Abstract
Phenothiazines are being repurposed for treatment of tuberculosis. We examined time-kill curves of thioridazine and first-line drugs against log-growth-phase and semidormant bacilli under acidic conditions and nonreplicating persistent Mycobacterium tuberculosis. While both the potency and the efficacy of first-line drugs declined dramatically as M. tuberculosis replication rates decreased, those of thioridazine improved. The mutation prevalence to 3 times the thioridazine MIC was <1 × 10−11, better than for ≥2 first-line drugs combined. Hollow fiber system studies revealed that the relationship between sterilizing effect and pharmacodynamic indices (PDI) was characterized by an r2 of 0.88 for peak/MIC, an r2 of 0.47 for the area under the concentration-time curve (AUC) to MIC, and an r2 of 0.14 for the cumulative percentage of a 24-h period that the drug concentration exceeds the MIC under steady-state pharmacokinetic conditions (%TMIC) at the end of the first week. However, the PDI linked to effect “wobbled” as the duration of therapy increased, so that by the fourth week the r2 was 0.88 for AUC/MIC, 0.78 for %TMIC, and 0.72 for peak/MIC. This “wobble” has implications on general pharmacokinetic/pharmacodynamic theory, whereby efficacy is linked to only one of the three PDIs in deterministic models. The potency changed 8.9-fold from the first to the fourth weeks. The non-protein-bound AUC/MIC associated with maximal kill at the end of therapy was 50.53 (protein binding = 99.5%). This thioridazine exposure was calculated to extinguish all three M. tuberculosis metabolic populations in human lungs in only 42.9 days of monotherapy. However, this concentration exceeds the 2- to 8-mg/liter thioridazine concentration in serum known to be lethal to humans. Therefore, the way forward for phenothiazine monotherapy that also reduces therapy duration is via synthesis of less toxic congeners.
INTRODUCTION
The treatment of tuberculosis is undergoing a tremendous change. Several new drugs have been introduced for the treatment of both drug-susceptible and drug-resistant tuberculosis, and several studies are ongoing to establish new regimens (1–4). In the meantime, standard dogmas critical to cure rates, such as directly observed therapy and how acquired drug resistance emerges, have been successfully challenged (5–8). These studies suggest that future treatments will radically differ from the current short-course regimen and will have a different underlying scientific basis. On the other hand, multidrug-resistant tuberculosis (MDR-TB) and extensively drug-resistant tuberculosis (XDR-TB) are becoming more established (9–11). Isolates termed “totally drug resistant” have also been described (12–15). A recent study demonstrated that XDR-TB patients had worse outcomes than MDR-TB patients and that XDR-TB with resistance to additional second-line drugs had worse outcomes than XDR-TB (14). Thus, despite several new drugs being introduced for the treatment of tuberculosis, the paradox is that there are still classes of patients who are therapeutically destitute. This has necessitated the repurposing of nonantibiotic pharmacophores for tuberculosis treatment. Phenothiazines such as thioridazine (THI) and chlorpromazine are old antipsychotic agents first synthesized in 1951 by Paul Charpentier and used in the clinic in France a year later (16, 17). They have been known to have antituberculosis effects for several decades (18–20). The main drawbacks to their clinical use are the high rates of cardiac and neurological toxicity. In order to further develop them and their congeners for clinical antimycobacterial use that minimizes toxicity while maximizing efficacy, detailed pharmacokinetic-pharmacodynamics (PK/PD) will be necessary.
PK/PD studies are used to identify the drug exposures best able to kill Mycobacterium tuberculosis (21–27). Such exposures can then be compared to those associated with toxicity, allowing for choice of an optimal dose that is nontoxic. In addition, dose-scheduling studies also identify the PK/PD indices (PDIs) linked to efficacy, which is determined as one of 3 parameters: the ratio of peak concentration of drug in serum (Cmax) to the MIC (Cmax/MIC), the ratio of the area under the concentration-time curve (AUC) to the MIC (AUC/MIC), or the cumulative percentage of a 24-h period that the drug concentration exceeds the MIC under steady-state pharmacokinetic conditions (%TMIC) (28, 29). This too can be compared to the dose schedule associated with the least toxicity, allowing for dose schedules that maximize efficacy while minimizing toxicity (30). Moreover, since PDIs linked to effect are similar across a pharmacophore, the parameters identified for THI would be the same as for its less toxic congeners. Here, we identified the PDI linked to THI efficacy in our in vitro hollow fiber system model of tuberculosis (HFM-TB).
MATERIALS AND METHODS
Bacteria.
M. tuberculosis H37Rv (ATCC 27294) stock cultures were stored at −80°C in 10% glycerol and 90% Middlebrook 7H9 broth with 10% oleic acid, albumin, dextrose, and catalase [OADC] (here termed “broth”). For each study, the M. tuberculosis stock was thawed and incubated in broth at 37°C for 4 days under shaking conditions and 5% CO2 to facilitate exponential-phase growth.
Drugs.
THI, isoniazid, rifampin, and pyrazinamide were purchased from Sigma-Aldrich. All drugs, except rifampin, were first dissolved in sterile water and subsequently diluted in broth (without 10% OADC). Rifampin was initially diluted in dimethyl sulfoxide and then back diluted in broth to make a final dimethyl sulfoxide concentration of <1% as described in the past (24). All THI experiments were performed so as to avoid direct light, since phenothiazines are highly potent photosensitizers that cause photodynamic damage to cells, which would lead to exaggerated efficacy (31–33). All drugs were prepared fresh for each experiment.
MICs and mutation frequencies.
THI MICs were identified using the agar dilution method (34). Mutation frequencies to 2× and 3× MIC were performed on Middlebrook 7H10 agar (here termed “agar”) supplemented with THI. Two hundred microliters of M. tuberculosis cultures on day 4 of log-phase growth was inoculated onto each of 20 agar plates to isolate and count resistant isolates in 8.0 log10 CFU, 9.0 log10 CFU, and 11.0 log10 CFU. The cultures on agar were incubated for 4 weeks under 5% CO2 at 37°C, after which colonies were counted.
The prevalence of mutants resistant to “critical” concentrations of rifampin, isoniazid, and pyrazinamide was also identified. The critical concentrations of rifampin, isoniazid, and pyrazinamide were chosen based on standard susceptibility breakpoints (34). Two hundred microliters of M. tuberculosis on the fourth day of log-phase growth was then cultured on each of 20 drug-containing plates using bacillary densities of 107 CFU/ml for isoniazid and 108 CFU/ml for rifampin. For pyrazinamide studies, agar was acidified to a pH of 5.8 prior to addition of the drug, and 105 CFU/ml of M. tuberculosis was used. Cultures were incubated for 4 weeks, and colonies were counted.
Growth rates of different M. tuberculosis metabolic populations.
Growth rates of different M. tuberculosis populations were measured using 4 different methods: changes in optical density monitored using absorbance at 600 nm (OD600), fluorescence in the Live/Dead cell viability assay (Invitrogen), quantitation of CFU/ml on agar, and quantitation of ribosomes/CFU (35). RNA extraction was performed using the RNeasy minikit (Qiagen). Growth rates were examined for relative fluorescence units (RFU), CFU/ml, and OD600 by determining the slope per unit of time (hours or days).
