Abstract
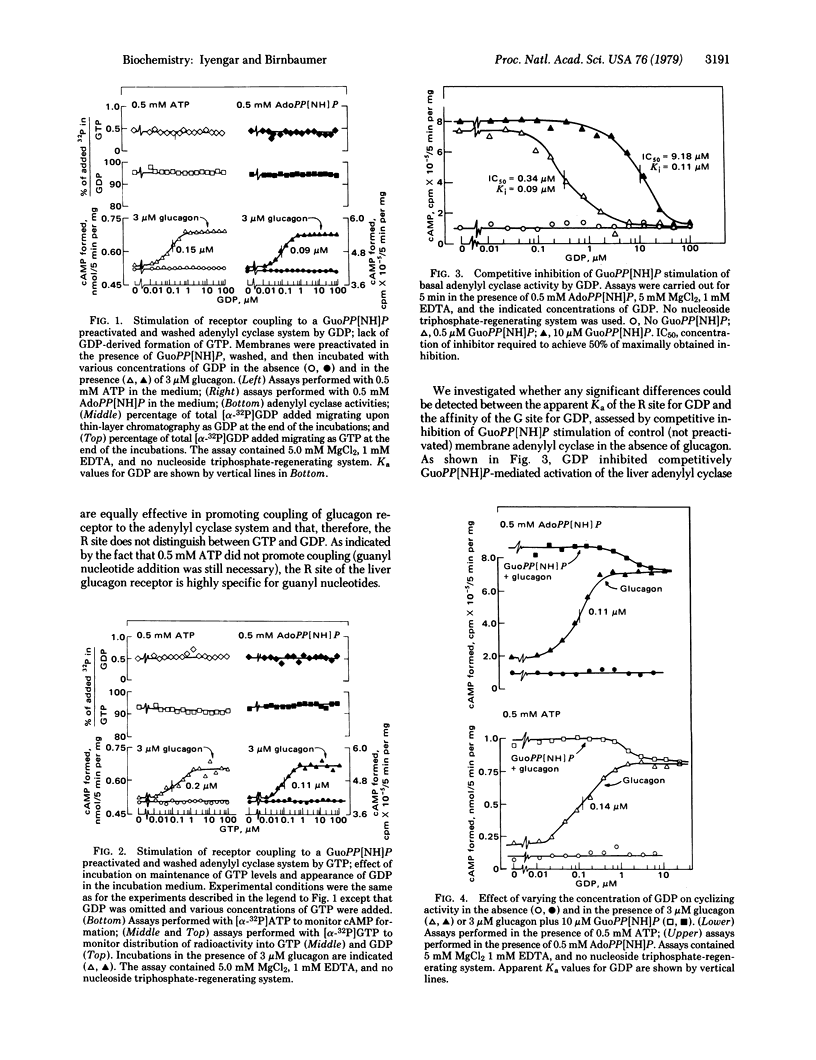
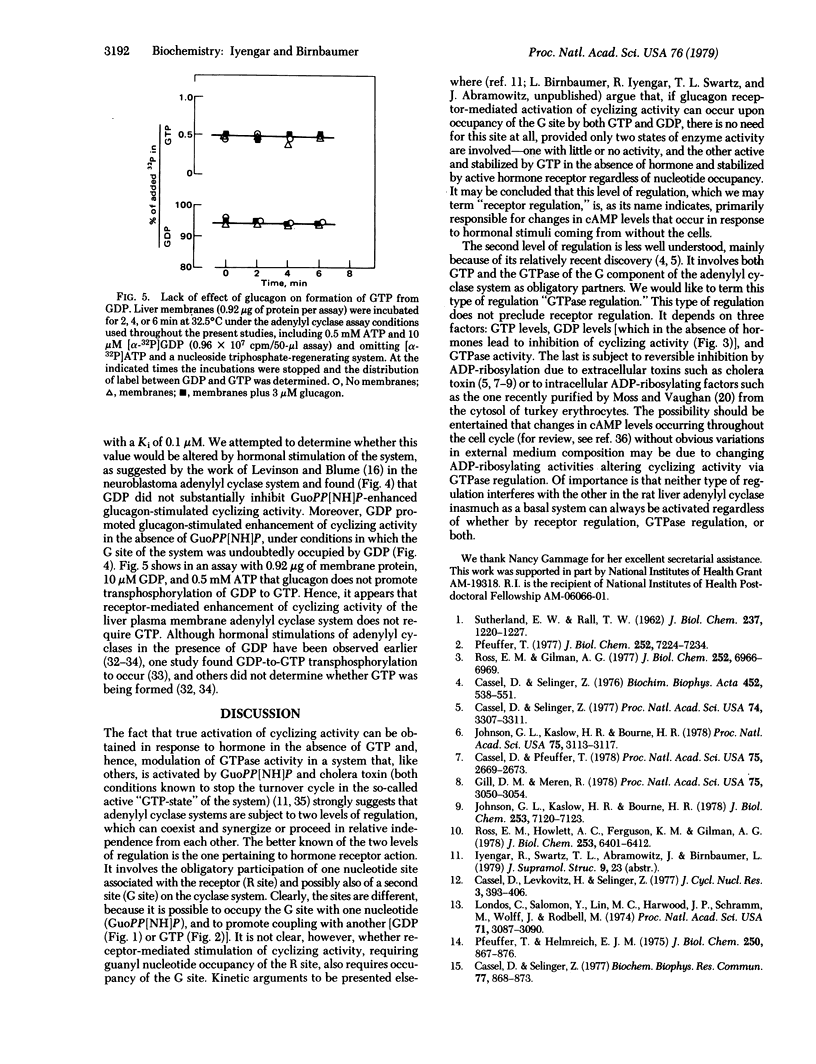
In rat liver plasma membranes preactivated with guanosine 5'-[beta,gamma-imido[triphosphate (GuoPP[NH]P), GDP promoted coupling of occupied glucagon receptor to adenylyl cyclase [adenylate cyclase; ATP, pyrophosphate-lyase (cyclizing), EC 4.6.1.1] with an apparent association constant Ka of 0.1-0.15 microM. The apparent Ka for the same effect of GTP was 0.2 microM. The effect of GDP was shown not to be due to GTP formed by putative transphosphorylation reaction(s) when ATP was present in the assay as substrate. In membranes not preactivated with GuoPP[NH]P, GDP both competitively inhibited GuoPP[NH]P stimulation of adenylyl cyclase (Ki 0.10 microM) and supported stimulation of cyclizing activity (apparent Ka 0.10 microM) by glucagon. These effects of GDP occurred in the absence of added GTP and in the absence of sufficient formation of GTP by putative transphosphorylation reaction(s) to account for them. It is concluded that two levels of regulation of liver adenylyl cyclase (cyclizing) activity must exit. One level is termed "receptor regulation"; it depends on occupancy of a receptor-related R site by nucleotide and is specific for either GDP or GTP. The second level of regulation is termed "GTPase regulation"; it is inhibited by GDP, depends on both GTP and GTPase, and accounts for activation of cyclizing activity by nonhydrolyzable analogs of GTP. The data suggest that both levels of regulation coexist and may synergize, one mediating responses to stimuli external to the cell (receptor regulation) and the other mediating stimuli of intracellular origin (GTPase regulation).
