Abstract
Rifampin is a potent inducer of cytochrome P450 (CYP) enzymes and transporters. Drug-drug interactions during tuberculosis treatment are common. Induction by rifapentine and rifabutin is understudied. Rifampin and rifabutin significantly induced CYP3A4 (80-fold and 20-fold, respectively) in primary human hepatocytes. The induction was concentration dependent. Rifapentine induced CYP3A4 in hepatocytes from 3 of 6 donors. Data were also generated for ABCB1, ABCC1, ABCC2, organic anion-transporting polypeptide 1B1 (OATP1B1), and OATP1B3. This work serves as a basis for further study of the extent to which rifamycins induce key metabolism and transporter genes.
TEXT
Tuberculosis is a major global health problem (1). Effective short-course therapy lasts for 6 months but requires rifampin, and clinically significant drug-drug interactions are common due to induction of cytochrome P450 3A4 (CYP3A4) and key drug transporters included herein (1–3). Hence, antiretroviral coadministration with tuberculosis treatment is particularly challenging. Besides CYP3A4, drug transporters can significantly alter the absorption and distribution of drugs. Many compounds used in human immunodeficiency virus treatment, particularly the protease inhibitors and nucleoside reverse transcriptase inhibitors, are transported by proteins such as ABCB1 (3), ABCC2 (4), organic anion-transporting polypeptide 1B1 (OATP1B1), and OATP1B3 (5, 6). The genes encoding these proteins are influential in the safety, efficacy, and disposition of many drugs. For example, the induction of ABCB1 by rifampin decreases the area under the curve (AUC) of efavirenz by 22% (2). Rifabutin is considered a less-potent inducer and is often used in place of rifampin for patients receiving antiretroviral drugs for human immunodeficiency virus to reduce the risk of drug interactions (7–9). The substitution of rifapentine for rifampin may reduce the treatment duration required for cure, but the induction potential of rifapentine is comparatively understudied (2, 10). The sterilizing activity of rifapentine is dose dependent in an established mouse model of tuberculosis, with eradication possible in 3 months or less when high-dose rifapentine is substituted for rifampin in a multidrug treatment regimen (11, 12). However, dose increases resulted in less-than-dose-proportional increases in rifapentine exposures (10, 13). In addition, the mean area under the concentration-time curve of oral midazolam, a CYP3A4 probe, decreased by 75% when coadministered with rifampin, compared to 92% when coadministered with rifapentine, each given at 10 mg/kg of body weight daily (14). We evaluated the in vitro induction of CYP3A4 and transporters by rifampin, rifabutin, and rifapentine in primary human hepatocyte samples from six donors. Other studies have previously investigated the induction of CYP activity by rifampin, rifabutin, and rifapentine (9) and the mRNA expression of drug transporters induced by rifampin (3, 6), but no studies have comprehensively compared the mRNA induction of CYPs and transporters in primary human hepatocytes with all 3 compounds in parallel.
Cryopreserved hepatocyte recovery medium (CHRM medium), Williams' E medium, plating and supplement medium, cryopreserved human hepatocytes, plating cocktail, maintenance cocktail, gene expression assays, and 96-well collagen-coated plates were purchased from Life Technologies (Paisley, Scotland, United Kingdom). All other chemicals were purchased from Sigma-Aldrich (Poole, Dorset, United Kingdom), unless otherwise indicated.
