Abstract
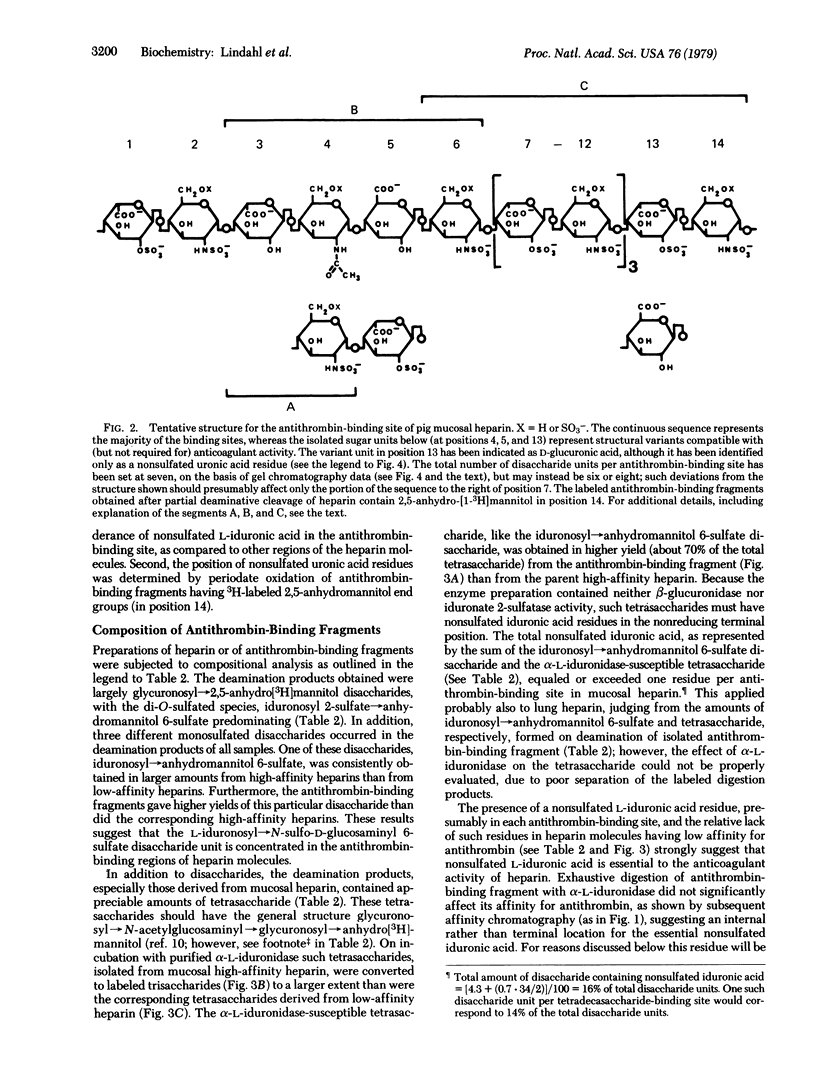
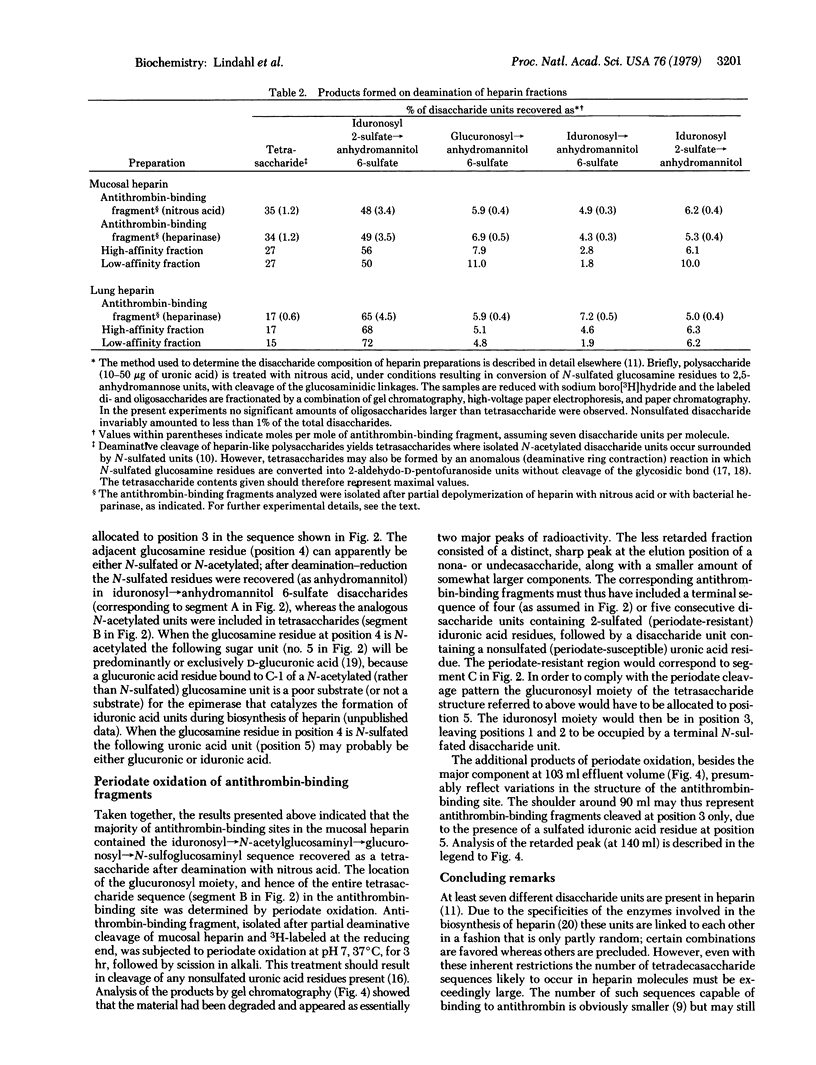
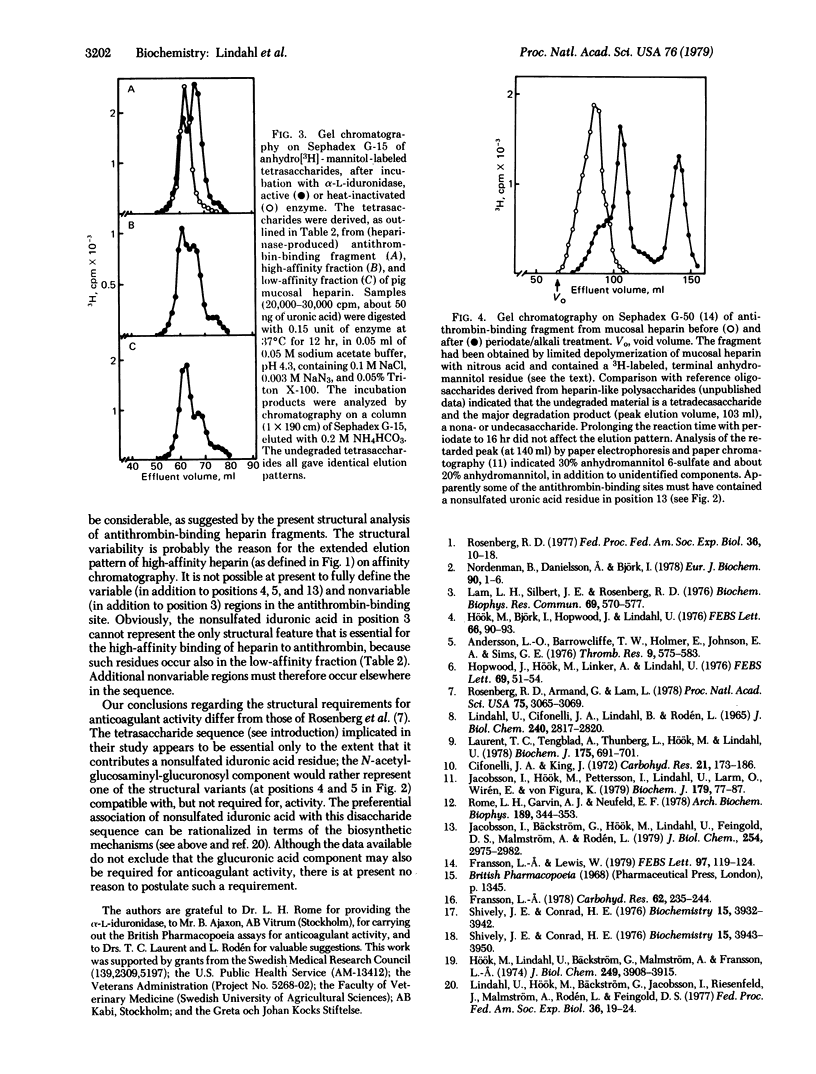
Heparin preparations from pig intestinal mucosa and from bovine lung were separated by chromatography on antithrombin-Sepharose into a high-affinity fraction (with high anticoagulant activity) and a low-affinity fraction (with low anticoagulant). Antithrombin-binding heparin fragments (12-16 monosaccharide units) were prepared, either by digesting a high-affinity heparin-antithrombin complex with bacterial heparinase or by partial deaminative cleavage of the unfractionated polysaccharide with nitrous acid followed by affinity chromatography on immobilized antithrombin. Compositional analysis based on separation and identification of deamination products reduced with sodium boro[3H]hydride showed that nonsulfated L-iduronic acid occurred in larger amounts in high-affinity heparin than in low-affinity heparin; furthermore, this component was concentrated in the antithrombin-binding regions of the high-affinity heparin molecules, amounting to approximately one residue per binding site. It is suggested that nonsulfated L-iduronic acid is essential for the anticoagulant activity of heparin. The location of the non-sulfated uronic acid in the antithrombin-binding site was determined by periodate oxidation of antithrombin-binding fragments containing a terminal 2,5-anhydro-D-[1-3H]mannitol unit. Tentative structures for antithrombin-binding sequences in heparin are proposed, including some structural variants believed to be compatible with, but not required for, activity.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersson L. O., Barrowcliffe T. W., Holmer E., Johnson E. A., Sims G. E. Anticoagulant properties of heparin fractionated by affinity chromatography on matrix-bound antithrombin iii and by gel filtration. Thromb Res. 1976 Dec;9(6):575–583. doi: 10.1016/0049-3848(76)90105-5. [DOI] [PubMed] [Google Scholar]