For studies of log-growth-phase M. tuberculosis, cultures on the fourth day of log growth phase were diluted to make a final concentration of 106 CFU/ml and then coincubated with THI. The cultures were then incubated under ambient air at 37°C under shaking conditions and protected from light. In the first study, the bacilli were coincubated with 0, 0.1, 1.0, 2.5, 5, 10, 20, 40, and 60 mg/liter of THI in duplicate and sampled on days 4 and 7. For all studies, samples were washed twice to remove THI and then serially diluted and cultured on agar. The cultures were incubated for 21 days, and colonies were counted. Based on the results of this study, a follow-up study was performed with THI concentrations of 0, 0.5, 1, 5, 10, 20, 40, and 60 mg/liter, in triplicate, and sampled on day 7 for quantitative cultures. In a third study, isoniazid concentrations of 0, 0.01, 0.03, 0.125, 0.5, 2, and 4 mg/liter, or rifampin concentrations of 0, 0.125, 0.250, 0.500, 0.750, 1, 1.25, 2, 4, and 8 mg/liter, or pyrazinamide concentrations of 0, 3.125, 5, 6.25, 10, 12.5, 25, 50, 100, 150, and 200 mg/liter were coincubated in duplicate with M. tuberculosis cultures on day 4 of log phase growth, and cultures were incubated under 5% CO2 and shaking conditions at 37°C. Cultures were sampled on day 7 for plating on agar for CFU/ml quantitation for all drugs, except those coincubated with pyrazinamide, which were incubated for 21 days prior to CFU quantitation.
Semidormant bacilli (SDB) under acidic conditions were generated in acidified Middlebrook 7H9 broth without OADC (here termed “acidified broth”), using methods described in prior studies (5, 26). Since progressively more acidic environments reduce the replication rates of the bacilli (26), we examined the effect of THI (pKa of 9.5) on bacilli growing slowly at three different pHs in acidified broth: 5.8, 6.0, or 6.8. Bacterial cultures on the 4th day of incubation at each pH were diluted in acidified broth to make a final bacillary density of 105 CFU/ml. The SDB were then incubated with THI concentrations of 0, 5, 10, 20, 40, and 60 mg/liter at 37°C under shaking conditions. The cultures were sampled on day 7 and quantitative cultures performed as described above. Rifampin, isoniazid, and pyrazinamide concentrations and coincubation, as well as quantitative cultures, were performed as described for log-growth-phase cultures above.
Nonreplicating persistent (NRP) M. tuberculosis cells were generated by inoculating 3.5 ml of 4.0 log10 CFU/ml M. tuberculosis into a 7-ml Vacutainer, together with 3.5 ml of air, and incubated at 37°C under shaking conditions, based on the Wayne and Hayes model (36). Changes in oxygen concentration were monitored by changes in the color of 1.5 mg/liter methylene blue in multiple sentinel tubes. Then, 0, 0.1, 1, 2.5, 5, 10, 20, 40, or 60 mg/liter of THI was added (without adding air or methylene blue color change) to triplicate Vacutainers and then incubated at 37°C under slow shaking. Pyrazinamide coincubation concentrations were as described for log-phase growth cultures, except that all cultures were under anaerobic conditions until plating on agar. The rifampin concentrations were as described for log-phase growth cultures; however, the highest concentration used was 32 mg/liter instead of 8 mg/liter. Similarly, for isoniazid the highest concentration tested was 16 mg/liter. Cultures were sampled on day 7, except for pyrazinamide, as described above.
HFM-TB studies.
Hollow fiber cartridges were purchased from Fibercell (Frederick, MD). Details of our HFM-TB model have been published in detail over the past decade (22–26). Two types of studies were performed, dose-effect and dose-scheduling studies, based on THI pharmacokinetics achieved in patients (37, 38). Dose-effect studies were performed with SDB M. tuberculosis. Each of seven hollow fiber systems (HFS) was dosed daily with one of seven THI exposures, shown in Table 1. To put this in context, 500 mg a day achieves a THI Cmax of 1.0 mg/liter and an AUC0–24 of 10.3 mg · h/liter; the pharmacokinetics were assumed to be linear. From these studies, the effective concentrations associated with either 20%, 50%, or 80% of maximal kill (EC20, EC50, and EC80) were calculated. Next, a dose-scheduling study was performed using SDB in order to identify the PDI linked to THI efficacy. THI EC20, EC50, and EC80 were administered as one of three dose schedules: a single dose once a week, the single dose equally divided into two and administered every 3.5 days, and the single dose equally divided into seven and administered daily (24, 26). The concentrations used for this study are shown in Table 2. The duration of therapy was 28 days. For each experiment, the central compartment was sampled 6 times over the first 24 h, and the samples were frozen at −80°C. The peripheral compartment of each HFM-TB was sampled on days 3, 7, 10, 14, 21, and 28 for the total M. tuberculosis population as well as the population growing on agar supplemented with 2× and 3× MIC of THI.
Table 1.
Daily dosing scheme in thioridazine dose-effect studies
| Dose no. | Cmax (μg/ml) | AUC0–24/MIC | %TMIC |
|---|---|---|---|
| 1 | 0 | 0 | 0 |
| 2 | 6.25 | 6.3 | 0 |
| 3 | 12.5 | 12.47 | 16.67 |
| 4 | 25 | 25.22 | 45.83 |
| 5 | 37.5 | 37.88 | 66.67 |
| 6 | 50 | 50.82 | 79.17 |
| 7 | 100 | 100.69 | 100 |
Table 2.
Dosing scheme in thioridazine dose-scheduling studies
| Dosing schedule | Cmax/MIC | AUC0–24/MIC | %TMIC |
|---|---|---|---|
| Control | 0 | 0 | 0 |
| Daily | 1.05 | 10.54 | 8 |
| Daily | 1.4 | 14.38 | 20.8 |
| Once a week | 10 | 14.38 | 15.46 |
| Daily | 2.5 | 25.22 | 45.83 |
| Twice a week | 8.75 | 25.22 | 29.76 |
| Once a week | 17.5 | 25.22 | 19.04 |
| Daily | 3.75 | 37.78 | 66.67 |
| Daily | 5.0 | 50.82 | 79.17 |
| Twice a week | 17.5 | 50.82 | 38.1 |
| Once a week | 35 | 50.82 | 23.21 |
| Daily | 10 | 100.69 | 100 |
Assays for identification of THI concentration.
Samples from the HFM-TB central compartment were analyzed for THI content using a qualified liquid chromatography-tandem mass spectrometry method. Samples were diluted with methanol, and 10 μl was injected directly without further processing and separated using a YMC hydrosphere C18 and guard column. The mobile phase consisted of 0.1% formic acid in methanol and 0.1% formic acid in deionized water. THI was detected using an API 3000 mass spectrometer, which was programmed in the multiple-reaction-monitoring mode and monitored the transition of the precursor ion m/z 371.2 to the product ion m/z 126.1. The method was accurate (±5%) and linear (r2 = 0.998) between 0.1 ng/ml and 1,000 ng/ml. Within- and between-day variation was <5%.
