 yr | Males, (9.27 × 103 × wt)/(6.68 × 103 + 216 × BMI) | |
| Females, (9.27 × 103 × wt)/(8.78 × 103 + 244 × BMI) | ||
| LBM2 (kg) | Wt < 50 kg | 3.8 × (0.0215 × wt0.6469 × ht0.7236) |
| Wt ≥ 50 kg | Males, 0.407 × wt + 0.267 × ht − 19.2 | |
| Females, 0.252 × wt + 0.473 × ht − 48.3 |
In formulas, weight is in kg, height is in cm, and age is in years. LBM1 and LBM2 were constrained to not exceed total body weight. BMIz values are z scores for subjects' body mass index values.
Obtaining estimates of lean body mass was complicated by the lack of LBM formulas applicable across the entire age range in this data set. Therefore, formulas were applied in a piecewise fashion according to age. To compute LBM1, formulas reported previously by Janmahasatian et al. (15), developed with adult subjects, were applied to patients at least 18 years of age, whereas formulas reported previously by Foster et al. (16), developed with children and adolescents, were applied to patients older than 5 but younger than 18 years of age. This formula requires that each child's body mass index (BMI) z score for age and gender be obtained. For the purposes of this analysis, BMI z scores corresponding to the CDC growth charts were computed by using a SAS macro available from the CDC (http://www.cdc.gov/nccdphp/dnpao/growthcharts/resources/sas.htm). As the formula by Foster et al. was not developed with very young children, patients 5 years of age and younger had LBM1 set to equal total body weight.
The second lean body mass estimation approach followed the method proposed previously by Peters et al. (17). This method is based on the underlying assumption that the relationship between extracellular fluid volume (ECV) and lean body mass is similar between adults and children. Peters et al. applied a proportionality constant linking ECV and lean body mass in adults to a pediatric ECV estimation formula described previously by Bird et al. (18). This yields a lean body mass formula applied in the present analysis to patients weighing <50 kg, a cutoff identified by Peters et al. For patients weighing 50 kg or more, a lean body mass formula derived by Boer (19), and utilized in the analysis by Peters et al., was applied.
Artesunate and DHA clearance and volume parameters were modeled with the various body size parameters by utilizing the following relationship:
where P is the typical value of a clearance or volume parameter, size is the value of a given body size descriptor for an individual, sizemedian is the median value of the descriptor in the full data set, and θ1 is the typical value of that parameter for a person with size equal to sizemedian. The value of θ2 was estimated as a free parameter or fixed to a set value. All estimated models are described in Table 3. For all body size descriptors, θ2 was estimated on all clearance and volume parameters for one model and set to 1 for an alternative, linear model. For weight, an additional allometric scaling model was tested with the exponent on body size set to 0.75 for clearance terms and 1.0 for volume terms. For purposes of model harmony, a single type of body size descriptor was used on all clearance and volume parameters in any given model. All models were evaluated by using both the full and pediatric data sets.
Table 3.
Body size models estimated using both full and pediatric data sets
The models incorporating body size descriptors as described above were evaluated per multiple criteria in order to identify a model to be carried forward for subsequent analyses. These criteria included the following: developmental and physiologic plausibility of the estimated parameter/body size descriptor relationships, overall model goodness-of-fit and estimate precision, goodness-of-fit equivalency across age strata, sensitivity of parameter estimates to inclusion of adolescent and adult data, and model complexity (i.e., number of estimated parameters).
Covariate modeling: full covariate model.
Once a model incorporating a body size descriptor was selected, additional covariate relationships of potential clinical interest were investigated in accordance with a full-covariate-model approach (20). These relationships included the effects of formulation on the absorption rate constant (ka) and the effect of gender, after accounting for body size, on all artesunate and DHA clearance and volume parameters. The effects of the granule formulation on ka were modeled as Popka = θ1 × (θ2)FORM, where Popka is the typical value of ka in the population, FORM is an indicator variable equal to 0 if patients were administered the tablet formulation and 1 if patients were administered the granule formulation, θ1 is the typical value of ka for patients administered the tablet formulation, and θ2 is the multiplicative factor describing the increase or decrease in ka associated with administration of the granule formulation.
The effects of gender on clearance and volume parameters were similarly modeled:
where sex is an indicator variable equal to 1 for males and 0 for females and θ3 is a multiplicative factor describing the effect of male gender on the clearance or volume parameter.
In order to obtain estimates for the magnitude of the covariate effects in the population, PsN was used to generate 500 bootstrap data sets from the full data set, with stratification by sampling type (extensive versus sparse) and formulation. The final full covariate model was fitted to these data sets. This process was then repeated using the pediatric data set. The distributions for the covariate effect estimates were then plotted by using the metrumrg R package (21).
Predictive checks.
After the full covariate models for both the full and pediatric data sets were obtained, PsN was used to perform numerical predictive checks (NPCs) and visual predictive checks (VPCs) for the analytes. For both NPC and VPC, 1,000 virtual observations at each sampling time point were simulated using the final parameter estimates. Due to the variability in the administered dose, prediction-correction was employed for VPC. NPC and VPC results were stratified per the following age categories: 5 years and younger, 6 years through 11 years, 12 years through 18 years, and >18 years. The latter two categories were not applicable to the pediatric data set evaluation. VPC results were visualized by using Xpose 4.4.0 (22). Categorical predictive checks for both artesunate and DHA were also implemented using PsN and Xpose. In this evaluation, the 95% confidence interval for the proportion of concentrations below the lower limit of quantification for a given analyte was calculated from simulations and compared to the observed proportion.
Covariate modeling: exploring trends.
After development of a full covariate model, conditional weighted residuals (CWRES) for artesunate and DHA and individual η estimates for parameters were plotted against various remaining covariates in the data sets. These included the following: age, baseline parasite count, baseline clinical laboratory findings (hemoglobin, hematocrit, and red blood cell count), baseline aspartate aminotransferase (AST) levels >1.5× the upper limit of normal (ULN), and baseline alanine aminotransferase (ALT) levels >1.5× the ULN. Covariates displaying potential and plausible relationships with parameters were tested for statistical significance by using forward addition (α = 0.05) followed by backward elimination (α = 0.01).
RESULTS
Data.
Data arising from 631 uncomplicated falciparum or vivax malaria patients were included in the full data set, with 274 patients <12 years of age also being included in the pediatric data set. The full data set contains data from 8 patients younger than 2 years, 266 patients 2 years through 11 years, 103 patients 12 years through 18 years, and 254 patients older than 18 years of age. A total of 1,490 observations were available for artesunate and DHA in the full data set, with 613 artesunate (41.2%) and 54 DHA (3.6%) concentrations being below the lower limit of quantification. For the pediatric data set, 786 observations were available, with 303 artesunate (38.6%) and 26 DHA (3.3%) concentrations being below the lower limit of quantification.
