Abstract
The Plasmodium falciparum genome is rich in regions of low amino acid complexity which evolve with few constraints on size. To explore the extent of diversity in these loci, we sequenced repeat regions in pfmdr1, pfmdr5, pfmdr6, pfmrp2, and the antigenic locus pfmsp8 in laboratory and cultured-adapted clinical isolates. We further assessed associations between the repeats and parasite in vitro responses to 7 antimalarials to determine possible adaptive roles of these repeats in drug tolerance. Our results show extensive repeat variations in the reference and clinical isolates in all loci. We also observed a modest increase in dihydroartemisinin activity in parasites harboring the pfmdr1 sequence profile 7-2-10 (reflecting the number of asparagine repeats, number of aspartate repeats, and number of asparagine repeats in the final series of the gene product) (P = 0.0321) and reduced sensitivity to chloroquine, mefloquine, quinine, and dihydroartemisinin in those with the 7-2-11 profile (P = 0.0051, 0.0068, 0.0011, and 0.0052, respectively). Interestingly, we noted an inverse association between two drugs whereby isolates with 6 asparagine repeats encoded by pfmdr6 were significantly more susceptible to piperaquine than those with 8 (P = 0.0057). Against lumefantrine, those with 8 repeats were, however, more sensitive (P = 0.0144). In pfmrp2, the 7-DNNNTS/NNNNTS (number of DNNNTS or NNNNTS motifs; underlining indicates dimorphism) repeat group was significantly associated with a higher lumefantrine 50% inhibitory concentration (IC50) (P = 0.008) than in those without. No associations were observed with pfmsp8. These results hint at the probable utility of some repeat conformations as markers of in vitro antimalarial response; hence, biochemical functional studies to ascertain their role in P. falciparum are required.
INTRODUCTION
The evolution and spread of multidrug resistant Plasmodium falciparum have prompted the adoption of artemisinin-based combination therapies (ACTs) as the first-line treatment in most countries where malaria is endemic (1). However, there is a growing body of evidence that the parasites are increasingly becoming less susceptible to the artemisinin-partnered drugs in southeast Asia (2, 3), and one report from Kenya documented a decline in response to artemisinin therapy (4), though this could have been attributed to declining immunity in the study area following a drop in transmission or effective control measures. Although the overall clinical efficacy of current regimens remains high in Africa, elucidation of any parasite genetic features that contribute to variation in sensitivity to these drugs is crucial for monitoring resistance, owing to the central role of these drugs in malaria control.
Transporter genes, especially of the ATP-binding cassette (ABC) superfamily, play a key role in determining drug resistance phenotypes in many biological systems. In P. falciparum, members of this superfamily couple ATP hydrolysis to the translocation of a wide range of drugs and other solutes across the food vacuole and plasma membrane of the parasite (5). Single-nucleotide polymorphisms (SNPs) and overexpression of particular members of this transporter group have been implicated in influencing this function, effectively modulating parasite responses to quinoline-related drugs as well as other inhibitors (6–8). However, these well-established genomic variations do not entirely explain the spectrum of responses observed in resistant parasites, implying that other polymorphisms within the genome may be involved in influencing parasite response to drugs (9). Furthermore, some resistance phenotypes in different Plasmodium species appear to be an aggregate of responses to mutations in multiple genes (10, 11). Consequently, multilocus genetic analyses and association studies on these transporters may provide more insights into understanding the parasite's tolerance to antimalarials.
The P. falciparum genome is rich in sequences encoding low-complexity amino acid regions (LCRs), with 87% of all the gene products containing at least one LCR, as opposed to an average of 65 to 70% in other eukaryotes (12). These regions generally comprise homopolymeric tracts of single amino acids or heteropolymers of short repetitive motifs (13) and exhibit increased polymorphism, especially if their genes are proximal to the high-recombination subtelomeric ends (14). Indeed, a number of studies have demonstrated the existence of repeat polymorphisms in some of the transporter genes. For instance, microsatellite length polymorphisms in the asparagine/aspartate-rich (Asn/Asp-rich) linker domain of the multidrug resistance protein 1, encoded by pfmdr1 (PF3D7_0523000), have been reported in samples from Africa, French Guiana, and Thailand (15–18). However, only two of these studies explored association between the genotypes observed with in vitro responses to drugs (15, 16). Interestingly, some of the repeat profiles observed in these studies were also associated with the proximal resistance-conferring mutations (N86Y and Y184F) on pfmdr1 (16, 19). In another report on multidrug resistance protein 6, encoded by pfmdr6 (PF3D7_1352100), length variation was observed in three different Asn-rich repeat loci among Asian isolates (20). In this study, the presence of 9 Asn residues in the polymorphic microsatellite region corresponding to amino acid positions 103 to 109 in 3D7 appeared to influence the parasite in vitro susceptibility to dihydroartemisinin (DHA). The coding sequences of two other ABC transporters, multidrug resistance protein 5, encoded by pfmdr5 (PF3D7_1339900), and drug resistance-associated protein 2, encoded by pfmrp2 (PF3D7_1229100), also contain repetitive amino acid motifs, with full-length analysis of pfmrp2 showing associations between some of these repeats with mefloquine (MEF) resistance in samples from Thailand (21). Though their corresponding gene sequences are clearly abundant in the parasite's genome, the functional significance of these repeats is still poorly understood owing to conflicting experimental evidence associating these variations with phenotype. For instance, while repeats in the microsatellite sequences of the sodium/proton exchanger, encoded by pfnhe1 (PF13_0019), have been shown to influence responses to quinine (QN) in different settings (22), variations in NIN and NI repeat motifs in the product of pfmdr6 were shown to have no association with drug sensitivity (20).
The present study therefore aimed at exploring the existence of repeat polymorphisms in P. falciparum LCRs (PfLCRs) in products of genes known to influence susceptibility to antimalarials and assess potential associations of these variations with in vitro responses to chloroquine (CQ), halofantrine (HLF), lumefantrine (LM), piperaquine (PQ), MEF, QN, and DHA. A detailed catalogue of the genes analyzed in this study is presented in Table 1. The antimalarials were chosen due to their clinical role as key components of current control strategies (23), while MEF, HLF, DHA, and QN were selected due to previous associations between their responses and variation in repeat sequences in transporter genes (15, 16, 20). We included CQ due to the modulatory effects of polymorphisms in pfmdr1 and the chloroquine resistance transporter gene, pfcrt (PF3D7_0709000), on its activity. The gene for merozoite surface protein 8, pfmsp8 (PF3D7_0502400), codes for a surface protein with a polymorphic Asn/Asp-rich domain in the N-terminal (24) and has no known involvement in shuttling of drugs and/or solutes across membranes. We included this gene in our analysis as a control, based on the hypothesis that variations in its Asn/Asp-rich region would also have no bearing on antimalarial response, and thus any associations with the ABC genes would by extension represent bona fide signals attributable to their transport roles.
Table 1.
Summary characteristics of the genes assessed in this study
| PlasmoDB ID | Gene | Chromosome | Annotated product description | No. of TM domains | Repeat position(s) | PfLCR family |
|---|---|---|---|---|---|---|
| PF3D7_0523000 | pfmdr1 | 5 | Multidrug resistance protein 1 | 12 | 643–661 | Poly-Asn |
| PF3D7_1229100 | pfmrp2 | 12 | ABC transporter (CT family) | 12 | 952–998 and 1160–1200 | High GC |
| PF3D7_1339900 | pfmdr5 | 13 | ABC transporter (MDR family) | 5 | 508–551 | High GC |
| PF3D7_1352100 | pfmdr6 | 13 | ABC transporter (heavy metal transporter family) | 12 | 103–109 | Poly-Asn |
| PF3D7_0502400 | pfmsp8 | 5 | Merozoite surface protein 8 | 0 | 164–180 and 189–195 | High GC |
The Plasmodium falciparum low-complexity region (PfLCR) family annotations assigned to various loci were adopted from the clusters described in reference 30, while the number of transmembrane (TM) domains is as predicted by the TMHMM2 algorithm. PfMSP8 is GPI anchored in the membrane and hence lacks transmembrane domains. The amino acid positions of the repeats are indicated relative to the 3D7 reference sequence from PlasmoDB (www.plasmodb.org).
