Abstract
The intracellular bacterial pathogen Listeria monocytogenes activates a robust type I interferon response upon infection. This response is partially dependent on the multidrug resistance (MDR) transporter MdrM and relies on cyclic-di-AMP (c-di-AMP) secretion, yet the functions of MdrM and cyclic-di-AMP that lead to this response are unknown. Here we report that it is not MdrM alone but a cohort of MDR transporters that together contribute to type I interferon induction during infection. In a search for a physiological function of these transporters, we revealed that they play a role in cell wall stress responses. A mutant with deletion of four transporter genes (ΔmdrMTAC) was found to be sensitive to sublethal concentrations of vancomycin due to an inability to produce and shed peptidoglycan under this stress. Remarkably, c-di-AMP is involved in this phenotype, as overexpression of the c-di-AMP phosphodiesterase (PdeA) resulted in increased susceptibility of the ΔmdrMTAC mutant to vancomycin, whereas overexpression of the c-di-AMP diadenylate cyclase (DacA) reduced susceptibility to this drug. These observations suggest a physiological association between c-di-AMP and the MDR transporters and support the model that MDR transporters mediate c-di-AMP secretion to regulate peptidoglycan synthesis in response to cell wall stress.
INTRODUCTION
Listeria monocytogenes is a Gram-positive, facultative, intracellular pathogen that invades a wide range of mammalian cells (1). Following internalization, the bacteria escape to the cell cytosol by secreting several virulence factors, primarily the pore-forming hemolysin, listeriolysin O (LLO). Once in the cytosol, L. monocytogenes replicates and spreads from cell to cell by recruiting host actin filaments (1). During infection, L. monocytogenes triggers a robust type I interferon response, as manifested by enhanced expression and secretion of the cytokine beta interferon (IFN-β) (2). This response was shown to be independent of Toll-like receptors but reliant on several innate immune signaling molecules (i.e., STING, TBK-1, and IRF3) (3–6). Remarkably, the bacteria must be replicating in the macrophages' cytosol to elicit a type I interferon response, as phagosomally trapped bacteria, such as an LLO-negative mutant, do not induce this response (2). A previous study aimed at identifying L. monocytogenes determinants involved in IFN-β activation identified multidrug resistance (MDR) transporters as modulators of the type I interferon response in vivo (7). Specifically, overexpression in bacteria of two closely related MDR transporters, MdrM and MdrT, was found to trigger enhanced induction of IFN-β by infected macrophages. However, only deletion of the mdrM gene resulted in reduced levels of IFN-β secreted by infected macrophages (7). This observation indicated that MdrM plays an active role during bacterial cytosolic growth that leads to induction of the type I interferon response.
MdrM and MdrT belong to the major facilitator superfamily (MFS) of MDR transporters and are closely related to the well-characterized MDR transporter, QacA, of Staphylococcus aureus (8). MDR transporters, such as QacA, are notorious for their ability to confer resistance to a wide variety of toxic compounds and drugs, including antibiotics, by utilizing proton motive force to actively extrude these compounds outside the cell (9). Accordingly, MdrM and MdrT were shown to be transcriptionally induced upon bacterial exposure to rhodamine 6G (R6G) and tetraphenylphosphonium (TPP), both well-known substrates of MDRs, and to confer resistance to cholic acid (7, 10). Nevertheless, none of these classical MDR functions could explain the observed role of these proteins in activating the innate immune system, implying they might possess distinct physiological roles during infection.
