Abstract
Beta-hemolytic Streptococcus agalactiae is the leading cause of bacteremia and invasive infections. These diseases are treated with β-lactams or macrolides, but the emergence of less susceptible and even fully resistant strains is a cause for concern. New bacteriophage lysins could be promising alternatives against such organisms. They hydrolyze the bacterial peptidoglycan at the end of the phage cycle, in order to release the phage progeny. By using a bioinformatic approach to screen several beta-hemolytic streptococci, a gene coding for a lysin was identified on a prophage carried by Streptococcus dysgalactiae subsp. equisimilis SK1249. The gene product, named PlySK1249, harbored an original three-domain structure with a central cell wall-binding domain surrounded by an N-terminal amidase and a C-terminal CHAP domain. Purified PlySK1249 was highly lytic and bactericidal for S. dysgalactiae (2-log10 CFU/ml decrease within 15 min). Moreover, it also efficiently killed S. agalactiae (1.5-log10 CFU/ml decrease within 15 min) but not several streptococcal commensal species. We further investigated the activity of PlySK1249 in a mouse model of S. agalactiae bacteremia. Eighty percent of the animals (n = 10) challenged intraperitoneally with 106 CFU of S. agalactiae died within 72 h, whereas repeated injections of PlySK1249 (45 mg/kg 3 times within 24 h) significantly protected the mice (P < 0.01). Thus, PlySK1249, which was isolated from S. dysgalactiae, demonstrated high cross-lytic activity against S. agalactiae both in vitro and in vivo. These encouraging results indicated that PlySK1249 might represent a good candidate to be developed as a new enzybiotic for the treatment of systemic S. agalactiae infections.
INTRODUCTION
Streptococcus agalactiae, or group B Streptococcus (GBS) (1), is a common inhabitant of the gastrointestinal and genital tracts, with an asymptomatic-carriage rate of 9% to 30% in adults. It is the leading cause of invasive neonatal infections in children (2), in whom it may cause bacteremia, pneumonia, and meningitis, with mortality rates ranging from 5% to 20% (3). In addition, GBS infection is now also increasingly reported in older children and adults, in whom it provokes severe sepsis often complicated by multiple deep-seated abscesses and sometimes endocarditis (4–6).
A major source of neonate GBS infection is asymptomatic vaginal carriage by the mother. Therefore, prevention guidelines aim at interfering with mother-to-child transmission at the time of birth and advocate parenteral administration of penicillin G or amoxicillin to GBS-colonized women 4 h before delivery (7). Recently, concerns regarding GBS isolates with decreased susceptibility to penicillin (8, 9) and even resistance to penicillin G and ceftriaxone have emerged (10). Moreover, in some U.S. states, the prevalence of GBS resistant to erythromycin and clindamycin has increased from 15.8% to 32.8% and 10.5% to 15%, respectively, between 1996 and 2003 (11). In parallel, Bergseng et al. (12) also reported erythromycin and clindamycin resistance in Norway, as well as an increase in the rate of mortality from neonatal GBS infections (from 6.5% in 1996 to 16.5% in 2005), which seemed to correlate with the drug resistance phenotype. This supports the necessity of searching for alternative compounds against drug-resistant bacteria in general and S. agalactiae in particular.
Bacteriophage lysins having peptidoglycan hydrolase activities represent one such compound (13). These enzymes are produced by both temperate and lytic phages in order to lyse their host cells and release their progeny at the end of their life cycle. Because purified lysins are able to reach and destroy the peptidoglycan of Gram-positive bacterial cells when added from the outside, they were successfully used either topically, to decontaminate colonized tissues, or parenterally, in cases of severe infections (13). Since a temperate bacteriophage integrates its genome into the host chromosome, we used a bioinformatic approach to screen sequenced bacterial genomes of beta-hemolytic streptococci in order to identify new bacteriophage lysin genes. Here we describe a new lysin encoded on a prophage-like region inserted into the genome of Streptococcus dysgalactiae subsp. equisimilis strain SK1249. The purified lysin, named PlySK1249, showed an uncommon architecture with a central cell wall-binding domain. It not only efficiently lysed and killed S. dysgalactiae but also had cross-lytic activity against GBS and several representatives of beta-hemolytic streptococci in vitro. Accordingly, PlySK1249 was very potent in curing mice suffering from GBS-induced bacteremia. Our results underline the cross-lytic activity of PlySK1249 between beta-hemolytic streptococci and suggest that it could constitute a new alternative for the prevention and the treatment of bacterial infections due to GBS.
MATERIALS AND METHODS
Bacterial strains and reagents.
Unless otherwise specified, chemicals used in this study were purchased from Sigma-Aldrich (St. Louis, MO). Restriction enzymes were obtained from Promega (Madison, WI), and primers were synthesized by Microsynth AG (Balgach, Switzerland). All strains used in this study (Table 1) were grown at 37°C with aeration (250-rpm shaking), except for Streptococcus pneumoniae, which was grown without aeration. Streptococci and enterococci were cultured in brain heart infusion (BHI) and plated on Mueller-Hinton agar containing 5% sheep blood (bioMérieux SA, Marcy l'Etoile, France) and BHI agar, respectively. Staphylococcus aureus was grown in tryptic soy broth (TSB) and plated as streptococci. Escherichia coli strains were grown in Luria-Bertani (LB) broth and plated on LB agar. Frozen stocks were made from cultures in the exponential phase of growth supplemented with 20% glycerol (vol/vol).
Table 1.