Rifampin, rifabutin, and rifapentine were prepared as 10 mM stocks in methanol and further diluted in hepatocyte medium to the required concentrations. Cryopreserved human hepatocytes (from 6 human donors) were thawed according to the manufacturer's instructions (15) and resuspended in William's E medium supplemented with plating cocktail (1 μM dexamethasone, a 1% solution of penicillin-streptomycin, 4 μg/ml insulin, 5% fetal bovine serum, 2 mM GlutaMAX, and 15 mM HEPES [Life Technologies, Paisley, United Kingdom]). Cell numbers and viability were assessed using trypan blue exclusion. Cells were seeded in 24-well plates precoated with collagen at a density of 2 × 105 cells per well and were incubated for 12 h at 37°C with 5% CO2 and 95% humidity. The medium was replaced with Williams' E medium supplemented with maintenance cocktail (0.1 μM dexamethasone, a 0.5% solution of penicillin-streptomycin, 2 mM GlutaMAX, 15 mM HEPES, 6.25 μg/ml human recombinant insulin, 6 μg/ml human transferrin, 6 μg/ml selenous acid, 1.25 μg/ml bovine serum albumin, and 5.35 μg/ml linoleic acid [Life Technologies, Paisley, United Kingdom]). Hepatocytes were incubated with rifampin, rifabutin, or rifapentine at concentrations spanning the therapeutic range (0.5, 5, and 10 μM) for 24 h; for rifampin, these concentrations have previously been shown to cause no toxicity (3). Control cells (0 μM) contained the same volume of methanol and hepatocyte medium. RNA was extracted using TRIzol reagent and reverse transcribed using the standard methodology recommended by Life Technologies. Gene expression analysis was conducted for OATP1B1, OATP1B3, ABCB1, ABCC1, ABCC2, and CYP3A4 by real-time PCR. Gene expression was normalized to that of the housekeeping gene, glyceraldehyde-3-phosphate dehydrogenase (GAPDH), and compared to that of the control (0 μM) using the comparative CT method (CT = 2−ΔΔCT; CT, cycle number at which the fluorescence in the reaction crosses the preset arbitrary threshold; ΔCT, difference between the CT target and reference; ΔΔCT, difference between the ΔCT of the test and the ΔCT of the preassigned control). The normality of the data was assessed using a Shapiro-Wilk test, and statistical analysis conducted using the paired t test or Wilcoxon signed-rank test for normally or nonnormally distributed data, respectively.
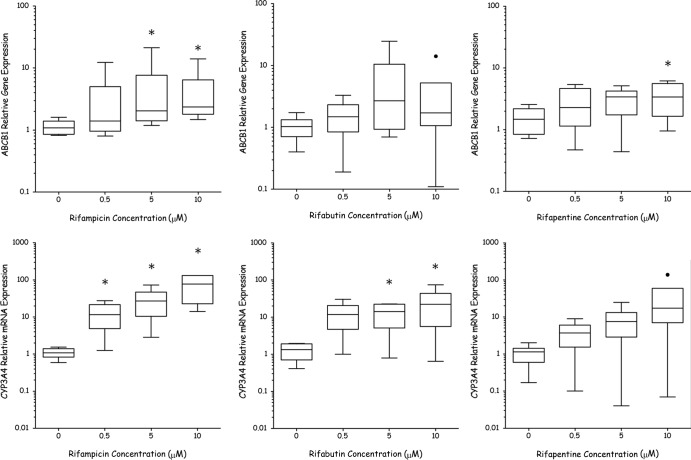
The effects of rifampin, rifabutin, and rifapentine on ABCB1 and CYP3A4 mRNA are shown in Fig. 1. The fold changes in gene expression for OATP1B1, OATP1B3, ABCC1, and ABCC2 when treated with rifampin, rifabutin, and rifapentine are shown in Table 1. Rifampin elicited significant upregulation of ABCB1 from 5 μM and of CYP3A4 from 0.5 μM. Concentration-dependent induction was observed for both genes, with the greatest induction observed at 10 μM (80-fold [P = 0.03] and 5-fold [P = 0.03] for CYP3A4 and ABCB1, respectively). Rifampin significantly upregulated OATP1B1 (2-fold [P = 0.03]) and ABCC2 (3-fold [P = 0.03]) at 10 μM (similar to the results of Haenisch et al., 2011). Rifabutin elicited a 20-fold upregulation in CYP3A4 gene expression (P = 0.05) and a 4-fold upregulation of OATP1B3 (P = 0.04) at 5 μM.
Fig 1.
Relative gene expression of cytochrome P450 isoenzyme 3A4 and ABCB1 in primary hepatocytes when incubated with rifampin (RIF), rifabutin (RBT), or rifapentine (RPT) at 0, 0.5, 5, and 10 μM. Data were normalized to results for GAPDH housekeeping gene and for control primary hepatocytes (0 μM) using the comparative CT method (CT = 2−ΔΔCT). Tukey box plot represents the means and interquartile ranges (IQR) (n = 6 donors completed in triplicate), with boxes showing IQR and whiskers representing <1.5 × IQR. Data outside 1.5 × IQR are labeled as outliers (•). An asterisk indicates a significant difference from the control (0 μM) by paired t test or Wilcoxon signed-rank test (P < 0.05).