It was recently proposed that MdrM and MdrT transporters extrude cyclic-di-AMP (c-di-AMP) during L. monocytogenes intracellular growth, which in turn activates infected macrophages to elicit the IFN-β response (11, 12). Indeed, c-di-AMP activates a robust type I interferon response when added exogenously; however, a physiological association between c-di-AMP and the MDR transporters was not established. Notably, several reports had indicated that c-di-AMP serves as a second messenger molecule that influences central cellular processes of bacteria: e.g., genome surveillance, response to cell wall stresses, and, more recently, peptidoglycan homeostasis (13–17). In bacteria, c-di-AMP is synthesized by diadenylate cyclase (DAC) using ATP as a substrate and, conversely, linearized to 5′-pApA by a specific c-di-AMP phosphodiesterase (PDE) (15). While it was shown that the level of c-di-AMP is largely dependent on the expression levels of DAC and PDE enzymes (15, 18), the mechanism coordinating the activity of these enzymes is not known. The prevalence of DAC domains among bacteria and archaea strengthens the premise that this c-di-AMP is fundamentally involved in microbial physiology (13). The L. monocytogenes genome contains both c-di-AMP dac and pde genes (dacA [lmo2120] and pdeA [lmo0052]). The dacA gene was shown to be essential for growth and to be the gene responsible for c-di-AMP production, while pdeA was shown to degrade c-di-AMP (11, 18).
In the present study, we aimed to identify a physiological association between L. monocytogenes MDR transporters and c-di-AMP. We discovered that MDR transporters play a role in the L. monocytogenes response to cell wall stress and found that this MDR function was linked to c-di-AMP production. More generally, this report furthers the understanding of the molecular mechanism whereby intracellular L. monocytogenes cells trigger type I interferon responses during infection.
MATERIALS AND METHODS
Bacterial strains, cells, growth media, and reagents.
L. monocytogenes strain 10403S was used as the wild-type (WT) strain and as the parental strain for all mutants generated in this work (Table 1). The Escherichia coli XL-1 Blue (Stratagene) and DH12 strains were used for vector propagation. E. coli strain SM-10 (19) was used for conjugative plasmid delivery to L. monocytogenes. L. monocytogenes strains were grown in brain heart infusion (BHI) (BD) medium or minimal medium (20) at 37°C, and E. coli strains were grown in Luria-Bertani (LB) medium (BD) at 37°C. For infection experiments, L. monocytogenes bacteria were grown overnight in BHI at 30°C without agitation. IPTG (isopropyl-β-d-1-thiogalactopyranoside) was purchased from Bio-Lab, Ltd. (Israel), penicillin G, vancomycin hydrochloride, and mutanolysin were purchased from Sigma, and c-di-AMP and c-di-GMP were purchased from the Biolog Institute (Germany). Primary bone marrow-derived (BMD) macrophages were isolated from 6- to 8-week-old female C57BL/6 mice (Harlan Laboratories, Ltd., Israel) and cultured as described previously (21). RAW264 macrophages were grown and maintained in Dulbecco's modified Eagle's medium (DMEM)-based media.
Table 1.
Genes similar to mdrM in L. monocytogenes strain 10403S based on protein sequence
| L. monocytogenes 10403S gene no. | L. monocytogenes EGDe gene identifier no. | % identity, % similarity of amino acid sequence | Intracellular inductiona | Gene name |
|---|---|---|---|---|
| LMRG_02976.6 | lmo1617 | Yes | mdrM | |
| LMRG_02679.6 | lmo2588 | 45, 65 | Yes | mdrT |
| LMRG_00200.6 | lmo0519 | 35, 60 | Yes | mdrA |
| LMRG_02080.6 | lmo0981 | 25, 46 | No | |
| LMRG_01853.6 | lmo2845 | 23, 44 | Yes | mdrB |
| LMRG_01880.6 | lmo2818 | 22, 41 | Yes | mdrC |
| LMRG_02296.6 | lmo0872 | 20, 38 | Yes | mdrD |
| LMRG_01872.6 | lmo2826 | 16, 33 | Yes | mdrE |
Based on microarray analysis (23).
Generation of L. monocytogenes in-frame deletion mutants.
Deletion mutants were generated by standard techniques using the pKSV7oriT vector, as described in reference 7. Plasmid pLIV2-mdrM was used for generation of 6×His-tagged MdrM and the F58V mutant (for primers, see Table S1C in the supplemental material).
Protein analysis by Western blotting.