MATERIALS AND METHODS
Global distribution of sequence diversity.
Sequence diversity was first assessed in the 5 genes (pfmdr1, pfmdr5, pfmdr6, pfmrp2, and pfmsp8) in 16 P. falciparum reference strains from different sources worldwide to determine the extent of divergence in these loci. We then analyzed these polymorphisms in culture-adapted clinical isolates and their associations with in vitro chemosensitivity profiles. The panel of reference isolates examined represents a geographically diverse set of isolates, with 4 from Africa (D6, RO33, Palo Alto, and Wellcome), 7 from Asia (T996, T9102, K1, FCC2, V1S, Dd2, and W2), 3 from South America (7G8, IT, and ITG; the latter two apparently have shared clonal history in culture), and 1 from Central America (HB3). 3D7 (cloned from NF54, an Amsterdam case of malaria presumably from an African mosquito) was grouped among the African strains.
Parasite adaptation and chemosensitivity profiling.
The 50% inhibitory concentration (IC50) data reported here were adopted from previous work by our group (25, 26). Briefly, P. falciparum parasites were collected from malaria patients as part of several clinical studies between 2005 and 2008 in Kilifi, Kenya, and in vitro adaptation was carried out as described elsewhere (25). Antimalarial activities were determined using radioisotopic [3H+]hypoxanthine incorporation, with at least two replicate experiments carried out and only averaged results with <30% variation considered. We present median IC50s, i.e., the inhibitory concentrations that kill 50% of parasites, and the corresponding interquartile ranges (IQRs). The multidrug resistant V1S and the drug-susceptible 3D7 laboratory lines were used as reference strains.
DNA preparation and PCR.
Parasite genomic DNA was extracted from dried filter paper blood spots using the boiling method (27) for the field isolates, while reference DNAs were obtained from the Malaria Research and Reference Reagent Resource (MR4) Centre (http://www.mr4.org/). The microsatellite domains of pfmdr1 and pfmdr6 (nucleotides 1927 to 1987 and 310 to 327, respectively) were amplified using primers and cycling conditions described elsewhere (16, 20). The pfmdr5 fragment was amplified by nested PCR using the primers MDR5F1 (forward) and MDR5R2 (reverse) for nest 1, followed by MDR5F1 (forward) and MDR5R1 (reverse) for nest 2. The nest 1 PCR conditions were as follows: primary denaturation at 94°C for 3 min, secondary denaturation at 94°C for 30 s, annealing for 30 s, and extension at 68°C for 30 s for 40 cycles, followed by a final 3-min extension at 68°C. The same conditions were used for nest 2 amplification. For the fragment comprising the sequence encoding the DNNNTS/NNNNTS (underlining indicates dimorphism) repeat in pfmrp2, we used the primers MRP_F1 (forward) and MRP_R1 (reverse), while the downstream locus harboring the DNNN repeat was amplified using MRP_F2 (forward) and MRP_R2 (reverse). The PCR conditions were similar for both fragments, with primary denaturation at 94°C for 3 min, secondary denaturation at 94°C for 30 s, annealing for 30 s, and extension at 68°C for 30 s for 40 cycles, followed by a final 3-min extension at 68°C. pfmsp8 was amplified using the primers PfMSP8_F1 (forward) and PfMSP8_R1 under the following conditions: primary denaturation at 94°C for 2 min, secondary denaturation at 94°C for 15 s, annealing for 30 s, and extension at 72°C for 2 min for 40 cycles followed by a final 7-min extension at 72°C. For all primer sequences, annealing temperatures, and fragment sizes, see Table S1 in the supplemental material.
Sequencing.
PCR products were purified using ethanol precipitation and sequenced using the amplification primers, BigDye Terminator v3.1 (Applied Biosystems, United Kingdom), and an ABI 3130xl capillary sequencer (Applied Biosystems, United Kingdom). Poor-quality sequences were either resequenced or discarded, and repeat polymorphisms were called if clean individual peaks were observed in the electropherogram. Mixed genotypes were noted but excluded from further analysis. Sequences were assembled and edited using SeqMan and aligned using MegAlign (Lasergene 7; DNASTAR, Madison, WI) and BioEdit version 7.0.9 to identify repeat polymorphisms in the LCRs.
Statistical analysis.
All statistical analyses were conducted using Stata version 11 (Stata, College Station, TX), while the graphs were drawn using GraphPad Prism (San Diego, CA). The nonparametric Mann-Whitney test was used to compare differences in drug responses between various groups of repeats. Only repeats with >10% frequency were examined against drug responses for between-group analysis. Linkage associations between repeat polymorphisms on different genes but the same chromosome were interrogated using Fisher's exact test. The significance level was assessed at 5% for all analyses.
Nucleotide sequence accession numbers.
A total of 343 sequences were analyzed for the clinical isolates and deposited in GenBank under the accession numbers KF277770 to KF277831 for pfmdr1, KF277832 to KF277889 for pfmdr5, KF277890 to KF277939 for pfmdr6, KF277940 to KF277997 for pfmsp8, KF277998 to KF278057 and KF278058 to KF278102 for pfmrp2.
RESULTS
Antimalarial responses against culture-adapted clinical isolates.
In brief, the median CQ, MEF, HLF, LM, PQ, QN and DHA IC50s were 101.9 nM, 12.0 nM, 9.0 nM, 33.2 nM, 54.0 nM, 189.3 nM and 2.3 nM, respectively, against V1S. Against 3D7, the IC50s were 5.1 nM, 15.4 nM, 16.9 nM, 103.9 nM, 46.9 nM, 27.1 nM and 2.1 nM, respectively. Among the clinical isolates, DHA was the most potent, with a median IC50 of 1.4 nM (IQR, 0.8 to 2.3 nM), while parasites were least susceptible to QN, with a median IC50 of 113.3 nM (IQR, 55.8 to 262.8 nM). The IC50 distribution for all the drugs against clinical and reference isolates is summarized in Table 2.
Table 2.
Summary of in vitro susceptibilities of P. falciparum clinical isolates and reference strains to 7 antimalarials
| Inhibitor | n | Median IC50 [IQR]a |
||
|---|---|---|---|---|
| Clinical isolates | V1S | 3D7 | ||
| Chloroquine (CQ) | 67 | 55.8 [16.8–93.0] | 101.9 [94.3–160.8] | 5.1 [4.4–5.9] |
| Mefloquine (MEF) | 53 | 34.5 [16.0–43.2] | 12.0 [5.7–12.1] | 15.4 [15.1–15.6] |
| Halofantrine (HLF) | 57 | 18.7 [10.1–37.7] | 10.0 [8.2–27.9] | 16.9 [15.0–18.8] |
| Lumefantrine (LM) | 67 | 97.6 [50.2–185.8] | 33.2 [29.3–45.6] | 103.9 [102.3–105.5] |
| Piperaquine (PQ) | 67 | 42.2 [23.5–67.2] | 54.0 [41.0–72.6] | 46.8 [46.1–47.6] |
| Quinine (QN) | 58 | 113.3 [55.8–262.8] | 189.3 [145.3–214.6] | 27.1 [17.8–36.3] |
| Dihydroartemisinin (DHA) | 67 | 1.4 [0.8–2.3] | 2.3 [2.1–2.8] | 2.1 [2.1–2.2] |
Values are in nanomolar units.