Bacterial strains, plasmids, and oligonucleotides used in this study
| Strain, plasmid, or oligonucleotide | Characteristics or origina | Source or reference |
|---|---|---|
| Bacterial strains | ||
| E. coli BL21(DE3)pLysS | F− ompT hsdSB (rB− mB−) dcm+ gal (DE3) pLysS (Camr) | Stratagene |
| E. coli BL21/pPlySK124915b | E. coli BL21(DE3)(pLysS) transformed with pPlySK124915b | This study |
| E. coli BL21/pPlySK124928a | E. coli BL21(DE3)(pLysS) transformed with pPlySK124928a | This study |
| S. dysgalactiae SK1249 | Human, hemoculture | |
| GBS FSL-S3-026 | Bovine | |
| GBS 17–2167 | Human, endocarditis | |
| GBS 532 | Human | |
| GBS GF | Human, hemoculture | |
| S. pyogenes ATCC 19615 | Human, sore throat | |
| S. gordonii DL1 | Human | |
| S. mutans ATCC 25175 | Human, carious dentine | |
| S. suis 19 | Porcine | |
| S. uberis ATCC 700407 | Bovine | |
| S. mutans ATCC 25175 | Human, carious dentine | |
| E. faecalis ATCC 29212 | Human, urine | |
| E. faecium D344 | Human | |
| S. pneumoniae D39 | Human | |
| S. aureus M32 | Bovine, subclinical mastitis | |
| S. aureus Laus102 | Human, healthy patient | |
| E. faecalis ATCC 29212 | Human, urine | |
| Plasmids | ||
| pET-15b | Expression vector; Ampr | Novagen |
| pET-28a | Expression vector; Kanr | Novagen |
| pPlySK124915b | pET-15b carrying PlySK1249 | This study |
| pPlySK124928a | pET-28a carrying PlySK1249 | This study |
| Oligonucleotides | ||
| plySK15bNdeIFw | 5′-GGAATTCCATATGGGAAAACATCTAGTCATTTGTGGTCATGGGCAAGGGCG-3′ | This study |
| plySK15bBamHIRv | 5′-CGCGGATCCTTAATGAAATTCTAAACCAACCAACAACTTTTCCAAGTTTAACTGTTCCAG-3′ | This study |
| plySK28aNcoIFw | 5′-GCATGCCATGGGAAAACATCTAGTGATTTGTGGACATGGGCAAGGACG-3′ | This study |
| plySK28aXhoIRv | 5′-GCCGCTCGAGTGAAATTCTAAACCAACCTACAACTTTTCCAAGTTTAACTGTTCCAG-3′ | This study |
| Universal T7 primer Fw | 5′-TAATACGACTCACTATAGGG-3′ | Microsynth |
| Universal T7 primer Rv | 5′-GCTAGTTATTGCTCAGCGG-3′ | Microsynth |
Ampr, ampicillin resistance; Camr, chloramphenicol resistance; Kanr kanamycin resistance. Restriction sites in oligonucleotides are underlined.
Cloning of PlySK1249.
The gene HMPREF9964_0831, potentially encoding a functional lysin, was identified using a bioinformatic approach and is referred to as PlySK1249 for convenience throughout this paper, as described in Results. To further clone PlySK1249, genomic DNA was prepared from S. dysgalactiae strain SK1249 using the DNeasy blood and tissue kit (Qiagen, Valencia, CA) according to the manufacturer's instructions. PlySK1249 was PCR amplified with specific primer pairs, i.e., plySK15bNdeIFw/plySK15bBamHIRv and plySK28aNcoIFw/plySK28aXhoIRv (Table 1). PCR products were digested with compatible restrictions endonucleases and ligated into expression vectors pET-15b and pET-28a (Merck KGaA, Darmstadt, Germany). Two plasmids, namely, pPlySK124915b and pPlySK124928a (Table 1), were obtained in which the His tag was located at either the 5′ or 3′ end of PlySK1249, respectively. Constructs were transformed in One Shot BL21(DE3)pLysS chemically competent E. coli cells (Life Technologies Europe B.V., Zug, Switzerland) and propagated on LB plates or broth supplemented with ampicillin (100 μg/ml) and chloramphenicol (25 μg/ml) for E. coli BL21/pPlySK124915b or kanamycin (30 μg/ml) and chloramphenicol (25 μg/ml) for E. coli BL21/pPlySK124928a. All constructs were validated by DNA sequencing using universal T7 primers (Table 1).
PlySK1249 expression and activity screening of the gene product.
In order to identify a clone expressing active PlySK1249 lysin, a protocol was adapted from the work of Schmitz et al. (14). Briefly, BL21/pPlySK124915b and BL21/pPlySK124928a transformants (Table 1) were replica plated on LB agar supplemented with the appropriate antibiotics and 0.4 mM isopropyl-1-thio-β-d-galactopyranoside (IPTG). Following overnight incubation at 37°C, colonies were exposed to chloroform vapors for 20 min. Permeabilized cells were further overlaid with 15 ml of molten soft agar containing autoclaved S. dysgalactiae SK1249 cells. Plates were incubated at 37°C and observed for clearing zones surrounding colonies for up to 24 h. To prepare molten soft agar, an overnight culture of S. dysgalactiae SK1249 was centrifuged, washed with 1 vol of 0.9% NaCl, and resuspended in 0.25 vol of PBS, pH 7.4. The cell suspension was further supplemented with granulated agar (7.5 g/liter), autoclaved for 15 min at 120°C, and stored at 4°C.
Purification of PlySK1249.
A starter culture from an E. coli colony carrying the BL21/pPlySK124928a construct and surrounded by a large lysis zone in the above-mentioned screening test was grown overnight in LB supplemented with kanamycin (30 μg/ml) and chloramphenicol (50 μg/ml). The following morning, 25 ml of the culture was diluted with 20 vol of preheated fresh LB and further grown at 37°C. At an optical density at 600 nm (OD600) of 0.7, the culture was induced for 20 h at 18°C by the addition of 0.4 mM IPTG. Cells were centrifuged, washed with 30 ml of 0.9% NaCl, and resuspended in 30 ml of binding buffer (20 mM imidazole, 20 mM phosphate buffer, 0.5 M NaCl [pH 7.4]). Aliquots of 15 ml were frozen at −80°C, thawed, and sonicated on ice (Sonopuls; Bandelin Electronics, Berlin, Germany). Cell debris was removed from pooled supernatants by centrifugation (15,000 rpm, 30 min, 4°C). Supernatant were further treated with 1 μg/ml RNase A and DNase I (Roche AG, Basel, Switzerland) for 45 min at 4°C and filtered through 0.45-μm Acrodisc filters (Pall, Ann Arbor, MI). The filtrate was applied to a 5-ml HisTrap HP column (GE Healthcare, Glattbrugg, Switzerland) previously equilibrated with binding buffer and coupled to an ÄKTA Prime apparatus (GE Healthcare). After a washing with 50 ml of buffer A (50 mM imidazole, 20 mM phosphate buffer, 0.5 M NaCl [pH 7.4]), His-tagged PlySK1249 was eluted in buffer B (500 mM imidazole, 20 mM phosphate buffer, 0.5 M NaCl [pH 7.4]). Imidazole was removed by extensive dialysis against lysin buffer (500 mM l-arginine, 50 mM phosphate buffer [pH 7.4]) using a membrane tubing (molecular weight cutoff [MWCO], 12,000 to 14,000; Spectra/Por, Rancho Dominguez, CA). PlySK1249 was analyzed on NuPage 4 to 12% bis-Tris gels (Invitrogen, Carlsbad, CA).
In vitro quantification of PlySK1249 activity against S. dysgalactiae: effect of bacterial growth phase and pH.