Pharmacokinetic and PK/PD modeling.
All THI concentrations were comodeled using Adapt 5 software (39). THI concentrations were modeled using a one-compartment pharmacokinetic model with first-order input and elimination. Pharmacokinetic parameter estimates identified were used to calculate exposures such as AUC, AUC/MIC, and %TMIC. The relationship between drug concentration and size of the M. tuberculosis population was analyzed using the inhibitory sigmoid Emax model in both Adapt 5 and GraphPad 5 software. The inhibitory sigmoid Emax model is a 4-parameter Hill-type equation described by equation 1:
| (1) |
where the effect is in CFU/ml, Econ is the M. tuberculosis burden without drug treatment, Emax is the maximal microbial kill or the efficacy of the drug in log10 CFU/ml, EC is the drug concentration, and H is the Hill factor. EC50 is the concentration associated with 50% of Emax and defines the potency of the drug.
RESULTS
The THI MIC for M. tuberculosis was 10 mg/liter based on the agar dilution method. There was a sharp cutoff point: 10 mg/liter THI agar plates had zero M. tuberculosis colonies compared to 4.98 ± 0.12 log10 CFU/ml on 5 mg/liter THI agar. When log-phase growth bacilli were incubated on agar with either 2× or 3× the THI MIC, none of 7.8 log10 CFU of bacilli grew on the agar in one experiment, none of 9 log10 CFU in the next experiment, and none of 11 log10 CFU in a third experiment. Thus, the prevalence of low-level THI-resistant mutants was <1 × 10−11. On the other hand, the prevalence of mutants resistant to rifampin was 1.15 × 10−6 (confidence interval [CI], 1.12 × 10−6 to 1.27 × 10−6), to isoniazid it was 5.40 × 10−6 (CI, 2.80 × 10−6 to 7.99 × 10−6), to 100 mg/liter of pyrazinamide it was 1.40 × 10−1 (CI, 1.14 × 10−1 to 1.67 × 10−1), and to 300 mg/liter of pyrazinamide it was 1.40 × 10−2 (CI, 1.30 × 10−2 to 1.50 × 10−2).
An examination of M. tuberculosis growth rates in our 3 different metabolic populations revealed the results shown in Table 3. The table demonstrates that log-growth-phase bacteria were growing at a rate multiplefold higher than that of SDB, that SDB growth at pH 5.8 differed only slightly from growth of NRP cells, and indeed that the last two were not statistically significant different from each other by some measures. These measures validate that our 3 metabolic populations grow at different rates, and measures such as the number of ribosomes/CFU demonstrate that the stable CFU/ml in SDB and NRP are not due to a balance of death and growth but to a shiftdown to reduced or no growth.
Table 3.
Different measures of growth rates of the three Mycobacterium tuberculosis metabolic populations
| Assay (unit) | Log-phase growth bacteria (95% CI) | Semidormant bacteria (95% CI) | NRP cells (95% CI) |
|---|---|---|---|
| Live/dead slope (RFU/h) | 60.72 (33.76–87.68) | 7.48 (3.06–11.90) | 4.72 (−0.56–10.01) |
| OD600 (×10−4 units/h) | 20.79 (12.03–29.55) | 1.98 (0.35–3.62) | 1.12 (−0.48–2.73) |
| Log10 CFU/ml/day (slope) | 0.069 (0.059–0.079) | 0.013 (0.007–0.018) | 0.005 (0.001–0.009) |
| Ribosomes/CFU (10−5) | 4.021 (3.204–4.838) | 2.555 (1.340–3.770) | 1.723 (1.294–2.153) |
Next, we examined the microbial kill of the 3 different M. tuberculosis metabolic populations by the first-line antituberculosis drugs, as well as by THI, in the test tube. Inhibitory sigmoid Emax parameters from these studies are shown in Table 4. First, maximal kill (Emax) of THI matched that of rifampin for NRP cells and pyrazinamide against SDB at low pH but was just slightly lower than that of isoniazid against log-growth-phase bacteria. Thus, THI is a drug that kills all 3 populations. Second, THI potency (EC50) was not altered by the different growth rates, as opposed to rifampin, isoniazid, and pyrazinamide, which were dramatically worse in NRP than in faster-growing bacteria. Third, the Hill factor or slope was no different by growth rate of the bacteria, suggesting that binding to drug targets of drug “receptors” does not change by growth rate or metabolic state.
Table 4.
Inhibitory sigmoid Emax parameters for different bacillary metabolic populations using static antibiotic concentrations
| Antibiotic and population | EC50 in mg/liter (95% CI) | Emax in log10 CFU/ml (95% CI) | Hill factor (95% CI) | r2 |
|---|---|---|---|---|
| Rifampin | ||||
| Log-phase growth cells | 0.67 (0.37–0.56) | 5.74 (5.00–6.48) | 1.90 (1.29–2.52) | 0.95 |
| SDB | 0.32 (0.29–0.36) | 5.24 (4.85–5.62) | 2.42 (1.91–2.93) | 0.98 |
| NRP cells | 10.47 (0–26.55) | 2.45 (0.90–4.00) | 0.88 (0.29–1.46) | 0.93 |
| Isoniazid | ||||
| Log-phase growth cells | 0.16 (0.06–0.26) | 5.72 (4.73–6.71) | 0.82 (0.49–1.15) | 0.93 |
| SDB | 0.12 (0.04–0.20) | 6.13 (5.20–7.05) | 0.48 (0.35–0.61) | 0.96 |
| NRP cells | >16 | 0 | 0 | NC |
| Pyrazinamide | ||||
| Log-phase growth cells | >200 | —b | — | — |
| SDB | 39.28 (0–87.56) | 2.38 (0–4.83) | 1.94 (0–5.42) | 0.85 |
| NRP cells | >200 | 0 | 0 | NC |
| Thioridazine | ||||
| Log-phase growth cells | 26.53 (22.67–30.39) | 3.66 (3.22–4.16) | 5.09 (2.93–7.25) | 0.98 |
| SDB pH 6.0 | 38.67 (12.84–60.0) | 4.74 (0–7.00) | 5.76 (−40.84–52.35) | 0.96 |
| SDB pH 5.8 | 16.37 (12.01–20.73) | 2.01 (1.55–2.47) | 4.54 (0.21–8.87) | >0.99 |
| NRP cells | 12.38 (5.27–19.49) | 2.05 (1.20–2.91) | 1.71 (0.13–3.29) | 0.98 |
Results are for 7-day incubation, except for pyrazinamide, for which incubation was 21 days. NC, not calculated.
For pyrazinamide effect against log-phase growth cells, Emax had not been achieved at the highest concentration tested, when kill was ∼1 log10 CFU/ml.