Base model.
The structural model ultimately selected included first-order artesunate absorption and a one-compartment model with first-order elimination for both artesunate and DHA. Importance sampling expectation maximization was the estimation method ultimately selected for modeling. Complete conversion of artesunate to DHA was assumed (23). The absorption rate constant (ka) for artesunate as well as the apparent clearance and volume of distribution for artesunate (CL/F and V2/F) and those for DHA (CLM/F and V3/F) were estimated. An IIV structure with covariance terms between all clearance and volume parameters was selected. Such a structure acknowledges that nonnegligible between-subject differences in artesunate bioavailability likely exist within the population. A discussion of alternative models evaluated during model development is provided in the supplemental material.
Covariate modeling: body size descriptor.
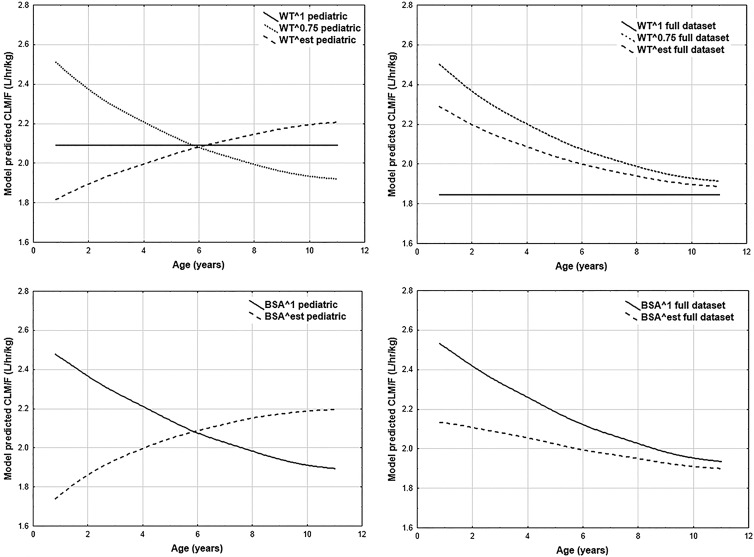
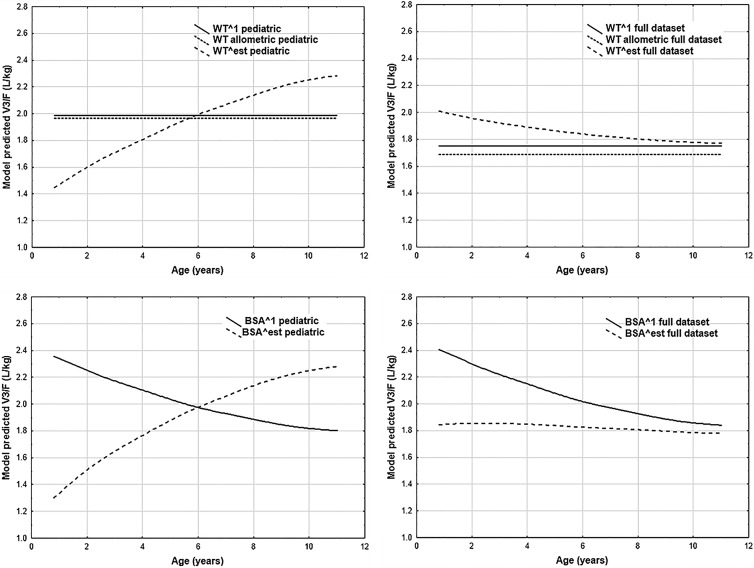
Since DHA is considered of greater clinical relevance than artesunate, the impact of incorporating various body size descriptors on DHA goodness-of-fit and DHA parameter estimates represented the principal focus of the body size descriptor modeling. Tables S1 and S2 in the supplemental material contain the point estimates and NONMEM-derived percent relative standard errors (%RSE) for CLM/F- and V3/F-related parameters, respectively. These point estimates were utilized to estimate the population-predicted CLM/F and V3/F values for each individual in the data set. As artemisinin derivative doses are typically expressed on a mg/kg basis, the population-predicted apparent clearance and volume of distribution values were converted to liters/h/kg and liters/kg, respectively. Plots of weight-adjusted DHA CLM/F and V3/F population-predicted values are given in Fig. 1 and 2, respectively, for models including weight or BSA.
Fig 1.
Best-fit lines for model-predicted typical DHA weight-normalized apparent clearance (CLM/F).
Fig 2.
Best-fit lines for model-predicted typical DHA weight-normalized apparent volume of distribution (V3/F).
Goodness-of-fit plots did not suggest superiority of any particular body size model, regardless of whether or not stratification by age was applied; therefore, this criterion did not inform model selection. Examination of the results from the models with estimated exponents revealed that the predicted CLM/F-versus-age and V3/F-versus-age curves for the pediatric data set models tended to differ, in some instances substantially, from their counterparts estimated with the full data set. Any interpretation of these observed differences is complicated by the imprecision associated with the estimates for the parameters in versions of these models derived from the pediatric data set; that is, due to the uncertainty in the parameter estimates, drawing conclusions based on the distinctions between the curves for the full and pediatric data sets would be inadvisable. Given this challenge, and the necessity of estimating four additional parameters, these models were not carried forward for use in further analyses.
The linear weight model was estimated with fairly good precision. However, the pediatric and full data sets yielded fairly different predicted CLM/F and V3/F values. The linear LBM1 model displays dramatic fluctuations across age, presumably due to the piecewise application of formulas used in LBM1 calculation. From a developmental plausibility perspective, such abrupt fluctuations are undesirable. In contrast, the linear LBM2 model did not display such fluctuations. Unfortunately, the parameter estimates for the full-data-set version of this linear LBM2 model displayed extremely poor precision, which supported rejection of this model.
The allometric scaling model and the linear BSA model were both estimated with adequate precision and displayed a relative insensitivity to inclusion or exclusion of adolescent and adult data. In actuality, the predicted CLM/F values, as shown in Fig. 1, were extremely similar between the two models, regardless of the data set employed. Ultimately, the allometric scaling and linear BSA models were deemed to be the best body size descriptor models, as judged per the prespecified criteria, from among the various models assessed. Given the similarities between the two models in the evaluation criteria, the most justifiable choice was to carry forward both models into further stages of modeling.
Full covariate model.
The full covariate model included gender on all clearance and volume parameters and formulation on ka. However, only unrealistically high estimates for the increase in ka associated with the granule formulation could be obtained. Ultimately, the granule ka was fixed to 10 h−1 to reflect the apparently quite rapid absorption of the formulation, with the tablet ka left to be freely estimated. Further discussion of this formulation effect is provided in the supplemental material.