Table 1.
Fold change in gene expression of hepatic influx and efflux transporters when hepatocytes were incubated with rifampin, rifabutin, or rifapentine at various concentrations
| Compound | Concn (μM) | Mean (range) fold change in mRNA expression compared to result for 0 μM treatmenta |
|||
|---|---|---|---|---|---|
| OATP1B1 | OATP1B3 | ABCC1 | ABCC2 | ||
| Rifampin | 0.5 | 5.19 (0.30–24.31) | 7.92 (0.73–29.79) | 2.15 (1.04–4.46) | 0.93 (0.28–1.44) |
| 5.0 | 1.51 (0.36–3.95) | 5.54 (1.02–19.89) | 1.53 (0.13–8.79) | 1.06 (0.77–1.83) | |
| 10.0 | 1.91 (0.55–4.77)* | 1.09 (0.41–2.10) | 1.78 (0.17–3.70) | 2.39 (0.49–7.31)* | |
| Rifabutin | 0.5 | 0.63 (0.11–1.69) | 2.00 (0.25–5.99) | 0.78 (0.35–1.91) | 1.19 (0.25–4.26) |
| 5.0 | 0.94 (0.13–2.95) | 3.58 (1.11–9.15)* | 2.69 (0.14–8.06) | 1.95 (0.22–12.01) | |
| 10.0 | 0.81 (0.38–2.05) | 3.06 (0.65–5.40) | 1.45 (0.34–2.53) | 1.89 (0.17–7.08) | |
| Rifapentine | 0.5 | 1.40 (0.52–3.08) | 1.46 (0.08–5.29) | 1.40 (0.20–4.25) | 1.19 (0.72–2.50) |
| 5.0 | 0.86 (0.41–1.48) | 1.07 (0.18–4.46) | 1.24 (0.10–2.01) | 1.52 (1.00–5.48) | |
| 10.0 | 1.37 (0.32–3.12) | 1.30 (0.11–6.60) | 2.23 (0.10–5.08) | 1.95 (0.65–3.93) | |
Change in relative gene expression of hepatic influx and efflux transporters when incubated with rifampin, rifabutin, or rifapentine at 0.5, 5, or 10 μM compared to the expression in control primary hepatocytes with no drug added. An asterisk indicates a significant difference from the control (0 μM) by paired t test or Wilcoxon signed-rank test (P < 0.05). OATP, organic anion-transporting polypeptide; ABC, ATP-binding cassette transporter.
When analyzed as the average of the results for the hepatocytes from the 6 donors, rifapentine did not significantly induce the expression of CYP3A4, but significant induction was observed in hepatocytes from 3 of 6 donors when analyzed individually. ABCB1 was the only gene significantly induced by rifapentine (4-fold [P = 0.04]) at 10 μM. The results herein suggest the hierarchy of the rifamycins' potency as CYP3A4 inducers to be rifampin > rifabutin > rifapentine, while previous studies found that rifapentine was more potent than rifabutin (8). However, both studies agree that rifampin is the most-potent inducer of CYP3A4.
Consistent with all primary hepatocyte studies, great interdonor variability was observed (16). However, concentration-dependent responses were seen for most genes (including CYP3A4). This work highlights the extent to which rifampin induces CYP3A4-mediated metabolism and the transport of compounds compared to the induction by rifapentine, a potential alternative. Larger increases in CYP3A4 mRNA expression were observed when hepatocytes were treated with rifampin than with rifabutin and rifapentine at the same micromolar concentration. In contrast to the data herein, a study with healthy volunteers found that the AUC of midazolam was decreased 17% more when coadministered with rifapentine than with rifampin (14). However, at standard daily doses, the average rifampin, rifabutin, and rifapentine concentrations are approximately 2.3 μM, 0.3 μM, and 15.7 μM, respectively (8, 17, 18). Of particular note, the plasma concentrations of rifabutin in patients are comparatively low, and this may help rationalize the limited induction by rifabutin seen clinically. Thus, there is a spectrum of levels of induction by rifampin, rifabutin, and rifapentine, but the data should be interpreted in the context of differences in plasma concentrations seen clinically. The data should also be interpreted in the context that concentrations in hepatocytes and/or gut may exceed those found in the plasma of patients. The unbound plasma concentration is often used to estimate clinical drug interactions. However, protein binding can be dependent on health status. For example, rifampin is 87 to 91% bound in healthy individuals but 84 to 88% bound in tuberculosis-infected individuals (19). Nonetheless, it should be noted that several previous investigations have shown that effects at the mRNA level are not always translated to activity (3, 7, 20).