PlySK1249 activity was measured by following the decrease in turbidity of a solution of S. dysgalactiae SK1249 cells resuspended in lysis buffer (40 mM phosphate buffer, 200 mM NaCl [pH 7.4]). Briefly, bacterial cells were liquid cultured overnight, subcultured (1:100) to an OD600 of ∼0.4 the next morning, and harvested by centrifugation before being washed with 0.9% NaCl and finally resuspended in lysis buffer to an OD600 of 1. Activity was measured by mixing 150 μl of the bacterial cell suspension with 150 μl of serial 2-fold dilutions of PlySK1249 in 96-well microtiter plates. Serial dilutions of the enzyme were done in lysin buffer. The decrease in OD600 was monitored each min for 1 h with an EL808 absorbance microplate reader run with Gen5 software (BioTek, Winooski, VT) and set to 37°C. The well in which a decrease in the optical density of half in 15 min was observed was defined as containing one unit (1 U) of purified enzyme (15). In order to test the effect of the growth phase on the susceptibility of S. dysgalactiae to PlySK1249, subcultured cells were harvested at OD600 of 0.13, 0.48, 0.85, and 1.02 before being exposed to purified lysin (final concentration, 3.3 U/ml). To test the effect of pH on the PlySK1249 activity, S. dysgalactiae cell suspensions and PlySK1249 solutions were both prepared in buffers with pH values ranging from 4.0 to 9.0. Cells were then exposed to PlySK1249 (final concentration, 3.3 U/ml), and the decrease in OD600 was monitored after 15 min incubation at 37°C. All reactions were performed in triplicate.
PlySK1249 host range.
In order to determine the PlySK1249 host range, subcultures of different bacterial species (Table 1) were harvested at an OD600 of ∼0.4 and processed as described above before being exposed to purified PlySK1249 (final concentration, 3.3 U/ml). The decrease in OD600 was monitored after 15 min incubation at 37°C. All reactions were performed in triplicate.
In vitro time-kill assays.
To determine bacterial viability, time-kill assays were performed in triplicate by exposing either a solution of S. dysgalactiae SK1249 at 109 CFU/ml or a solution of GBS clinical strain 17-2167 at 5 × 108 CFU/ml resuspended in lysin buffer to purified PlySK1249 (final concentration, 3.3 U/ml) at 37°C. At different times over 1 h of incubation, 100-μl aliquots were collected, serially diluted in 10 ml 0.9% ice-cold NaCl, and plated for colony counts. Viable colonies were enumerated after 24 h of incubation at 37°C.
Transmission electron microscopy (TEM).
S. dysgalactiae SK1249 was grown to an OD600 of 0.4, centrifuged, and resuspended in lysis buffer to an OD600 of 1.0. Then 150 μl of this bacterial suspension was mixed with 150 μl of PlySK1249 (final concentration, 3.3 U/ml). After 15 min of incubation at 37°C, 1 μl of this solution was adsorbed on a glow-discharged copper 200-mesh grid coated with Formvar-carbon (EMS, Hatfield, PA) during 1 min at room temperature (RT). The grid was then washed with two drops of distilled water followed by staining with 1% of uranyl acetate in H2O (Sigma, St. Louis, MO) for 1 min. Excess uranyl acetate was removed with blotting paper, and the grid was dried for 10 min before image acquisition. Micrographs were taken with an FEI CM100 transmission electron microscope (FEI, Eindhoven, The Netherlands) at an acceleration voltage of 80 kV and 11,000× magnification (pixel size of 0.93 nm) with a TVIPS TemCam-F416 digital camera (TVIPS GmbH, Gauting, Germany).
PlySK1249 therapeutic trials in mice.
Animal experimentation was carried out in strict accordance with the recommendations of the Swiss Federal Act on Animal Protection, and the protocol was approved by the Committee on the Ethics of Animal Experiments of the Consumer and Veterinary Affairs Department of the State of Vaud (permit number 2395). A total of 35 six-week-old CD1 Swiss female mice (Charles River Laboratories, L'Arbresle, France) with an average weight of 22 ± 1 g were used. In order to validate the bacteremic state of mice at the time of the initial treatment injection, i.e., 1 h after the bacterial challenge, the left kidney and spleen were removed aseptically from three mice 45 min after i.p injection of 106 CFU of GBS clinical strain 17-2167. Organs were homogenized in 1 ml of saline and briefly centrifuged, and supernatants were plated on blood agar plates to determine the number of viable organisms in tissues. For therapeutic experiments, animal sample sizes were estimated with the formula for dichotomous variables (16). In a first series of experiments conducted to evaluate the effect of a single bolus injection of PlySK1249, two groups of mice were injected intraperitoneally (i.p.) with 106 CFU of the GBS 17-2167. Group 1 (treatment group) received 22.5 mg/kg of PlySK1249 in 200 μl i.p. 1 h after the bacterial challenge, while group 2 (control group) received 200 μl of lysin buffer i.p. in parallel.
A second series of experiments was run to test the effect of dose escalation. Two groups of mice were injected i.p. with 106 CFU of the GBS 17-2167, with the treatment group receiving 45 mg/kg of PlySK1249 in 200 μl i.p. 2, 20, and 24 h after the bacterial challenge, while the control or untreated group received 200 μl of lysin buffer i.p at the same times. Survival curves were drawn, analyzed, and compared with log rank (Mantel-Cox) and Gehan-Breslow-Wilcoxon tests using GraphPad Prism version 5.00 for Windows (GraphPad Software, San Diego, CA).
Nucleotide sequence accession numbers.
The full genomic sequence of S. dysgalactiae SK1249 has been deposited in GenBank as a whole-genome shotgun sequencing project under accession number AFIN00000000. The sequence of PlySK1249 has been submitted to GenBank under accession number EGL49245.1.
RESULTS
Identification of prophage-like regions in the genome of S. dysgalactiae strain SK1249 and identification of PlySK1249.
Phage search tool (PHAST) (17), a web tool finder and annotator of prophage regions within bacterial genomes, found nine prophage-related regions in the sequenced genome of S. dysgalactiae SK1249 (http://phast.wishartlab.com/cgi-bin/change_to_html.cgi?num=AFIN00000000).
HMPREF9964_0831, encoding a potential lysin, was identified at chromosomal positions 550,441 to 551,910 within prophage-related region 2, annotated as a questionable prophage by PHAST (data not shown), and renamed PlySK1249 for convenience in this study. The analysis of the deduced gene product, named PlySK1249, revealed a putative 489-amino-acid LysM domain protein with a calculated molecular mass of ca. 53 kDa. PlySK1249 shares 98% and 80% identity with phage-associated cell wall hydrolases found in Streptococcus suis R61 (EHC01546.1) and S. agalactiae FSL S3-026 (ZP_11943978.1), respectively.
The predicted primary structure of PlySK1249 is depicted in Fig. 1. Conserved domain analysis using the Conserved Domains Database (CDD) (http://www.ncbi.nlm.nih.gov/Structure/cdd/cdd.shtml) indicated that PlySK1249 harbors three putative functional domains: (i) an N-terminal catalytic domain with predicted amidase activity; (ii) a central LysM cell wall-binding domain that shares 38% identity (100% coverage) with a similar domain found in the S. aureus LytN autolysin (18), and (iii) a C-terminal catalytic domain that shares 27% (71% coverage) and 33% (40% coverage) identity with the cysteine histidine-dependent aminohydrolase/peptidase (CHAP) domains of the LytN autolysin (18) and the B30 lysin (19, 20), respectively. The conserved cysteine and histidine residues of the CHAP domain were found at positions 353 and 414 of the PlySK1249 sequence (see Fig. S1 in the supplemental material).
