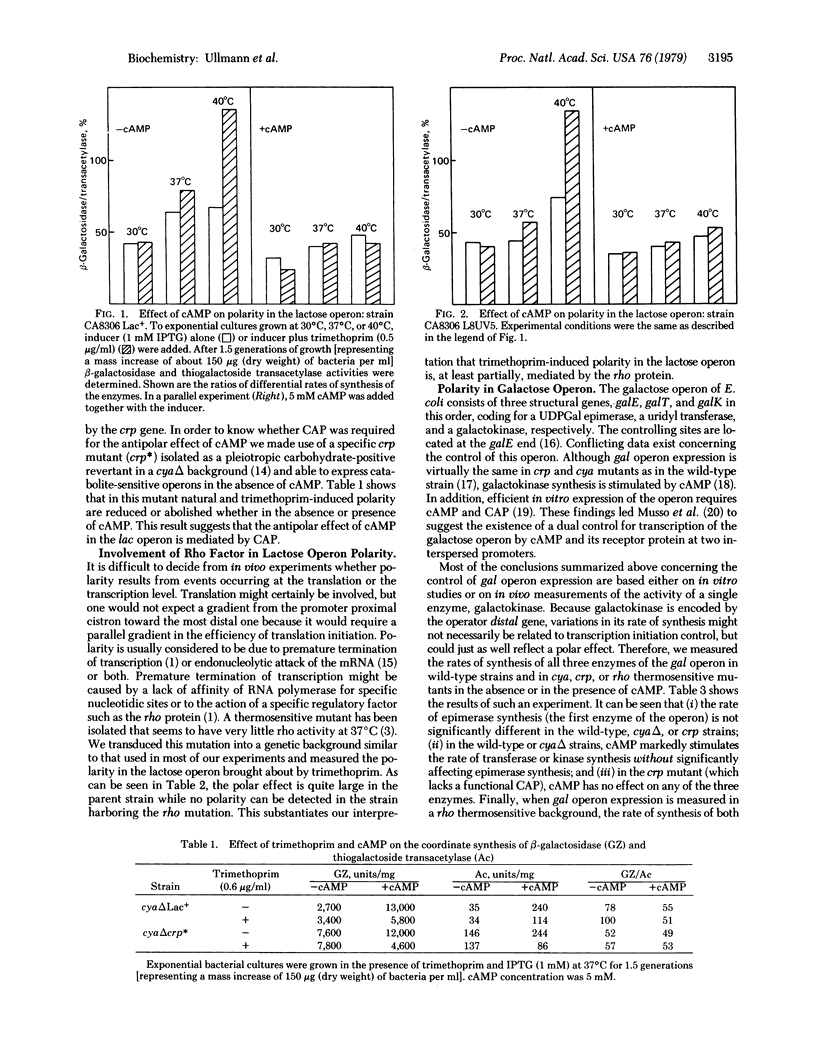
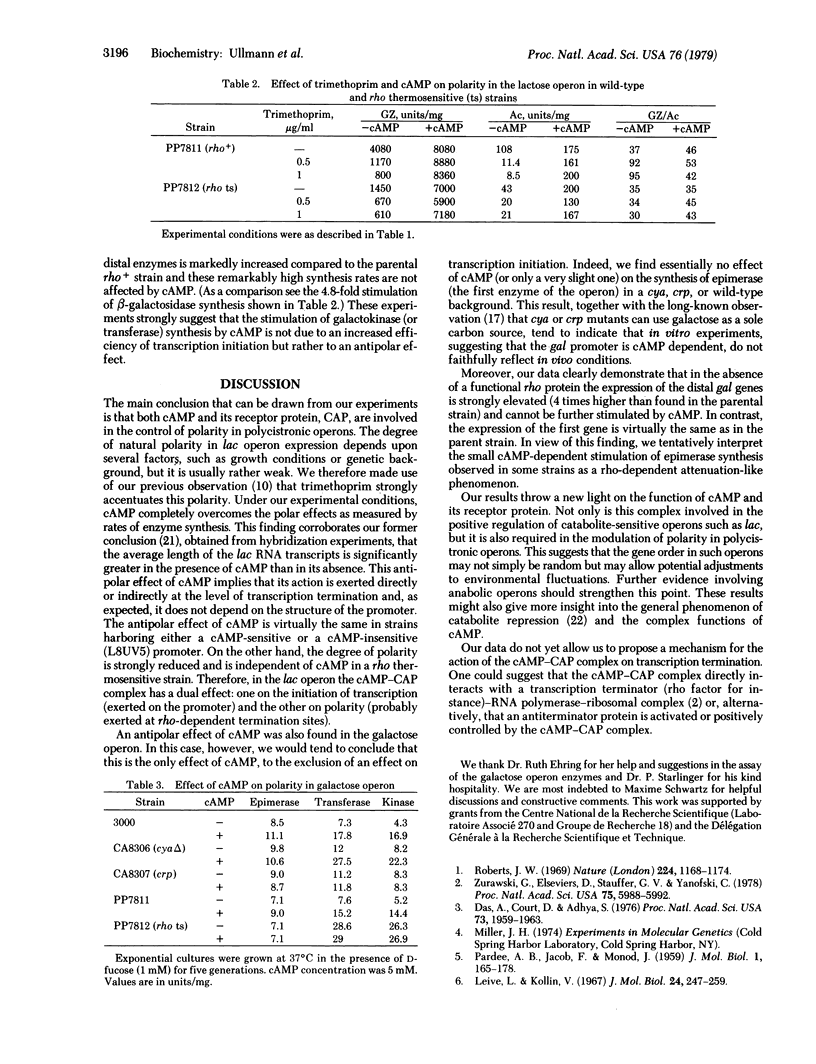
Abstract
The degree of natural polarity in the lactose and galactose operons of Escherichia coli is affected by adenosine 3',5'-cyclic monophosphate (cAMP). This effect, mediated by the cAMP receptor protein, is exerted at sites distinct from the promoter. Experiments performed with a mutant bearing a thermosensitive rho factor activity indicate that cAMP relieves polarity by interfering with transcription termination. Conflicting results in the literature concerning the role of cAMP receptor protein and cAMP in galactose operon expression can be reconciled by the findings that cAMP stimulates the expression of operator distal genes without significantly affecting the proximal genes. Therefore, it appears necessary to reevaluate the classification of the galactose operon as exhibiting cAMP-mediated catabolite repression at the level of transcription initiation.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arditti R., Grodzicker T., Beckwith J. Cyclic adenosine monophosphate-independent mutants of the lactose operon of Escherichia coli. J Bacteriol. 1973 May;114(2):652–655. doi: 10.1128/jb.114.2.652-655.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BUTTIN G. M'ECANISMES R'EGULATEURS DANS LA BIOSYNTH'ESE DES ENZYMES DU M'ETABOLISME DU GALACTOSE CHEZ ESCHERICHIA COLI K12. I. LA BIOSYNTH'ESE INDUITE DE LA GALACTOKINASE ET L'INDUCTION SIMULTAN'EE DE LA S'EQUENCE ENZYMATIQUE. J Mol Biol. 1963 Aug;7:164–182. doi: 10.1016/s0022-2836(63)80044-3. [DOI] [PubMed] [Google Scholar]
- Das A., Court D., Adhya S. Isolation and characterization of conditional lethal mutants of Escherichia coli defective in transcription termination factor rho. Proc Natl Acad Sci U S A. 1976 Jun;73(6):1959–1963. doi: 10.1073/pnas.73.6.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imamoto F., Schlessinger D. Bearing of some recent results on the mechanisms of polarity and messenger RNA stability. Mol Gen Genet. 1974;135(1):29–38. doi: 10.1007/BF00433898. [DOI] [PubMed] [Google Scholar]
- Leive L., Kollin V. Synthesis, utilization and degradation of lactose operon mRNA in Escherichia coli. J Mol Biol. 1967 Mar 14;24(2):247–259. doi: 10.1016/0022-2836(67)90330-0. [DOI] [PubMed] [Google Scholar]
- Musso R. E., Di Lauro R., Adhya S., de Crombrugghe B. Dual control for transcription of the galactose operon by cyclic AMP and its receptor protein at two interspersed promoters. Cell. 1977 Nov;12(3):847–854. doi: 10.1016/0092-8674(77)90283-5. [DOI] [PubMed] [Google Scholar]