- Bäckström G., Hök M., Lindahl U., Feingold D. S., Malmström A., Rodén L., Jacobsson I. Biosynthesis of heparin. Assay and properties of the microsomal uronosyl C-5 epimerase. J Biol Chem. 1979 Apr 25;254(8):2975–2982. [PubMed] [Google Scholar]
- Cifonelli J. A., King J. The distribution of 2-acetamido-2-deoxy-D-glucose residues in mammalian heparins. Carbohydr Res. 1972 Feb;21(2):173–186. doi: 10.1016/s0008-6215(00)82144-8. [DOI] [PubMed] [Google Scholar]
- Fransson L. A., Lewis W. Relationship between anticoagulant activity of heparin and susceptibility to periodate oxidation. FEBS Lett. 1979 Jan 1;97(1):119–123. doi: 10.1016/0014-5793(79)80065-4. [DOI] [PubMed] [Google Scholar]
- Hopwood J., Hök M., Linker A., Lindahl U. Anticoagulant activity of heparin: isolation of antithrombin-binding sites. FEBS Lett. 1976 Oct 15;69(1):51–54. doi: 10.1016/0014-5793(76)80651-5. [DOI] [PubMed] [Google Scholar]
- Hök M., Björk I., Hopwood J., Lindahl U. Anticoagulant activity of heparin: separation of high-activity and low-activity heparin species by affinity chromatography on immobilized antithrombin. FEBS Lett. 1976 Jul 1;66(1):90–93. doi: 10.1016/0014-5793(76)80592-3. [DOI] [PubMed] [Google Scholar]
- Hök M., Lindahl U., Bäckström G., Malmström A., Fransson L. Biosynthesis of heparin. 3. Formation of iduronic acid residues. J Biol Chem. 1974 Jun 25;249(12):3908–3915. [PubMed] [Google Scholar]
- Jacobsson I., Hök M., Pettersson I., Lindahl U., Larm O., Wirén E., von Figura K. Identification of N-sulphated disaccharide units in heparin-like polysaccharides. Biochem J. 1979 Apr 1;179(1):77–87. doi: 10.1042/bj1790077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LINDAHL U., CIFONELLI J. A., LINDAHL B., RODEN L. THE ROLE OF SERINE IN THE LINKAGE OF HEPARIN TO PROTEIN. J Biol Chem. 1965 Jul;240:2817–2820. [PubMed] [Google Scholar]