Fig 1.
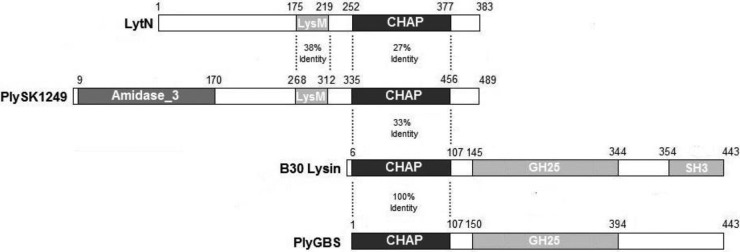
Schematic representation of relevant lysins. MurNac-LAA, amidase catalytic domain; CHAP, cysteine histidine-dependent aminohydrolase/peptidase catalytic domain; GH25, glycoside hydrolase family 25 catalytic domain; LysM and SH3, substrate binding domains. Amino acid identities are presented between the considered domains.
Cloning of PlySK1249 and screening of PlySK1249 lytic activity.
PlySK1249 was successfully amplified by PCR from purified genomic DNA of S. dysgalactiae SK1249 and cloned into pET-15b and pET-28a expression vectors (leading to the constructs pPlySK124915b and pPlySK124928a, respectively). Constructs were transformed into E. coli BL21 cells (Table 1), and transformants were recovered on LA plates supplemented with antibiotics as described in Materials and Methods. Following IPTG induction, chloroform permeabilization, and overlay with soft agar containing autoclaved S. dysgalactiae SK1249 cells, lysis zones developed around BL21/pPlySK124928a colonies within 12 h of incubation (see Fig. S2 in the supplemental material). In contrast, no lysis zones were observed around BL21/pet28a and BL21/pPlySK124915b colonies (Fig. S2 and data not shown, respectively). One BL21/pPlySK124928a colony, surrounded by a large halo, was selected and further grown in order to purify PlySK1249.
Purification of PlySK1249.
We obtained 8 mg of PlySK1249 from 1 liter of culture by a single-step passage of the crude extract, obtained after sonication, on a 5-ml HisTrap HP column. PlySK1249 was expressed, was soluble, and migrated at the expected molecular mass of 53 kDa on a 4 to 12% Novex bis-Tris gel (Fig. 2, lanes 2.1, 2.2, and 3.1). A purity of ca. 90% was achieved (Fig. 2, lane 3.1; also, see Fig. S3 in the supplemental material). Purified PlySK1249 was stored at 4°C in lysin buffer (500 mM l-arginine, 50 mM phosphate buffer [pH 7.4]) until further use.
Fig 2.
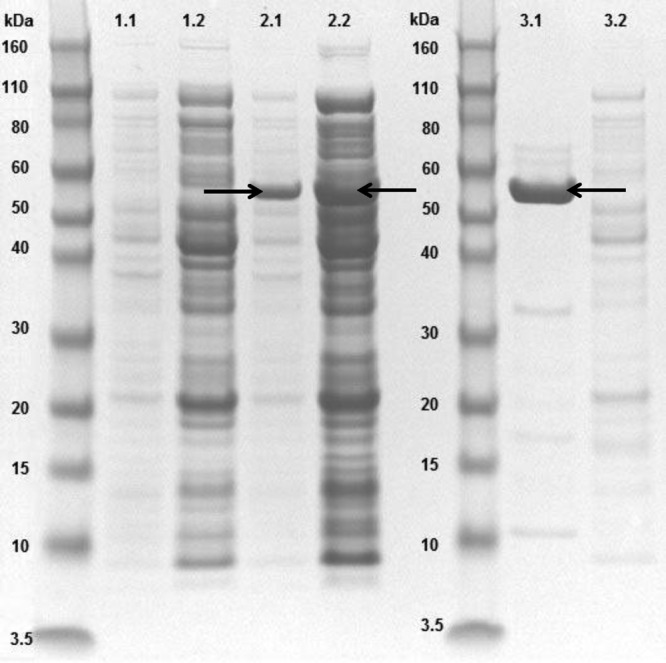
Electrophoretic profile of overexpressed PlySK1249. Lanes: 1.1 and 1.2, noninduced total and cytoplasmic fractions, respectively; 2.1 and 2.2, induced total and cytoplasmic fractions, respectively; 3.1 and 3.2, purified PlySK1249 and column flowthrough, respectively. Arrows indicate overexpressed PlySK1249. Molecular masses of the Novex Sharp standard are given.
In vitro quantification of PlySK1249 activity; effect of bacterial growth phase and pH.
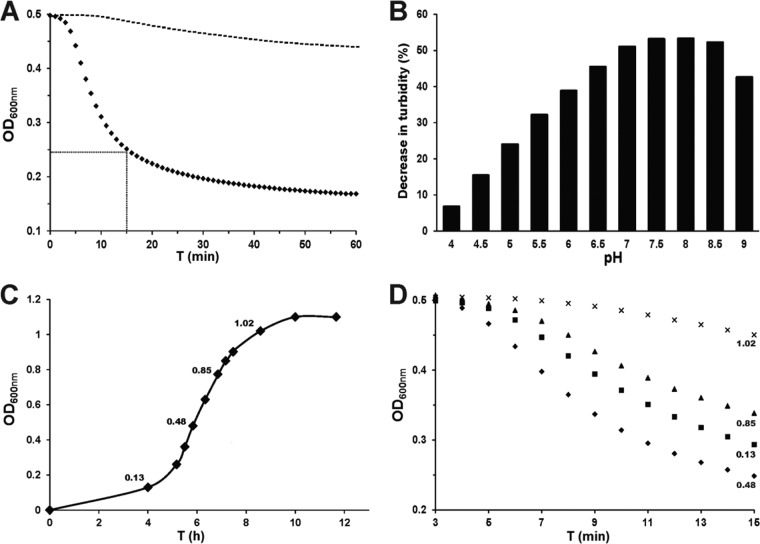
The antibacterial activity of purified lysins is commonly quantified by determining their capacity to decrease the turbidity of a suspension of bacterial cells over time. In such turbidity assays, 1 U of enzyme is defined as the amount of enzyme that results in a 50% drop in turbidity (OD600) in 15 min and at 37°C in a suspension of bacterial cells harvested in mid-logarithmic growth phase. Using this definition, which is commonly accepted, the calculated specific activity was 1 U/μg, which was equivalent to 50 U/nmol of PlySK1249 (Fig. 3A).
Fig 3.