An ATP binding cassette transporter (ABC), ABCB1, is a transmembrane efflux protein responsible for the removal of a broad range of bile acids, lipids, and xenobiotics from hepatocytes (21). Rifampin and rifapentine significantly induced ABCB1; hence, an enhanced clearance of coadministered substrates may be predicted. Also, rifampin, rifapentine, ethambutol, and isoniazid are all substrates of ABCB1 (7, 22, 23), suggesting a potential role in autoinduction and potential effects between drugs within a Mycobacterium tuberculosis treatment regimen. A significant upregulation of OATP1B1 mRNA was also observed with 10 μM rifampin. Given that rifampin is in itself a substrate for OATP1B1 (24) and that polymorphisms within the SLCO1B1 gene affect rifampin pharmacokinetics (25), these data indicate an involvement of OATP1B1 in the reported rifampin autoinduction (26).
Current clinical trials are investigating high-dose daily rifapentine (Tuberculosis Trials Consortium Study 29X [27]) and high-dose rifampin (PanACEA Consortium [28]) as potential regimens to shorten tuberculosis treatment. In vitro results suggest that increasing the concentration of rifapentine may lead to clinically relevant drug-drug interactions mediated through ABCB1. Boeree et al. (28) found that 35 mg/kg of rifampin daily was safe and well tolerated over 14 days and that early bactericidal activity increased with increasing dose, with no apparent plateau. Trials are now being planned to assess the activity of high-dose rifampin over 8 weeks. Our data suggest that concentration-dependent induction should be considered when interpreting the results of ongoing trials of higher-dose rifampin and rifapentine, since it cannot be assumed that maximum autoinduction is achieved at standard doses. With the absence of an apparent ceiling as drug exposure increases (28), rifampin doses above those used in the clinic today may lead to significant and highly variable drug-drug interactions, which is of considerable clinical concern.
ACKNOWLEDGMENTS
This work was funded by a supplement from the Division of AIDS (DAIDS) to grant 5U01AI069465. B.W. is funded by AstraZeneca (grant AZ217862) and the Biotechnology and Biosciences Research Council (grant BXR10976). K.D. is supported by NIH (grant K23AI080842).
We thank LifeTechnologies, Paisley, United Kingdom, for their support with access to primary human hepatocytes.
The authors report no conflicts of interest.
Footnotes
Published ahead of print 23 September 2013
REFERENCES
- 1. World Health Organization 2012. Global tuberculosis report 2012. World Health Organization, Geneva, Switzerland: http://www.who.int/tb/publications/global_report/gtbr12_main.pdf [Google Scholar]
- 2. Niemi M, Backman JT, Fromm MF, Neuvonen PJ, Kivisto KT. 2003. Pharmacokinetic interactions with rifampicin: clinical relevance. Clin. Pharmacokinet. 42:819–850 [DOI] [PubMed] [Google Scholar]
- 3. Martin P, Riley R, Back DJ, Owen A. 2008. Comparison of the induction profile for drug disposition proteins by typical nuclear receptor activators in human hepatic and intestinal cells. Br. J. Pharmacol. 153:805–819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Huisman MT, Smit JW, Crommentuyn KM, Zelcer N, Wiltshire HR, Beijnen JH, Schinkel AH. 2002. Multidrug resistance protein 2 (MRP2) transports HIV protease inhibitors, and transport can be enhanced by other drugs. AIDS 16:2295–2301 [DOI] [PubMed] [Google Scholar]