Global LCR variation in reference strains.
We genotyped LCRs in 5 genes in 16 laboratory strains, and as expected, there was extensive sequence variation in all the genes (Table 3). We identified 7 distinct pfmdr1 microsatellite sequence profiles, with most samples having sequences encoding 7 tandem Asn residues followed by 2 Asp residues and then a final series of Asn residues which varied in number; hence, the profiles were designated 7-2-n. The 7-2-09 and 7-2-11 profiles were the most dominant, at a frequency of 25% each. The 7-2-10, 7-0-02, and 7-2-06 profiles were unique to 3D7, Palo Alto, and FCC2, respectively, while ITG and the Wellcome strain shared the 7-2-07 profile. The Ghanaian strain, RO-33, had the 8-2-09 allele, which was previously shown to be in circulation predominantly among West African (and not East African) parasite populations (19). Strikingly, all strains from Africa (5/5) had different alleles at this locus, while 57% in Asia shared alleles. We identified 4 different alleles in pfmdr5, with only the shorter DNNN repeat appearing to be polymorphic. Fifty percent of the strains had 5 DNNN repeats coupled with a single DHHNDHNNDNNN motif (thus designated 5-1), with a higher degree of allele sharing (83%) again being observed among Asian strains. Sequences from pfmdr6 clustered into 5 allelic groups in which the numbers of Asn repeats ranged between 4 and 10. The 9-repeat alleles observed in strains from the China-Myanmar border (20) was observed only in HB3, not any of the Asian strains. The DNNNTS/NNNNTS repeat domain of pfmrp2 showed less divergence than the DNNN region, which had numbers of repeats ranging between 6 and 9. Since it has been argued that LCRs in P. falciparum possibly seed and grow neutrally with no particular adaptive function (12), it was prudent to run a control analysis on a gene neither coding for a drug/metabolite transporter nor conventionally associated with differential antimalarial response. There were 2 repeat motifs in pfmsp8, NDD and DDNDDNG, with most strains having 5 NDD repeats and a single copy of the longer DDNDDNG motif; hence, they were given a 5-1 designation. Overall, our results corroborate findings from other studies involving reference strains (17, 24, 28) and are also in line with publicly available whole-genome sequence data from the Wellcome Trust Sanger Institute (http://www.sanger.ac.uk/Projects/P_falciparum), PlasmoDB (www.plasmodb.org), and the Broad Institute of Harvard (http://www.broadinstitute.org/annotation/genome/plasmodium_falciparum_spp/Regions.html), thus affirming the fidelity of our sequence analyses.
Table 3.
P. falciparum strains sampled and corresponding genotypes of the repeat regionsa
| Origin | Strain | Repeat region profile forb: |
|||||
|---|---|---|---|---|---|---|---|
| pfmdr1 (N-D-N) | pfmdr5 (DNNN-DHHNDHNNDNNN) | pfmdr6 (N repeats) |
pfmrp2 |
pfmsp8 (NDD-DDNDDNG) | |||
| DNNNTS | DNNN | ||||||
| Africac | |||||||
| Netherlands | 3D7 | 7-2-10 | 8-1 | 6 | 8 | 9 | 5-1 |
| Ghana | RO33 | 8-2-09 | 8-1 | ND | 7 | 7 | 2-2 |
| Sierra Leone | D6 | 7-2-09 | 7-1 | ND | 7 | 8 | 5-1 |
| Nigeria | Wellcome | 7-2-07 | 5-1 | 8 | 7 | 8 | 5-2 |
| Uganda | Palo Alto | 7-0-02 | 5-1 | 6 | 7 | 9 | 5-1 |
| South America | |||||||
| Brazil | 7G8 | 7-2-09 | 7-1 | ND | 4 | 7 | 5-1 |
| Brazil | ITG | 7-2-07 | ND | ND | ND | ND | ND |
| Brazil | IT | ND | 5-1 | 8 | 7 | 8 | ND |
| Asia | |||||||
| Thailand | T9102 | 7-2-11 | 5-1 | 4 | 7 | 6 | 5-0 |
| Thailand | K1 | 7-2-09 | 5-1 | 10 | 7 | 6 | ND |
| Indochina | W2 | 7-2-11 | ND | ND | ND | ND | ND |
| Indochina | Dd2 | 7-2-11 | 5-1 | 6 | 7 | 8 | 5-1 |
| Vietnam | V1S | 7-2-11 | 5-1 | 10 | 7 | 7 | 5-2 |
| Thailand | T996 | 8-2-09 | 7-1 | 6 | 7 | 9 | 5-1 |
| China | FCC2 | 7-2-06 | 5-1 | 8 | 7 | 7 | 5-1 |
| Central America | |||||||
| Honduras | HB3 | 7-2-09 | 3-1 | 9 | 7 | 7 | 6-1 |
Samples were obtained from various parts of the world where malaria is endemic.
The pfmdr1 genotype nomenclature describes the number of asparagine (N) repeats followed by aspartate (D) then a final series of asparagine repeats in an N-D-N order. For pfmdr5, the number of DNNN motifs is indicated followed by the number of DHHNDHNNDNNN units, while the values for pfmdr6 are the numbers of asparagines. The numbers of DNNNTS and DNNN motifs are shown for pfmrp2, while the pfmsp8 value is the number of NDD repeats followed by the number of DDNDDNG motifs. ND refers to loci for which there were no evaluable sequences.
3D7 was cloned from NF54, an Amsterdam case of malaria presumably from an African mosquito, and is therefore grouped with the African strains.
Sequence variation in clinical isolates and associations with IC50. (i) pfmdr1.
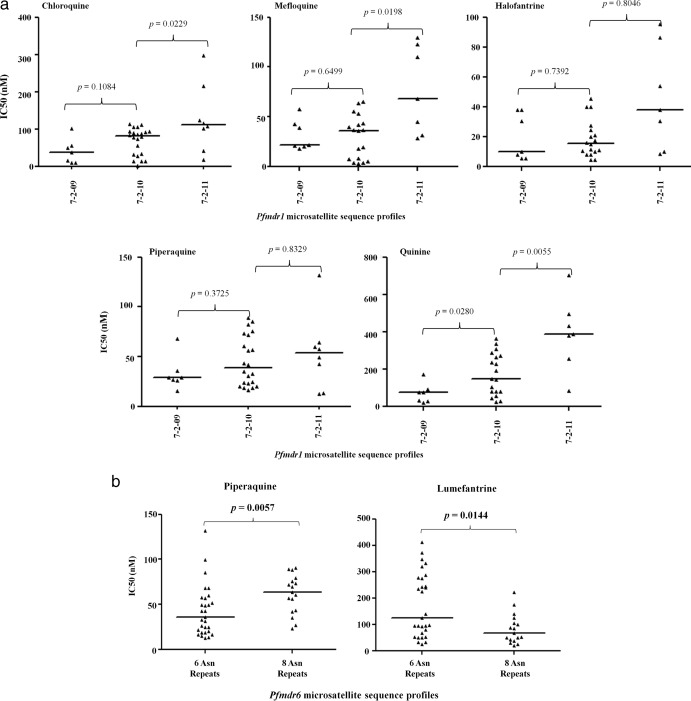
We obtained 65 complete sequences of the poly-Asn/Asp repeat region of pfmdr1, which broadly fell into 9 microsatellite groups, as shown in Table 4. The 7-2-10 group was the most predominant (occurring in 33.9% of the population), consistent with our previous observation in western Kenya (19). Three isolates each had the 7-0-02, 7-0-04, and 7-3-09 profiles, representing the lowest frequencies. Further analysis on the groups with >10% frequency revealed a significant but borderline increase in susceptibility to DHA in isolates that bore the 7-2-10 allele compared to those that did not (P = 0.0321; Mann-Whitney U test). Also, samples bearing the 7-2-11 allele appeared to be significantly more sensitive to LM (P = 0.0360; Mann-Whitney U test) and less susceptible to CQ, MEF, QN, and DHA than those that did not (P = 0.0052, 0.0065, 0.0011, and 0.0052, respectively; Mann-Whitney U test), as shown in Table 4. It is worth noting that 7-2-11 is the profile of the pfmdr1 allele found in Dd2, W2, and V1S, all of which have some known degree of in vitro resistance to multiple drugs (Dd2 is CQ and MEF resistant, while W2 and V1S are both resistant to CQ and QN). A possible indicator of structural/functional constraints, we also noted an increasing trend in median IC50s of 5 drugs (MEF, PQ, HLF, QN, and CQ) with corresponding increases in the latter series of Asn repeats; that is, 7-2-09 < 7-2-10 < 7-2-11 (Fig. 1a).