PlySK1249 in vitro activity. All experiments were done in OD600 turbidity assays using S. dysgalactiae SK1249 as the target. (A) The specific activity of PlySK1249 was 1 U/μg. One unit of activity was defined as the amount of lysin resulting in a 50% decrease in OD600 in 15 min (closed diamonds, PlySK1249; dotted line, controls). (B) The optimum pH was between 7 and 8.5, determined after a 15-min exposure of the cells to PlySK1249 (final concentration, 3.3 U/ml). (C and D) PlySK1249 was more active against bacteria in the early than the late stage of growth. The OD600 at which the cells were harvested before challenge with PlySK1249 (final concentration, 3.3 U/ml) are indicated.
Figure 3B depicts the pH activity profile in the presence of 3.3 U/ml of PlySK1249. The optimal pH was found to be between pH 7 and 8.5, with a ca. 50% decrease in OD600 achieved at these pH values in 15 min. The activity was lower at acidic and basic pHs, with, for instance, only a 7% OD600 decrease at pH 4.
It is well known that the activity of phage lysins is dependent on the growth phase the bacterial targets are in when they are challenged by the enzyme. In order to test this growth phase-dependent sensitivity to PlySK1249, aliquots of S. dysgalactiae SK1249 were harvested at different stages of growth (Fig. 3C) and further challenged by purified PlySK1249 in turbidity assays (Fig. 3D). We observed that bacterial cells harvested at early exponential and mid-exponential phases were much more susceptible to PlySK1249-induced lysis than cells harvested in the late exponential or stationary phase. For instance, while the turbidity of a solution of cells harvested in the exponential growth phase (i.e., at an OD600 of 0.48) decreased by 50% in 15 min, that of cells harvested in the early stationary growth phase decreased by only ca. 5% (i.e., at an OD600 of 1.02) (Fig. 3D).
Host range.
We used the turbidity assay to further determine the activity of PlySK1249 against several different bacterial species. All beta-hemolytic species tested (S. dysgalactiae, GBS, and Streptococcus pyogenes) were susceptible to PlySK1249, with some strain-to-strain variation for GBS (Fig. 4). Indeed, while a ca. 65% turbidity decrease was observed for the GBS strain FSL-03, only a ca. 15% decrease was achieved under the same conditions with strain 532. Interestingly, Streptococcus uberis and Streptococcus suis were also susceptible, with ca. 20% and ca. 30% decreases in turbidity, respectively. PlySK1249 was active against Streptococcus gordonii strain DL1 (ca. 45% turbidity decrease) but not against Streptococcus mutans, S. pneumoniae, S. aureus, Enterococcus faecalis, or Enterococcus faecium (<10% turbidity decreases in all cases) (Fig. 4).
Fig 4.
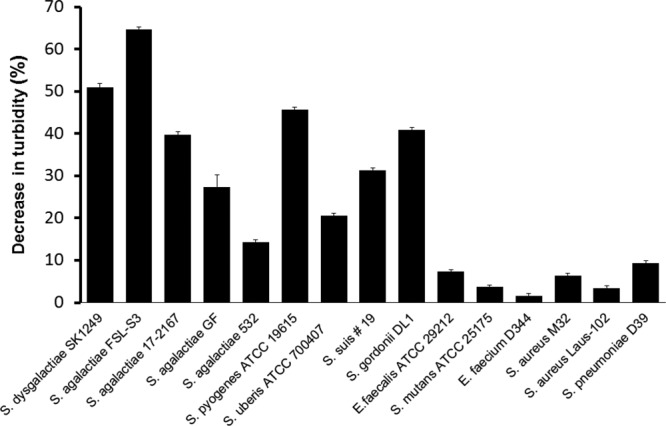
Host range. All experiments were done in turbidity assays in the presence of purified PlySK1249 (final concentration, 3.3 U/ml) and bacterial cells harvested in mid-exponential growth phase (i.e., at an OD600 of ∼0.5), which had been previously washed and adjusted to an OD600 of ∼0.5 in lysis buffer. Values are means and standard deviations from triplicates.
In vitro time-kill assays.
In order to determine if the observed loss of turbidity resulted from cell bursting and subsequent death, we further tested the viability of bacterial cells exposed to PlySK1249 in time-kill experiments (Fig. 5) and observed the effect of lysin on the cells by TEM (Fig. 6). To this end, a suspension of S. dysgalactiae SK1249 harvested in mid-log phase was challenged with 3.3 U/ml of lysin and plated for numeration at different incubation times (Fig. 5). Our data showed a ca. 2-log CFU/ml decrease in 15 min, which was in agreement with the observed decrease in turbidity. Similarly, a ca. 2-log10 CFU/ml decrease in 15 min was achieved with the same amount of PlySK1249 for the GBS clinical strain 17-2167, which was used in the mouse model of GBS-induced bacteremia. In parallel, TEM imaging of aliquots of unchallenged (Fig. 6A) and challenged (Fig. 6B) S. dysgalactiae cells revealed that exposure to PlySK1249 led to cytoplasm extrusion, resulting in ghost cells.
Fig 5.
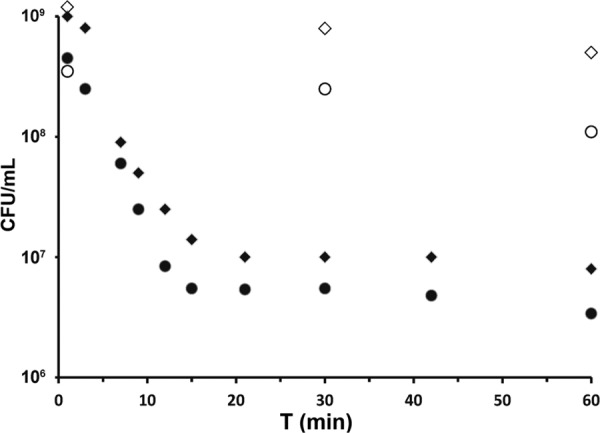
In vitro time-kill assays. S. dysgalactiae SK1249 (closed diamonds) and S. agalactiae clinical strain 17-2167 (closed circles) were exposed to PlySK1249 (final concentration, 3.3 U/ml). Controls (open diamonds and circles) were exposed to lysin buffer only. Each dot represents the mean of triplicates.
Fig 6.