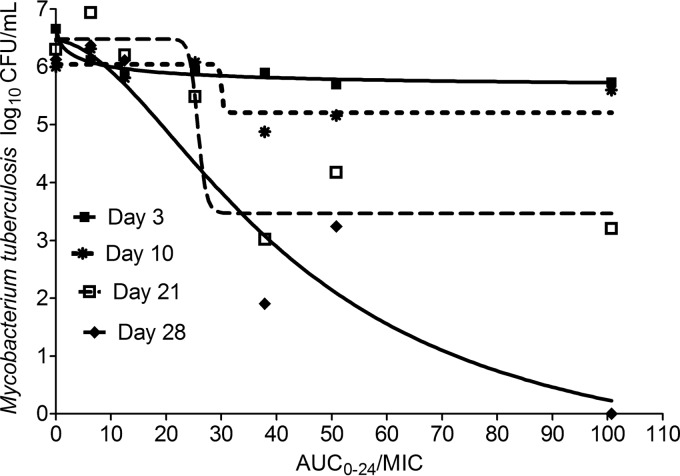
Next, we performed PK/PD studies in the HFM-TB using SDB at pH 5.8. The THI pharmacokinetic parameters achieved in the HFM-TB were a systemic clearance of 0.61 ± 0.50 liter/h/kg, a volume of distribution of 3.61 ± 2.45 liter/kg and a ka (absorption constant) of 0.94 ± 0.27/h. The relationship between drug exposure and microbial kill is shown in Fig. 1, with total drug exposure expressed as AUC/MIC. The EC50 increased 8.9-fold, from an AUC0-24/MIC of 4.84 (r2 = 0.96) on day 3 to 42.86 (r2 = 0.91) on day 28. Thus, the potency changed with time. The relationship between exposure and effect on day 28 can be described by equation 2 (where effect is in CFU/ml and r2 is 0.91):
| (2) |
Fig 1.
Thioridazine dose-effect studies in the hollow fiber system. The data demonstrate the change in thioridazine dose-effect as duration of therapy increases and a shift in EC50.
Next, we performed dose-scheduling studies using SDB at pH 5.8. The PDI linked to optimal efficacy is shown in Table 5. The table demonstrates that during the first 14 days, M. tuberculosis kill was most closely linked to THI Cmax/MIC ratios. The relationship between free, unbound fraction Cmax (fCmax)/MIC and M. tuberculosis bacterial burden at the end of the first dosing interval (week 1) was as described by equation 3 (where effect is in CFU/ml):
| (3) |
Table 5.
Dose-scheduling study results for thioridazinea
| Parameter | Value for therapy with duration of: |
||||
|---|---|---|---|---|---|
| 7 days | 10 days | 14 days | 21 days | 28 days | |
| Cmax/MIC | 0.88 | 0.86 | 0.79 | 0.52 | 0.72 |
| AUC/MIC | 0.47 | 0.46 | 0.50 | 0.83 | 0.88 |
| %TMIC | 0.14 | 0.21 | 0.17 | 0.47 | 0.78 |
Values in boldface indicate PDI linked to microbial kill.
At the end of the second dosing interval (i.e., at the 2-week time point), the r2 for Cmax/MIC versus bacterial burden was already declining (Table 5), and the relationship was as described by equation 4:
| (4) |
where effect is in CFU/ml.
Unexpectedly, after day 14, the PDI that was best explanatory for M. tuberculosis kill was the AUC0–24/MIC ratio and no longer the Cmax/MIC ratio. Indeed, the r2 for sterilizing effect and %TMIC started to improve on day 21 and exceeded that for Cmax/MIC by day 28, although AUC0–24/MIC was still the most important (Table 5). This indicates a “wobble” between the PDIs as duration of therapy increases. The relationship between antimicrobial effect (in CFU/ml) and THI exposure on day 21 is described by equation 5:
| (5) |
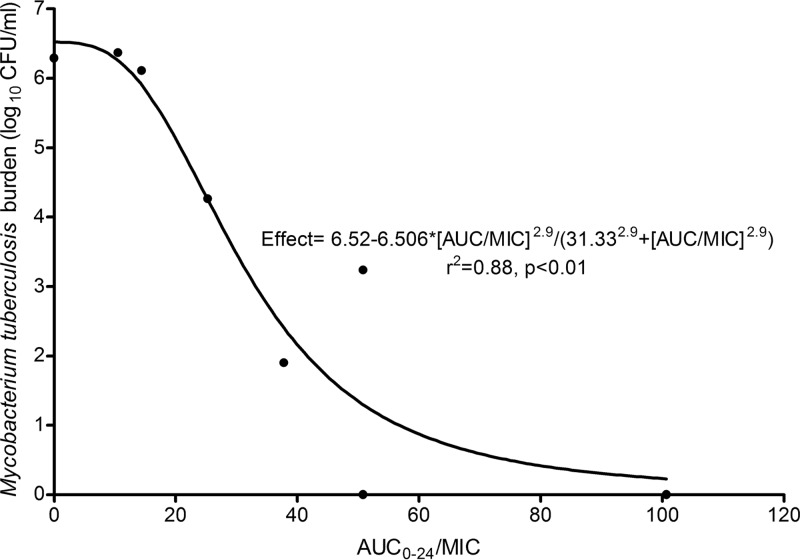
At the end of the study, on day 28, the relationship between THI exposure and effect (in CFU/ml) was as shown in Fig. 2 and as calculated by equation 6:
| (6) |
Fig 2.
Thioridazine inhibitory Emax curve at the end of 4 weeks in hollow fiber studies.
From equation 6, we calculated the non-protein-bound EC80 as an AUC/MIC of 50.53, which we used to examine the time to complete sterilization.
For time to complete sterilization, the total bacterial burden expected in lung lesions was first calculated. Pyrazinamide, at 2 g per day, has a sterilizing effect rate (slope) of 0.114 (95% CI, 0.085 to 0.143) log10 CFU/ml/day, starting after 2 days of therapy, in patients and the HFM-TB (26, 40). Thus, the total bacillary burden of the subpopulation killed by pyrazinamide from days 2 to 56 in patients calculates to an upper limit estimate of 7.72 log10 CFU/ml. The THI EC80 exposure, which is an AUC/MIC of 50.53 calculated from HFM-TB studies' equation 6 above, had a sterilizing effect rate of 0.18 log10 CFU/ml/day. This drug exposure would lead to total sterilization of SDB population in 42.9 days, as opposed to the 56 days of pyrazinamide currently needed to eradicate this population. If, on the other hand, our calculation of the total microbial population was an underestimate by up to 2 log10 CFU/ml (i.e., if the total bacillary burden was 9.72 log10 CFU/ml), THI EC80 would still be able to wipe out that bacterial burden of SDB in 54 days. Given that the efficacy rates and potency of THI against SDB are virtually similar to those against NRP cells in the time-kill studies in test tubes (Table 4) and the size of NRP is much less than SDB (41–43), NRP would be extinguished in a shorter time.