Table 4.
Summary of the genotypes for the 5 genes analyzed among the clinical isolates
| Gene (motif) | Genotypea | nb | Frequency (%) |
Pc |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Chloroquine | Mefloquine | Halofantrine | Lumefantrine | Piperaquine | Quinine | Dihydroartemisinin | ||||
| pfmdr1 (N-D-N) | 7-0-01 | 19 | 29.2 | 0.3058 | 0.2217 | 0/7552 | 0.1706 | 0.3339 | 0.8615 | 0.9540 |
| 7-2-09 | 7 | 10.8 | 0.1330 | 0.9040 | 0.2702 | 0.6568 | 0.2042 | 0.0287 | 0.6415 | |
| 7-2-10 | 22 | 33.9 | 0.3749 | 0.3559 | 0.1658 | 0.1078 | 0.8137 | 0.4667 | 0.0369 | |
| 7-2-11 | 8 | 12.3 | 0.0052 | 0.0065 | 0.1157 | 0.0360 | 0.8574 | 0.0011 | 0.0052 | |
| 7-0-02 | 1 | 1.5 | ||||||||
| 7-0-04 | 1 | 1.5 | ||||||||
| 7-2-06 | 2 | 3.1 | ||||||||
| 7-2-07 | 4 | 6.2 | ||||||||
| 7-3-09 | 1 | 1.5 | ||||||||
| Total | 65 | 100 | ||||||||
| pfmdr5 (DNNN-DHHNDHNNDNNN) | 5-1 | 19 | 32.8 | 0.8019 | 0.5080 | 0.7359 | 0.0173 | 0.8694 | 0.2763 | 0.8762 |
| 6-1 | 6 | 10.3 | 0.8518 | 0.3590 | 0.9392 | 0.1723 | 0.9199 | 0.9328 | 0.7195 | |
| 7-1 | 15 | 25.9 | 0.1739 | 0.4883 | 0.3919 | 0.9631 | 0.1570 | 0.8075 | 0.6772 | |
| 8-1 | 10 | 17.2 | 0.3662 | 0.5330 | 0.8651 | 0.0838 | 0.6635 | 0.6381 | 0.8148 | |
| 4-1 | 1 | 1.7 | ||||||||
| 4-2 | 1 | 1.7 | ||||||||
| 5-2 | 2 | 3.5 | ||||||||
| 9-1 | 4 | 6.9 | ||||||||
| Total | 58 | 100 | ||||||||
| pfmdr6 (N repeats) | 6 | 29 | 52.7 | 0.2755 | 0.5680 | 0.5540 | 0.0085 | 0.0463 | 0.8761 | 0.9869 |
| 8 | 17 | 30.4 | 0.8237 | 0.5797 | 0.9647 | 0.0363 | 0.0036 | 0.9399 | 0.7824 | |
| 10 | 2 | 3.6 | ||||||||
| 4 | 1 | 1.8 | ||||||||
| 7 | 3 | 5.4 | ||||||||
| 9 | 4 | 7.1 | ||||||||
| Total | 56 | 100 | ||||||||
| pfmrp2 (DNNNTS) | 10 | 7 | 13 | 0.5726 | 0.2562 | 0.6148 | 0.0781 | 0.1780 | 0.3112 | 0.8629 |
| 7 | 23 | 38.3 | 0.6212 | 0.6697 | 0.2371 | 0.0080 | 0.8612 | 0.6362 | 0.2976 | |
| 8 | 17 | 28.3 | 0.7366 | 0.3661 | 0.5463 | 0.4262 | 0.7740 | 0.0754 | 0.6226 | |
| 9 | 9 | 15 | 0.5691 | 0.8343 | 0.7653 | 0.8930 | 0.1886 | 0.9128 | 0.4314 | |
| 4 | 1 | 1.7 | ||||||||
| 5 | 1 | 1.7 | ||||||||
| 6 | 2 | 3.3 | ||||||||
| Total | 56 | 100 | ||||||||
| pfmrp2 (DNNN) | 7 | 20 | 43.5 | 0.7733 | 0.4720 | 0.9103 | 0.1436 | 0.9823 | 0.6522 | 0.6576 |
| 8 | 13 | 28.3 | 0.1607 | 0.0233 | 0.7431 | 0.1273 | 0.2998 | 0.1511 | 0.5023 | |
| 9 | 9 | 19.6 | 0.8294 | 0.3601 | 0.3727 | 0.8294 | 0.8294 | 0.7024 | 0.5180 | |
| 10 | 2 | 4.4 | ||||||||
| 11 | 1 | 2.2 | ||||||||
| 6 | 1 | 2.2 | ||||||||
| Total | 46 | 100 | ||||||||
| pfmsp8 (NDD) | 6 | 50 | 86.2 | 0.1554 | 0.5536 | 0.2191 | 0.6040 | 0.4706 | 0.1144 | 0.9910 |
| 3 | 3 | 5.2 | ||||||||
| 5 | 1 | 1.7 | ||||||||
| 7 | 2 | 3.5 | ||||||||
| 9 | 2 | 3.5 | ||||||||
| Total | 58 | 100 | ||||||||
| pfmsp8 (DDNDDNG) | 1 | 53 | 91.4 | 0.2282 | 0.1166 | 0.4968 | 0.7711 | 0.9669 | 0.5264 | 0.8571 |
| 2 | 5 | 8.6 | ||||||||
| Total | 58 | 100 | ||||||||
Underlining indicates profiles with >10% allele frequencies.
Number of samples bearing each genotype.
We used Mann-Whitney's test to compare IC50s for parasites bearing these genotypes versus those that do not, and significant results (P < 0.05) are in bold. This analysis was only done for genotypes with allele frequencies of >10%.
Fig 1.
(a) Plots showing a trend of apparent increase in tolerance to 5 antimalarials with corresponding increases in the second series of Asn repeats in the pfmdr1 linker repeat region. The increase from 7-2-09 to 7-2-10 and from 7-2-10 to 7-2-11 was tested for significance using Mann-Whitney's test (P < 0.05). (b) Inverse association between the 6- and 8-Asn-repeat groups in pfmdr6 in relation to piperaquine (PQ) and lumefantrine (LM) activity. This association was assessed using Mann-Whitney's test for significance (P < 0.05).
(ii) pfmdr5.
