Abstract
The use of therapeutic drug monitoring (TDM) to optimize beta-lactam dosing in critically ill patients is growing in popularity, although there are limited data describing the potential impact of altered protein binding on achievement of target concentrations. The aim of this study was to compare the measured unbound concentration to the unbound concentration predicted from published protein binding values for seven beta-lactams using data from blood samples obtained from critically ill patients. From 161 eligible patients, we obtained 228 and 220 plasma samples at the midpoint of the dosing interval and trough, respectively, for ceftriaxone, cefazolin, meropenem, piperacillin, ampicillin, benzylpenicillin, and flucloxacillin. The total and unbound beta-lactam concentrations were measured using validated methods. Variabilities in both unbound and total concentrations were marked for all antibiotics, with significant differences being present between measured and predicted unbound concentrations for ceftriaxone and for flucloxacillin at the mid-dosing interval (P < 0.05). The predictive performance for calculating unbound concentrations using published protein binding values was poor, with bias for overprediction of unbound concentrations for ceftriaxone (83.3%), flucloxacillin (56.8%), and benzylpenicillin (25%) and underprediction for meropenem (12.1%). Linear correlations between the measured total and unbound concentrations were observed for all beta-lactams (R2 = 0.81 to 1.00; P < 0.05) except ceftriaxone and flucloxacillin. The percent protein binding of flucloxacillin and the plasma albumin concentration were also found to be linearly correlated (R2 = 0.776; P < 0.01). In conclusion, significant differences between measured and predicted unbound drug concentrations were found only for the highly protein-bound beta-lactams ceftriaxone and flucloxacillin. However, direct measurement of unbound drug in research and clinical practice is suggested for selected beta-lactams.
INTRODUCTION
Infections are common in intensive care units (ICU), with over 50% of patients considered infected at any one time (1). Morbidity and mortality rates remain high among critically ill patients with infection, and antibiotics are the single most effective intervention for reducing mortality rates (2–7). Complicating the likelihood of achieving effective antibiotic therapy are the effects of the complex disease processes that critically ill patients undergo and the associated significant effects on antibiotic pharmacokinetics (PK). For the commonly used family of antibiotics, the beta-lactams, these PK changes, including those relating to altered protein binding, have been shown in numerous studies to result in high proportions of critically ill patients manifesting subtherapeutic or toxic concentrations when standard dosing approaches are used (7–12). Given the association between therapeutic antibiotic exposure and improved patient outcomes (13, 14), the use of therapeutic drug monitoring (TDM) to optimize beta-lactam exposures has been proposed as potentially useful for dose optimization in critically ill patients (8, 15–18).
The existing reports for beta-lactam TDM have all used total antibiotic concentrations for determining the need for dose adjustment, which is problematic given that the unbound concentration of antibiotic is responsible for bacterial killing. The accuracy of such an approach is unclear. Given that hypoalbuminemia occurs in approximately 40% of critically ill patients (19, 20), the potentially negative effects of altered protein binding of beta-lactam antibiotics may be common (21–24). It is likely that direct measurement of unbound antibiotic concentrations should be preferred to the calculation of unbound concentrations from published protein binding values because such calculations may not reflect the unbound beta-lactam plasma concentration in a critically ill patient and therefore reduces the likelihood of optimized dosing. An understanding of protein binding of beta-lactams in this challenging patient population is essential to ensure optimal clinical dose adjustment.
Given the uncertainty regarding protein binding changes in critically ill patients, the aim of this study was to compare the measured unbound concentrations with the unbound concentrations predicted from published protein binding values for seven beta-lactam antibiotics (ceftriaxone, cefazolin, meropenem, piperacillin, ampicillin, benzylpenicillin, and flucloxacillin).
MATERIALS AND METHODS
Patient selection.
This observational study was conducted as part of a beta-lactam TDM program for critically ill patients at a 27-bed tertiary referral ICU. Beta-lactam TDM is provided as a part of routine clinical care in this unit. Approval to collect these data was granted by the local institutional review board (Royal Brisbane and Women's Hospital Human Research Ethics Committee).
Ten antibiotics are included in the routine TDM service: ampicillin, benzylpenicillin, dicloxacillin, flucloxacillin, piperacillin, ceftriaxone, cephalothin, cefazolin, meropenem, and ertapenem (25, 27). Patients were eligible for inclusion in this analysis if they were >18 years old, were receiving one of the selected study antibiotic(s), and were expected to remain on the treatment for the next 24 h. The empirical dosing regimen and subsequent dose adjustment were undertaken by the treating physician in consultation with the clinical pharmacist.