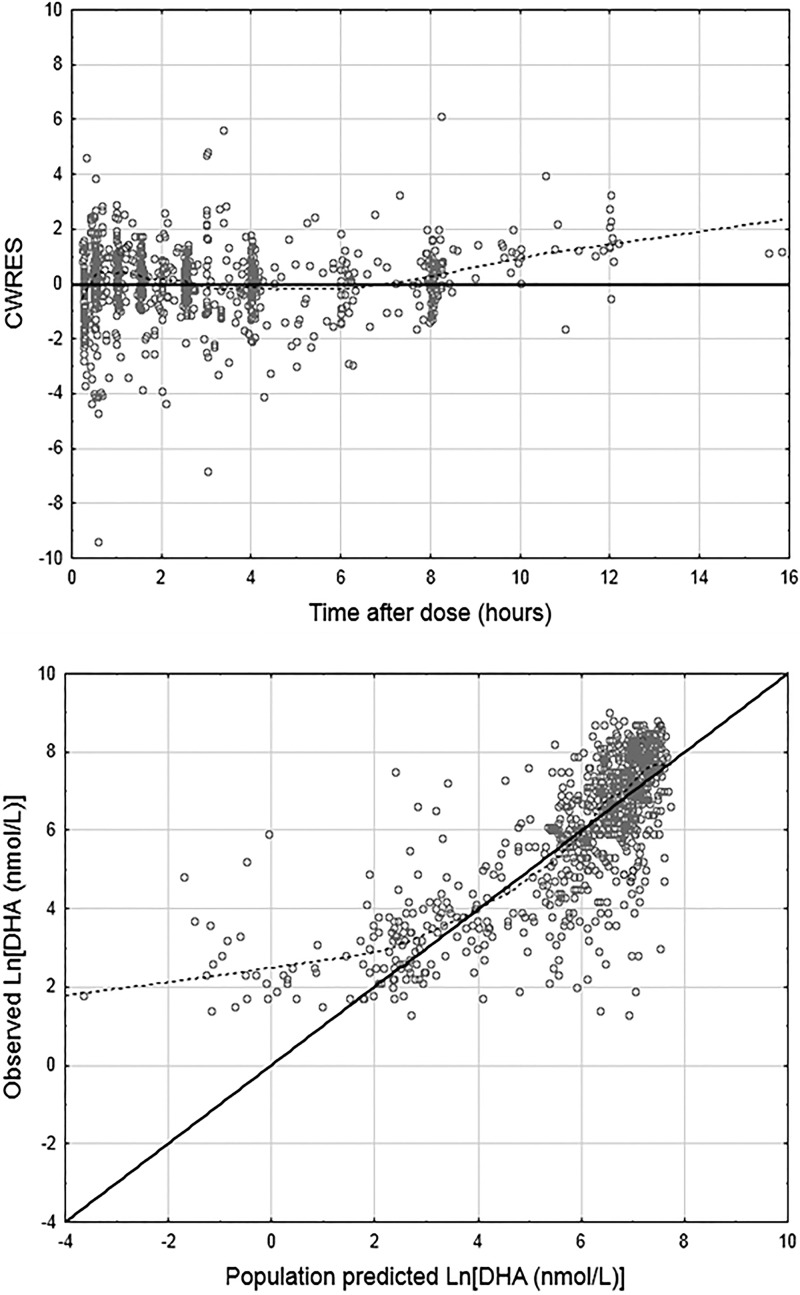
The parameter estimates for the full covariate models with this modified ka coding, and with the gender effects estimated on the clearance and volume parameters, are given in Table 4 for the allometric scaling and the linear BSA models, with analogous parameter estimates from the full population models given in Table S3 in the supplemental material. IIV covariance estimates for the various models are given in Table S4 in the supplemental material. The 95% confidence intervals for the effects, determined through 500 bootstrap runs, are plotted for CLM/F and V3/F in Fig. 3 and 4, respectively, with plots for the artesunate parameters provided in Fig. S1 and S2 in the supplemental material. Plots of DHA CWRES versus time after dose and population-predicted DHA versus observed DHA concentrations for the pediatric linear BSA model are given in Fig. 5. The analogous plots for the allometric scaling model (not shown) were essentially identical.
Table 4.
Summary of the results obtained from the allometric scaling model and linear BSA model as implemented with the pediatric data seta
| Parameter | Allometric scaling |
Linear BSA |
||
|---|---|---|---|---|
| Model estimate (bootstrap %RSE) | Bootstrap 95% CI | Model estimate (bootstrap %RSE) | Bootstrap 95% CI | |
| CL/F (liters/h) | 923 (9.41) | 765, 1,101 | 884 (9.40) | 732, 1,052 |
| V2/F (liters) | 1,130 (16.6) | 794, 1,538 | 884 (16.6) | 617, 1,208 |
| CLM/F (liters/h) | 65.1 (8.54) | 55.2, 77.5 | 62.3 (8.56) | 52.8, 74.3 |
| V3/F (liters/h) | 79.1 (12.1) | 61.6, 101 | 63.9 (12.0) | 49.6, 80.9 |
| ka (h−1) | 2.46 (20.7) | 1.92, 3.92 | 2.27 (19.8) | 1.78, 3.54 |
| Effect of gender on CL/F | 1.07 (12.0) | 0.826, 1.34 | 1.08 (11.9) | 0.829, 1.35 |
| Effect of gender on V2/F | 1.06 (22.9) | 0.738, 1.72 | 1.06 (23.3) | 0.745, 1.70 |
| Effect of gender on CLM/F | 1.05 (10.6) | 0.861, 1.29 | 1.05 (10.7) | 0.858, 1.30 |
| Effect of gender on V3/F | 0.891 (17.6) | 0.600, 1.21 | 0.927 (17.2) | 0.612, 1.26 |
| IIV-CL/F | 0.279 (22.4) | 0.165, 0.412 | 0.278 (22.5) | 0.160, 0.407 |
| IIV-V2/F | 0.830 (21.1) | 0.499, 1.19 | 0.827 (22.6) | 0.498, 1.22 |
| IIV-CLM/F | 0.248 (25.7) | 0.147, 0.408 | 0.258 (25.8) | 0.154, 0.428 |
| IIV-V3/F | 0.414 (29.7) | 0.192, 0.643 | 0.424 (29.3) | 0.201, 0.642 |
| IIV-ka | 0.987 (50.8) | 0.548, 2.55 | 0.975 (50.0) | 0.547, 2.46 |
| Residual variability (σ2) for AS | 0.586 (29.2) | 0.510, 1.19 | 0.583 (29.5) | 0.509, 1.19 |
| Residual variability (σ2) for DHA | 0.876 (15.5) | 0.575, 1.13 | 0.872 (15.6) | 0.574, 1.13 |
CL/F, V2/F, and ka are artesunate apparent clearance, apparent volume of distribution, and absorption rate constant, respectively. CLM/F and V3/F are DHA apparent clearance and apparent volume of distribution, respectively. CI, confidence interval.