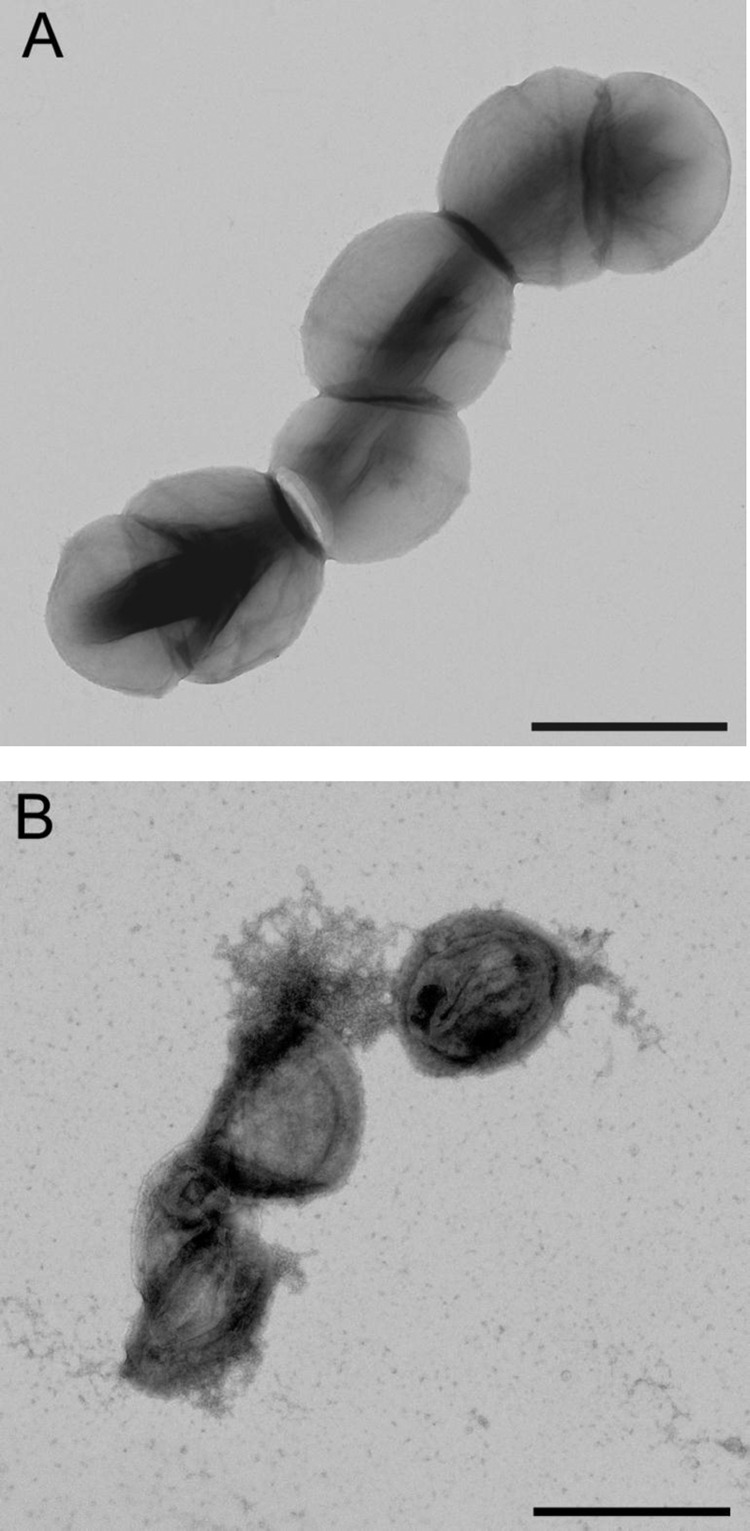
Visualization of PlySK1249 lytic activity by TEM (magnification, ×11,000). (A) S. dysgalactiae SK1249 treated with lysis buffer was not disturbed. (B) S. dysgalactiae SK1249 treated for 15 min with PlySK1249 (final concentration, 3.3 U/ml) showed cytoplasmic extrusion and ghost cells. Scale bar, 1 μm.
PlySK1249 efficacy in a mouse model of GBS-induced bacteremia.
The therapeutic potential of PlySK1249 was tested in a mouse model of GBS-induced bacteremia. To determine the natural history of early infection, three mice were challenged i.p. with 106 CFU of GBS 17-2167 in 100 μl of 0.9% NaCl. Animals were euthanized 1 h postinfection, and various organs were tested for viable GBS. We found >105 CFU/g in the spleen and kidneys (data not shown), demonstrating that extensive bacterial seeding had already occurred in the organs at this time point. On this basis, PlySK1249 treatment was started 1 h after bacterial challenge in all subsequent experiments.
In a first series of therapeutic experiments, mice were infected by i.p. injection of 106 CFU of GBS 17-2167 in 100 μl of 0.9% NaCl and treated i.p. 1 h later with either a single dose of 22.5 mg/kg PlySK1249 in 100 μl lysin buffer (8 animals) or 100 μl of lysin buffer alone (7 animals). Figure 7A shows that PlySK1249 improved survival at 48 h and 72 h in this single-dose experiment. However, the difference from untreated controls was not statistically significant (P > 0.05). Therefore, a second series of experiments was attempted in which the total dosage and the frequency of PlySK1249 injections were increased. Animals received three injections of 45 mg/kg of PlySK1249 at 2, 20, and 24 h after bacterial challenge (Fig. 7B, arrows). This multidose treatment significantly improved survival at any time. Indeed, while only 2/10 (20%) of mice were still alive 5 days after bacterial challenge in the control group, 8/10 (80%) of mice survived during the same period in the PlySK1249 treatment group (P < 0.01) (Fig. 7B).
Fig 7.
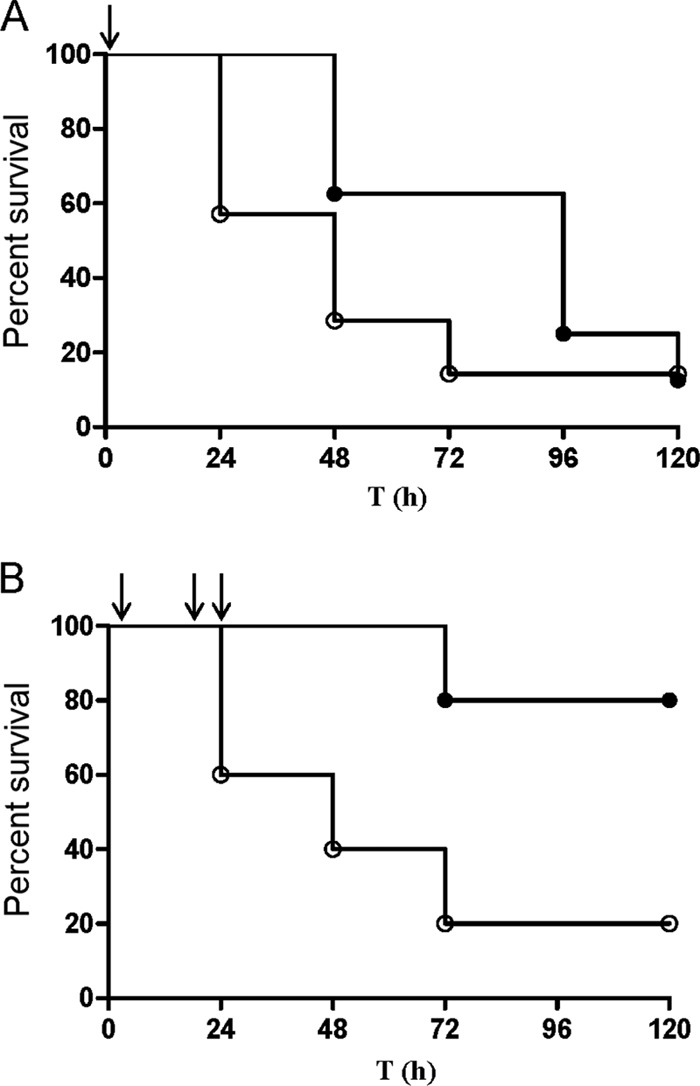
Therapeutic effect of PlySK1249 in a mouse model of GBS-induced bacteremia. (A) CD1 Swiss mice were i.p. injected with 106 CFU of the GBS clinical strain 17-2167. At 1 h postinfection (black arrow), animals received an i.p. injection of 22.5 mg/kg PlySK1249 (closed circles). A control group received lysin buffer only (open circles). (B) Mice were i.p. injected with 106 CFU of the GBS clinical strain 17-2167. At 2, 20, and 24 h postinfection (arrows), animals received an i.p. injection of 45 mg/kg, i.e., a total of 135 mg/kg over the first 24 h, of PlySK1249 (closed circles). A control group received lysin buffer only (open circles). Mice were monitored for survival over a period of 5 days, and results were plotted in Kaplan-Meier survival curves. Survival curves were compared with the log rank (Mantel-Cox) and Gehan-Breslow-Wilcoxon tests.