Various demographic and clinical data were collected to describe the patient sample, including age, gender, weight, and plasma albumin and creatinine concentrations. Data for creatinine clearance on the sampling day were also collected, which were estimated from plasma creatinine concentrations by using the Cockcroft-Gault equation (26).
Sampling.
As per the TDM protocol, blood samples were obtained at the assumed PK “steady state,” defined as sampling after administration of at least four prior doses. For intermittent dosing, two plasma samples were obtained, the first one at the midpoint of the dosing interval and the second one immediately prior to redosing (trough concentration). For continuous infusion, plasma samples were taken after at least 4 half-lives. Patients treated with more than one study antibiotic were eligible to provide more than one set of blood samples on the same day.
Beta-lactam assays.
Plasma total and unbound concentrations of beta-lactams were determined by using two different validated high-performance liquid chromatography (HPLC) assays, which were reported previously (25, 27). Briefly, to measure total beta-lactam concentrations, plasma samples were extracted with a known amount of internal standard and deproteinated with acetonitrile. The supernatant was added to chloroform, and the aqueous phase was analyzed by using HPLC. The concentration ranges of the standard curves were 1 to 500 mg/liter for all antibiotics (except for meropenem [1 to 250 mg/liter]). The coefficients of variation for interassay and intra-assay precision were <10%, and the accuracy was within 6% for all antibiotics. To measure unbound drug concentrations, plasma samples were filtered by using an Amicon Ultra 0.5-ml 30,000-molecular-weight-cutoff centrifugal filter device. The ultrafiltrates were mixed with morpholineethanesulfonic acid (MES) buffer (pH 6.6) and analyzed by using HPLC. The concentration ranges of the standard curves were 0.1 to 50 mg/liter for all antibiotics (except for piperacillin [0.1 to 100 mg/liter]). The coefficients of variation for interassay and intra-assay precision were <10%, and the accuracy was within 10% for all antibiotics.
Calculation of unbound concentrations from total concentrations.
Unbound concentrations of antibiotics were calculated from total concentrations by using published percent binding data, as shown in Table 1. In view of the saturable concentration-dependent protein binding kinetics of ceftriaxone, unbound concentrations of ceftriaxone were calculated from total concentrations (Ctot) by using the following equation (28):
where the capacity constant (nP) is 517 mol/liter and the binding affinity constant (kaff) is 0.0367 liters/mol.
Table 1.
Published percentages of protein binding for the study antibiotics
Statistics.
All continuous data were reported as means and standard deviations, or medians and interquartile ranges, as appropriate. Continuous data were analyzed by using the Student t test. Correlation between total and unbound antibiotic concentrations and covariations between continuous demographic and clinical variables with antibiotic concentrations and percentages of protein binding were determined by using linear regression. Bland-Altman plots were constructed with GraphPad (version 6.0a; GraphPad Software Inc.) to assess the agreement between calculated unbound beta-lactam concentrations and measured unbound concentrations. Percent transformation was performed to increase the normality of the data for the constructions of Bland-Altman plots, as evaluated by Spearman's rank correlation coefficient (ρ). Bias, 95% limits of agreement, and corresponding 95% confidence intervals were calculated as previously described (29). P values of <0.05 were considered significant.
RESULTS
One hundred sixty-one patients were eligible for analysis, and their demographic and clinical characteristics are shown in Table 2. Patients were typically male and older than 50 years of age, with a serum albumin concentration below the normal range on the day of sampling. Renal function, as described by plasma creatinine concentrations, was highly variable. Forty-two patients had TDM performed on more than one occasion, and one patient received two beta-lactams simultaneously. Two hundred twenty-eight and 220 mid-dosing and trough samples were assayed, respectively. Due to the limited sensitivity of the assay for total concentrations below 1 mg/liter, 3 mid-dosing-interval concentrations and 23 trough concentrations reported as <1 mg/liter were excluded from the analysis. The total number of samples included in the analyses described below was 422. Seventeen patients received continuous renal replacement therapy (CRRT) on the day of sampling.
Table 2.
Demographics and clinical characteristics of the studied patientsa
Plasma creatinine and plasma albumin concentrations were measured on the day of sampling; other parameters were measured upon admission. IQR, interquartile range; BMI, body mass index.
Creatinine clearance was estimated by using the Cockcroft-Gault formula.