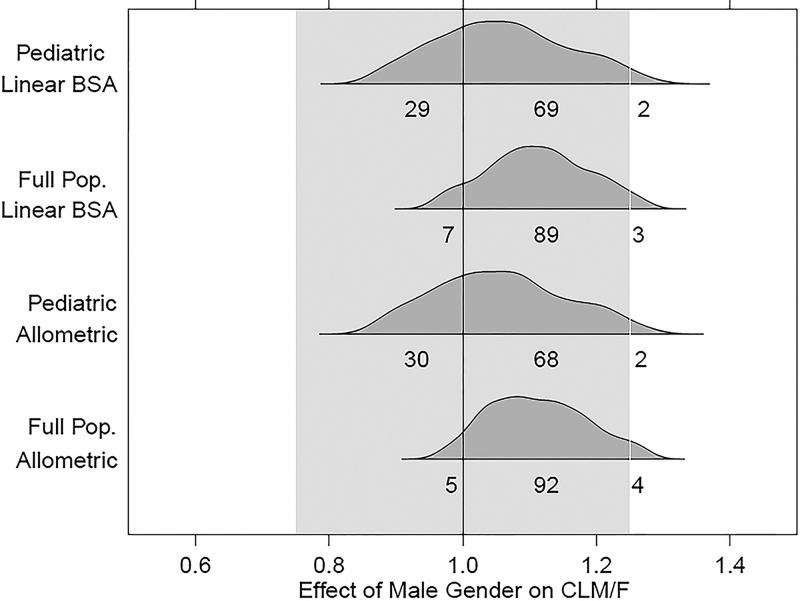
Fig 3.
Covariate-effect plots for effect of gender on CLM/F. Distributions correspond to the 2.5th to 97.5th percentiles for gender effects obtained from the bootstrap results from each model.
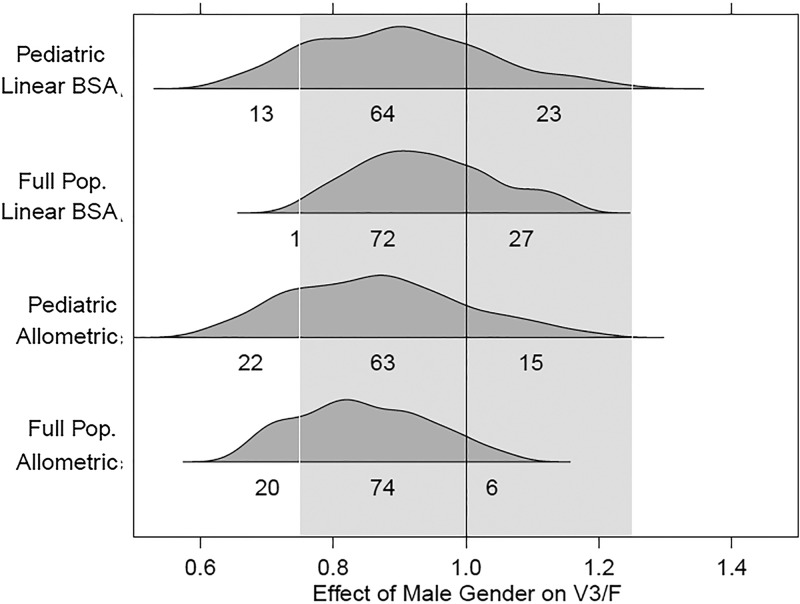
Fig 4.
Covariate effect plots for effect of gender on V3/F. Distributions correspond to the 2.5th to 97.5th percentiles for gender effects obtained from the bootstrap results from each model.
Fig 5.
Goodness-of-fit plots for DHA in the full covariate model. The solid lines are lines of identity. The broken lines are smoothing lines. Both plots represent the pediatric linear BSA model. CWRES, conditional weighted residuals.
Predictive checks.
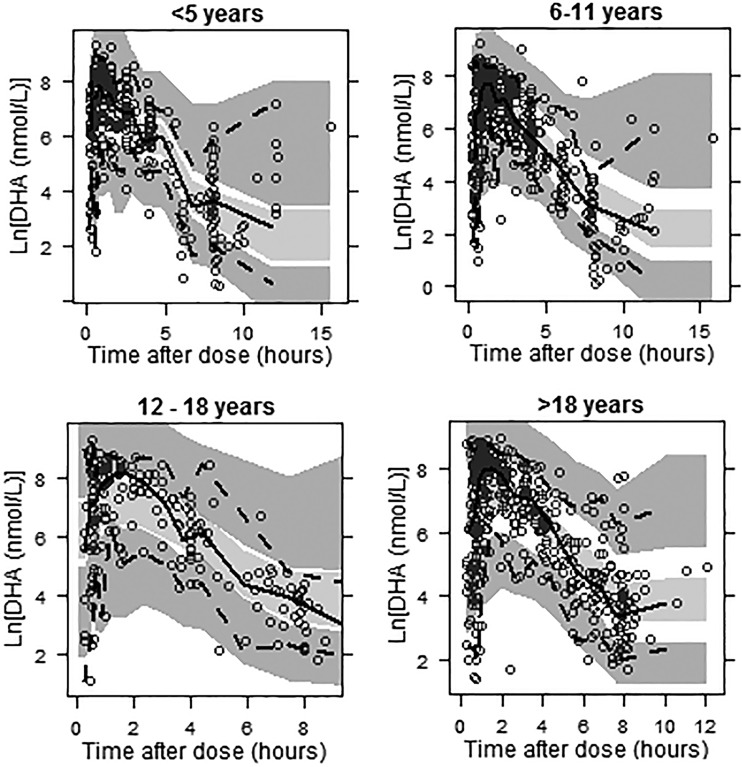
The full covariate models were used to perform VPCs stratified by age. The DHA VPCs for the full-population linear BSA model are given in Fig. 6. DHA VPCs for the remaining models were quite similar. The percentages of concentrations below the 5th percentile and above the 95th percentile of the simulated concentrations are given in Table S5 in the supplemental material. Although these results do not indicate a clear difference in predictive ability for the two body size models, the stratification does indicate that predictive ability is superior for patients 18 years of age and younger. This is an acceptable result given that the target population to be described by the analysis is pediatric patients.
Fig 6.
VPC plots for DHA stratified by age for the full-population linear BSA model. The open circles represent the observed concentrations, the solid lines represent the median of the observed data, the dashed lines represent the 5th and 95th percentiles for the observed data, and the shaded areas represent the 95% confidence intervals surrounding the simulation-derived prediction intervals (5th, 50th, and 95th percentiles) obtained from the simulations.