DISCUSSION
Searching for potential new phage lysins against beta-hemolytic streptococci, we focused on PlySK1249 of S. dysgalactiae SK1249 because of its unusual predicted three-domain structure, in which the binding domain lay between the two catalytic domains. Moreover, PlySK1249 was also interesting from the functional point of view, as it demonstrated cross-lytic activity against several beta-hemolytic streptococci (plus a selection of other streptococci) and could successfully prevent mortality in a mouse model of GBS bacteremia.
First, from the structure point of view, the PlySK1249 lysin was different from the four anti-GBS lysins that have been described so far. These include lysin B30 (isolated from GBS bacteriophage B30) (19), PlyGBS (21), and LambdaSa1 and LambdaSa2, which were isolated from GBS as well (22). Like PlySK1249, lysin B30 and PlyGBS carried two different catalytic domains. However, the primary structure and the organization of these domains were quite different, as shown in Fig. 1. For instance, although the three enzymes contained a CHAP (for “cysteine histidine-dependent amidohydrolase/peptidase”) endopeptidase domain, the second functional domain of PlySK1249 was predicted to be an amidase, whereas that of lysin B30 and PlyGBS was a glycoside hydrolase of the GH25 family (19, 21). Likewise, PlySK1249 was also different from LambdaSa1 and LambdaSa2 lysins, which harbored different catalytic and binding domains (22). Finally, although some hydrolytic functions were shared among these lysin species, their overall amino acid homology was low, i.e., <40%.
Second, from the structure-function point of view, it was interesting that double-layer screening assays detected lytic activities only around E. coli transformed with C-terminally His-tagged pPlySK124928a constructs, not those transformed with N-terminally His-tagged pPlySK124915b constructs. This suggests that either the product of N-terminally His-tagged constructs was not expressed or the His tag positioned at the N-terminal end (but not the C-terminal end) of PlySK1249 abrogated the enzyme lytic activity. Although we did not work out the definitive answer to this question, it was previously shown that His tagging can affect enzymatic functions (23). Thus, it is quite possible that the N-terminal amidase domain of PlySK1249 carried the major part of the enzyme's lytic activity and that this function was affected by the His tag. As a consequence, any further development of this enzyme as a potential antimicrobial agent should take this point into consideration.
Third, from the functional point of view, some salient aspects of the PlySK1249 activity are relevant here, including pH and growth phase dependency. The optimal pH-activity relationship is well known for enzymatic activities in general and lysins in particular. For instance, lysin B30 and PlyGBS are mostly active at pH 4.5 to 6 and 4 to 6, respectively (19, 21), the optimal pH of the major autolysin LytA is around 7 (24), and the optimal pH for PlySK1249 is between 7.0 and 8.5. Beyond academic interest, these differences may have pharmacokinetic/pharmacodynamic (PK/PD) implications the development of these proteins for medical purposes. Indeed, an optimal pH of 4.5 might be more adapted to GBS decolonization of the vagina, where the surrounding pH is acidic, as shown for the PlyGBS lysin (21). Therefore, lysins with neutral optimal pH, such as PlySK1249, might have to be delivered in an appropriate buffering carrier to circumvent this issue. In contrast, PlySK1249 would be more suitable in any other infected area, especially for parenteral therapy of severe GBS infection (although the case of abscess environments, which are slightly acidic, should be tested as well). Regarding growth dependency, lysis induced by PlySK1249 (as determined in vitro) was faster in cells in the exponential growth phase than cells in stationary phase. Similar observations were reported for other lysins, including the B30 lysin (19). A greater susceptibility of the nascent peptidoglycan was also shown for PlyGBS, since lysin-induced extrusion and rupture of the cytoplasmic membrane occurred during cell division in the region of the septum (21). The pertinence of these observations for potential in vivo treatment success is not known. Bacteria at infected sites are likely to span the whole array between vegetative and dormant cells. However, the observation is conceptually similar to that made with the very large majority of antibiotics, which are active on growing but not dormant cells. Yet they do confer treatment success. Thus, the definite proof of efficacy must come from in vivo experimentation.
Toward this end, we tested the therapeutic potential of PlySK249 in a mouse model of GBS-induced bacteremia. While <20% of untreated control animals survived, 80% survival was observed in the animals treated with PlySK1249. Therapeutic efficacy was dose dependent, which is compatible with in vivo observations made with other lysins, such as the anti-pneumococcal lysin Cpl-1 (25). Moreover, although we did not perform sophisticated PK/PD measurements in these experiments, it is worth noting that the specific activity of PlySK1249 (1 U/μg) was in the same range as that of Cpl-1 (15).
Taken together, the present results demonstrate the in vitro efficacy and the proof of concept for successful in vivo therapy against GBS with the new PlySK249 lysin. Moreover, PlySK1249 represent a new lysin with cross-species lytic activity (26) against several beta-hemolytic streptococci, including GBS, and some other streptococcal species. This makes PlySK1249 a good candidate for more thorough preclinical testing. Larger-scale antimicrobial screening should be performed to broaden the information on its spectrum. In addition, more in vivo studies could be carried out in relevant situations before human trials are initiated, for instance, in the veterinarian field against bovine- and swine-associated pathogenic streptococci.