The dosage ranges for the prescribed antibiotics are shown in Table 3. Variabilities in dosing regimens and resultant concentrations were observed, with marked deviations of predicted concentrations from the line of identity, in particular for piperacillin and benzylpenicillin at both sampling times and for trough concentrations of meropenem, ceftriaxone (Fig. 1a), and flucloxacillin. Measured unbound concentrations tended to be higher than predicted for ceftriaxone at all concentrations and for piperacillin, benzylpenicillin, and flucloxacillin especially at higher concentrations. However, measured unbound concentrations were only significantly higher (P < 0.05) than predicted for ceftriaxone at both sampling times and for flucloxacillin at the mid-dosing interval. The percentage of unbound piperacillin was significantly higher at the trough time point in patients receiving CRRT (82.5%, versus 69.6% for non-CRRT patients; P < 0.01). No significant relationship was observed for other studied beta-lactams.
Table 3.
Dosage ranges for studied beta-lactams
Fig 1.
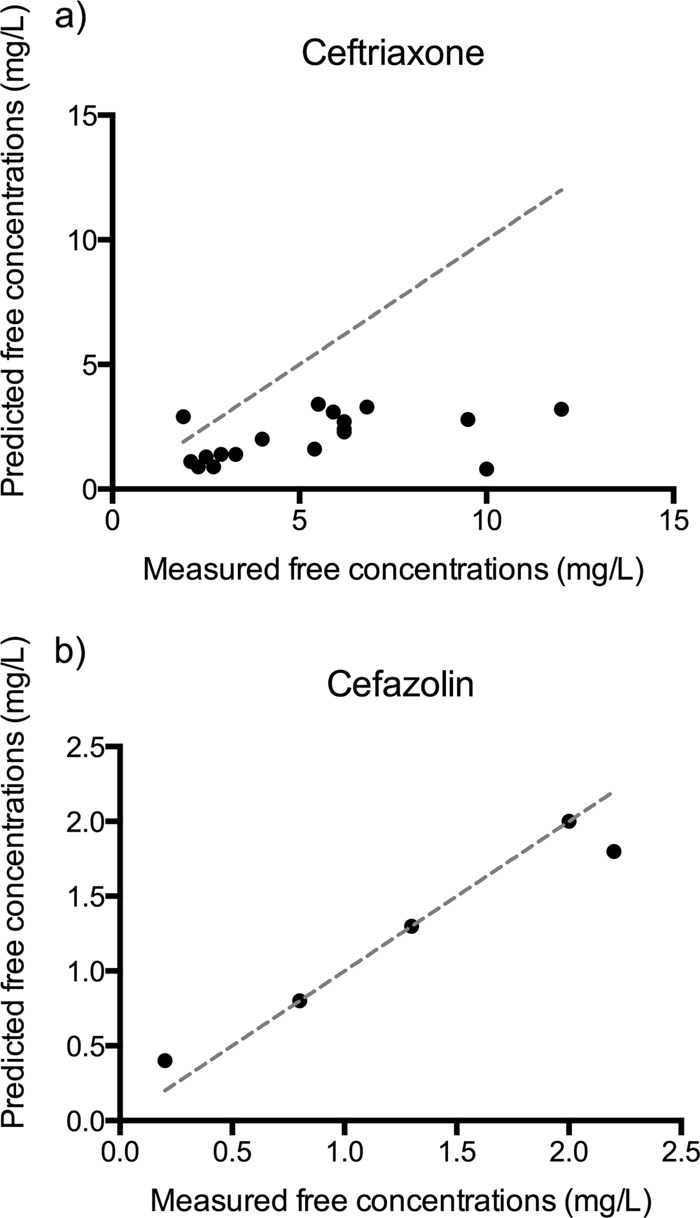
Linear correlation between measured and predicted unbound trough concentrations of ceftriaxone (a) and cefazolin (b) (R2 = 0.96; P = 0.003). The x = y plots are shown as gray dashed lines.
A linear correlation between the measured and predicted unbound concentrations was established for all studied beta-lactams except ceftriaxone and flucloxacillin. Linear regression correlations between total and unbound concentrations described using an R2 value were between 0.81 and 1.00 for beta-lactams (P ≤ 0.001 for all except cefazolin [P = 0.003]) (Fig. 1b). A nonlinear correlation between the measured and predicted unbound concentrations was observed for flucloxacillin (R2 = 0.91).
