Abstract
We describe the pharmacokinetics of raltegravir of a preterm newborn after short-course treatment of the mother tested HIV + the day of delivery. At age 1 month, the circulating concentration of raltegravir in the newborn was 29 ng/ml (the IC95 of RAL against HIV-1 is 15 ng/ml). Raltegravir should therefore be considered a potential transplacental postexposure prophylaxis for HIV-1 and an alternative to the use of boosted lopinavir in this context.
TEXT
Raltegravir (RAL), first inhibitor of the integrase of HIV-1, is increasingly used as an emergency treatment for untreated women infected with HIV-1 discovered in late pregnancy or for preterm delivery. RAL can sharply decrease the HIV-1 viral load (1) and combined with other antiretrovirals helps minimize the risk of mother to child transmission (MTCT). If the mother's HIV-positive status is discovered late, control of viral load at delivery is compromised. In such cases, the newborn, especially if premature, is at high risk of being infected with HIV-1 (2), so post exposure treatment should be strengthened. Unfortunately, the use of boosted protease inhibitors (PIs) in preterm newborns is associated with life-threatening side effects. They should not be used or only with extreme caution (3).
RAL is well tolerated by adults, and targets HIV-1 pre-integration: it is therefore a possible alternative to PIs for neonates. We describe here the transplacental pharmacokinetics of RAL in a premature newborn after short-course treatment of the mother.
A 30-year-old woman was admitted to our hospital at 30 weeks gestation with premature rupture of membranes and contractions. An HIV test was positive and the woman reported that she had never taken any antiretrovirals. The following antiretroviral treatment was administered to the mother 4 h after her arrival and after having provided inform consent: 245 mg tenofovir/200 mg emtricitabine/600 mg darunavir/100 mg ritonavir/400 mg RAL + single dose nevirapine, and an intraveinous zidovudine drip. The viral load of the mother was 14000 copies/ml and the CD4 cell count was 80/mm3. Genotyping showed a wild-type HIV-1 sub type B.
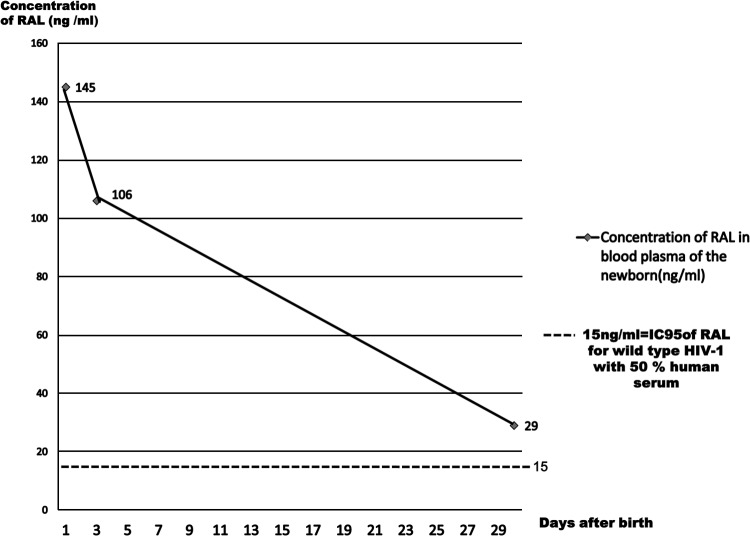
Betamethasone was also given to the mother to mature the lungs of the fetus. Delivery was by Cesarean section, 36 h after hospitalization, 2 h after the third intake of 400 mg RAL + 600 mg darunavir/100 mg ritonavir. The baby weighed 3.1 pounds (1420g) and was 15.7 in. in length (40 cm). The trough concentration of RAL in cord blood was determined by an ultra Performance LC–tandem-mass spectrometry (UPLC-MS/MS) method (Acquity UPLC-Acquity tandem quadruple detector [TQD]) after sample pretreatment (limit of quantification [LOQ], ∼1 ng/ml) and was 145 ng/ml. The RAL concentration in maternal plasma was not determined (no sample). Cord blood was negative for proviral DNA as assessed with a real-time PCR test kit for POL and LTR sequences (Pasteur Cerba, lower limit of detection: 10 copies/million PBMC).
Postexposure prophylaxis of the newborn was started the same day with 2 mg/kg twice a day of zidovudine + lamivudine enhanced with nevirapine 2 mg/kg/week; lopinavir + ritonavir syrup was not used because of the risk of adrenal dysfunction in premature newborns (3). At age 2 days, the trough plasma concentration of RAL in the newborn was 106 ng/ml. The neonate was treated for icteria with phototherapy from age 1 day. The newborn lost the ability to feed after 1 week due to complications of ventilation and was referred to another hospital for intensive care on the 7th day of life; oral antiretrovirals were discontinued in the intensive care unit, and zidovudine was administered intravenously until 4 weeks of life. The trough concentration of RAL at age 1 month was 29 ng/ml, above the limit of efficacy (the 95% inhibitory concentration [IC95] of RAL against HIV-1 is 15 ng/ml in 50% human serum).