Figures S3 and S4 in the supplemental material display the categorical VPC results for artesunate and DHA, respectively, for the full-population linear BSA model; alternative models yielded similar results. Per this evaluation, the proportion of artesunate concentrations below the lower limit of quantification is underpredicted by the model. However, for DHA, the simulated and actual proportions are well aligned, suggesting that failure to incorporate concentrations below the lower limit of quantification into the modeling did not bias predictions for DHA, the analyte of primary interest.
Covariate modeling: exploring trends.
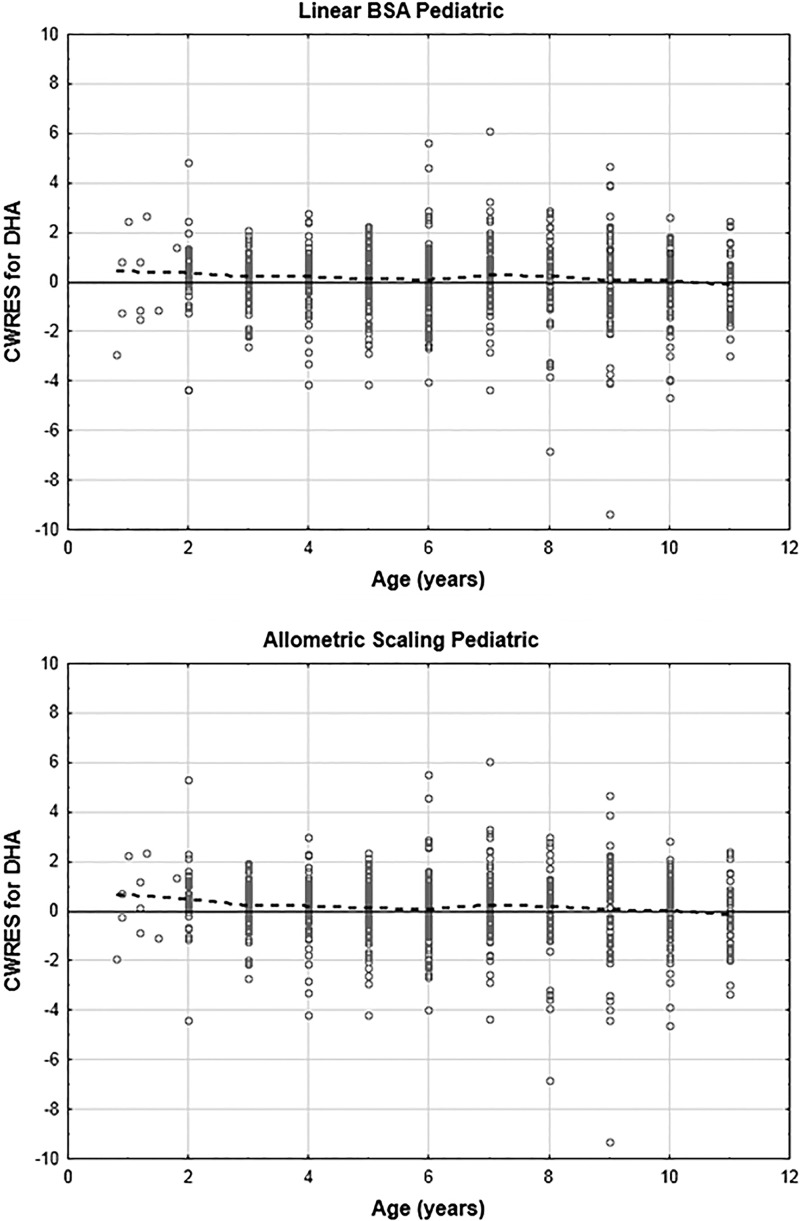
The plots of DHA CWRES versus age for patients 12 years of age and younger are given in Fig. 7. There appears to be a slight trend toward underestimation of DHA concentrations for very young patients. For artesunate, this trend was also apparent (plots not shown). Plots of estimated η values versus age indicated a trend toward negative η values for multiple parameters (CL/F, CLM/F, and V3/F) for patients 4 years of age and younger; that is, these plots suggested that the patients may have lower actual parameter values than predicted by the model. Given that this trend appears to be acting across multiple parameters, a likely explanation may be that the patients are displaying higher bioavailability than accounted for by the model. To investigate this possibility, F1 was set to 1 for patients older than 4 years of age, with relative bioavailability estimated for patients 4 years of age and younger. This did result in statistically significant differences (P < 0.01) in all models and data sets.
Fig 7.
Plots of DHA conditional weighted residuals (CWRES) versus age for pediatric linear BSA and allometric scaling models.
Major potential confounding factors related to this finding must be considered, however. For example, among patients 4 years of age and younger, 77% of the patients were administered granules, compared to 26% of patients aged 5 to 11 years. Considering only the pediatric data set, for patients 4 years of age and younger, 91% of the artesunate and 86% of the DHA concentrations used in modeling were obtained on day 1, with the remainder being obtained on day 3. In contrast, for patients between 5 and 11 years of age, 71% of the artesunate and 63% of the DHA concentrations were obtained on day 1. Therefore, the modeled increase in bioavailability in this age group could reflect a bioavailability effect of acute illness or of the granule formulation. Unfortunately, neither of these effects was previously successfully modeled. To avoid spuriously ascribing an effect to age which, in actuality, likely might have stemmed from other causes, full evaluation of models including this effect was not pursued, and the effect was not included in the final models.
DISCUSSION
The intent of this analysis was to describe the population pharmacokinetics of artesunate and DHA in pediatric patients, utilizing data from 631 pediatric, adolescent, and adult uncomplicated malaria patients participating in phase II and III clinical trials for the combination agent pyronaridine tetraphosphate-artesunate. To this end, a parent metabolite base model with first-order artesunate absorption and a one-compartment model with first-order elimination for both artesunate and DHA was developed. Various methods for incorporating body size descriptors into clearance and volume parameters were assessed, and two highly similar models, a linear BSA model and an allometric scaling model, were ultimately selected as the optimal body size models. Building upon these two models, the effects of gender on the clearance and volume parameters were evaluated by using a full-covariate-model approach; although the covariate effect estimates were sufficiently imprecise to preclude drawing any definitive conclusions, the findings could be considered tentatively consistent with the lack of a clinically relevant effect of gender on DHA apparent clearance. Finally, in this analysis, it was found that modeling with a data set including adolescent and adult data allowed for increased precision in estimation of parameters without introducing any meaningful bias in the point estimates for these parameters.
Physiologic basis for models.