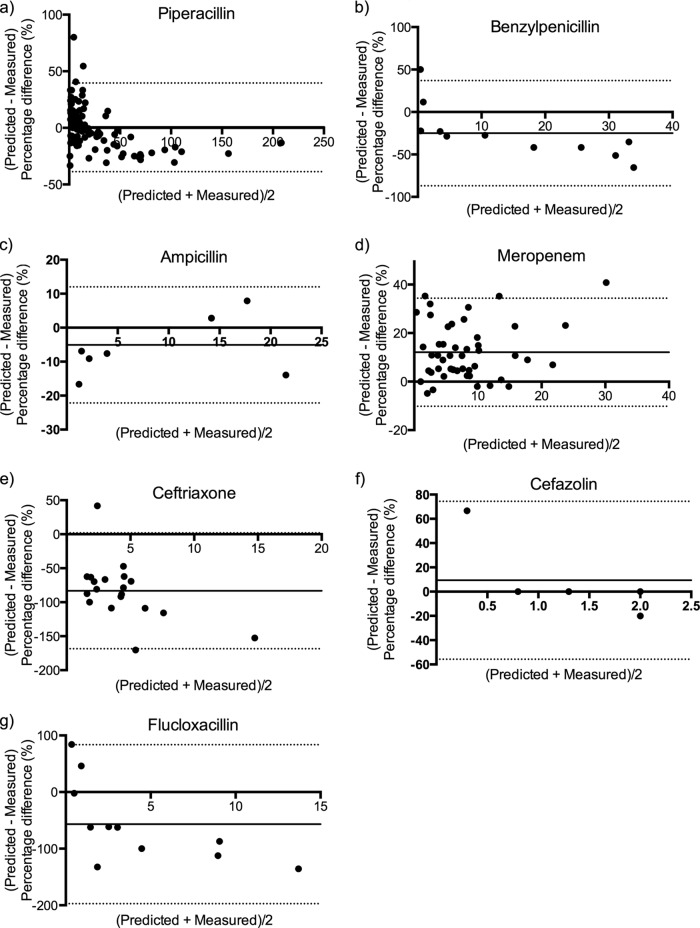
The predictive performance of the calculated unbound beta-lactam trough concentrations was assessed by Bland-Altman plots, as shown in Fig. 2 and Table 4. Biases in calculated unbound concentrations were observed for ceftriaxone, flucloxacillin, and benzylpenicillin, where actual (measured) unbound concentrations were underpredicted. For meropenem and cefazolin, the calculated unbound concentrations were biased for overpredicting unbound concentrations. The 95% limits of agreement for calculated unbound concentrations as a predictor of measured unbound concentrations were wide for the majority of studied beta-lactams.
Fig 2.
Bland-Altman plots of relative difference (percentage of measured unbound concentrations) against the mean of predicted and measured unbound concentrations for piperacillin (n = 94; ρ = −0.51; P < 0.01) (a), ampicillin (n = 8; ρ = 0.42; P = 0.30) (b), benzylpenicillin (n = 11; ρ = −0.92; P < 0.01) (c), meropenem (n = 49; ρ = 0.02; P = 0.91) (d), ceftriaxone (n = 19; ρ = −0.43; P = 0.07) (e), cefazolin (n = 5; ρ = −0.80; P < 0.01) (f), and flucloxacillin (n = 11; ρ = −0.81; P < 0.01) (g). The biases and 95% limits of agreement are shown as solid and broken horizontal lines, respectively.
Table 4.
Performance evaluation of calculated unbound concentrations as a predictor of measured unbound concentrations using bias, 95% limits of agreement, and associated confidence intervals, as determined by Bland-Altman plotsa
| Antibiotic | Bias (%) | 95% CI of bias | 95% limit of agreement | 95% CI (lower limit) | 95% CI (upper limit) |
|---|---|---|---|---|---|
| Piperacillin | 0.43 | −6.6–7.4 | −34.7, 39.6 | −41.7–−27.7 | 33.6–46.6 |
| Ampicillin | −5.08 | −15.5–5.4 | −22.2, 12.0 | −32.7–−11.7 | 1.5–22.5 |
| Benzylpenicillin | −25.00 | −57.4–7.4 | −86.97, 37.0 | −119.3–−54.6 | 4.6–69.4 |
| Meropenem | 12.08 | 6.6–17.6 | −10.2, 34.4 | −15.7–−4.7 | 28.8–39.9 |
| Ceftriaxone | −83.30 | −117.2–−49.4 | −168.5, 1.9 | −202.4–−134.6 | −32.0–35.8 |
| Cefazolin | 9.33 | −41.1–59.7 | −55.7, 74.4 | −106.1–−5.3 | 24.0–124.8 |
| Flucloxacillin | −56.80 | −130.1–16.5 | −197.2, 83.6 | −270.5–−123.9 | 10.3–156.9 |
Data are presented as percentages relative to percentage protein binding. CI, confidence interval.
No significant associations were found between the percentage of binding and albumin concentrations for any of the beta-lactams studied except flucloxacillin (R2 = 0.76; P < 0.01). For cefazolin, the relationship between percentage of protein binding and albumin concentrations was not analyzed due to inadequate albumin concentration data available for those subjects.
