Abstract
The opportunistic human pathogen Enterococcus faecium overproduces the low-affinity PBP5. In clinical strains, mutations in PBP5 further reduce its acylation rate by β-lactams. Previous studies have reported that ceftaroline had poor inhibitory activity against β-lactam-resistant E. faecium strains. In this study, we show that ceftaroline exhibits killing activity against our laboratory-derived ampicillin-resistant E. faecium mutant that overproduces a wild-type PBP5 and that ceftaroline inactivates PBP5 much faster than benzylpenicillin and faster than ceftobiprole.
TEXT
β-Lactam antibiotics (penicillins, cephalosporins, carbapenems, and monobactams) represent the most important group of drugs prescribed to treat bacterial infections. They form stable acyl-enzymes with their targets, the membrane-bound d, d-transpeptidases, which are essential enzymes in peptidoglycan biosynthesis. These proteins are usually referred to as penicillin-binding proteins (PBPs) (1–3). The presence or overproduction of lower-affinity PBPs is responsible for the resistance of Gram-positive cocci against β-lactam antibiotics. It is known that the opportunistic human pathogen Enterococcus faecium overproduces the low-affinity PBP5 and that mutations further reduce the acylation rate of PBP5 by altering its amino acid sequence (4, 5). Ceftaroline is a cephalosporin with broad-spectrum activity against Gram-positive organisms, including methicillin-resistant Staphylococcus aureus (MRSA), and many common Gram-negative bacteria except those producing extended-spectrum β-lactamase (ESBL) or AmpC β-lactamase (6–8). The Staphylococcus aureus low-affinity PBP2a is closely related to PBP5 (9). It is inhibited by ceftaroline, the active metabolite of the prodrug ceftaroline fosamil, which was recently approved by the U.S. Food and Drug Administration (FDA) for use in adult patients with acute bacterial skin and skin-structure infections and community-acquired bacterial pneumonia and more recently approved by the European Medicines Agency (EMA) for similar indications (10, 11). Previous studies have reported that ceftaroline had poor inhibitory activity against β-lactam-resistant E. faecium strains, all of which were expected to possess a PBP5 protein (6, 12). To better understand these differences, we have characterized the interaction between a wild-type form of PBP5 and ceftaroline.
Ceftaroline MIC values determined by the microdilution method (13) for E. faecium D63r and D63 strains (5) were 2 and 0.25 mg · liter−1, respectively. These values contrasted with those reported by others (10–12), who have found that most ampicillin-resistant E. faecium clinical isolates were resistant to ceftaroline and concluded that ceftaroline had little activity against most isolates of E. faecium. The killing effects (14) of ceftaroline on E. faecium D63 and D63r strains were studied by exposing exponentially growing cultures of both strains to increasing concentrations of antibiotics corresponding to 1 and 4 times their respective MICs (Fig. 1). Ceftaroline showed killing effects (1 log drop after 24 h) for concentrations higher than the MICs. We determined the 50% inhibitory concentration (IC50) values of ceftaroline, benzylpenicillin, cefepime, and ceftazidime on the various PBPs of E. faecium D63 and D63r by using purified membrane preparations and fluorescent ampicillin (5, 15). Titration of the PBPs by ceftaroline (Fig. 2 and Table 1) revealed that at twice the MIC values, all high-molecular-mass PBPs were inhibited by the antibiotic (Fig. 2) and that the low-molecular-mass PBP6, a d, d-carboxypeptidase (16), was not affected. This inhibition pattern is completely different from that obtained with benzylpenicillin, for which PBP5 is the most resistant PBP (17). The IC50s for ceftaroline on PBP5 were 1.40 ± 0.09 mg/liter and 0.7 mg/liter when determined with membrane preparations and the purified sPBP5, respectively. The kinetic parameters governing the acylation of PBP5 by ceftaroline were determined by using the sPBP5 (a soluble PBP5 from which the N-terminal membrane anchoring peptide was removed), which was overproduced and purified as previously described, except that the molecular sieve step was eliminated (18). The kinetics model used has been described previously (17). On the basis of the data shown in Fig. 3, the second-order rate constant k+2/K for ceftaroline was 950 ± 70 M−1 s−1, i.e., 50 to 100 times higher than the value reported for benzylpenicillin (15 to 24 M−1 s−1), which indicates that ceftaroline inactivates sPBP5 much faster than benzylpenicillin (5) and faster than ceftobiprole, another anti-MRSA cephalosporin (k+2/K = 110 ± 10 M−1 s−1 [13]). While the inhibitory activity of ceftaroline for E. faecium PBP5 is significant, the k+2/K rate constant for sPBP5 is 15 to 25 times lower than those determined for MRSA PBP2a (23,600 ± 2,000 M−1 s−1 [unpublished data]) produced and purified in our laboratory (19) and for the penicillin-resistant Streptococcus pneumoniae PBP2x (12,600 M−1 s−1) (20). The sPBP5-ceftaroline adduct was very stable. Indeed, no free enzyme could be detected after a 4-hour incubation of the isolated acyl-enzyme at 37°C.