DISCUSSION
There are five important findings in our study. First, it has been known since the early days of penicillin and streptomycin use 60 years ago that rapidly dividing cells are easier to kill with chemotherapy than slowly growing ones and that NRP cells are the most difficult to kill (44–46). This is the principle that underlies the basis of current short-course chemotherapy. In reactivation pulmonary tuberculosis, M. tuberculosis organisms are believed to exist as one of three growth-rate-defined metabolic populations. Log-phase growth bacteria are rapidly killed by isoniazid within 2 to 5 days, and SDB are killed slowly by pyrazinamide over 8 weeks, while NRP cells are killed over 6 months by rifamycins (40, 41, 47–50). This necessitates multidrug therapy. Here, we found that the thioridazine effect was independent of these growth rates. For a drug whose mechanism of kill is inhibition of ATP synthesis and collapse of proton motive force, both necessary in replicating as well as NRP cells, it may matter little whether the bacillus is replicating or not (51). This suggests that the effectiveness of an antibiotic against M. tuberculosis is driven more by its own mechanism of effect than by rates of bacterial replication. Accordingly, antituberculosis agents such as isoniazid, pyrazinamide, ethambutol, streptomycin, and rifampin that were discovered earlier happened to belong to pharmacophores whose mechanisms of effect involve inhibiting biochemical pathways required for bacterial replication. Their patterns of kill thus became the normative one, and with that came the historical categorization of M. tuberculosis metabolic populations as log-phase growth, “semidormant,” and “nonreplicating persistent” cells.
Second, during the early development of current antituberculosis regimens, acquired drug resistance was a major limitation of monotherapy, which necessitated the use of multiple-drug therapy (52–54). Thus, currently, “prevention of the emergence of drug resistance is defined as the ability of a drug to prevent selection of mutants resistant to the companion drug” (55). The explanation has been that the average mutation rates to each of the antituberculosis drugs range from 10−5 to 3 × 10−8, which means that the probability of chromosomal mutations to a single drug arising is near certainty given total lung bacterial burdens of up to 109 CFU. However, the probability of mutations arising to two or more drugs is very small given that it is the product of the mutation rates for each drug. We show, however, that the prevalence of mutants resistant to THI concentrations just above the MIC was better than that for combinations of some first-line antituberculosis drugs. Thus, THI and its congeners could offer an unusually high hurdle for acquired drug resistance, so that it should now be theoretically possible to design antituberculosis drugs that can suppress resistance for themselves without the need for companion drugs.
Third, the PDI linked to efficacy is often assumed to be a single one, often chosen based on the highest r2 or Akaike information criteria for the inhibitory sigmoid Emax model fit (28, 29, 56). It is these deterministic relationships that make PK/PD science a powerful tool for drug development. Nevertheless, these relationships are often derived based on short-term therapy in bacteria with doubling times of only 20 min, which are wiped out by antibiotics within a few days. M. tuberculosis grows “in slow motion” compared to these other bacteria (∼72 times slower than Escherichia coli when in log-phase growth and ∼720 times slower for semidormant M. tuberculosis). This low growth rate and the poor kill rates of antituberculosis drugs allowed us the practical interrogation of basic PK/PD theory. Moreover, the HFM-TB model has the major advantage of being amenable to repetitive sampling of the same microbial population at multiple points, difficult to achieve in other preclinical models in which sampling is a terminal procedure. This system eliminates the possibility that the change in PK/PD effect linked to efficacy could be due to different metabolic populations of M. tuberculosis predominating at different periods of the therapy, given that we inoculated only SDB whose status and growth rates we validated at each point in the repetitive sampling scheme. Moreover, since no resistant subpopulation arose, this cannot be due to a switch between the PDI linked to microbial kill and that associated with resistance emergence. Furthermore, we sampled the HFM-TB at the end of each 1-week dosing schedule; earlier sampling can introduce bias by examining effect prior to achievement of the full AUC in the least intermittent regimens. Thus, the switch from Cmax/MIC linked sterilizing effect during the first 2 weeks to AUC/MIC is likely not an artifact. This brings up two obvious questions. First, if the PDI best linked to effect wobbles as the duration of therapy increases, when then is it the best time to identify that relationship? Second, at which time point is it best to calculate optimal drug exposures such as EC80 and EC90? This is because the concentration-effect curve changed with time such that the EC50 increased 8.9-fold; values such as the EC80 would also change with time. Prudence would suggest choosing the inhibitory sigmoid Emax at the point with the highest EC50 for optimal dose design. That, however, remains unresolved. On the other hand, we suspect that the wobble reflects that THI has several different mechanisms of effect, each one being prominent at different times during the long duration of M. tuberculosis therapy. Indeed, since systems pharmacology studies have begun to demonstrate that most therapeutic molecules have many different mechanisms of effect due to target promiscuity (57), it is possible that this concept of a wobbling PDI may apply to many more antibiotics used as chemotherapy characterized by long duration.
Fourth, we identified the relationship between THI concentration and efficacy, which enabled us to identify the optimal AUC/MIC exposure (EC80) of 50.53. If this concentration were achieved in tuberculosis lung lesions in patients, we calculate that based on relative kill rates, THI monotherapy would be able to wipe out all three M. tuberculosis metabolic populations in less than 8 weeks. We based these calculations on the size of the SDB population, which had an upper bound of 7.72 log10 CFU/ml, based on pyrazinamide kill rates as well as on the finding that both the EC50 and the Emax of THI against SDB and NRP cells were virtually the same. Moreover, even if the SDB population was 9.72 log10 CFU/ml, the bacterial population would still be wiped out in less than 8 weeks of therapy. Thus, phenothiazines represent an attractive pharmacophore for shortening therapy duration with a single drug, if patients can tolerate the optimal concentrations.
Finally, the EC80 AUC/MIC of 50.53 was derived in the HFM-TB for sterilizing effect, which uses no protein in the broth; thus, this is a non-protein-bound concentration. THI protein binding in human serum is due to α1-glycoprotein and is 99.5% (58, 59). Thus, the EC80 translates to a total AUC/MIC ratio of 10,106. In our laboratory M. tuberculosis strain, we found that the THI MIC was 10 mg/liter. If, on the other hand, one assumed a generously lower MIC of 0.1 mg/liter in clinical isolates, this would still be associated with an average concentration (Cavg) (Cavg = AUC/24 h) of 4.2 mg/liter. A THI Cavg of 1 mg/liter in serum is associated with toxicity to patients; 2 to 8 mg/liter is lethal (60). This means that doses that are optimal for M. tuberculosis kill will likely be toxic to patients. On the other hand, autopsy studies of patients who died of phenothiazine overdose, as well as pharmacokinetic studies in numerous animal species, demonstrate that concentrations in tissues such as lungs are multiplefold higher than in serum (61–63). Thus, it may be that the drug is concentrated at the site of pulmonary tuberculosis, so that the EC80 could be achieved at lower doses than those that are lethal to humans. Nevertheless, the common MIC in clinical isolates is likely to be higher than 0.1 mg/liter and indeed has been reported as ranging between 6 and 32 mg/liter (64). Finally, the EC80 target concentrations that we derived will also be useful as design specification of less toxic but equally efficacious congeners. Given the short time to extinction of SDB and NRP cells, such congeners would be able to shorten therapy to less than 2 months. We believe that it is in such congeners that greater prospects for use of phenothiazines as monotherapy antituberculosis agents lie.