DISCUSSION
In this study of critically ill patients, we compared the observed unbound concentration of beta-lactam antibiotics with the unbound concentration predicted by using published protein binding values. These data confirm the high variability and in some cases the unpredictability of unbound beta-lactam concentrations in critically ill patients. The present work is unique in terms of the number of antibiotics studied and the evaluation of predictive performance for calculating unbound beta-lactam concentrations in critically ill patients by using published protein binding values.
The efficacy of beta-lactams has been well defined according to the time that the unbound (or free) concentration exceeds the MIC (fT>MIC) of the bacterial pathogen. Traditionally, most assays used in PK studies of critically ill patients measure total beta-lactam antibiotic concentrations and subsequently calculate unbound concentrations from published plasma protein binding data that have been obtained from non-critically-ill patient groups.
Since variations in protein binding and the prevalence of hypoalbuminemia among critically ill patients have been observed in other studies (21, 25, 30), there is increasing concern regarding the accuracy of this estimation, especially for highly protein-bound drugs in critically ill patients. Since the time course of unbound beta-lactam concentrations is more relevant than the total concentration, direct measurement of the unbound fraction has been suggested to have potential advantage in antibiotic dose optimization for critically ill patients. In this study, we utilized a rapid and inexpensive assay for measurement of unbound beta-lactam concentrations in clinical practice. The data presented again demonstrate severely altered PK of beta-lactams in critically ill patients.
As expected, in our cohort of critically ill patients with low plasma albumin concentrations (mean, 24.5 g/liter), significant differences between predicted (from total concentrations) and measured unbound concentrations were found for the highly protein-bound antibiotics ceftriaxone and flucloxacillin. The mean percentage of protein binding for ceftriaxone (87.3 to 87.7%) determined in this study lies within the lower limits (83 to 96%) found in healthy volunteers (31) yet is similar to that found in a group of surgical critically ill patients (85.5 to 91.5%) (32). However, when we used a saturable model of ceftriaxone protein binding with published binding parameters to predict unbound concentrations from our total concentration data, the percentage of protein binding was approximated to be around 95%, suggesting an overestimation of protein binding by the model when applied to our patient cohort. Nevertheless, no significant correlation between albumin concentrations and the unbound fraction of ceftriaxone was found in this study. On the other hand, a correlation between albumin concentrations and the unbound fraction was found for flucloxacillin, as observed previously in cohorts of septic neonates and adult critically ill patients, with both groups having lower-than-normal plasma albumin concentrations (30, 33). Of note, significant differences between measured and calculated unbound flucloxacillin concentrations were found only at the higher concentrations at the mid-dosing time point but not at the trough time point. This may reflect the nonlinearity of protein binding at high concentrations and, thus, poor prediction of unbound values in this range.
A reduction of the unbound fraction for meropenem from 98% in healthy volunteers to a median of <90% was observed among our patients. Despite this fact, there was no significant difference between measured and predicted unbound meropenem concentrations, possibly due to its relatively low fraction of protein binding, where a small change in the percentage of binding would alter the unbound drug concentrations only minimally (34, 42). Our data demonstrate that plasma protein binding of beta-lactams in critically ill patients is highly variable, and a correlation with albumin concentration exists only for selected agents. Although linear correlations between total and unbound concentrations exist for some of the studied beta-lactams, the predictive performance of calculated unbound concentrations was of concern in terms of underdosing, especially for piperacillin at low concentrations (<50 mg/liter) and for meropenem, where unbound concentrations were consistently underpredicted at a limited but considerable magnitude. Another important finding from this analysis was that for ceftriaxone, benzylpenicillin, and flucloxacillin, the observed unbound concentration was higher than the predicted concentration, meaning that dose adjustments based on the low predicted unbound concentrations may not always be required. Dose adjustments for possible concentration-related adverse events may also not be accurate in this context. The unbound concentration assay used in this study is an inexpensive and convenient means to overcome the limitation of predicting unbound drug concentrations under these circumstances.
Limitations of the study.
First, the variability of clinical conditions and interventions that can vary beta-lactam PK in critically ill patients as well as the small cohort of patients and small number of samples available for the analysis of some antibiotics (namely, cefazolin, benzylpenicillin, and flucloxacillin) could be considered limitations of this study. Nevertheless, this is the largest data set of unbound measurements of these drugs. Second, the assay used in this study has limited sensitivity for total beta-lactam concentrations of <1 mg/liter, such that conversion from total to unbound concentrations was not established for this low concentration range. Finally, the 95% limits of agreement determined by Bland-Altman plots depend on the assumption that the differences between the two measurement methods are constant throughout the range of measurements and follow a Gaussian distribution. Although percent transformation (or logarithmic transformation [data not shown]) improved the distribution of our data, the percent differences between predicted and measured unbound concentrations still significantly varied with the means of the two measurements for benzylpenicillin, flucloxacillin, piperacillin, and cefazolin. However, the analysis used provides sufficient information on bias and precision of calculating unbound concentrations from total measured concentrations to conclude whether the predictive performance of the calculated unbound concentrations is adequate in the clinical context.