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Birnbaumer L., Torres H. N., Flawiá M. M., Fricke R. F. Improved methods for determination of guanylyl cyclase activity and synthesis of [alpha-32P]GTP. Anal Biochem. 1979 Feb;93(1):124–133. [PubMed] [Google Scholar]
- Birnbaumer L., Yang P. C., Hunzicker-Dunn M., Bockaert J., Duran J. M. Adenylyl cyclase activities in ovarian tissues. I. Homogenization and conditions of assay in graafian follicles and corpora lutea of rabbits, rats, and pigs: regulation by ATP, and some comparative properties. Endocrinology. 1976 Jul;99(1):163–184. doi: 10.1210/endo-99-1-163. [DOI] [PubMed] [Google Scholar]
- Birnbaumer L., Yang P. C. Studies on receptor-mediated activation of adenylyl cyclases. III. Regulation by purine nucleotides of the activation of adenylyl cyclases from target organs for prostaglandins, luteinizing hormone, neurohypophyseal hormones and catecholamines. Tissue- and hormone-dependent variations. J Biol Chem. 1974 Dec 25;249(24):7867–7873. [PubMed] [Google Scholar]
- Bockaert J., Hunzicker-Dunn M., Birnbaumer L. Hormone-stimulated desensitization of hormone-dependent adenylyl cyclase. Dual action of luteninizing hormone on pig graafian follicle membranes. J Biol Chem. 1976 May 10;251(9):2653–2663. [PubMed] [Google Scholar]
- Cassel D., Levkovitz H., Selinger Z. The regulatory GTPase cycle of turkey erythrocyte adenylate cyclase. J Cyclic Nucleotide Res. 1977 Dec;3(6):393–406. [PubMed] [Google Scholar]
- Cassel D., Pfeuffer T. Mechanism of cholera toxin action: covalent modification of the guanyl nucleotide-binding protein of the adenylate cyclase system. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2669–2673. doi: 10.1073/pnas.75.6.2669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassel D., Selinger Z. Activation of turkey erythrocyte adenylate cyclase and blocking of the catecholamine-stimulated GTPase by guanosine 5'-(gamma-thio) triphosphate. Biochem Biophys Res Commun. 1977 Aug 8;77(3):868–873. doi: 10.1016/s0006-291x(77)80058-2. [DOI] [PubMed] [Google Scholar]
- Cassel D., Selinger Z. Catecholamine-stimulated GTPase activity in turkey erythrocyte membranes. Biochim Biophys Acta. 1976 Dec 8;452(2):538–551. doi: 10.1016/0005-2744(76)90206-0. [DOI] [PubMed] [Google Scholar]
- Cassel D., Selinger Z. Mechanism of adenylate cyclase activation by cholera toxin: inhibition of GTP hydrolysis at the regulatory site. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3307–3311. doi: 10.1073/pnas.74.8.3307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassel D., Selinger Z. Mechanism of adenylate cyclase activation through the beta-adrenergic receptor: catecholamine-induced displacement of bound GDP by GTP. Proc Natl Acad Sci U S A. 1978 Sep;75(9):4155–4159. doi: 10.1073/pnas.75.9.4155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark R. B. Endogenous GTP and the regulation of epinephrine stimulation of adenylate cyclase. J Cyclic Nucleotide Res. 1978 Apr;4(2):71–85. [PubMed] [Google Scholar]
- Friedman D. L., Johnson R. A., Zeilig C. E. The role of cyclic nucleotides in the cell cycle. Adv Cyclic Nucleotide Res. 1976;7:69–114. [PubMed] [Google Scholar]
- Gill D. M., Meren R. ADP-ribosylation of membrane proteins catalyzed by cholera toxin: basis of the activation of adenylate cyclase. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3050–3054. doi: 10.1073/pnas.75.7.3050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanoune J., Lacombe M. L., Pecker F. The epinephrine-sensitive adenylate cyclase of rat liver plasma membranes. Role of guanyl nucleotides. J Biol Chem. 1975 Jun 25;250(12):4569–4574. [PubMed] [Google Scholar]
- Iyengar R., Swartz T. L., Birnbaumer L. Coupling of glucagon receptor to adenylyl cyclase. Requirement of a receptor-related guanyl nucleotide binding site for coupling of receptor to the enzyme. J Biol Chem. 1979 Feb 25;254(4):1119–1123. [PubMed] [Google Scholar]
- Johnson G. L., Kaslow H. R., Bourne H. R. Genetic evidence that cholera toxin substrates are regulatory components of adenylate cyclase. J Biol Chem. 1978 Oct 25;253(20):7120–7123. [PubMed] [Google Scholar]