Fig 1.
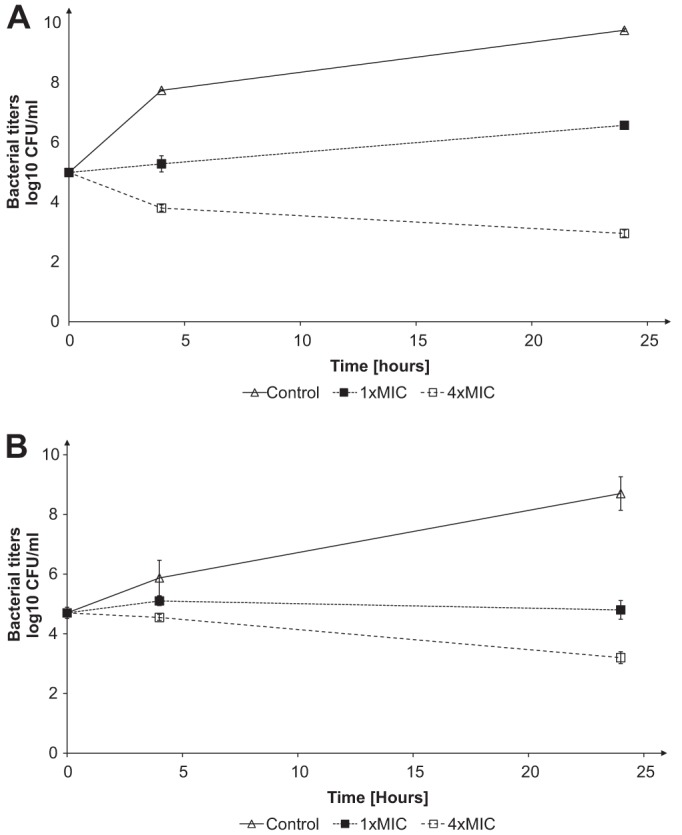
Drug binding studies for ceftaroline with D63 and D63r membrane fractions. MICs of D63 (A) and D63r (B) for ceftaroline were 0.25 mg/liter and 2 mg/liter, respectively. The surviving bacteria were counted after 0, 4, and 24 h of incubation at 37°C by subculturing serial dilutions (at least 10-fold, to minimize drug carryover). For all data, the standard deviations were below 10% of the mean.
Fig 2.