There are several limitations to our study. First, we did not consider intracellular M. tuberculosis bacilli, which are 20% of those in pulmonary tuberculosis cavities (65). However, phenothiazines such as THI are known to be concentrated multiplefold inside mammalian cells (66). Thus, THI may be effective at concentrations lower than those that are toxic for extracellular bacilli. Second, our studies were performed using HFM-TB, which does not mimic many aspects of human physiology. Thus, the clinical meaning of our results could be limited. However, the quantitative predictions of the HFS model of tuberculosis have been shown to be fairly accurate as judged by clinical findings undertaken after the fact; they have also been shown to correlate well with findings in clinical studies performed prior to use of this technology (5–8, 23, 25, 67, 68). Thus, our results likely have clinical relevance. Third, as discussed above, if we had continued therapy beyond 28 days, say up to 2 months, it could be that a different PDI such as %TMIC could have been identified for the latter period. This possibility cannot be discounted.
In summary, THI represents a pharmacophore that is equally effective whether M. tuberculosis is replicating or not, under hypoxia or not. The mutation frequency to even 3× MIC is low, which means that the hurdle for acquired drug resistance is relatively high. THI exhibited an unusual phenomenon, which was that the PDI linked to sterilizing effect changed with time. Nevertheless, at optimal concentrations, THI would be expected to wipe out all 3 metabolic populations of M. tuberculosis in less than 2 months, as monotherapy. Unfortunately, the concentrations likely to achieve that would be fatal to patients.
ACKNOWLEDGMENTS
This work was supported by the National Institute of General Medical Sciences of the National Institutes of Health New Innovator Award DP2 OD001886-01 and the National Institute of Allergy and Infectious Diseases award R01AI079497.
Footnotes
Published ahead of print 16 September 2013
REFERENCES
- 1. Andries K, Verhasselt P, Guillemont J, Gohlmann HW, Neefs JM, Winkler H, Van Gestel J, Timmerman P, Zhu M, Lee E, Williams P, de Chaffoy D, Huitric E, Hoffner S, Cambau E, Truffot-Pernot C, Lounis N, Jarlier V. 2005. A diarylquinoline drug active on the ATP synthase of Mycobacterium tuberculosis. Science 307:223–227 [DOI] [PubMed] [Google Scholar]
- 2. Diacon AH, Pym A, Grobusch M, Patientia R, Rustomjee R, Page-Shipp L, Pistorius C, Krause R, Bogoshi M, Churchyard G, Venter A, Allen J, Palomino JC, De Marez T, van Heeswijk RP, Lounis N, Meyvisch P, Verbeeck J, Parys W, De Beule K, Andries K, Mc Neeley DF. 2009. The diarylquinoline TMC207 for multidrug-resistant tuberculosis. N. Engl. J. Med. 360:2397–2405 [DOI] [PubMed] [Google Scholar]
- 3. Conde MB, Efron A, Loredo C, De Souza GR, Graca NP, Cezar MC, Ram M, Chaudhary MA, Bishai WR, Kritski AL, Chaisson RE. 2009. Moxifloxacin versus ethambutol in the initial treatment of tuberculosis: a double-blind, randomised, controlled phase II trial. Lancet 373:1183–1189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Diacon AH, Dawson R, Hanekom M, Narunsky K, Maritz SJ, Venter A, Donald PR, van Niekerk C, Whitney K, Rouse DJ, Laurenzi MW, Ginsberg AM, Spigelman MK. 2010. Early bactericidal activity and pharmacokinetics of PA-824 in smear-positive tuberculosis patients. Antimicrob. Agents Chemother. 54:3402–3407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Srivastava S, Pasipanodya JG, Meek C, Leff R, Gumbo T. 2011. Multidrug-resistant tuberculosis not due to noncompliance but to between-patient pharmacokinetic variability. J. Infect. Dis. 204:1951–1959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Pasipanodya JG, Srivastava S, Gumbo T. 2012. Meta-analysis of clinical studies supports the pharmacokinetic variability hypothesis for acquired drug resistance and failure of antituberculosis therapy. Clin. Infect. Dis. 55:169–177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Pasipanodya JG, Gumbo T. 2013. A meta-analysis of self-administered versus directly observed therapy effect on microbiologic failure, relapse, and acquired drug resistance in tuberculosis patients. Clin. Infect. Dis. 57:21–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Pasipanodya J, McIlleron H, Burger A, Wash PA, Smith P, Gumbo T. 2013. Serum drug concentrations predictive of pulmonary tuberculosis outcomes. J. Infect. Dis. 208:1464–1473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dalton T, Cegielski P, Akksilp S, Asencios L, Campos CJ, Cho SN, Erokhin VV, Ershova J, Gler MT, Kazennyy BY, Kim HJ, Kliiman K, Kurbatova E, Kvasnovsky C, Leimane V, Van der Walt M, Via LE, Volchenkov GV, Yagui MA, Kang H, Akksilp R, Sitti W, Wattanaamornkiet W, Andreevskaya SN, Chernousova LN, Demikhova OV, Larionova EE, Smirnova TG, Vasilieva IA, Vorobyeva AV, Barry CE, III, Cai Y, Shamputa IC, Bayona J, Contreras C, Bonilla C, Jave O, Brand J, Lancaster J, Odendaal R, Chen MP, Diem L, Metchock B, Tan K, Taylor A, Wolfgang M, Cho E, Eum SY, Kwak HK, Lee J, Lee J, Min S, Degtyareva I, Nemtsova ES, Khorosheva T, Kyryanova EV, Egos G, Perez MT, Tupasi T, Hwang SH, Kim CK, Kim SY, Lee HJ, Kuksa L, Norvaisha I, Skenders G, Sture I, Kummik T, Kuznetsova T, Somova T, Levina K, Pariona G, Yale G, Suarez C, Valencia E, Viiklepp P. 2012. Prevalence of and risk factors for resistance to second-line drugs in people with multidrug-resistant tuberculosis in eight countries: a prospective cohort study. Lancet 380:1406–1417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hoffner S. 2012. Unexpected high levels of multidrug-resistant tuberculosis present new challenges for tuberculosis control. Lancet 380:1367–1369 [DOI] [PubMed] [Google Scholar]
- 11. Zhao Y, Xu S, Wang L, Chin DP, Wang S, Jiang G, Xia H, Zhou Y, Li Q, Ou X, Pang Y, Song Y, Zhao B, Zhang H, He G, Guo J, Wang Y. 2012. National survey of drug-resistant tuberculosis in China. N. Engl. J. Med. 366:2161–2170 [DOI] [PubMed] [Google Scholar]
- 12. Udwadia ZF, Amale RA, Ajbani KK, Rodrigues C. 2012. Totally drug-resistant tuberculosis in India. Clin. Infect. Dis. 54:579–581 [DOI] [PubMed] [Google Scholar]