In summary, this is the first paper that directly compares measured total and unbound beta-lactam antibiotic concentrations in critically ill patients. We found a high variability in beta-lactam concentrations and plasma protein binding in a cohort of critically ill patients. A correlation between percent protein binding and plasma albumin concentrations was observed only for flucloxacillin. Given the variability of unbound beta-lactam concentrations in critically ill patients and the clinical importance of unbound drug concentrations, utilization of an inexpensive and convenient assay for determination of unbound drug concentrations in research and clinical practice is suggested.
Footnotes
Published ahead of print 30 September 2013
REFERENCES
- 1. Vincent J-L, Rello J, Marchall JC, Silva E, Anzueto A, Martin CD, Moreno R, Lipman J, Gomersall CD, Sakr Y, Reinhart K, EPIC II Group of Investigators. 2009. International study of the prevalence and outcomes of infection in intensive care units. J. Am. Med. Assoc. 302:2323–2329 [DOI] [PubMed] [Google Scholar]
- 2. Kollef M, Sherman G, Ward S, Fraser V. 1999. Inadequate antimicrobial treatment of infections: a risk factor for hospital mortality among critically ill patients. Chest 115:462–474 [DOI] [PubMed] [Google Scholar]
- 3. Garnacho-Montero J, Garcia-Garmendia JL, Barrero-Almodovar A, Jimenez-Jimenez F, Perez-Paredes C, Ortiz-Leyba C. 2003. Impact of adequate empirical antibiotic therapy on the outcome of patients admitted to the intensive care unit with sepsis. Crit. Care Med. 31:2742–2751 [DOI] [PubMed] [Google Scholar]
- 4. Harbarth S, Garbino J, Pugin J, Romand JA, Lew D, Pittet D. 2003. Inappropriate initial antimicrobial therapy and its effect on survival in a clinical trial of immunomodulating therapy for severe sepsis. Am. J. Med. 115:529–535 [DOI] [PubMed] [Google Scholar]
- 5. MacArthur R, Miller M, Albertson T. 2004. Adequacy of early empiric antibiotic treatment and survival in severe sepsis: experience from the MONARCS trial. Clin. Infect. Dis. 38:284–288 [DOI] [PubMed] [Google Scholar]
- 6. Eagye KJ, Kuti JL, Dowzicky M, Nicolau DP. 2007. Empiric therapy for secondary peritonitis: a pharmacodynamic analysis of cefepime, ceftazidime, ceftriaxone, imipenem, levofloxacin, piperacillin/tazobactam, and tigecycline using Monte Carlo simulation. Clin. Ther. 29:889–899 [DOI] [PubMed] [Google Scholar]
- 7. Yost RJ, Cappelletty DM, RECEIPT Study Group. 2011. The Retrospective Cohort of Extended-Infusion Piperacillin-Tazobactam (RECEIPT) study: a multicenter study. Pharmacotherapy 31:767–775 [DOI] [PubMed] [Google Scholar]
- 8. Roberts JA, Ulldemolins M, Roberts MS, McWhinney B, Ungerer J, Paterson DL, Lipman J. 2010. Therapeutic drug monitoring of beta-lactams in critically ill patients: proof of concept. Int. J. Antimicrob. Agents 36:332–339 [DOI] [PubMed] [Google Scholar]
- 9. Taccone FS, Cotton F, Roisin S, Vincent J-L, Jacobs F. 2012. Optimal meropenem concentrations to treat multidrug-resistant Pseudomonas aeruginosa septic shock. Antimicrob. Agents Chemother. 56:2129–2131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Verdier MC, Tribut O, Tattevin P, Michelet C, Bentue-Ferrer D. 2011. Assessment of interindividual variability of plasma concentrations after administration of high doses of intravenous amoxicillin or cloxacillin in critically ill patients. J. Chemother. 23:277–281 [DOI] [PubMed] [Google Scholar]
- 11. Zelenitsky SA, Ariano RE, Zhanel GG. 2011. Pharmacodynamics of empirical antibiotic monotherapies for an intensive care unit (ICU) population based on Canadian surveillance data. J. Antimicrob. Chemother. 66:343–349 [DOI] [PubMed] [Google Scholar]