We identified 8 different allelic groups in the 58 isolates successfully genotyped at pfmdr5 (Table 4). The insertions/deletions of repeats in this gene occur within an ∼180-bp region of the open reading frame, with the sequences corresponding to the shorter DNNN repeat ranging from 4 to 9 and the longer DHHNDHNNDNNN motif existing as a single- or double-copy motif. Most isolates (32.8%) had the 5-1 allele, while 4-1 and 4-2 were less frequent, being observed in one isolate each. Two other isolates, bearing the 5-2 allele, also had two repeats of the longer DHHNDHNNDNNN motif. Analyses between in vitro responses to all the test drugs and the repeat groups with >10% frequency yielded a significant association only between the 5-1 allele and increased LM IC50 (P = 0.0173; Mann-Whitney U test).
(iii) pfmdr6.
Six different poly-Asn repeat profiles were detected in pfmdr6, including one that had not previously been described. These microsatellite sequences correspond to nucleotides 310 to 327 (relative to 3D7) and ranged from 4 to 10 Asn repeats. The proportion of samples with 6 Asn repeats was highest in our analysis (52.7%), contrary to results of a recent analysis on the same locus on Asian isolates in which 8 repeats was predominant, occurring in 76.5% of the isolates (20). A winnowed analysis on repeat groups 6 and 8 (>10% allele frequency) revealed that isolates harboring the 3D7-like allele (6 Asn repeats) were significantly less susceptible to LM (P = 0.0085; Mann-Whitney's U test) than those that did not but exhibited only borderline sensitivity to PQ (P = 0.0463; Mann-Whitney's U test). Conversely, we observed a significant association between 8 repeats and reduced susceptibility to PQ (P = 0.0036; Mann-Whitney's U test) but only a modest increase in sensitivity to LM (P = 0.0363; Mann-Whitney's U test), as shown in Table 4. Further between-group comparison showed that samples with 6 repeats were significantly more sensitive to PQ (P = 0.0057; Mann-Whitney's U test) but less susceptible to LM (P = 0.0144) than the variants with 8 repeats (Fig. 1b). The association reported by Wang et al. (20) between 9 Asn repeats and reduced sensitivity to DHA was not observed in our population (P = 0.8114; Mann-Whitney's U test).
(iv) pfmrp2.
Sequence data were successfully generated for 65 samples bearing the hexapeptide motif DNNNTS or its alternative NNNNTS and 46 harboring the DNNN repeat in pfmrp2. We identified 7 and 6 distinct DNNNTS/NNNNTS and DNNN repeat profiles, respectively, with most isolates having 7 copies of the DNNNTS/NNNNTS (38.3%) and DNNN (43.5%) repeats (Table 4). The number of DNNNTS/NNNNTS repeats ranged from 4 to 10 (median = 7; 13.0% of the isolates), whereas that of DNNN repeats ranged from 6 to 11 (median = 9; 12.0% of the isolates). In the association analysis, the presence of 7 DNNNTS/NNNNTS repeats was significantly associated with higher LM IC50s compared with the rest of the groups (P = 0.008; Mann-Whitney's U test). The same trend persisted when the 7-repeat group was compared against the 10-repeat group (P = 0.0174; Mann-Whitney's U test), though caution should be exercised when interpreting this result due to the considerably low numbers in the latter group (n = 7). We did not find any associations with the DNNN motif other than increased MEF IC50s in variants with 8 repeats (n = 11) compared to those with the wild-type 3D7-like 9 repeats (n = 7) (P = 0.0164; Mann-Whitney's U test).
(v) pfmsp8.
In the 59 complete pfmsp8 sequences we obtained, the number of NDD repeats ranged between 3 and 9, with most isolates (86.2%) harboring 6 repeats (Table 4). The longer DDNDDNG motif was present in a dimorphic form, with isolates bearing either 1 or 2 copies of the motif. The majority of the samples (91.4%) bore the 3D7-like single repeat, while only 5 isolates had an extra copy of the repeat. As expected, we did not observe any significant associations between either of the repeat groups and drug responses.
Chromosomal linkages among different repeats.
Due to the degeneracy in transporter loci modulating drug responses (the activities of particular classes of drugs seem to be influenced by a familiar cast of genes), it is likely that the chromosomal proximity of the genes for any set of transporters performing the same function(s) would broaden the parasite's capacity to extrude toxic compounds. We therefore assessed linkage between repeat groups on the same chromosome and those on different chromosomes. We observed a linkage between sequences for 6 Asn repeats on pfmdr6 and the 5-1 repeat polymorphism on pfmdr5 (odds ratio, 16; 95% confidence interval [CI], 1.03 to 835.1; P = 0.0235; Fisher's exact test) on chromosome 13. However, we did not find any significant association between repeats on any of the other genes. We further analyzed the linkage between combinations of alleles of different genes and their association with drug responses. The linked alleles (5-1 repeats on pfmdr5 and 6 Asn repeats on pfmdr6) together showed a significant increase in LM IC50 (P = 0.0145; Mann-Whitney's U test). Notably, we also observed decreased drug sensitivities in combinations involving the 7-2-11 pfmdr1 allele. For example, 7-2-11 together with 6 pfmdr6-encoded Asn repeats yielded significant elevation in CQ, MEF, HLF, LM, and QN IC50s (P = 0.0259, 0.0039, 0.0300, 0.0212, and 0.0091, respectively; Mann-Whitney's U test), while strains with the 5-1 pfmdr5 repeat profile and the 7-2-11 profile exhibited significant increases in CQ, MEF, QN, and DHA IC50s (P = 0.0003, 0.0172, 0.0005, and 0.0059, respectively; Mann-Whitney's U test). However, these changes were not significant compared with the effect of the individual alleles, and the numbers in these combined categories were indeed low.
DISCUSSION
The P. falciparum genome comprises regions of reduced amino acid complexity which have been shown to be polymorphic (29, 30) but whose functional utilities still remain contentious. However, the link between microsatellite repeat polymorphisms in these regions in transporter genes and antimalarial response has been previously reported in pfnhe1 and QN resistance in this population (26). We evaluated both the extent of repeat sequence diversity in these regions in four genes encoding putative transporters (plus an antigenic locus) and their associations with in vitro antimalarial response. We confirmed the existence of remarkable plasticity in the LCRs of the parasite's genome, with high-level polymorphism being observed in the transporter genes and the locus encoding a surface protein. We also report associations between some of the repeat patterns within these LCRs and in vitro responses to clinically important antimalarials.
The extensive variations at the 5 loci further attest to the existence of minimal size constraints in the evolution of these regions (31) and a high recombination rate at chromosomal subtelomeres (where all the genes examined lie) generating such high diversity (32). All the African strains had distinct pfmdr1 microsatellite profiles and less allele sharing in the other loci, while most strains from Asia shared sequence profiles in a number of loci, reinforcing evidence of the existence of higher diversity in Africa than Asia (33), presumably due to more recombination (32, 34). Notably, T9102, Dd2, and V1S, which are all of southeast Asian ancestry, had the same pfmdr1, pfmdr5, and pfmrp2 microsatellite profiles. However, we reiterate that these are laboratory-cloned parasite lines whose genotypes may not necessarily be accurate representations of the parasite population circulating in the field.