In this analysis, the clearances of artesunate and DHA were described by using relationships with either weight or BSA but not with patient age. The choice to not incorporate the covariate of age a priori with body size, as well as to not assess for an age effect in the full covariate model, essentially rested on two assumptions. The first assumption was that across the studied patient population, the hepatic clearances of artesunate and DHA would be dependent on the rate of hepatic blood flow rather than intrinsic clearance. The second assumption was that the developmental changes in hepatic blood flow could be satisfactorily accounted for by using clearance-body size descriptor relationships. Additionally, it should be noted that no claim is being made that these assumptions would, or would not, be applicable to patients <2 years of age. As there were only eight such patients in the data set, all with sparse sampling data, no attempt was made in the analysis to derive and justify pharmacokinetic findings appropriate for this age group.
Evidence for the assumption that the analyte clearances will display hepatic blood flow-limited kinetics among patients as young as 2 years of age can be found in a previous study by Nealon et al. (24). This study included an assessment of artesunate and DHA pharmacokinetics following i.v. administration of artesunate to children with severe malaria. The two subgroups of children in the study had median ages of 36 and 21 months. Pooled pharmacokinetic findings from the two patient subgroups indicated median artesunate and DHA clearance values of 46 ml/kg/min and 25 ml/kg/min, respectively, with substantial individual variability being associated with both estimates. As a point of reference, the clearance of indocyanine green (25), a probe substrate for hepatic blood flow, had a mean value of 15.6 ml/kg/min (standard deviation, 7.3 ml/kg/min) among patients younger than 10 years of age. Allowing for the observed variability in both the i.v. artesunate pharmacokinetic findings and hepatic blood flow estimates, it appears that artesunate and DHA clearances are not limited by intrinsic clearance even in children as young as 21 months of age.
Further evidence that intrinsic clearance does not limit artesunate and DHA clearances even in young children can be obtained from in vivo and in vitro findings for agents with analogous metabolic profiles. With regard to artesunate, pediatric pharmacokinetic findings for agents undergoing esterase-mediated hydrolysis, such as oseltamivir and remifentanil, are indicative of efficient esterase activity for patients at, and even prior to, 1 year of age (26, 27). With regard to DHA, various in vivo studies with morphine, a probe substrate for UGT2B7, have indicated achievement of adult UGT2B7 activity well prior to 2 years of age (28). Similar conclusions were reached following an in vitro investigation of epirubicin glucuronidation by UGT2B7 in pediatric and adult liver microsomes (29).
The pediatric i.v. artesunate results, coupled with the findings related to the ontogeny of the individual metabolizing enzymes, provide support for the assumption that artesunate and DHA hepatic clearance will be limited by hepatic blood flow among patients at least 2 years of age. Granting this assumption, clearly a body size descriptor-clearance relationship appropriately modeling changes in hepatic blood flow would account for the developmental pattern of artesunate and DHA clearances. Hepatic blood flow is proportional to liver volume; liver volume, expressed per kg of total body weight, is higher in younger children than in older children (30). Therefore, for agents with clearance dependent on hepatic blood flow, pediatric clearance values, expressed per kg of total body weight, decline as children mature. Liver volume, when normalized to BSA, but not to total body weight, is constant over the pediatric age range (30, 31). In actuality, a general nonlinear trend between age and per-kg clearance in pediatric subjects is approximated by both the linear BSA model and the allometric scaling models, and both models have been extensively employed in pediatric pharmacokinetic analyses.
The potential clinical implications of this nonlinear trend in per-kg clearance do merit attention. For example, with the allometric scaling model, given patients administered equivalent mg/kg doses, a typical 10-kg patient's expected DHA exposure (area under the concentration-time curve [AUC]) would be approximately one-quarter or one-third lower than that of a 35-kg or a 60-kg patient, respectively. Put another way, given a 60-kg patient administered 3 mg/kg/day artesunate, a 10-kg patient would need to receive, on average, 4.5 mg/kg/day to attain a similar exposure. Of course, such estimates of exposure differences reflect expected population values; within a given pair of individuals, exposure differences are far less predictable.
Covariate model: gender effects.
For the full covariate model, the effect intervals for all of the gender-parameter relationships crossed 1.0, indicating a lack of statistically significant effects. No interval, regardless of parameter, model, or data set employed, had a bootstrap 95% confidence interval contained entirely within the interval of 0.75 to 1.25 for a clinically irrelevant effect. Essentially, the results indicated that insufficient information was available to conclusively judge any effect as being clinically relevant or irrelevant. It is worth noting, however, that for CLM/F, the large bulk of the effect estimate distribution for each model fell within the 0.75-to-1.25 no-effect region. This would appear to offer some evidence for the lack of a clinically relevant gender effect on CLM/F. For strictly predictive purposes, more parsimonious models without the gender effects would be justifiable.
The standard interval of 0.75 to 1.25 was used to indicate a clinically relevant effect of a covariate on a parameter. This interval was adopted because, at the time of this analysis, a clear relationship between concentrations and clinical efficacy has remained largely undefined for the artemisinin derivatives (32). However, were such a relationship to be determined, the full covariate modeling in the present analysis could be reinterpreted. For example, if a threshold DHA AUC for efficacy were known, the CLM/F gender effect results could be used to estimate the probability that being male would result in a failure to meet that target. More generally, additional pharmacodynamic information could be used to adjust the default limits of 0.75 to 1.25 to artesunate-specific values.
Pharmacokinetic comparisons.
One of the studies (study 1) included in the present analysis was previously analyzed by using noncompartmental methods (7). The mean apparent DHA clearance from the noncompartmental analysis, approximated from subgroup means, was 2.4 liters/kg/h. For an average weight of 18.5 kg, the full population and pediatric allometric scaling models yielded a prediction of 2.1 liters/kg/h. Given that this value reflects a population prediction for an average weight, this model-predicted value is adequately similar to the noncompartmental findings.
The population pharmacokinetics of oral artesunate in pediatric patients was previously examined by Stepniewska et al., who studied artesunate pharmacokinetics following oral artesunate administration to uncomplicated falciparum malaria patients between 6 months and 5 years of age in Burkina Faso (33). Artesunate and DHA pharmacokinetic data were obtained from 70 children who received artesunate and amodiaquine, with samples taken once in the first dosing period and once in the third dosing period. Pharmacokinetic modeling was conducted using DHA concentrations as well as total antimalarial activity. The DHA concentration data were fit to a one-compartment model. Those authors estimated a DHA apparent clearance of 0.636 liters/h/kg for the first dosing period, with a substantial increase of 0.760 liters/h/kg associated with the third dosing period, yielding a day 3 apparent clearance rate of approximately 1.4 liters/h/kg.