We confirm the previous finding (4) that RAL crosses the placenta. Although the child was only exposed to RAL prenatally, the concentration of RAL in the newborn's plasma at age 1 month remained above the IC95 for wild-type HIV 1. RAL is only eliminated by the liver, mainly by UGT 1A1 activity (5), known to be immature in preterm newborns (6), and this may explain the slow clearance. There may also have been enterohepatic recirculation of RAL as suggested recently by Clarke and colleagues (4) and this may lead to clearance of the drug slowing after few days (Fig. 1). Note that the mother did not breast feed: the neonate was hospitalized in intensive care during its first month of life. There were no other treatments that could have resulted in drug-drug interaction with RAL, except nevirapine which has a long half-life, but any such interaction would have been expected to decrease the concentration of RAL (7). The diagnostic test for Gilbert's syndrome was not performed. The half-life of RAL in adults is 9 h (5), and an estimated 1 to 4 days in newborn infants (4); our case indicates that it is approximately 7 days in premature newborns. The baby tolerated the treatment well. As 60% of RAL in the body is associated with albumin, there is a theoretical risk of kernicterus; our patient was treated with phototherapy for an excessively high level of bilirubin.
Fig 1.
Pharmacokinetics of RAL in blood plasma of the newborn.
Treatment with zidovudine was discontinued after 6 weeks. The baby was seen regularly and showed a normal development and remained negative for proviral DNA at age 5 months. To our knowledge, this is the first description of the pharmacokinetics of RAL in a premature neonate during the month following birth. More data are needed to confirm these findings beyond this single case. Nevertheless, RAL should be considered a potential transplacental postexposure prophylaxis for HIV-1: it is metabolized only slowly in premature newborns. Thus, if the mother is treated with RAL the drug passes to the fetus, and remains active in the newborn. This effect may be significant, particularly in view of the need to avoid the use of boosted lopinavir in premature neonates. The duration of treatment of the newborn with zidovudine + lamivudine should be therefore adjusted according to RAL plasma concentrations to prevent the risk of selection of resistant strains in cases of contamination.
ACKNOWLEDGMENTS
We thank Johanna Moutoncarpin (midwife), Syvianne Verron (pediatric nurse), and Marthe Demonlis (biologist).
Footnotes
Published ahead of print 30 September 2013
REFERENCES
- 1. Markowitz M, Morales-Ramirez JO, Nguyen BY, Kovacs CM, Steigbigel RT, Cooper DA, Liporace R, Schwartz R, Isaacs R, Gilde LR, Wenning L, Zhao J, Teppler H. 2006. Antiretroviral activity, pharmacokinetics, and tolerability of MK-0518, a novel inhibitor of HIV-1 integrase, dosed as monotherapy for 10 days in treatment-naive HIV-1-infected individuals. J. Acquir.Immune DeficSyndr. 43:509–515 [DOI] [PubMed] [Google Scholar]
- 2. Mandelbrot L, Mayaux MJ, Bongain A, Berrebi A, Moudoub-Jeanpetit Y, Bénifla JL, Ciraru-Vigneron N, Le Chenadec J, Blanche S, Delfraissy JF. 1996. Obstetric factors and mother-to-child transmission of human immunodeficiency virus type 1: the French perinatal cohorts. SEROGEST French Pediatric HIV Infection Study Group. Am. J. Obstet. Gynecol. 175(Pt 1):661–7 [DOI] [PubMed] [Google Scholar]
- 3. Simon A, Warszawski J, Kariyawasam D, Le Chenadec J, Benhammou V, Czernichow P, Foissac F, Laborde K, Tréluyer JM, Firtion G, Layouni I, Munzer M, Bavoux F, Polak M, Blanche S, ANRS French Perinatal Cohort Study Group 2011. Association of prenatal and postnatal exposure to lopinavir-ritonavir and adrenal dysfunction among uninfected infants of HIV-infected mothers. JAMA 306:70–8. 10.1001/jama.2011.915 [DOI] [PubMed] [Google Scholar]
- 4. Clarke D, Acosta E, Rizk M, Bryson Y, Spector S, Mofenson L, Teppler H, Welebob C, Handelsman E, Mirochnick M, IMPAACT P1097 Study Team 2013. RAL pharmacokinetics and safety in neonates (IMPAACT P1097), poster 974. Abstr. 20th Conf. Retroviruses Opportunistic Infect. (CROI 2013), 3 to 6 March 2013, Atlanta, GA http://www.retroconference.org/2013b/Abstracts/47397.htm [Google Scholar]
- 5. Iwamoto M, Wenning LA, Petry AS, Laethem M, De Smet M, Kost JT, Merschman SA, Strohmaier KM, Ramael S, Lasseter KC, Stone JA, Gottesdiener KM, Wagner JA. 2008. Safety, tolerability, and pharmacokinetics of raltegravir after single and multiple doses in healthy subjects. Clin. Pharmacol. Ther. 83:293–9 [DOI] [PubMed] [Google Scholar]
- 6. Shogo J, Miyagi, Abby Collier C. 2011. The development of UDP-glucuronosyltransferases 1A1 and 1A6 in the pediatric liver. Drug Metab. Dispos. 39:912–919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Montrucchio C, Calcagno A, Lanzafame M. 2013. Pharmacokinetics of the dual NRTI- and protease inhibitor-sparing regimen raltegravir plus nevirapine in HIV-1+ patients, abstr 536. Abstr. 20th Conf. Retroviruses Opportunistic Infect. (CROI 2013), 3 to 6 March 2013, Atlanta, GA [Google Scholar]