Only a few reports have examined the associations between microsatellite polymorphisms in pfmdr1and drug phenotypes (15, 16), as most have instead focused on the contribution of SNPs and copy number variations. The number of pfmdr1 microsatellite profiles noted here was comparable to that which our group observed in a recent analysis of samples from western Kenya (19), with the marginally higher diversity in the latter study likely being due to superior sampling (n = 83). The increase in susceptibility to DHA in the 7-2-10 group observed here was also noted in Gambia, though with the 8-2-8 profile, which was among the most dominant in that study (16). We argue that this association, together with the linkage between the 7-2-11 allele with reduced susceptibility to DHA, perhaps only mirrors quantitative differences in IC50s and is unlikely to be clinically significant, since no clinical resistance was reported in a recent trial on DHA-PQ (Artekin) in this population (4). This contention is further strengthened by the observation that DHA was equipotent against the resistant strain VIS (median IC50, 2.3 nM) and the sensitive strain 3D7 (median IC50, 2.1 nM). Nonetheless, this marker could prove crucial in profiling DHA responses should future studies support these associations. In relation to function, there is persuasive evidence to suggest that the linker region of pfmdr1 might be adaptive. For instance, certain members of the ABC superfamily, like cystic fibrosis transmembrane conductance regulator and P glycoprotein, also contain linker regions (35, 36) involved in protein kinase-mediated phosphorylation and regulation of substrate specificity (37). Additionally, this region in the yeast a-factor transporter (Ste6) mediates ubiquitination and controls protein turnover (38), two processes associated with drug tolerance in Plasmodium species (39, 40). It is therefore not unreasonable to posit that any variations from the wild-type 7-2-10 sequence of this region would influence the extrusion of drugs. Instructively, transmembrane proteins are malleable at the linker region (41), and consequently the variant 7-2-11 is likely to disturb a conformational flexibility essential for motion, possibly due to steric hindrance. Therefore, though this allele seems to be associated with reduced sensitivity to multiple drugs alone or in combination, it is likely that it may also simultaneously interfere with the translocation of essential metabolites. This may explain its low frequency in our population and even lower in Sudan, Burkina Faso, and western Kenya (19).
The heavy metal transporter encoded by pfmdr6 has been previously shown to be a target of drug selection (7, 9), and there is recent evidence that microsatellite polymorphisms in this locus influence DHA sensitivity (20). The reduction in LM susceptibility observed against 6 Asn repeats was notable, since it is the phenotype associated with 3D7, which also bears 6 Asn repeats at this locus. Interestingly, there was a significant association between 8 repeats and reduced susceptibility to PQ but a converse increase in sensitivity to LM. This trend is reminiscent of the inverse association between in vitro responses to LM and CQ, a structural analog of PQ. The exact mechanism of action of PQ is unknown but is likely to mirror that of CQ, which has been demonstrated to also inhibit mitochondrial iron acquisition in Saccharomyces cerevisiae (42). Since pfmdr6 is speculated to be involved in mitochondrial iron transport (43), it is conceivable that PQ exerts its cytotoxicity through a similar mode, and therefore, the inverse association between CQ and PQ versus LM is likely due to a functional relationship. The association previously reported with Asian isolates between 9 Asn repeats and DHA was not observed in our population. This discordance could be because it is a region-specific trend which might not necessarily hold true elsewhere. Indeed, a similar discrepancy in association between African and Asian isolates has been reported in pfnhe1 microsatellite polymorphisms and their association with parasite responses to QN (44).
PfMRP2 is localized on the plasma membrane (45), with two full-length analyses of the gene revealing the presence of length polymorphisms, one between nucleotides 705 and 726 (46) and another beginning from nucleotide 2337, with respect to the 3D7 sequence (21). The observed elevation in LM IC50 in the 7-DNNNTS/NNNNTS-repeat group and in MEF IC50 in the group with the 8 DNNN repeats may reflect a preference in activity of this locus against aryl-amino alcohols but not unrelated antimalarials. PfMDR5 also localizes on the parasite's plasma membrane (45), reinforcing its putative designation as a drug/metabolite exporter. To the best of our knowledge, this is the first study exploring the involvement of this transporter in antimalarial resistance. We contend that the association observed between a pfmdr5 5-1 repeat and LM could be an extended effect of the strong association with the 6-Asn-encoding allele in pfmdr6 due to the chromosomal linkage between them. The antigenic pfmsp8 is a highly conserved locus, with only slight length variations restricted to indels in the Asn/Asp repeat-rich domain (24). Since it encodes a surface protein with no established role in drug/metabolite transport, the lack of association between its repeats and drug responses was unsurprising. The precise role of the poly-Asn/Asp domain in this gene is, however, unclear and, like some antigens with repetitive amino acid sequences, could involve diverting the immune response from functionally important epitopes (47).
Loci under similar selective pressures are likely affected by common physiological factors and are probably responsible for shared phenotypes. Nonrandom distribution of variability in functional classes of genes has been demonstrated in P. falciparum (48), and indeed, our data showed linkage between polymorphisms on pfmdr5 and pfmdr6. The lack of linkage between pfmdr1 and pfmsp8 is likely due to different selective pressures (antimalarials and immunity, respectively) on the two genes. Though our results reveal compounded effects in drug response when some alleles were analyzed together, much of these could actually be residual signals from the singular alleles, further confirming the oligogenic nature of antimalarial resistance.
In summary, we have shown the global existence of sequence diversity in PfLCRs in laboratory and culture-adapted clinical isolates. We have also demonstrated that particular microsatellite repeat sequences are associated with differential responses to DHA, MEF, LM, QN, and PQ, all crucial antimalarials. As a limitation, this study did not query whether these associations are a direct consequence of the variations within these LCRs or the sheer proximity of some of these regions to resistance-conferring loci. For instance, our group recently reported linkage between the 7-2-10 pfmdr1 sequence with the pfmdr1_86Y variant in Africa (19), while pfmrp2 and pfmdr6 comprise SNPs close to the repetitive domains analyzed here (28, 49). Therefore, the possible influence of these mutations due to hitchhiking cannot be ignored. If indeed adaptive, the widespread variation in these loci is puzzling, since one expects high sequence conservation owing to the expensive fitness costs that accompany genomic changes on essential loci. However, it is also possible that these costs are offset elsewhere in the genome. Also, our parasite population is markedly sensitive to DHA (mean IC50, 1.9 nM); hence, the absence of discernible sensitive and resistance parasite groups limited confident interpretation of some results. We therefore emphasize caution in interpretation of these results owing to the modest associations and low numbers, especially in the analysis of combined alleles. Overall, these findings highlight the need for functional biochemical studies to ascertain the role of these repeats in P. falciparum.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by a Wellcome Trust/Association of Physicians of Great Britain and Ireland scholarship awarded to J.O. and Malaria Capacity Development Consortium re-entry grant to L.I.O.-O.
A.N. is thankful to King Fahd University of Petroleum and Minerals for personal support. We thank the Director of the Kenya Medical Research Institute for permission to publish the article.
Footnotes
Published ahead of print 30 September 2013
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AAC.01465-13.