Considering a patient with a weight of 13 kg, an approximate average weight for patients in the study by Stepniewska et al., the population-predicted CLM/F would be 2.2 liters/h/kg for a female patient per the allometric scaling model (pediatric or full data set) and 2.4 liters/h/kg for a male patient. Clearly, the estimated apparent clearance in the study by Stepniewska et al., particularly for day 1, is substantially lower than the model-estimated apparent clearance from the present analysis. The difference is unlikely to be due in any large part to the failure of the model to account for a simple effect of acute infection on day 1 pharmacokinetics. After all, as described above, noncompartmental results of study 1, which were derived entirely from day 1 samples, are consistent with model-estimated clearance predictions. However, a more complex disease effect, dependent on the severity of infection and the age of the patients, could perhaps be operating. The median parasite count for patients in the pediatric data set in the present analysis was 10,341, whereas the median parasite counts for the two cohorts of the study by Stepniewska et al. were 29,000 and 30,000. In a recent analysis of artesunate pharmacokinetics in pregnant women, it was observed that women who were moderately unwell displayed significantly higher combined exposure to artesunate and DHA than women who were mildly unwell (14). This dynamic could account for some of the discrepancies observed for day 1 clearance estimates. Furthermore, the median ages in the two cohorts of the study by Stepniewska et al. were 3.1 and 2.7 years, compared to 7 years in the pediatric data set of the present analysis. It is not inconceivable that pediatric patients might experience a more dramatic physiologic response to acute illness than older patients, resulting in a more pronounced disease effect on artesunate and DHA pharmacokinetics. Further investigation would be required to characterize such a potential interaction effect between age and acute illness.
Inclusion of adult data.
Throughout this analysis, models were evaluated by using two data sets, one with the full age range of patients and another including patients only younger than 12 years of age. The intention of this parallel modeling approach was to allow for utilization of additional data, which could bolster model stability and estimate precision, while simultaneously checking for possible estimate biases in covariate-parameter relationships introduced through inclusion of data from nonpediatric patients. Indeed, the full-data-set models did allow for more precise estimation of gender effects, as well as multiple other parameters, than their pediatric counterparts. However, the point estimates of the final models for essentially all of the parameters were quite similar, suggesting that bias was not introduced through inclusion of the adolescent and adult data. Further discussion of this aspect of the analysis is provided in the supplemental material.
Conclusions.
Overall, the results of the present analysis indicate that the pharmacokinetics of artesunate and DHA following oral artesunate administration can be described for pediatric patients using either an allometric scaling or linear BSA model, a finding consistent with the likely tight relationship between hepatic blood flow and artesunate-DHA clearance. Furthermore, the analysis demonstrated that when utilizing these body size models, adolescent and adult pharmacokinetic data could be included in the modeling data set to enhance parameter estimate precision. Limitations of the data set used in this analysis include the relatively mild infection experienced by a majority of the patients and the minimal number of patients <2 years of age. Both the allometric scaling and linear BSA models predict that, for the same mg/kg artesunate dose, younger children are expected to have lower DHA exposure than older children or adults. The extent to which this pattern can be extrapolated to children younger than 2 years of age is dependent on the relative influences of hepatic blood flow and metabolizing enzyme maturation as well as any interaction between age and an acute disease effect. Further investigation clearly is required, and should be undertaken, to elucidate these various dynamics. Given the high risk of malaria-related mortality experienced by young children, there is clearly a significant need for such investigations.
Supplementary Material
ACKNOWLEDGMENTS
Funding for this study was provided by Medicines for Malaria Ventures and Shin Poong Pharmaceuticals.
Footnotes
Published ahead of print 16 September 2013
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AAC.00635-13.
REFERENCES
- 1. World Health Organization 2012. World malaria report: 2012. World Health Organization, Geneva, Switzerland: http://www.who.int/malaria/publications/world_malaria_report_2012/wmr2012_full_report.pdf [Google Scholar]
- 2. World Health Organization 2011. Guidelines for the treatment of malaria, 2nd ed, rev 1 World Health Organization, Geneva, Switzerland: http://whqlibdoc.who.int/publications/2010/9789241547925_eng.pdf [Google Scholar]
- 3. Morris CA, Duparc S, Borghini-Fuhrer I, Jung D, Shin CS, Fleckenstein L. 2011. Review of the clinical pharmacokinetics of artesunate and its active metabolite dihydroartemisinin following intravenous, intramuscular, oral or rectal administration. Malar. J. 10:263. 10.1186/1475-2875-10-263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mercer AE, Sarr Sallah M. 2011. The pharmacokinetic evaluation of artemisinin drugs for the treatment of malaria in paediatric populations. Expert Opin. Drug Metab. Toxicol. 7:427–439 [DOI] [PubMed] [Google Scholar]
- 5. Pawluk SA, Wilby KJ, Ensom MH. 2013. Pharmacokinetic profile of artemisinin derivatives and companion drugs used in artemisinin-based combination therapies for the treatment of Plasmodium falciparum malaria in children. Clin. Pharmacokinet. 52:153–167 [DOI] [PubMed] [Google Scholar]
- 6. International Conference on Harmonisation 2000. Clinical investigation of medicinal products in the pediatric population. International Conference on Harmonisation, Geneva, Switzerland: http://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Efficacy/E11/Step4/E11_Guideline.pdf [PubMed] [Google Scholar]
- 7. Ramharter M, Kurth F, Schreier AC, Nemeth J, Glasenapp I, Belard S, Schlie M, Kammer J, Koumba PK, Cisse B, Mordmuller B, Lell B, Issifou S, Oeuvray C, Fleckenstein L, Kremsner PG. 2008. Fixed-dose pyronaridine-artesunate combination for treatment of uncomplicated falciparum malaria in pediatric patients in Gabon. J. Infect. Dis. 198:911–919 [DOI] [PubMed] [Google Scholar]