- 13. Migliori GB, Centis R, D'Ambrosio L, Spanevello A, Borroni E, Cirillo DM, Sotgiu G. 2012. Totally drug-resistant and extremely drug-resistant tuberculosis: the same disease? Clin. Infect. Dis. 54:1379–1380 [DOI] [PubMed] [Google Scholar]
- 14. Migliori GB, Sotgiu G, Gandhi NR, Falzon D, DeRiemer K, Centis R, Hollm-Delgado MG, Palmero D, Perez-Guzman C, Vargas MH, D'Ambrosio L, Spanevello A, Bauer M, Chan ED, Schaaf HS, Keshavjee S, Holtz TH, Menzies D. 2013. Drug resistance beyond XDR-TB: results from a large individual patient data meta-analysis. Eur. Respir. J. 42:169–179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Klopper M, Warren RM, Hayes C. 2013. Emergence and spread of extensively and totally drug-resistant tuberculosis, South Africa. Emerg. Infect. Dis. 19:449–455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Charpentier P, Gailliot P, Jacobs R. 1952. Recherches sur les diméthylaminopropyl-N phénothiazines substituées. C. R. Acad. Sci. 235:59–60 [Google Scholar]
- 17. Ban TA. 2007. Fifty years chlorpromazine: a historical perspective. Neuropsychiatr. Dis. Treat. 3:495–500 [PMC free article] [PubMed] [Google Scholar]
- 18. Molnar J, Beladi I, Foldes I. 1977. Studies on antituberculotic action of some phenothiazine derivatives in vitro. Zentralbl. Bakteriol. Orig. A 239:521–526 [PubMed] [Google Scholar]
- 19. Crowle AJ, Douvas GS, May MH. 1992. Chlorpromazine: a drug potentially useful for treating mycobacterial infections. Chemotherapy 38:410–419 [DOI] [PubMed] [Google Scholar]
- 20. Amaral L, Udwadia Z, Abbate E, van Soolingen D. 2012. The added effect of thioridazine in the treatment of drug-resistant tuberculosis. Int. J. Tuberc. Lung Dis. 16:1706–1708 [DOI] [PubMed] [Google Scholar]
- 21. Jayaram R, Shandil RK, Gaonkar S, Kaur P, Suresh BL, Mahesh BN, Jayashree R, Nandi V, Bharath S, Kantharaj E, Balasubramanian V. 2004. Isoniazid pharmacokinetics-pharmacodynamics in an aerosol infection model of tuberculosis. Antimicrob. Agents Chemother. 48:2951–2957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gumbo T, Louie A, Deziel MR, Parsons LM, Salfinger M, Drusano GL. 2004. Selection of a moxifloxacin dose that suppresses drug resistance in Mycobacterium tuberculosis, by use of an in vitro pharmacodynamic infection model and mathematical modeling. J. Infect. Dis. 190:1642–1651 [DOI] [PubMed] [Google Scholar]
- 23. Gumbo T, Louie A, Deziel MR, Drusano GL. 2005. Pharmacodynamic evidence that ciprofloxacin failure against tuberculosis is not due to poor microbial kill but to rapid emergence of resistance. Antimicrob. Agents Chemother. 49:3178–3181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gumbo T, Louie A, Deziel MR, Liu W, Parsons LM, Salfinger M, Drusano GL. 2007. Concentration-dependent Mycobacterium tuberculosis killing and prevention of resistance by rifampin. Antimicrob. Agents Chemother. 51:3781–3788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gumbo T, Louie A, Liu W, Brown D, Ambrose PG, Bhavnani SM, Drusano GL. 2007. Isoniazid bactericidal activity and resistance emergence: integrating pharmacodynamics and pharmacogenomics to predict efficacy in different ethnic populations. Antimicrob. Agents Chemother. 51:2329–2336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gumbo T, Siyambalapitiyage Dona CS, Meek C, Leff R. 2009. Pharmacokinetics-pharmacodynamics of pyrazinamide in a novel in vitro model of tuberculosis for sterilizing effect: a paradigm for faster assessment of new antituberculosis drugs. Antimicrob. Agents Chemother. 53:3197–3204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Jayaram R, Gaonkar S, Kaur P, Suresh BL, Mahesh BN, Jayashree R, Nandi V, Bharat S, Shandil RK, Kantharaj E, Balasubramanian V. 2003. Pharmacokinetics-pharmacodynamics of rifampin in an aerosol infection model of tuberculosis. Antimicrob. Agents Chemother. 47:2118–2124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Craig WA. 1998. Pharmacokinetic/pharmacodynamic parameters: rationale for antibacterial dosing of mice and men. Clin. Infect. Dis. 26:1–10 [DOI] [PubMed] [Google Scholar]
- 29. Ambrose PG, Bhavnani SM, Rubino CM, Louie A, Gumbo T, Forrest A, Drusano GL. 2007. Pharmacokinetics-pharmacodynamics of antimicrobial therapy: it's not just for mice anymore. Clin. Infect. Dis. 44:79–86 [DOI] [PubMed] [Google Scholar]
- 30. Drusano GL, Ambrose PG, Bhavnani SM, Bertino JS, Nafziger AN, Louie A. 2007. Back to the future: using aminoglycosides again and how to dose them optimally. Clin. Infect. Dis. 45:753–760 [DOI] [PubMed] [Google Scholar]
- 31. Viola G, Latterini L, Vedaldi D, Aloisi GG, Dall'Acqua F, Gabellini N, Elisei F, Barbafina A. 2003. Photosensitization of DNA strand breaks by three phenothiazine derivatives. Chem. Res. Toxicol. 16:644–651 [DOI] [PubMed] [Google Scholar]
- 32. Elisei F, Latterini L, Aloisi GG, Mazzucato U, Viola G, Miolo G, Vedaldi D, Dall'Acqua F. 2002. Excited-state properties and in vitro phototoxicity studies of three phenothiazine derivatives. Photochem. Photobiol. 75:11–21 [DOI] [PubMed] [Google Scholar]
- 33. Wilson B, Fernandez MJ, Lorente A, Grant KB. 2008. Synthesis and DNA interactions of a bis-phenothiazinium photosensitizer. Org. Biomol. Chem. 6:4026–4035 [DOI] [PubMed] [Google Scholar]
- 34. Clinical and Laboratory Standards Institute 2003. Susceptibility testing of mycobacteria, nocardiae, and other aerobic actinomycetes; approved standard. Clinical and Laboratory Standards Institute, Wayne, PA: [PubMed] [Google Scholar]
- 35. Ellard GA. 1991. The use of RNA/DNA ratio measurements to assess rifampicin-induced growth inhibition of Escherichia coli. J. Antimicrob. Chemother. 28:347–355 [DOI] [PubMed] [Google Scholar]
- 36. Wayne LG, Hayes LG. 1996. An in vitro model for sequential study of shiftdown of Mycobacterium tuberculosis through two stages of nonreplicating persistence. Infect. Immun. 64:2062–2069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Muusze RG, Vanderheeren FA. 1977. Plasma levels and half lives of thioridazine and some of its metabolites. II. Low doses in older psychiatric patients. Eur. J. Clin. Pharmacol. 11:141–147 [DOI] [PubMed] [Google Scholar]