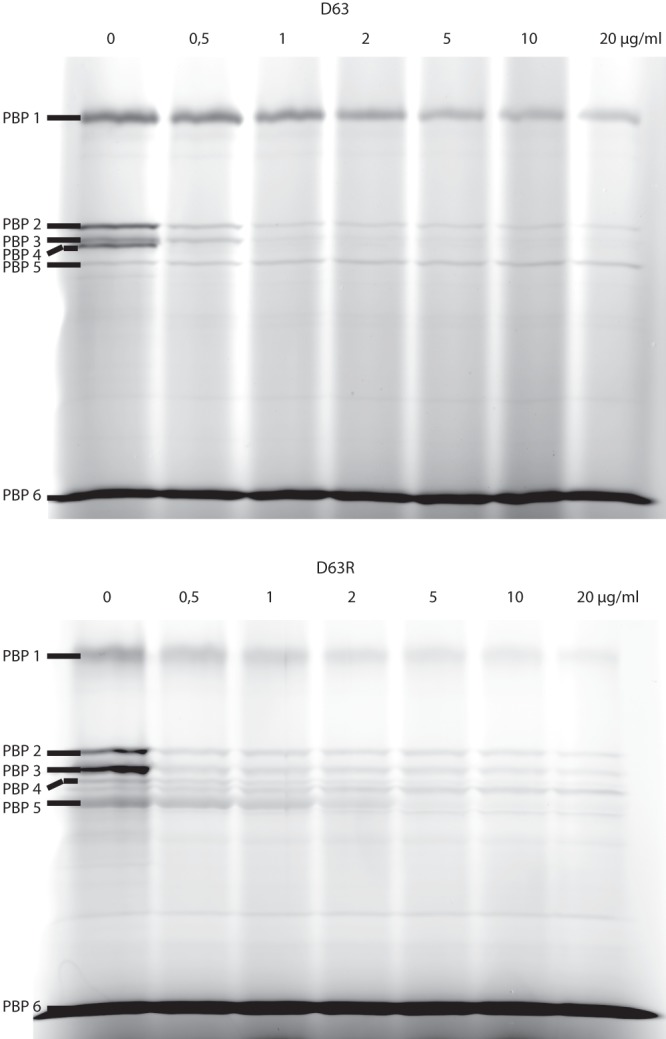
PBP binding studies for ceftaroline with membrane fractions from D63 and D63r. The concentration of ceftaroline in the competition assay with fluorescent ampicillin is indicated in μg/ml.
Table 1.
Inhibition of PBPs from E. faecium D63 and D63r strains
| Strain | Antibiotic | IC50a (mg/liter) for PBP: |
MIC (mg/liter) | |||||
|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | |||
| D63r | Benzylpenicillin | 1.6 ± 0.4 | 0.06 ± 0.01 | 0.6 ± 0.3 | 8 ± 5 | 55 ± 15 | 2 ± 1 | 70 |
| Cefepime | 1.7 ± 0.6 | 0.6 ± 0.3 | 0.5 ± 0.1 | >200 | >200 | >50 | >100 | |
| Ceftazidime | 0.9 ± 0.6 | 0.5 ± 0.3 | 0.04 ± 0.01 | >200 | >200 | >50 | >100 | |
| Ceftobiprole | 0.7 ± 0.1 | 0.2 ± 0.1 | 0.5 ± 0.1 | 1.8 ± 0.2 | 1 ± 0.2 | >16 | 8 | |
| Ceftaroline | 0.63 ± 0.09 | 0.07 ± 0.01 | 0.06 ± 0.01 | 0.97 ± 0.03 | 1.38 ± 0.1 | >50 | 2 | |
| D63 | Benzylpenicillin | 0.9 ± 0.7 | 0.1 ± 0.07 | 1.3 ± 0.6 | 10 ± 8 | 75 ± 25 | 1.5 ± 1 | 5 |
| Ceftobiprole | 0.6 ± 0.2 | 0.2 ± 0.1 | 0.6 ± 0.2 | 1.5 ± 0.3 | 0.7 ± 0.1 | >16 | 2 | |
| Ceftaroline | 0.64 ± 0.04 | 0.07 ± 0.01 | 0.06 ± 0.01 | 0.212 ± 0.03 | 0.53 ± 0.03 | >50 | 0.25 | |
Concentration of β-lactam antibiotic that reduced binding of fluorescent ampicillin by 50% compared to a control containing no drug. IC50s for D63r PBP5 against antibiotics other than ceftaroline were previously published (17).
Fig 3.
