Abstract
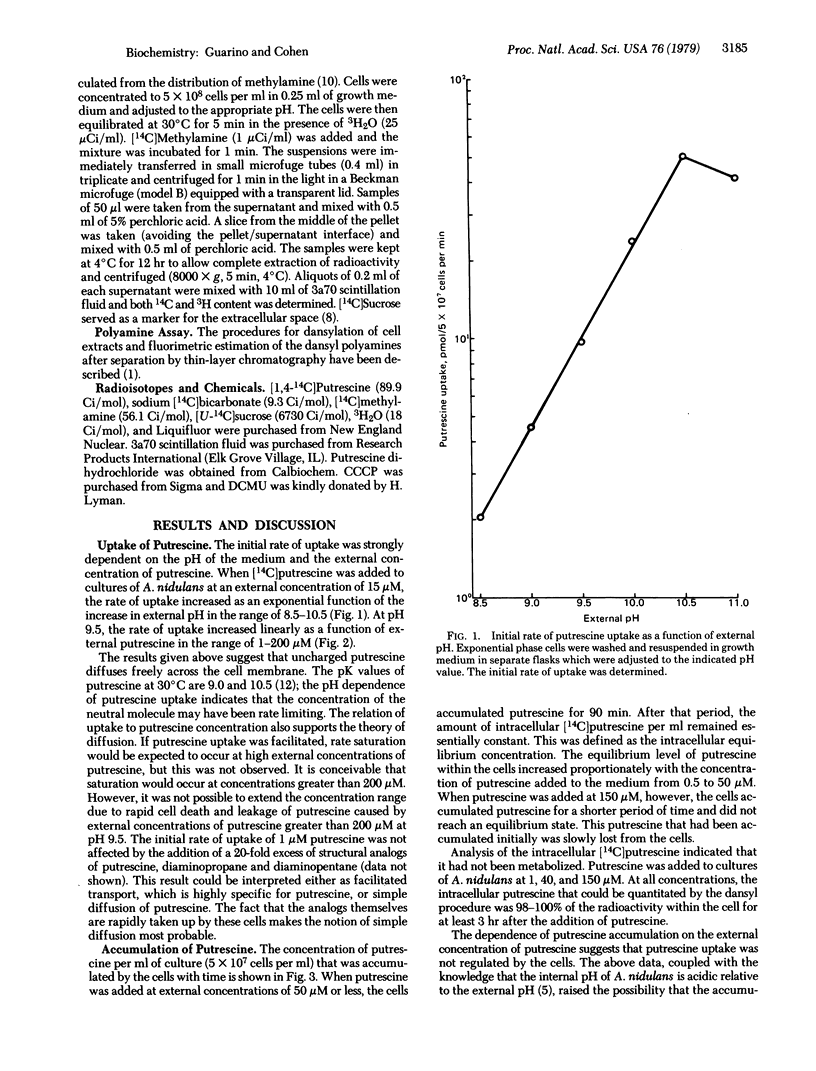
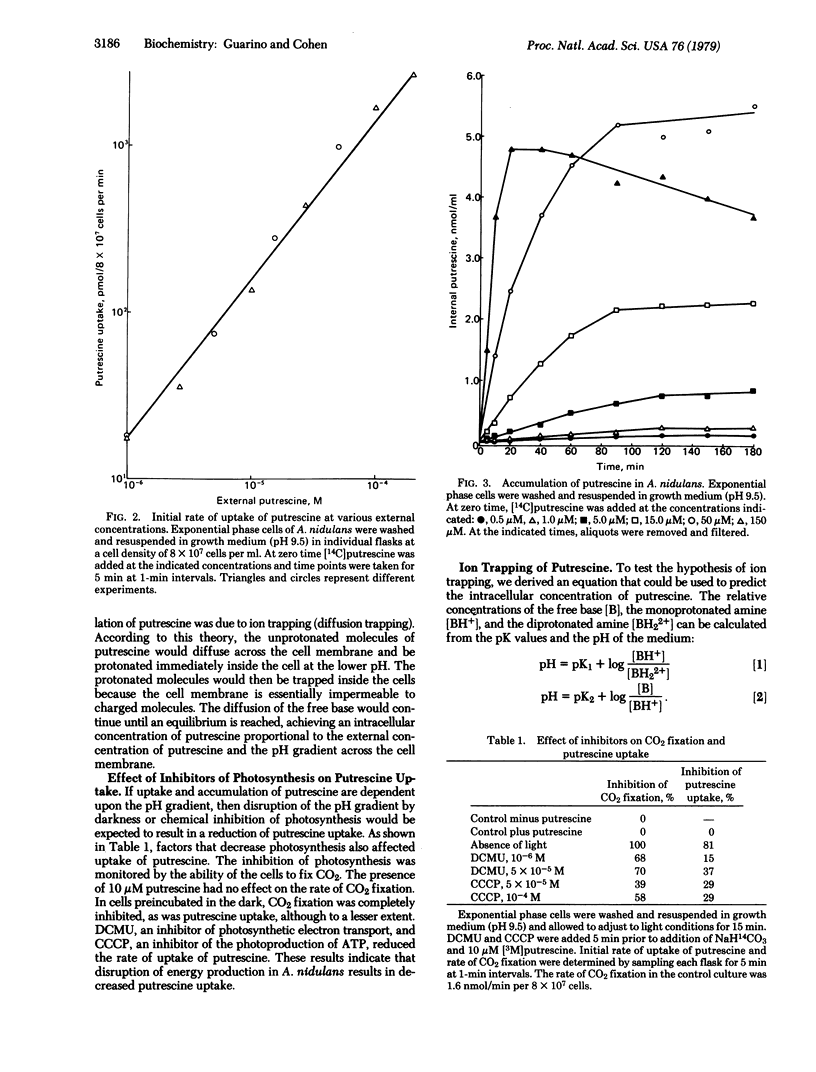
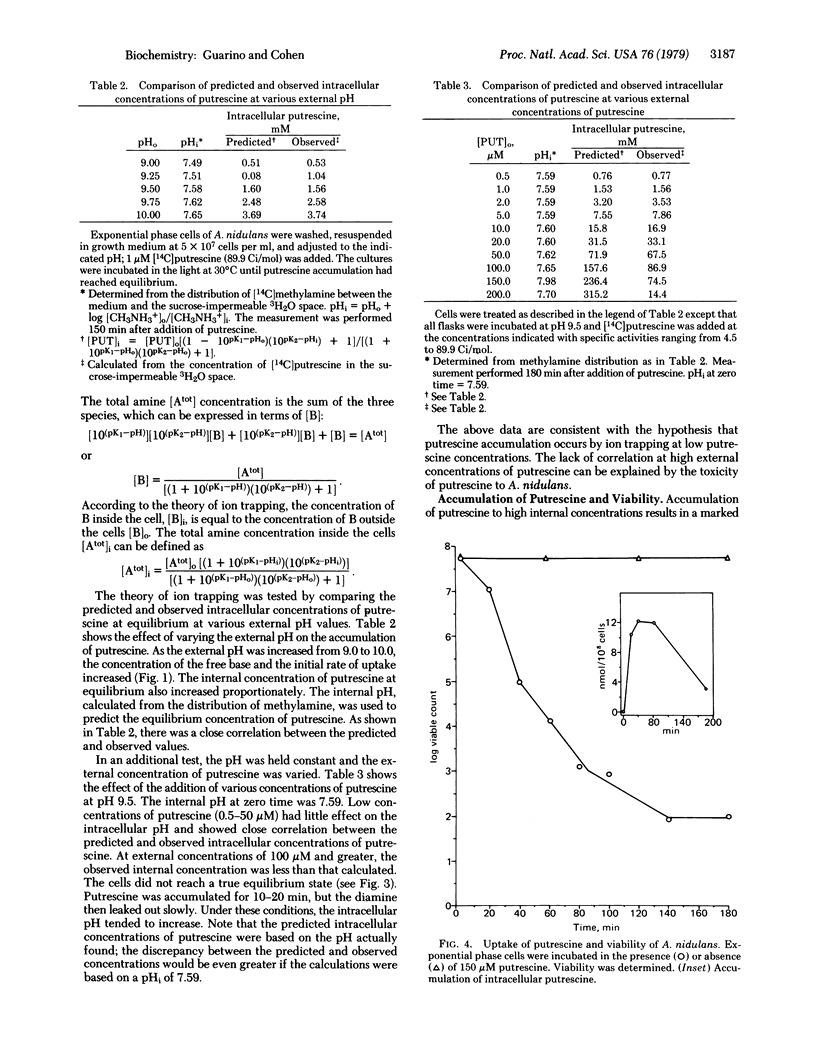
The rate of uptake of putrescine by Anacystis nidulans has been shown to depend on the external pH and the extracellular concentration of putrescine. Accumulation of exogenous putrescine was also proportional to the concentration of putrescine in the medium, suggesting that putrescine uptake was not subject to cellular regulation. An equation was derived to test the hypothesis that putrescine accumulation was due to ion trapping. Comparison of the predicted and observed intracellular concentrations of putrescine under various conditions showed a close correlation in support of the hypothesis of ion trapping. Under conditions leading to cell death (e.g., 150 microM putrescine, pH 9.8), the correlation did not hold as a result of leakage of accumulated putrescine.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Falkner G., Horner F. pH Changes in the Cytoplasm of the Blue-Green Alga Anacystis nidulans Caused by Light-dependent Proton Flux into the Thylakoid Space. Plant Physiol. 