- Lam L. H., Silbert J. E., Rosenberg R. D. The separation of active and inactive forms of heparin. Biochem Biophys Res Commun. 1976 Mar 22;69(2):570–577. doi: 10.1016/0006-291x(76)90558-1. [DOI] [PubMed] [Google Scholar]
- Laurent T. C., Tengblad A., Thunberg L., Hök M., Lindahl U. The molecular-weight-dependence of the anti-coagulant activity of heparin. Biochem J. 1978 Nov 1;175(2):691–701. doi: 10.1042/bj1750691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindahl U., Hök M., Bäckström G., Jacobsson I., Riesenfeld J., Malmström A., Rodén L., Feingold D. S. Structure and biosynthesis of heparin-like polysaccharides. Fed Proc. 1977 Jan;36(1):19–24. [PubMed] [Google Scholar]
- Nordenman B., Danielsson A., Björk I. The binding of low-affinity and high-affinity heparin to antithrombin. Fluorescence studies. Eur J Biochem. 1978 Sep 15;90(1):1–6. doi: 10.1111/j.1432-1033.1978.tb12567.x. [DOI] [PubMed] [Google Scholar]
- Rome L. H., Garvin A. J., Neufeld E. F. Human kidney alpha-L-iduronidase: purification and characterization. Arch Biochem Biophys. 1978 Aug;189(2):344–353. doi: 10.1016/0003-9861(78)90221-7. [DOI] [PubMed] [Google Scholar]
- Rosenberg R. D., Armand G., Lam L. Structure-function relationships of heparin species. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3065–3069. doi: 10.1073/pnas.75.7.3065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg R. D. Chemistry of the hemostatic mechanism and its relationship to the action of heparin. Fed Proc. 1977 Jan;36(1):10–18. [PubMed] [Google Scholar]
- Shively J. E., Conrad H. E. Formation of anhydrosugars in the chemical depolymerization of heparin. Biochemistry. 1976 Sep 7;15(18):3932–3942. doi: 10.1021/bi00663a005. [DOI] [PubMed] [Google Scholar]
- Shively J. E., Conrad H. E. Nearest neighbor analysis of heparin: identification and quantitation of the products formed by selective depolymerization procedures. Biochemistry. 1976 Sep 7;15(18):3943–3950. doi: 10.1021/bi00663a006. [DOI] [PubMed] [Google Scholar]