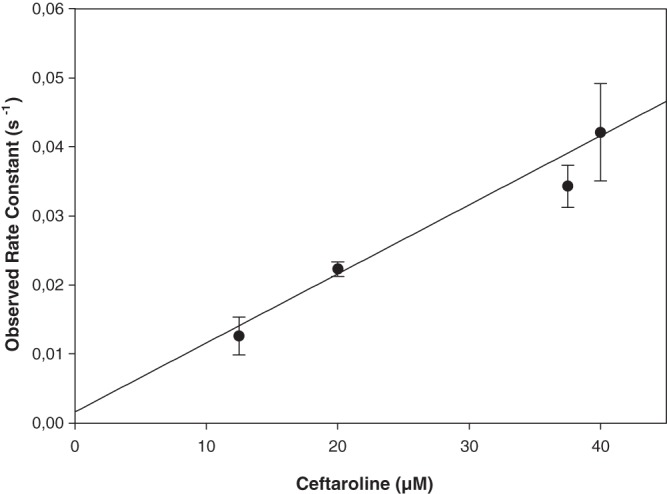
Variation of the pseudo-first-order inactivation rate constant (ka) as a function of ceftaroline concentration. The opening of the ceftaroline β-lactam ring due to the acylation reaction was monitored by recording the decrease of absorbance at 300 nm (Δε = 4,330 M−1 cm−1) with a Specord 200 spectrophotometer (Analytik Jena, Germany), at 30°C in 10 mM phosphate buffer (pH 7.0) with 10 μM sPBP5.
We have shown that ceftaroline inactivates the low-affinity E. faecium PBP5 more efficiently than benzylpenicillin. It exhibits bacterial killing against our laboratory-derived ampicillin-resistant E. faecium mutant that overproduces the wild-type PBP5. Such a profile differs from those of most E. faecium clinical isolates, which were reported as resistant to all β-lactams, including ceftaroline (6). Like other cephalosporins, ceftaroline is not indicated for treatment of enterococcal infections. It is likely that most E. faecium clinical isolates produce mutant PBP5 forms with reduced affinity. This suggests that simple overexpression of wild-type PBP5 is sufficient to moderately increase the MIC for ceftaroline but that amino acid substitutions in the protein are necessary to confer high-level resistance. Among the β-lactams tested to date, ceftaroline has the highest affinity for wild-type PBP5, which makes it a potentially useful tool for PBP5 studies.
ACKNOWLEDGMENTS
We acknowledge the support of the Belgian Federal Government (IUAP program P7/44 iPROS). This study was supported by Forest Laboratories, Inc. Scientific Therapeutics Information, Inc., provided editorial coordination, which was funded by Forest Research Institute, Inc.
Ceftaroline powder was supplied by Forest Laboratories, Inc.
Forest Laboratories, Inc., had no involvement in the design, collection, analysis, interpretation of data, and decision to present these results.
Footnotes
Published ahead of print 23 September 2013
REFERENCES
- 1. Ghuysen JM. 1988. Bacterial active-site serine penicillin-interactive proteins and domains: mechanism, structure, and evolution. Rev. Infect. Dis. 10:726–732 [DOI] [PubMed] [Google Scholar]
- 2. Waxman DJ, Strominger JL. 1983. Penicillin-binding proteins and the mechanism of action of beta-lactam antibiotics. Annu. Rev. Biochem. 52:825–869 [DOI] [PubMed] [Google Scholar]
- 3. Sauvage E, Kerff F, Terrak M, Ayala JA, Charlier P. 2008. The penicillin-binding proteins: structure and role in peptidoglycan biosynthesis. FEMS Microbiol. Rev. 32:234–258 [DOI] [PubMed] [Google Scholar]
- 4. Rice LB, Carias LL, Hutton-Thomas R, Sifaoui F, Gutmann L, Rudin SD. 2001. Penicillin-binding protein 5 and expression of ampicillin resistance in Enterococcus faecium. Antimicrob. Agents Chemother. 45:1480–1486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zorzi W, Zhou XY, Dardenne O, Lamotte J, Raze D, Pierre J, Gutmann L, Coyette J. 1996. Structure of the low-affinity penicillin-binding protein 5 PBP5fm in wild-type and highly penicillin-resistant strains of Enterococcus faecium. J. Bacteriol. 178:4948–4957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ge Y, Biek D, Talbot GH, Sahm DF. 2008. In vitro profiling of ceftaroline against a collection of recent bacterial clinical isolates from across the United States. Antimicrob. Agents Chemother. 