1976 Dec;58(6):717–718. doi: 10.1104/pp.58.6.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginzburg D., Padan E., Shilo M. Effect of cyanophage infection on CO2 photoassimilation in Plectonema boryanum. J Virol. 1968 Jul;2(7):695–701. doi: 10.1128/jvi.2.7.695-701.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heldt H. W., Sauer F. The inner membrane of the chloroplast envelope as the site of specific metabolite transport. Biochim Biophys Acta. 1971 Apr 6;234(1):83–91. doi: 10.1016/0005-2728(71)90133-2. [DOI] [PubMed] [Google Scholar]
- Kano K., Oka T. Polyamine transport and metabolism in mouse mammary gland. General properties and hormonal regulation. J Biol Chem. 1976 May 10;251(9):2795–2800. [PubMed] [Google Scholar]
- Lajtha A., Sershen H. Substrate specificity of uptake of diamines in mouse brain slices. Arch Biochem Biophys. 1974 Dec;165(2):539–547. doi: 10.1016/0003-9861(74)90280-x. [DOI] [PubMed] [Google Scholar]
- Padan E., Schuldiner S. Energy transduction in the photosynthetic membranes of the cyanobacterium (blue-green alga) P-lectonema boryanum. J Biol Chem. 1978 May 10;253(9):3281–3286. [PubMed] [Google Scholar]
- Padan E., Zilberstein D., Rottenberg H. The proton electrochemical gradient in Escherichia coli cells. Eur J Biochem. 1976 Apr 1;63(2):533–541. doi: 10.1111/j.1432-1033.1976.tb10257.x. [DOI] [PubMed] [Google Scholar]
- Ramakrishna S., Guarino L., Cohen S. S. Polyamines of Anacystis nidulans and metabolism of exogenous spermidine and spermine. J Bacteriol. 1978 Jun;134(3):744–750. doi: 10.1128/jb.134.3.744-750.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHILO M., SHILO M. The mechanism of lysis of Prymnesium parvum by weak electrolytes. J Gen Microbiol. 1962 Dec;29:645–658. doi: 10.1099/00221287-29-4-645. [DOI] [PubMed] [Google Scholar]
- Tabor C. W., Tabor H. Transport systems for 1,4-diaminobutane, spermidine, and spermine in Escherichia coli. J Biol Chem. 1966 Aug 25;241(16):3714–3723. [PubMed] [Google Scholar]