REFERENCES
- 1. Bosman A, Mendis KN. 2007. A major transition in malaria treatment: the adoption and deployment of artemisinin-based combination therapies. Am. J. Trop. Med. Hyg. 77:193–197 [PubMed] [Google Scholar]
- 2. Dondorp AM, Nosten F, Yi P, Das D, Phyo AP, Tarning J, Lwin KM, Ariey F, Hanpithakpong W, Lee SJ, Ringwald P, Silamut K, Imwong M, Chotivanich K, Lim P, Herdman T, An SS, Yeung S, Singhasivanon P, Day NP, Lindegardh N, Socheat D, White NJ. 2009. Artemisinin resistance in Plasmodium falciparum malaria. N. Engl. J. Med. 361:455–467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Noedl H, Se Y, Schaecher K, Smith BL, Socheat D, Fukuda MM. 2008. Evidence of artemisinin-resistant malaria in western Cambodia. N. Engl. J. Med. 359:2619–2620 [DOI] [PubMed] [Google Scholar]
- 4. Borrmann S, Sasi P, Mwai L, Bashraheil M, Abdallah A, Muriithi S, Fruhauf H, Schaub B, Pfeil J, Peshu J, Hanpithakpong W, Rippert A, Juma E, Tsofa B, Mosobo M, Lowe B, Osier F, Fegan G, Lindegardh N, Nzila A, Peshu N, Mackinnon M, Marsh K. 2011. Declining responsiveness of Plasmodium falciparum infections to artemisinin-based combination treatments on the Kenyan coast. PLoS One 6:e26005. 10.1371/journal.pone.0026005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cowman AF, Karcz S. 1993. Drug resistance and the P-glycoprotein homologues of Plasmodium falciparum. Semin. Cell Biol. 4:29–35 [DOI] [PubMed] [Google Scholar]
- 6. Ekland EH, Fidock DA. 2007. Advances in understanding the genetic basis of antimalarial drug resistance. Curr. Opin. Microbiol. 10:363–370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mu J, Ferdig MT, Feng X, Joy DA, Duan J, Furuya T, Subramanian G, Aravind L, Cooper RA, Wootton JC, Xiong M, Su XZ. 2003. Multiple transporters associated with malaria parasite responses to chloroquine and quinine. Mol. Microbiol. 49:977–989 [DOI] [PubMed] [Google Scholar]
- 8. Reed MB, Saliba KJ, Caruana SR, Kirk K, Cowman AF. 2000. Pgh1 modulates sensitivity and resistance to multiple antimalarials in Plasmodium falciparum. Nature 403:906–909 [DOI] [PubMed] [Google Scholar]
- 9. Anderson TJ, Nair S, Qin H, Singlam S, Brockman A, Paiphun L, Nosten F. 2005. Are transporter genes other than the chloroquine resistance locus (pfcrt) and multidrug resistance gene (pfmdr) associated with antimalarial drug resistance? Antimicrob. Agents Chemother. 49:2180–2188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kinga Modrzynska K, Creasey A, Loewe L, Cezard T, Trindade Borges S, Martinelli A, Rodrigues L, Cravo P, Blaxter M, Carter R, Hunt P. 2012. Quantitative genome re-sequencing defines multiple mutations conferring chloroquine resistance in rodent malaria. BMC Genomics 13:106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Veiga MI, Ferreira PE, Jornhagen L, Malmberg M, Kone A, Schmidt BA, Petzold M, Bjorkman A, Nosten F, Gil JP. 2011. Novel polymorphisms in Plasmodium falciparum ABC transporter genes are associated with major ACT antimalarial drug resistance. PLoS One 6:e20212. 10.1371/journal.pone.0020212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. DePristo MA, Zilversmit MM, Hartl DL. 2006. On the abundance, amino acid composition, and evolutionary dynamics of low-complexity regions in proteins. Gene 378:19–30 [DOI] [PubMed] [Google Scholar]
- 13. Wootton JC, Federhen S. 1996. Analysis of compositionally biased regions in sequence databases. Methods Enzymol. 266:554–571 [DOI] [PubMed] [Google Scholar]
- 14. Haerty W, Golding GB. 2011. Increased polymorphism near low-complexity sequences across the genomes of Plasmodium falciparum isolates. Genome Biol. Evol. 3:539–550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Basco LK, Le Bras J, Rhoades Z, Wilson CM. 1995. Analysis of pfmdr1 and drug susceptibility in fresh isolates of Plasmodium falciparum from subsaharan Africa. Mol. Biochem. Parasitol. 74:157–166 [DOI] [PubMed] [Google Scholar]
- 16. Duraisingh MT, Jones P, Sambou I, von Seidlein L, Pinder M, Warhurst DC. 2000. The tyrosine-86 allele of the pfmdr1 gene of Plasmodium falciparum is associated with increased sensitivity to the anti-malarials mefloquine and artemisinin. Mol. Biochem. Parasitol. 108:13–23 [DOI] [PubMed] [Google Scholar]
- 17. Legrand E, Yrinesi J, Ekala MT, Peneau J, Volney B, Berger F, Bouchier C, Bertani S, Musset L, Meynard JB, Mercereau-Puijalon O. 2012. Discordant temporal evolution of Pfcrt and Pfmdr1 genotypes and Plasmodium falciparum in vitro drug susceptibility to 4-aminoquinolines after drug policy change in French Guiana. Antimicrob. Agents Chemother. 56:1382–1389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wilson CM, Volkman SK, Thaithong S, Martin RK, Kyle DE, Milhous WK, Wirth DF. 1993. Amplification of pfmdr 1 associated with mefloquine and halofantrine resistance in Plasmodium falciparum from Thailand. Mol. Biochem. Parasitol. 57:151–160 [DOI] [PubMed] [Google Scholar]
- 19. Okombo J, Zongo I, Gadalla N, Bousema T, Beshir K, Roper C, Hallett R, Ochola-Oyier LI, Sutherland CJ. 2013. The polymorphic linker domain of Pfmdr1 is associated with resistance-conferring mutations in Plasmodium falciparum populations from East and West Africa. Antimicrob. Agents Chemother. 57:4595–4598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wang Z, Parker D, Meng H, Wu L, Li J, Zhao Z, Zhang R, Fan Q, Wang H, Cui L, Yang Z. 2012. In vitro sensitivity of Plasmodium falciparum from China-Myanmar border area to major ACT drugs and polymorphisms in potential target genes. PLoS One 7:e30927. 10.1371/journal.pone.0030927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Nogueira F, Lopes D, Alves AC, Rosario VED. 2008. Plasmodium falciparum drug resistance protein (MRP) gene expression under chloroquine and mefloquine challenge. J. Cell Anim. Biol. 2:10–20 [Google Scholar]
- 22. Menard D, Andriantsoanirina V, Khim N, Ratsimbasoa A, Witkowski B, Benedet C, Canier L, Mercereau-Puijalon O, Durand R. 2013. Global analysis of Plasmodium falciparum Na+/H+ exchanger (pfnhe-1) allele polymorphism and its usefulness as a marker of in vitro resistance to quinine. Int. J. Parasitol. Drugs Drug Resist. 3:8–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Four Artemisinin-Based Combinations (4ABC) Study Group 2011. A head-to-head comparison of four artemisinin-based combinations for treating uncomplicated malaria in African children: a randomized trial. PLoS Med. 8:e1001119. 10.1371/journal.pmed.1001119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Black CG, Wu T, Wang L, Hibbs AR, Coppel RL. 2001. Merozoite surface protein 8 of Plasmodium falciparum contains two epidermal growth factor-like domains. Mol. Biochem. Parasitol. 114:217–226 [DOI] [PubMed] [Google Scholar]