- 12. Udy AA, Varghese JM, Altukroni M, Briscoe S, McWhinney B, Ungerer J, Lipman J, Roberts JA. 2012. Sub-therapeutic initial β-lactam concentrations in select critically ill patients. Chest 142:30–39 [DOI] [PubMed] [Google Scholar]
- 13. Ariano RE, Nyhlen A, Donnelly JP, Sitar DS, Harding GK, Zelenitsky SA. 2005. Pharmacokinetics and pharmacodynamics of meropenem in febrile neutropenic patients with bacteremia. Ann. Pharmacother. 39:32–38 [DOI] [PubMed] [Google Scholar]
- 14. McKinnon PS, Paladino JA, Schentag JJ. 2008. Evaluation of area under the inhibitory curve (AUIC) and time above the minimum inhibitory concentration (T>MIC) as predictors of outcome for cefepime and ceftazidime in serious bacterial infections. Int. J. Antimicrob. Agents 31:345–351 [DOI] [PubMed] [Google Scholar]
- 15. Roberts JA, Hope WW, Lipman J. 2010. Therapeutic drug monitoring of beta-lactams for critically ill patients: unwarranted or essential? Int. J. Antimicrob. Agents 35:419–420 [DOI] [PubMed] [Google Scholar]
- 16. Hayashi Y, Lipman J, Udy AA, Ng M, McWhinney B, Ungerer J, Lust K, Roberts JA. 2013. Beta-lactam therapeutic drug monitoring in the critically ill: optimising drug exposure in patients with fluctuating renal function and hypoalbuminaemia. Int. J. Antimicrob. Agents 41:162–166 [DOI] [PubMed] [Google Scholar]
- 17. Patel BM, Paratz J, See NC, Muller MJ, Rudd M, Paterson DL, Briscoe S, Ungerer J, McWhinney BC, Lipman J, Roberts JA. 2012. Therapeutic drug monitoring of beta-lactam antibiotics in burns patients—a one-year prospective study. Ther. Drug Monit. 34:160–164 [DOI] [PubMed] [Google Scholar]
- 18. Smith N, Freebairn R, Park M, Wallis S, Roberts J, Lipman J. 2012. Therapeutic drug monitoring when using cefepime in CRRT: seizures associated with cefepime. Crit. Care Resusc. 14:312–315 [PubMed] [Google Scholar]
- 19. Kushner I. 1982. The phenomenon of the acute phase response. Ann. N. Y. Acad. Sci. 389:39–48 [DOI] [PubMed] [Google Scholar]
- 20. SAFE Study Investigators, Finfer S, Bellomo R, McEvoy S, Lo S, Myburgh J, Neal B, Norton R. 2006. Effect of baseline serum albumin concentration on outcome of resuscitation with albumin or saline in patients in intensive care units: analysis of data from the Saline versus Albumin Fluid Evaluation (SAFE) study. BMJ 333:1044–1049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Joynt GM, Lipman J, Gomersall CD, Young RJ, Wong ELY, Gin T. 2001. The pharmacokinetics of once-daily dosing of ceftriaxone in critically ill patients. J. Antimicrob. Chemother. 47:421–429 [DOI] [PubMed] [Google Scholar]
- 22. Merrikin DJ, Briant J, Rolinson GN. 1983. Effect of protein binding on antibiotic activity in vivo. J. Antimicrob. Chemother. 11:233–238 [DOI] [PubMed] [Google Scholar]
- 23. Mimoz O, Soreda S, Padoin C, Tod M, Petitjean O, Benhamou D. 2000. Ceftriaxone pharmacokinetics during iatrogenic hydroxyethyl starch-induced hypoalbuminemia. Anesthesiology 93:735–743 [DOI] [PubMed] [Google Scholar]
- 24. Brink AJ, Richards GA, Schillack V, Kiem S, Schentag JJ. 2009. Pharmacokinetics of once-daily dosing of ertapenem in critically ill patients with severe sepsis. Int. J. Antimicrob. Agents 33:432–436 [DOI] [PubMed] [Google Scholar]
- 25. Briscoe S, McWhinney B, Lipman J, Roberts JA, Ungerer JP. 2012. A method for determining the free (unbound) concentration of ten beta-lactam antibiotics in human plasma using high performance liquid chromatography with ultraviolet detection. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 907:178–184 [DOI] [PubMed] [Google Scholar]
- 26. Cockcroft D, Gault M. 1976. Prediction of creatinine clearance from serum creatinine. Nephron 16:31–41 [DOI] [PubMed] [Google Scholar]