- Johnson G. L., Kaslow H. R., Bourne H. R. Reconstitution of cholera toxin-activated adenylate cyclase. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3113–3117. doi: 10.1073/pnas.75.7.3113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura N., Nagata N. The requirement of guanine nucleotides for glucagon stimulation of adenylate cyclase in rat liver plasma membranes. J Biol Chem. 1977 Jun 10;252(11):3829–3835. [PubMed] [Google Scholar]
- Levinson S. L., Blume A. J. Altered guanine nucleotide hydrolysis as basis for increased adenylate cyclase activity after cholera toxin treatment. J Biol Chem. 1977 Jun 10;252(11):3766–3774. [PubMed] [Google Scholar]
- Lin M. C., Welton A. F., Berman M. F. Essential role of GTP in the expression of adenylate cyclase activity after cholera toxin treatment. J Cyclic Nucleotide Res. 1978 Jun;4(3):159–168. [PubMed] [Google Scholar]
- Londos C., Salomon Y., Lin M. C., Harwood J. P., Schramm M., Wolff J., Rodbell M. 5'-Guanylylimidodiphosphate, a potent activator of adenylate cyclase systems in eukaryotic cells. Proc Natl Acad Sci U S A. 1974 Aug;71(8):3087–3090. doi: 10.1073/pnas.71.8.3087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss J., Vaughan M. Isolation of an avian erythrocyte protein possessing ADP-ribosyltransferase activity and capable of activating adenylate cyclase. Proc Natl Acad Sci U S A. 1978 Aug;75(8):3621–3624. doi: 10.1073/pnas.75.8.3621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeuffer T. GTP-binding proteins in membranes and the control of adenylate cyclase activity. J Biol Chem. 1977 Oct 25;252(20):7224–7234. [PubMed] [Google Scholar]
- Pfeuffer T., Helmreich E. J. Activation of pigeon erythrocyte membrane adenylate cyclase by guanylnucleotide analogues and separation of a nucleotide binding protein. J Biol Chem. 1975 Feb 10;250(3):867–876. [PubMed] [Google Scholar]
- Pohl S. L., Birnbaumer L., Rodbell M. The glucagon-sensitive adenyl cyclase system in plasma membranes of rat liver. I. Properties. J Biol Chem. 1971 Mar 25;246(6):1849–1856. [PubMed] [Google Scholar]
- Rendell M. S., Rodbell M., Berman M. Activation of hepatic adenylate cyclase by guanyl nucleotides. Modeling of the transient kinetics suggests an "excited" state of GTPase is a control component of the system. J Biol Chem. 1977 Nov 25;252(22):7909–7912. [PubMed] [Google Scholar]
- Rodbell M., Krans H. M., Pohl S. L., Birnbaumer L. The glucagon-sensitive adenyl cyclase system in plasma membranes of rat liver. IV. Effects of guanylnucleotides on binding of 125I-glucagon. J Biol Chem. 1971 Mar 25;246(6):1872–1876. [PubMed] [Google Scholar]
- Ross E. M., Gilman A. G. Resolution of some components of adenylate cyclase necessary for catalytic activity. J Biol Chem. 1977 Oct 25;252(20):6966–6969. [PubMed] [Google Scholar]
- Ross E. M., Howlett A. C., Ferguson K. M., Gilman A. G. Reconstitution of hormone-sensitive adenylate cyclase activity with resolved components of the enzyme. J Biol Chem. 1978 Sep 25;253(18):6401–6412. [PubMed] [Google Scholar]
- Ross E. M., Maguire M. E., Sturgill T. W., Biltonen R. L., Gilman A. G. Relationship between the beta-adrenergic receptor and adenylate cyclase. J Biol Chem. 1977 Aug 25;252(16):5761–5775. [PubMed] [Google Scholar]
- SUTHERLAND E. W., RALL T. W., MENON T. Adenyl cylase. I. Distribution, preparation, and properties. J Biol Chem. 1962 Apr;237:1220–1227. [PubMed] [Google Scholar]
- Salomon Y., Londos C., Rodbell M. A highly sensitive adenylate cyclase assay. Anal Biochem. 1974 Apr;58(2):541–548. doi: 10.1016/0003-2697(74)90222-x. [DOI] [PubMed] [Google Scholar]
- Svoboda M., Robberecht P., Christophe J. Deactivation of persistently activated pancreatic adenylate cyclase. Evidence of uncoupling of hormone receptors and enzyme effector in the persistently activated state, and of the presence of two guanyl nucleotide regulatory sites. FEBS Lett. 1978 Aug 15;92(2):351–356. doi: 10.1016/0014-5793(78)80785-6. [DOI] [PubMed] [Google Scholar]
- Welton A. F., Lad P. M., Newby A. C., Yamamura H., Nicosia S., Rodbell M. Solubilization and separation of the glucagon receptor and adenylate cyclase in guanine nucleotide-sensitive states. J Biol Chem. 1977 Sep 10;252(17):5947–5950. [PubMed] [Google Scholar]