- 5. Su Y, Zhang X, Sinko PJ. 2004. Human organic anion-transporting polypeptide OATP-A (SLC21A3) acts in concert with P-glycoprotein and multidrug resistance protein 2 in the vectorial transport of saquinavir in Hep G2 cells. Mol. Pharm. 1:49–56 [DOI] [PubMed] [Google Scholar]
- 6. Dixit V, Hariparsad N, Li F, Desai P, Thummel KE, Unadkat JD. 2007. Cytochrome P450 enzymes and transporters induced by anti-human immunodeficiency virus protease inhibitors in human hepatocytes: implications for predicting clinical drug interactions. Drug Metab. Dispos. 35:1853–1859 [DOI] [PubMed] [Google Scholar]
- 7. Baciewicz AM, Chrisman CR, Finch CK, Self TH. 2013. Update on rifampin, rifabutin, and rifapentine drug interactions. Curr. Med. Res. Opin. 29:1–12 [DOI] [PubMed] [Google Scholar]
- 8. Burman WJ, Gallicano K, Peloquin C. 2001. Comparative pharmacokinetics and pharmacodynamics of the rifamycin antibacterials. Clin. Pharmacokinet. 40:327–341 [DOI] [PubMed] [Google Scholar]
- 9. Li AP, Reith MK, Rasmussen A, Gorski JC, Hall SD, Xu L, Kaminski DL, Cheng LK. 1997. Primary human hepatocytes as a tool for the evaluation of structure-activity relationship in cytochrome P450 induction potential of xenobiotics: evaluation of rifampin, rifapentine and rifabutin. Chem. Biol. Interact. 107:17–30 [DOI] [PubMed] [Google Scholar]
- 10. Dooley K, Flexner C, Hackman J, Peloquin CA, Nuermberger E, Chaisson RE, Dorman SE. 2008. Repeated administration of high-dose intermittent rifapentine reduces rifapentine and moxifloxacin plasma concentrations. Antimicrob. Agents Chemother. 52:4037–4042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Rosenthal IM, Tasneen R, Peloquin CA, Zhang M, Almeida D, Mdluli KE, Karakousis PC, Grosset JH, Nuermberger EL. 2012. Dose-ranging comparison of rifampin and rifapentine in two pathologically distinct murine models of tuberculosis. Antimicrob. Agents Chemother. 56:4331–4340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Rosenthal IM, Zhang M, Williams KN, Peloquin CA, Tyagi S, Vernon AA, Bishai WR, Chaisson RE, Grosset JH, Nuermberger EL. 2007. Daily dosing of rifapentine cures tuberculosis in three months or less in the murine model. PLoS Med. 4:e344. 10.1371/journal.pmed.0040344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sterling TR, Villarino ME, Borisov AS, Shang N, Gordin F, Bliven-Sizemore E, Hackman J, Hamilton CD, Menzies D, Kerrigan A, Weis SE, Weiner M, Wing D, Conde MB, Bozeman L, Horsburgh CR, Chaisson RE. 2011. Three months of rifapentine and isoniazid for latent tuberculosis infection. N. Engl. J. Med. 365:2155–2166 [DOI] [PubMed] [Google Scholar]
- 14. Dooley KE, Bliven-Sizemore EE, Weiner M, Lu Y, Nuermberger EL, Hubbard WC, Fuchs EJ, Melia MT, Burman WJ, Dorman SE. 2012. Safety and pharmacokinetics of escalating daily doses of the antituberculosis drug rifapentine in healthy volunteers. Clin. Pharmacol. Ther. 91:881–888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Invitrogen 2012. Thawing and plating hepatocytes protocol. Invitrogen-Life Technologies, Carlsbad, CA. http://www.invitrogen.com/site/us/en/home/References/protocols/drug-discovery/adme-tox-protocols/thawing-and-plating-hepatocytes-protocol.html#4
- 16. Hewitt NJ, Lechon MJ, Houston JB, Hallifax D, Brown HS, Maurel P, Kenna JG, Gustavsson L, Lohmann C, Skonberg C, Guillouzo A, Tuschl G, Li AP, LeCluyse E, Groothuis GM, Hengstler JG. 2007. Primary hepatocytes: current understanding of the regulation of metabolic enzymes and transporter proteins, and pharmaceutical practice for the use of hepatocytes in metabolism, enzyme induction, transporter, clearance, and hepatotoxicity studies. Drug Metab. Rev. 39:159–234 [DOI] [PubMed] [Google Scholar]