- 25. Mwai L, Kiara SM, Abdirahman A, Pole L, Rippert A, Diriye A, Bull P, Marsh K, Borrmann S, Nzila A. 2009. In vitro activities of piperaquine, lumefantrine, and dihydroartemisinin in Kenyan Plasmodium falciparum isolates and polymorphisms in pfcrt and pfmdr1. Antimicrob. Agents Chemother. 53:5069–5073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Okombo J, Kiara SM, Rono J, Mwai L, Pole L, Ohuma E, Borrmann S, Ochola LI, Nzila A. 2010. In vitro activities of quinine and other antimalarials and pfnhe polymorphisms in Plasmodium isolates from Kenya. Antimicrob. Agents Chemother. 54:3302–3307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bereczky S, Martensson A, Gil JP, Farnert A. 2005. Short report: rapid DNA extraction from archive blood spots on filter paper for genotyping of Plasmodium falciparum. Am. J. Trop. Med. Hyg. 72:249–251 [PubMed] [Google Scholar]
- 28. Veiga MI, Ferreira PE, Schmidt BA, Ribacke U, Bjorkman A, Tichopad A, Gil JP. 2010. Antimalarial exposure delays Plasmodium falciparum intra-erythrocytic cycle and drives drug transporter genes expression. PLoS One 5:e12408. 10.1371/journal.pone.0012408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bowman S, Lawson D, Basham D, Brown D, Chillingworth T, Churcher CM, Craig A, Davies RM, Devlin K, Feltwell T, Gentles S, Gwilliam R, Hamlin N, Harris D, Holroyd S, Hornsby T, Horrocks P, Jagels K, Jassal B, Kyes S, McLean J, Moule S, Mungall K, Murphy L, Oliver K, Quail MA, Rajandream MA, Rutter S, Skelton J, Squares R, Squares S, Sulston JE, Whitehead S, Woodward JR, Newbold C, Barrell BG. 1999. The complete nucleotide sequence of chromosome 3 of Plasmodium falciparum. Nature 400:532–538 [DOI] [PubMed] [Google Scholar]
- 30. Zilversmit MM, Volkman SK, DePristo MA, Wirth DF, Awadalla P, Hartl DL. 2010. Low-complexity regions in Plasmodium falciparum: missing links in the evolution of an extreme genome. Mol. Biol. Evol. 27:2198–2209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wootton JC. 1994. Non-globular domains in protein sequences: automated segmentation using complexity measures. Comput. Chem. 18:269–285 [DOI] [PubMed] [Google Scholar]
- 32. Mu J, Awadalla P, Duan J, McGee KM, Joy DA, McVean GA, Su XZ. 2005. Recombination hotspots and population structure in Plasmodium falciparum. PLoS Biol. 3:e335. 10.1371/journal.pbio.0030335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wootton JC, Feng X, Ferdig MT, Cooper RA, Mu J, Baruch DI, Magill AJ, Su XZ. 2002. Genetic diversity and chloroquine selective sweeps in Plasmodium falciparum. Nature 418:320–323 [DOI] [PubMed] [Google Scholar]
- 34. Conway DJ, Roper C, Oduola AM, Arnot DE, Kremsner PG, Grobusch MP, Curtis CF, Greenwood BM. 1999. High recombination rate in natural populations of Plasmodium falciparum. Proc. Natl. Acad. Sci. U. S. A. 96:4506–4511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Riordan J. 1993. The cystic fibrosis transmembrane conductance regulator. Annu. Rev. Physiol. 55:609–630 [DOI] [PubMed] [Google Scholar]
- 36. van Veen HW, Konings WN. 1997. Multidrug transporters from bacteria to man: similarities in structure and function. Semin. Cancer Biol. 8:183–191 [DOI] [PubMed] [Google Scholar]
- 37. Sato T, Kodan A, Kimura Y, Ueda K, Nakatsu T, Kato H. 2009. Functional role of the linker region in purified human P-glycoprotein. FEBS J. 276:3504–3516 [DOI] [PubMed] [Google Scholar]
- 38. Kolling R, Losko S. 1997. The linker region of the ABC-transporter Ste6 mediates ubiquitination and fast turnover of the protein. EMBO J. 16:2251–2261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Deplaine G, Lavazec C, Bischoff E, Natalang O, Perrot S, Guillotte-Blisnick M, Coppee JY, Pradines B, Mercereau-Puijalon O, David PH. 2011. Artesunate tolerance in transgenic Plasmodium falciparum parasites overexpressing a tryptophan-rich protein. Antimicrob. Agents Chemother. 55:2576–2584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Hunt P, Afonso A, Creasey A, Culleton R, Sidhu AB, Logan J, Valderramos SG, McNae I, Cheesman S, do Rosario V, Carter R, Fidock DA, Cravo P. 2007. Gene encoding a deubiquitinating enzyme is mutated in artesunate- and chloroquine-resistant rodent malaria parasites. Mol. Microbiol. 65:27–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Cordes FS, Bright JN, Sansom MS. 2002. Proline-induced distortions of transmembrane helices. J. Mol. Biol. 323:951–960 [DOI] [PubMed] [Google Scholar]
- 42. Emerson LR, Nau ME, Martin RK, Kyle DE, Vahey M, Wirth DF. 2002. Relationship between chloroquine toxicity and iron acquisition in Saccharomyces cerevisiae. Antimicrob. Agents Chemother. 46:787–796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Pattaradilokrat S, Mu J, Awadalla P, Su X-Z. 2013. Genome diversity and applications in genetic studies of human malaria parasites Plasmodium falciparum and Plasmodium vivax, p 59–89 In Carlton J. (ed), Malaria parasites: comparative genomics, evolution and molecular biology. Caister Publishing Press, Norfolk, United Kingdom [Google Scholar]
- 44. Andriantsoanirina V, Menard D, Rabearimanana S, Hubert V, Bouchier C, Tichit M, Bras JL, Durand R. 2010. Association of microsatellite variations of Plasmodium falciparum Na+/H+ exchanger (Pfnhe-1) gene with reduced in vitro susceptibility to quinine: lack of confirmation in clinical isolates from Africa. Am. J. Trop. Med. Hyg. 82:782–787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kavishe RA, van den Heuvel JM, van de Vegte-Bolmer M, Luty AJ, Russel FG, Koenderink JB. 2009. Localization of the ATP-binding cassette (ABC) transport proteins PfMRP1, PfMRP2, and PfMDR5 at the Plasmodium falciparum plasma membrane. Malaria J. 8:205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Robinson T, Campino SG, Auburn S, Assefa SA, Polley SD, Manske M, MacInnis B, Rockett KA, Maslen GL, Sanders M, Quail MA, Chiodini PL, Kwiatkowski DP, Clark TG, Sutherland CJ. 2011. Drug-resistant genotypes and multi-clonality in Plasmodium falciparum analysed by direct genome sequencing from peripheral blood of malaria patients. PLoS One 6:e23204. 10.1371/journal.pone.0023204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Anders RF, Coppel RL, Brown GV, Kemp DJ. 1988. Antigens with repeated amino acid sequences from the asexual blood stages of Plasmodium falciparum. Prog. Allergy 41:148–172 [PubMed] [Google Scholar]
- 48. Kidgell C, Volkman SK, Daily J, Borevitz JO, Plouffe D, Zhou Y, Johnson JR, Le Roch K, Sarr O, Ndir O, Mboup S, Batalov S, Wirth DF, Winzeler EA. 2006. A systematic map of genetic variation in Plasmodium falciparum. PLoS Pathog. 2:e57. 10.1371/journal.ppat.0020057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Miotto O, Almagro-Garcia J, Manske M, Macinnis B, Campino S, Rockett KA, Amaratunga C, Lim P, Suon S, Sreng S, Anderson JM, Duong S, Nguon C, Chuor CM, Saunders D, Se Y, Lon C, Fukuda MM, Amenga-Etego L, Hodgson AV, Asoala V, Imwong M, Takala-Harrison S, Nosten F, Su XZ, Ringwald P, Ariey F, Dolecek C, Hien TT, Boni MF, Thai CQ, Amambua-Ngwa A, Conway DJ, Djimde AA, Doumbo OK, Zongo I, Ouedraogo JB, Alcock D, Drury E, Auburn S, Koch O, Sanders M, Hubbart C, Maslen G, Ruano-Rubio V, Jyothi D, Miles A, O'Brien J, Gamble C, et al. 2013. Multiple populations of artemisinin-resistant Plasmodium falciparum in Cambodia. Nat. Genet. 45:648–655 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.