52:3398–3407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sader HS, Fritsche TR, Jones RN. 2008. Antimicrobial activities of ceftaroline and ME1036 tested against clinical strains of community-acquired methicillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 52:1153–1155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sader HS, Fritsche TR, Kaniga K, Ge Y, Jones RN. 2005. Antimicrobial activity and spectrum of PPI-0903M (T-91825), a novel cephalosporin, tested against a worldwide collection of clinical strains. Antimicrob. Agents Chemother. 49:3501–3512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Goffin C, Ghuysen JM. 1998. Multimodular penicillin-binding proteins: an enigmatic family of orthologs and paralogs. Microbiol. Mol. Biol. Rev. 62:1079–1093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Moisan H, Pruneau M, Malouin F. 2010. Binding of ceftaroline to penicillin-binding proteins of Staphylococcus aureus and Streptococcus pneumoniae. J. Antimicrob. Chemother. 65:713–716 [DOI] [PubMed] [Google Scholar]
- 11. Villegas-Estrada A, Lee M, Hesek D, Vakulenko SB, Mobashery S. 2008. Co-opting the cell wall in fighting methicillin-resistant Staphylococcus aureus: potent inhibition of PBP 2a by two anti-MRSA beta-lactam antibiotics. J. Am. Chem. Soc. 130:9212–9213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mushtaq S, Warner M, Ge Y, Kaniga K, Livermore DM. 2007. In vitro activity of ceftaroline (PPI-0903M, T-91825) against bacteria with defined resistance mechanisms and phenotypes. J. Antimicrob. Chemother. 60:300–311 [DOI] [PubMed] [Google Scholar]
- 13. Clinical and Laboratory Standards Institute 2006. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically, 7th ed. Clinical and Laboratory Standards Institute, Wayne, PA [Google Scholar]
- 14. Small PM, Chambers HF. 1990. Vancomycin for Staphylococcus aureus endocarditis in intravenous drug users. Antimicrob. Agents Chemother. 34:1227–1231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zhao G, Meier TI, Kahl SD, Gee KR, Blaszczak LC. 1999. Bocillin FL, a sensitive and commercially available reagent for detection of penicillin-binding proteins. Antimicrob. Agents Chemother. 43:1124–1128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. el Kharroubi A, Piras G, Jacques P, Szabo I, Van Beeumen J, Coyette J, Ghuysen JM. 1989. Active-site and membrane topology of the DD-peptidase/penicillin-binding protein no. 6 of Enterococcus hirae (Streptococcus faecium) A.T.C.C. 9790. Biochem. J. 262:457–462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Henry X, Amoroso A, Coyette J, Joris B. 2010. Interaction of ceftobiprole with the low-affinity PBP 5 of Enterococcus faecium. Antimicrob. Agents Chemother. 54:953–955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sauvage E, Kerff F, Fonze E, Herman R, Schoot B, Marquette JP, Taburet Y, Prevost D, Dumas J, Leonard G, Stefanic P, Coyette J, Charlier P. 2002. The 2.4-A crystal structure of the penicillin-resistant penicillin-binding protein PBP5fm from Enterococcus faecium in complex with benzylpenicillin. Cell. Mol. Life Sci. 59:1223–1232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lemaire S, Glupczynski Y, Duval V, Joris B, Tulkens PM, Van Bambeke F. 2009. Activities of ceftobiprole and other cephalosporins against extracellular and intracellular (THP-1 macrophages and keratinocytes) forms of methicillin-sensitive (MSSA) and methicillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 53:2289–2297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zervosen A, Zapun A, Frere JM. 2013. Inhibition of Streptococcus pneumoniae penicillin-binding protein 2x and Actinomadura R39 DD-peptidase activities by ceftaroline. Antimicrob. Agents Chemother. 57:661–663 [DOI] [PMC free article] [PubMed] [Google Scholar]