- 27. McWhinney B, Wallis SC, Hillister T, Roberts JA, Lipman J, Ungerer J. 2010. Analysis of 12 beta-lactam antibiotics in human plasma by HPLC with ultraviolet detection. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 878:2036–2043 [DOI] [PubMed] [Google Scholar]
- 28. Garot D, Respaud R, Lanotte P, Simon N, Mercier E, Ehrmann S, Perrotin D, Dequin P-F, Le Guellec C. 2011. Population pharmacokinetics of ceftriaxone in critically ill septic patients: a reappraisal. Br. J. Clin. Pharmacol. 72:758–767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bland JM, Altman DG. 2003. Applying the right statistics: analyses of measurement studies. Ultrasound Obstet. Gynecol. 22:85–93 [DOI] [PubMed] [Google Scholar]
- 30. Ulldemolins M, Roberts JA, Wallis SC, Rello J, Lipman J. 2010. Flucloxacillin dosing in critically ill patients with hypoalbuminaemia: special emphasis on unbound pharmacokinetics. J. Antimicrob. Chemother. 65:1771–1778 [DOI] [PubMed] [Google Scholar]
- 31. Stoeckel K, McNamara PJ, Brandt R, Plozza-Nottebrock H, Ziegler WH. 1981. Effects of concentration-dependent plasma protein binding on ceftriaxone kinetics. Clin. Pharmacol. Ther. 29:650–657 [DOI] [PubMed] [Google Scholar]
- 32. Heinemeyer G, Link J, Weber W, Meschede V, Roots I. 1990. Clearance of ceftriaxone in critical care patients with acute renal failure. Intensive Care Med. 16:448–453 [DOI] [PubMed] [Google Scholar]
- 33. Pullen J, Stolk LML, Degraeuwe PLJ, van Tiel FH, Neef C, Zimmerman LJI. 2007. Protein binding of flucloxacillin in neonates. Ther. Drug Monit. 29:279–283 [DOI] [PubMed] [Google Scholar]
- 34. Wise R, Gillett AP, Cadge B, Durham SR, Baker S. 1980. The influence of protein binding upon tissue fluid levels of six beta-lactam antibiotics. J. Infect. Dis. 124:77–82 [DOI] [PubMed] [Google Scholar]
- 35. Pfizer 2012. Piperacillin sodium and tazobactam sodium (Zosyn) product information. Pfizer, New York, NY [Google Scholar]
- 36. Craig WA. 1997. The pharmacology of meropenem, a new carbapenem antibiotic. Clin. Infect. Dis. 24(Suppl 2):S266–S275. 10.1093/clinids/24.Supplement_2.S266 [DOI] [PubMed] [Google Scholar]
- 37. Adnan S, Paterson DL, Lipman J, Kumar S, Li J, Rudd M, Roberts JA. 2012. Pharmacokinetics of beta-lactam antibiotics in patients with intra-abdominal disease: a structured review. Surg. Infect. 13:9–17 [DOI] [PubMed] [Google Scholar]
- 38. Burgess DS, Frei CR, Lewis JS, II, Fiebelkorn KR, Jorgensen JH. 2007. The contribution of pharmacokinetic-pharmacodynamic modelling with Monte Carlo simulation to the development of susceptibility breakpoints for Neisseria meningitidis. Clin. Microbiol. Infect. 13:33–39 [DOI] [PubMed] [Google Scholar]
- 39. van Kralingen S, Taks M, Diepstraten J, van de Garde EM, van Dongen EP, Wiezer MJ, van Ramshorst B, Vlaminckx B, Deneer VH, Knibbe CA. 2011. Pharmacokinetics and protein binding of cefazolin in morbidly obese patients. Eur. J. Clin. Pharmacol. 67:985–992 [DOI] [PubMed] [Google Scholar]
- 40. Petri WA., Jr 2012. Penicillins, cephalosporins, and other beta-lactam antibiotics, p 1477–1504 In Brunton LL, Chabner B, Knollman B. (ed), Goodman and Gilman's the pharmacological basis of therapeutics, 12th ed. McGraw-Hill Medical, New York, NY [Google Scholar]
- 41. Chambers HF. 2009. Penicillins and beta-lactam inhibitors, p 309–322 In Mandell GL, Bennett JE, Dolin R. (ed), Mandell, Douglas, and Bennett's principles and practice of infectious diseases, 7th ed, vol 1 Elsevier Inc, Philadelphia, PA [Google Scholar]
- 42. Roberts JA, Pea F, Lipman J. 2013. The clinical relevance of plasma protein binding changes. Clin. Pharmacokinet. 52:1–8 [DOI] [PubMed] [Google Scholar]