- 38. Chakraborty BS, Midha KK, McKay G, Hawes EM, Hubbard JW, Korchinski ED, Choc MG, Robinson WT. 1989. Single dose kinetics of thioridazine and its two psychoactive metabolites in healthy humans: a dose proportionality study. J. Pharm. Sci. 78:796–801 [DOI] [PubMed] [Google Scholar]
- 39. D'Argenio DZ, Schumitzky A, Wang X. 2009. ADAPT 5 user's guide: pharmacokinetic/pharmacodynamic systems analysis software. Biomedical Simulations Resource, Los Angeles, CA [Google Scholar]
- 40. Jindani A, Dore CJ, Mitchison DA. 2003. Bactericidal and sterilizing activities of antituberculosis drugs during the first 14 days. Am. J. Respir. Crit. Care Med. 167:1348–1354 [DOI] [PubMed] [Google Scholar]
- 41. Mitchison DA. 1979. Basic mechanisms of chemotherapy. Chest 76:771–781 [DOI] [PubMed] [Google Scholar]
- 42. Mitchison DA. 1985. The action of antituberculosis drugs in short-course chemotherapy. Tubercle 66:219–225 [DOI] [PubMed] [Google Scholar]
- 43. Hu Y, Coates AR, Mitchison DA. 2006. Sterilising action of pyrazinamide in models of dormant and rifampicin-tolerant Mycobacterium tuberculosis. Int. J. Tuberc. Lung Dis. 10:317–322 [PubMed] [Google Scholar]
- 44. Bigger JW. 1944. Treatment of staphylococcal infections with penicillin by intermittent sterilization. Lancet 244:497–500 [Google Scholar]
- 45. Lewis K. 2007. Persister cells, dormancy and infectious disease. Nat. Rev. Microbiol. 5:48–56 [DOI] [PubMed] [Google Scholar]
- 46. Smith DG, Waksman SA. 1947. Tuberculostatic and tuberculocidal properties of streptomycin. J. Bacteriol. 54:253–261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. McCune RM, Lee SH, Deuschle K, McDermott W. 1957. Ineffectiveness of isoniazid in modifying the phenomenon of microbial persistence. Am. Rev. Tuberc. 76:1106–1109 [DOI] [PubMed] [Google Scholar]
- 48. McCune RM, Feldmann FM, Lambert HP, McDermott W. 1966. Microbial persistence. I. The capacity of tubercle bacilli to survive sterilization in mouse tissues. J. Exp. Med. 123:445–468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. McCune RM, Feldmann FM, McDermott W. 1966. Microbial persistence. II. Characteristics of the sterile state of tubercle bacilli. J. Exp. Med. 123:469–486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. McDermott W, Tompsett R. 1954. Activation of pyrazinamide and nicotinamide in acidic environments in vitro. Am. Rev. Tuberc. 70:748–754 [DOI] [PubMed] [Google Scholar]
- 51. Rao SP, Alonso S, Rand L, Dick T, Pethe K. 2008. The protonmotive force is required for maintaining ATP homeostasis and viability of hypoxic, nonreplicating Mycobacterium tuberculosis. Proc. Natl. Acad. Sci. U. S. A. 105:11945–11950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Yeager RL, Munroe WG, Dessau FI. 1952. Pyrazinamide (aldinamide*) in the treatment of pulmonary tuberculosis. Am. Rev. Tuberc. 65:523–546 [PubMed] [Google Scholar]
- 53. Anonymous 1948. Streptomycin treatment of pulmonary tuberculosis. Br. Med. J. 30:769–782 [PMC free article] [PubMed] [Google Scholar]
- 54. Selkon JB, Devadatta S, Kulkarni KG, Mitchison DA, Narayana AS, Nair CN, Ramachandran K. 1964. The emergence of isoniazid-resistant cultures in patients with pulmonary tuberculosis during treatment with isoniazid alone or isoniazid plus PAS. Bull. World Health Org. 31:294. [PMC free article] [PubMed] [Google Scholar]
- 55. Rieder HL. 2002. Interventions for tuberculosis control and elimination. International Union Against Tuberculosis and Lung Diseases, Paris, France [Google Scholar]
- 56. Craig WA. 2007. Pharmacodynamics of antimicrobials: general concepts and applications, p 1–19 In Nightangle CH, Ambrose PG, Drusano GL, Murakawa T. (ed), Antimicrobial pharmacodynamics in theory and practice, 2nd ed, vol 44 Informa Healthcare U. S. A., Inc., New York, NY [Google Scholar]
- 57. Brown JB, Okuno Y. 2012. Systems biology and systems chemistry: new directions for drug discovery. Chem. Biol. 19:23–28 [DOI] [PubMed] [Google Scholar]
- 58. Nyberg G, Axelsson R, Martensson E. 1978. Binding of thioridazine and thioridazine metabolites to serum proteins in psychiatric patients. Eur. J. Clin. Pharmacol. 14:341–350 [DOI] [PubMed] [Google Scholar]
- 59. Nyberg G, Martensson E. 1982. Binding of thioridazine and thioridazine metabolites to serum proteins. An in vitro study. Naunyn Schmiedebergs Arch. Pharmacol. 319:189–196 [DOI] [PubMed] [Google Scholar]
- 60. Mylan Institutional Inc 2011. Thioridazine hydrochloride. Mylan Institutional Inc., Morgantown, WV [Google Scholar]
- 61. Dinovo EC, Bost RO, Sunshine I, Gottschalk LA. 1978. Distribution of thioridazine and its metabolites in human tissues and fluids obtained postmortem. Clin. Chem. 24:1828–1830 [PubMed] [Google Scholar]
- 62. Hackman CR, Rosengard S, Vapaatalo H. 1970. Tissue distribution of chlorpromazine studied by microautoradiography. Eur. J. Pharmacol. 9:59–66 [DOI] [PubMed] [Google Scholar]
- 63. Forrest FM, Forrest IS, Roizin L. 1963. Clinical, biochemical and post mortem studies on a patient treated with chlorpromazine. Agressologie 4:259–265 [PubMed] [Google Scholar]
- 64. Thanacoody HKR. 2007. Thioridazine: resurrection as an antimicrobial agent? Br. J. Clin. Pharmacol. 64:566–574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Eum SY, Kong JH, Hong MS, Lee YJ, Kim JH, Hwang SH, Cho SN, Via LE, Barry CE., III 2010. Neutrophils are the predominant infected phagocytic cells in the airways of patients with active pulmonary TB. Chest 137:122–128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Daniel WA, Wojcikowski J. 1999. The role of lysosomes in the cellular distribution of thioridazine and potential drug interactions. Toxicol. Appl. Pharmacol. 158:115–124 [DOI] [PubMed] [Google Scholar]
- 67. Pasipanodya J, Gumbo T. 2011. An oracle: antituberculosis pharmacokinetics-pharmacodynamics, clinical correlation, and clinical trial simulations to predict the future. Antimicrob. Agents Chemother. 55:24–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pasipanodya J, Hanna D, Romero K, Gumbo T.Abstr. 53rd Intersci. Conf. Antimicrob. Agents Chemother., abstr A-294.2013. [Google Scholar]