Supplementary Material
ACKNOWLEDGMENTS
We thank Mogens Killian, Marisa Haenni, and John McCullers for having kindly provided us with S. dysgalactiae SK1249, S. suis 19, and S. pneumoniae D39, respectively. We are grateful to José M. Entenza and Shawna E. McCallin for very fruitful discussions and comments on the manuscript.
Footnotes
Published ahead of print 7 October 2013
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AAC.01701-13.
REFERENCES
- 1. Lancefield RC. 1933. A serological differentiation of human and other groups of hemolytic streptococci. J. Exp. Med. 57:571–595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Phares CR, Lynfield R, Farley MM, Mohle-Boetani J, Harrison LH, Petit S, Craig AS, Schaffner W, Zansky SM, Gershman K, Stefonek KR, Albanese BA, Zell ER, Schuchat A, Schrag SJ. 2008. Epidemiology of invasive group B streptococcal disease in the United States, 1999–2005. JAMA 299:2056–2065 [DOI] [PubMed] [Google Scholar]
- 3. Money DM, Dobson S. 2004. The prevention of early-onset neonatal group B streptococcal disease. J. Obstet. Gynaecol. Can. 26:826–840 [DOI] [PubMed] [Google Scholar]
- 4. Muller AE, Oostvogel PM, Steegers EA, Dorr PJ. 2006. Morbidity related to maternal group B streptococcal infections. Acta Obstet. Gynecol. Scand. 85:1027–1037 [DOI] [PubMed] [Google Scholar]
- 5. Jackson LA, Hilsdon R, Farley MM, Harrison LH, Reingold AL, Plikaytis BD, Wenger JD, Schuchat A. 1995. Risk factors for group B streptococcal disease in adults. Ann. Intern. Med. 123:415–420 [DOI] [PubMed] [Google Scholar]
- 6. Yanai H, Hamasaki H, Tsuda N, Adachi H, Yoshikawa N, Moriyama S, Masui Y, Mishima S. 2012. Group B streptococcus infection and diabetes: a review. J. Microbiol. Antimicrob. 4:1–5 [Google Scholar]
- 7. Centers for Disease Control and Prevention 2010. Prevention of perinatal group B streptococcal disease. Morb. Mortal. Wkly. Rep. 59:1–4 [Google Scholar]
- 8. Kimura K, Suzuki S, Wachino J, Kurokawa H, Yamane K, Shibata N, Nagano N, Kato H, Shibayama K, Arakawa Y. 2008. First molecular characterization of group B streptococci with reduced penicillin susceptibility. Antimicrob. Agents Chemother. 52:2890–2897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dahesh S, Hensler ME, Van Sorge NM, Gertz RE, Jr, Schrag S, Nizet V, Beall BW. 2008. Point mutation in the group B streptococcal pbp2x gene conferring decreased susceptibility to beta-lactam antibiotics. Antimicrob. Agents Chemother. 52:2915–2918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sendi P, Zimmerli W. 2012. Comment on: Prosthetic hip joint infection with a Streptococcus agalactiae isolate not susceptible to penicillin G and ceftriaxone. J. Antimicrob. Chemother. 67:1050–1051 [DOI] [PubMed] [Google Scholar]
- 11. Castor ML, Whitney CG, Como-Sabetti K, Facklam RR, Ferrieri P, Bartkus JM, Juni BA, Cieslak PR, Farley MM, Dumas NB, Schrag SJ, Lynfield R. 2008. Antibiotic resistance patterns in invasive group B streptococcal isolates. Infect. Dis. Obstet. Gynecol. 2008:727505. 10.1155/2008/727505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bergseng H, Afset JE, Radtke A, Loeseth K, Lyng RV, Rygg M, Bergh K. 2009. Molecular and phenotypic characterization of invasive group B streptococcus strains from infants in Norway 2006–2007. Clin. Microbiol. Infect. 15:1182–1185 [DOI] [PubMed] [Google Scholar]
- 13. Fischetti VA. 2008. Bacteriophage lysins as effective antibacterials. Curr. Opin. Microbiol. 11:393–400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Schmitz JE, Schuch R, Fischetti VA. 2010. Identifying active phage lysins through functional viral metagenomics. Appl. Environ. Microbiol. 76:7181–7187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Loeffler JM, Djurkovic S, Fischetti VA. 2003. Phage lytic enzyme Cpl-1 as a novel antimicrobial for pneumococcal bacteremia. Infect. Immun. 71:6199–6204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Dell RB, Holleran S, Ramakrishnan R. 2002. Sample size determination. ILAR J. 43:207–213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zhou Y, Liang Y, Lynch KH, Dennis JJ, Wishart DS. 2011. PHAST: a fast phage search tool. Nucleic Acids Res. 39:W347–W352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Frankel MB, Hendrickx AP, Missiakas DM, Schneewind O. 2011. LytN, a murein hydrolase in the cross-wall compartment of Staphylococcus aureus, is involved in proper bacterial growth and envelope assembly. J. Biol. Chem. 286:32593–32605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Pritchard DG, Dong S, Baker JR, Engler JA. 2004. The bifunctional peptidoglycan lysin of Streptococcus agalactiae bacteriophage B30. Microbiology 150:2079–2087 [DOI] [PubMed] [Google Scholar]
- 20. Baker JR, Liu C, Dong S, Pritchard DG. 2006. Endopeptidase and glycosidase activities of the bacteriophage B30 lysin. Appl. Environ. Microbiol. 72:6825–6828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Cheng Q, Nelson D, Zhu S, Fischetti VA. 2005. Removal of group B streptococci colonizing the vagina and oropharynx of mice with a bacteriophage lytic enzyme. Antimicrob. Agents Chemother. 49:111–117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Pritchard DG, Dong S, Kirk MC, Cartee RT, Baker JR. 2007. LambdaSa1 and LambdaSa2 prophage lysins of Streptococcus agalactiae. Appl. Environ. Microbiol. 73:7150–7154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Arnau J, Lauritzen C, Petersen GE, Pedersen J. 2006. Current strategies for the use of affinity tags and tag removal for the purification of recombinant proteins. Protein Expr. Purif 48:1–13 [DOI] [PubMed] [Google Scholar]
- 24. Holtje JV, Tomasz A. 1976. Purification of the pneumococcal N-acetylmuramyl-l-alanine amidase to biochemical homogeneity. J. Biol. Chem. 251:4199–4207 [PubMed] [Google Scholar]
- 25. Entenza JM, Loeffler JM, Grandgirard D, Fischetti VA, Moreillon P. 2005. Therapeutic effects of bacteriophage Cpl-1 lysin against Streptococcus pneumoniae endocarditis in rats. Antimicrob. Agents Chemother. 49:4789–4792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gilmer DB, Schmitz JE, Euler CW, Fischetti VA. 2013. Novel bacteriophage lysin with broad lytic activity protects against mixed infection by Streptococcus pyogenes and methicillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 57:2743–2750 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.