- 17. Acocella G. 1978. Clinical pharmacokinetics of rifampicin. Clin. Pharmacokinet. 3:108–127 [DOI] [PubMed] [Google Scholar]
- 18. Skinner MH, Blaschke TF. 1995. Clinical pharmacokinetics of rifabutin. Clin. Pharmacokinet. 28:115–125 [DOI] [PubMed] [Google Scholar]
- 19. Boman G, Ringberger VA. 1974. Binding of rifampicin by human plasma proteins. Eur. J. Clin. Pharmacol. 7:369–373 [DOI] [PubMed] [Google Scholar]
- 20. Ohtsuki S, Schaefer O, Kawakami H, Inoue T, Liehner S, Saito A, Ishiguro N, Kishimoto W, Ludwig-Schwellinger E, Ebner T, Terasaki T. 2012. Simultaneous absolute protein quantification of transporters, cytochromes P450, and UDP-glucuronosyltransferases as a novel approach for the characterization of individual human liver: comparison with mRNA levels and activities. Drug Metab. Dispos. 40:83–92 [DOI] [PubMed] [Google Scholar]
- 21. Shugarts S, Benet LZ. 2009. The role of transporters in the pharmacokinetics of orally administered drugs. Pharm. Res. 26:2039–2054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Yimer G, Ueda N, Habtewold A, Amogne W, Suda A, Riedel KD, Burhenne J, Aderaye G, Lindquist L, Makonnen E, Aklillu E. 2011. Pharmacogenetic and pharmacokinetic biomarker for efavirenz based ARV and rifampicin based anti-TB drug induced liver injury in TB-HIV infected patients. PLoS One 6:e27810. 10.1371/journal.pone.0027810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Crews KR, Hicks JK, Pui CH, Relling MV, Evans WE. 2012. Pharmacogenomics and individualized medicine: translating science into practice. Clin. Pharmacol. Ther. 92:467–475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Choi MK, Jin QR, Choi YL, Ahn SH, Bae MA, Song IS. 2011. Inhibitory effects of ketoconazole and rifampin on OAT1 and OATP1B1 transport activities: considerations on drug-drug interactions. Biopharmaceut. Drug Dispos. 32:175–184 [DOI] [PubMed] [Google Scholar]
- 25. Chigutsa E, Visser ME, Swart EC, Denti P, Pushpakom S, Egan D, Holford NH, Smith PJ, Maartens G, Owen A, McIlleron H. 2011. The SLCO1B1 rs4149032 polymorphism is highly prevalent in South Africans and is associated with reduced rifampin concentrations: dosing implications. Antimicrob. Agents Chemother. 55:4122–4127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Smythe W, Khandelwal A, Merle C, Rustomjee R, Gninafon M, Bocar Lo M, Sow OB, Olliaro PL, Lienhardt C, Horton J, Smith P, McIlleron H, Simonsson USH. 2012. A semimechanistic pharmacokinetic-enzyme turnover model for rifampin autoinduction in adult tuberculosis patients. Antimicrob. Agents Chemother. 56:2091–2098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Jindani AMH, Charalambous S, Mungofa S, Zizhou S, van Dijk J, Shepherd J, Phillips P P, Nunn A, Mitchison D, Trial Team RIFAQUIN 2013. A multicentre randomized clinical trial to evaluate high-dose rifapentine with a quinolone for treatment of pulmonary TB: The RIFAQUIN Trial, abstr 147LB 20th Conf. Retroviruses Opportun. Infect. (CROI), Atlanta, GA, 3 to 6 March 2013 [Google Scholar]
- 28. Boeree MAD, Dawson R, Venter A, du Bois J, Narunsky K, Hoelscher M, Gillespie S, Phillips P, Aarnoutse R. 2013. What is the “right” dose of rifampin?, abstr 148LB 20th Conf. Retroviruses Opportun. Infect. (CROI), Atlanta, GA, 3 to 6 March 2013 [Google Scholar]
- 29. Haenisch S, Laechelt S, Bruckmueller H, Werk A, Noack A, Bruhn O, Remmler C, Casscorbi I. 2011. Down-regulation of ATP-binding cassette C2 protein expression in HepG2 cells after rifampicin treatment is mediated by microRNA-379. Mol. Pharmacol. 80:314–320 [DOI] [PubMed] [Google Scholar]