- Nisseley S. P., Anderson W. B., Gottesman M. E., Perlman R. L., Pastan I. In vitro transcription of the gal operon requires cyclic adenosine monophosphate and cyclic adenosine monophosphate receptor protein. J Biol Chem. 1971 Aug 10;246(15):4671–4678. [PubMed] [Google Scholar]
- Petersen H. U., Joseph E., Ullmann A., Danchin A. Formylation of initiator tRNA methionine in procaryotic protein synthesis: in vivo polarity in lactose operon expression. J Bacteriol. 1978 Aug;135(2):453–459. doi: 10.1128/jb.135.2.453-459.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts J. W. Termination factor for RNA synthesis. Nature. 1969 Dec 20;224(5225):1168–1174. doi: 10.1038/2241168a0. [DOI] [PubMed] [Google Scholar]
- Rothman-Denes L. B., Hesse J. E., Epstein W. Role of cyclic adenosine 3',5'-monophosphate in the in vivo expression of the galactose operon of Escherichia coli. J Bacteriol. 1973 Jun;114(3):1040–1044. doi: 10.1128/jb.114.3.1040-1044.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabourin D., Beckwith J. Deletion of the Escherichia coli crp gene. J Bacteriol. 1975 Apr;122(1):338–340. doi: 10.1128/jb.122.1.338-340.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao M., Schweiger M. Stimulation of galactokinase synthesis in Escherichia coli by adenosine 3',5'-cyclic monophosphate. J Bacteriol. 1970 Apr;102(1):138–141. doi: 10.1128/jb.102.1.138-141.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanner B. L., Kodaira R., Neidhardt F. C. Regulation of lac operon expression: reappraisal of the theory of catabolite repression. J Bacteriol. 1978 Dec;136(3):947–954. doi: 10.1128/jb.136.3.947-954.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetekam W., Staack K., Ehring R. DNA-dependent in vitro synthesis of enzymes of the galactose operon of Escherichia coli. Mol Gen Genet. 1971;112(1):14–27. doi: 10.1007/BF00266928. [DOI] [PubMed] [Google Scholar]
- Wetekam W., Staack K., Ehring R. Relief of polarity in DNA-dependent cell-free synthesis of enzymes of the galactose operon of Escherichia coli. Mol Gen Genet. 1972;116(3):258–276. doi: 10.1007/BF00269770. [DOI] [PubMed] [Google Scholar]
- Zurawski G., Elseviers D., Stauffer G. V., Yanofsky C. Translational control of transcription termination at the attenuator of the Escherichia coli tryptophan operon. Proc Natl Acad Sci U S A. 1978 Dec;75(12):5988–5992. doi: 10.1073/pnas.75.12.5988. [DOI] [PMC free article] [PubMed] [Google Scholar]