- 8. Kayentao K, Doumbo OK, Penali LK, Offianan AT, Bhatt KM, Kimani J, Tshefu AK, Kokolomami JH, Ramharter M, de Salazar PM, Tiono AB, Ouedraogo A, Bustos MD, Quicho F, Borghini-Fuhrer I, Duparc S, Shin CS, Fleckenstein L. 2012. Pyronaridine-artesunate granules versus artemether-lumefantrine crushed tablets in children with Plasmodium falciparum malaria: a randomized controlled trial. Malar. J. 11:364. 10.1186/1475-2875-11-364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Naik H, Murry DJ, Kirsch LE, Fleckenstein L. 2005. Development and validation of a high-performance liquid chromatography-mass spectroscopy assay for determination of artesunate and dihydroartemisinin in human plasma. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 816:233–242 [DOI] [PubMed] [Google Scholar]
- 10. Bauer RJ. 2011. NONMEM users guide: introduction to NONMEM 7.2.0. ICON Development Solutions, Ellicott City, MD: ftp://nonmem.iconplc.com/Public/nonmem7/Release_Notes_Plus/nm720.pdf [Google Scholar]
- 11. Lindbom L, Pihlgren P, Jonsson EN. 2005. PsN-Toolkit—a collection of computer intensive statistical methods for non-linear mixed effect modeling using NONMEM. Comput. Methods Programs Biomed. 79:241–257 [DOI] [PubMed] [Google Scholar]
- 12. Keizer RJ, van Benten M, Beijnen JH, Schellens JH, Huitema AD. 2011. Pirana and PCluster: a modeling environment and cluster infrastructure for NONMEM. Comput. Methods Programs Biomed. 101:72–79 [DOI] [PubMed] [Google Scholar]
- 13. Beal SL. 2001. Ways to fit a PK model with some data below the quantification limit. J. Pharmacokinet. Pharmacodyn. 28:481–504 [DOI] [PubMed] [Google Scholar]
- 14. McGready R, Phyo AP, Rijken MJ, Tarning J, Lindegardh N, Hanpithakpon W, Than HH, Hlaing N, Zin NT, Singhasivanon P, White NJ, Nosten F. 2012. Artesunate/dihydroartemisinin pharmacokinetics in acute falciparum malaria in pregnancy: absorption, bioavailability, disposition and disease effects. Br. J. Clin. Pharmacol. 73:467–477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Janmahasatian S, Duffull SB, Ash S, Ward LC, Byrne NM, Green B. 2005. Quantification of lean bodyweight. Clin. Pharmacokinet. 44:1051–1065 [DOI] [PubMed] [Google Scholar]
- 16. Foster BJ, Platt RW, Zemel BS. 2012. Development and validation of a predictive equation for lean body mass in children and adolescents. Ann. Hum. Biol. 39:171–182 [DOI] [PubMed] [Google Scholar]
- 17. Peters AM, Snelling HL, Glass DM, Bird NJ. 2011. Estimation of lean body mass in children. Br. J. Anaesth. 106:719–723 [DOI] [PubMed] [Google Scholar]
- 18. Bird NJ, Henderson BL, Lui D, Ballinger JR, Peters AM. 2003. Indexing glomerular filtration rate to suit children. J. Nucl. Med. 44:1037–1043 [PubMed] [Google Scholar]
- 19. Boer P. 1984. Estimated lean body mass as an index for normalization of body fluid volumes in humans. Am. J. Physiol. 247:F632–F636 [DOI] [PubMed] [Google Scholar]
- 20. Ravva P, Gastonguay MR, Tensfeldt TG, Faessel HM. 2009. Population pharmacokinetic analysis of varenicline in adult smokers. Br. J. Clin. Pharmacol. 68:669–681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bergsma TT, Knebel W, Fisher J, Gillespie WR, Riggs MM, Gibiansky L, Gastonguay MR. 2013. Facilitating pharmacometric workflow with the metrumrg package for R. Comput. Methods Programs Biomed. 109:77–85 [DOI] [PubMed] [Google Scholar]
- 22. Jonsson EN, Karlsson MO. 1999. Xpose—an S-PLUS based population pharmacokinetic/pharmacodynamic model building aid for NONMEM. Comput. Methods Programs Biomed. 58:51–64 [DOI] [PubMed] [Google Scholar]
- 23. Ilett KF, Ethell BT, Maggs JL, Davis TM, Batty KT, Burchell B, Binh TQ, Thu LTA, Hung NC, Pirmohamed M, Park BK, Edwards G. 2002. Glucuronidation of dihydroartemisinin in vivo and by human liver microsomes and expressed UDP-glucuronosyltransferases. Drug Metab. Dispos. 30:1005–1012 [DOI] [PubMed] [Google Scholar]
- 24. Nealon C, Dzeing A, Muller-Romer U, Planche T, Sinou V, Kombila M, Kremsner PG, Parzy D, Krishna S. 2002. Intramuscular bioavailability and clinical efficacy of artesunate in Gabonese children with severe malaria. Antimicrob. Agents Chemother. 46:3933–3939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Evans WE, Relling MV, de Graaf S, Rodman JH, Pieper JA, Christensen ML, Crom WR. 1989. Hepatic drug clearance in children: studies with indocyanine green as a model substrate. J. Pharm. Sci. 78:452–456 [DOI] [PubMed] [Google Scholar]
- 26. Oo C, Hill G, Dorr A, Liu B, Boellner S, Ward P. 2003. Pharmacokinetics of anti-influenza prodrug oseltamivir in children aged 1-5 years. Eur. J. Clin. Pharmacol. 59:411–415 [DOI] [PubMed] [Google Scholar]
- 27. Ross AK, Davis PJ, Dear GD, Ginsberg B, McGowan FX, Stiller RD, Henson LG, Huffman C, Muir KT. 2001. Pharmacokinetics of remifentanil in anesthetized pediatric patients undergoing elective surgery or diagnostic procedures. Anesth. Analg. 93:1393–1401 [DOI] [PubMed] [Google Scholar]
- 28. de Wildt SN, Kearns GL, Leeder JS, van den Anker JN. 1999. Glucuronidation in humans. Pharmacogenetic and developmental aspects. Clin. Pharmacokinet. 36:439–452 [DOI] [PubMed] [Google Scholar]
- 29. Zaya MJ, Hines RN, Stevens JC. 2006. Epirubicin glucuronidation and UGT2B7 developmental expression. Drug Metab. Dispos. 34:2097–2101 [DOI] [PubMed] [Google Scholar]
- 30. Murry DJ, Crom WR, Reddick WE, Bhargava R, Evans WE. 1995. Liver volume as a determinant of drug clearance in children and adolescents. Drug Metab. Dispos. 23:1110–1116 [PubMed] [Google Scholar]
- 31. Urata K, Kawasaki S, Matsunami H, Hashikura Y, Ikegami T, Ishizone S, Momose Y, Komiyama A, Makuuchi M. 1995. Calculation of child and adult standard liver volume for liver transplantation. Hepatology 21:1317–1321 [PubMed] [Google Scholar]
- 32. Saunders D, Khemawoot P, Vanachayangkul P, Siripokasupkul R, Bethell D, Tyner S, Se Y, Rutvisuttinunt W, Sriwichai S, Chanthap L, Lin J, Timmermans A, Socheat D, Ringwald P, Noedl H, Smith B, Fukuda M, Teja-Isavadharm P. 2012. Pharmacokinetics and pharmacodynamics of oral artesunate monotherapy in patients with uncomplicated Plasmodium falciparum malaria in western Cambodia. Antimicrob. Agents Chemother. 56:5484–5493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Stepniewska K, Taylor W, Sirima SB, Ouedraogo EB, Ouedraogo A, Gansane A, Simpson JA, Morgan CC, White NJ, Kiechel JR. 2009. Population pharmacokinetics of artesunate and amodiaquine in African children. Malar. J. 8:200. 10.1186/1475-2875-8-200 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.