Overnight cultures were diluted 1:100 and grown to an optical density at 600 nm (OD600) of 1 U and then supplemented with 0.25 mM IPTG when indicated. Bacteria were harvested, treated with 50 U of mutanolysin for 1 h, and sonicated in a mixture of 20 mM Tris-HCl (pH 8), 0.5 M NaCl, 1 mM EDTA, and 1 mM phenylmethylsulfonyl fluoride (PMSF). After removal of cell debris, membranes were collected by ultracentrifugation. Membrane fractions (50 μg of protein) were then subjected to 12.5% SDS-PAGE and blotted for His tag detection using India HisProbe-horseradish peroxidase (HRP) (Pierce) and the ECL enhanced chemiluminescence reagent.
Bacterial growth curves.
Overnight cultures were adjusted to an OD600 of 0.03 in 20 ml fresh BHI broth, supplemented when indicated with 0.25 mM IPTG, 0.08 μg ml−1 penicillin G, 1 μg ml−1 vancomycin, or 3 μg ml−1 c-di-AMP. For bacterial RNA extraction, bacteria were grown at 37°C to an OD600 of 0.4 and then supplemented with 3 μg ml−1 lincomycin, 50 μM rhodamine 6 G (R6G), 1 μg ml−1 vancomycin, and 0.08 μg ml−1 penicillin G for 2 h or centrifuged and resuspended in: minimal medium (pH 5) (lactic acid), minimal medium with 10 mM H2O2, or defined minimal medium (pH 5) with 10 mM H2O2 for 30 min. For microscopy, bacteria were grown similarly and supplemented with 1 μg ml−1 vancomycin for 2 h. Growth curves in the presence of drugs were performed in a Synergy HT Biotek plate reader at 37°C with continuous shaking and monitoring of the OD600 every 15 min for 24 h. Of note, bacterial growth in the plate reader is different from that in flasks with respect to the OD levels that are measured. In each experiment, growth conditions are indicated.
L. monocytogenes intracellular growth in cells.
Intracellular growth curves were performed as described previously (22). Briefly, 2 × 106 cells were seeded on a petri dish with glass coverslips and infected with 8 × 106 bacteria. At 0.5 h postinfection (p.i.), cells were washed, and at 1 h p.i., gentamicin was added. At each time point, cells from 3 coverslips were lysed and CFU were counted. For bacterial gene expression of intracellularly grown L. monocytogenes cells, 25 × 106 BMD macrophages were infected with 1 × 108 bacteria and lysed in 20 ml of ice-cold water at 6 h p.i., and the released bacteria were collected on 0.45-μm-pore hydroxyapatite (HA) filters (Millipore, catalog no. HAWP04700).
Gene expression analysis.
RNA was purified from bacteria in mid-log-phase growth in BHI or from infected cells using standard phenol-chloroform extraction methods. RNA from intracellularly grown bacteria was amplified using the MessageAmp II (Ambion) bacterial RNA amplification kit according to the manufacturer's instructions. RNA of infected macrophages was extracted using TRIzol reagent according to standard protocols. In all cases, 1 μg of RNA was reverse transcribed to cDNA using an Applied Biosystems high-capacity reverse transcription kit. Real-time quantitative PCR (RT-qPCR) was performed on 10 ng of cDNA using SYBR green with the Step-One Plus RT-PCR system (Applied Biosystems). The transcription of bacterial genes was normalized using 16S rRNA or the rpoB gene, and that of macrophage cytokines was normalized using the glyceraldehyde 3-phosphate dehydrogenase gene (gpdh). Statistical analysis was performed using the StepOne V2.1 software. Error bars represent 95% confidence intervals; in a case where the error bars of two samples do not overlap, the P value is ≪0.01. Primer sequences are described in Table S1A and B in the supplemental material. The complete intracellular expression profile of L. monocytogenes 10403S was published separately (23).
β-Galactosidase MUG assay for mdrC transcription.
Overnight cultures of WT L. monocytogenes pPL2-PmdrClacZ and ΔmdrMTA pPL2-PmdrClacZ cells were adjusted to an OD600 of 0.05. Cultures were grown in 96-well black plates (200 μl) with a clear bottom to an OD600 of ∼0.4 at 37°C. Next, the plates were centrifuged for 10 min at 3,800 rpm, supernatants were aspirated, and the cells were washed twice with phosphate-buffered saline (PBS). Two hundred microliters of ABT buffer (60 mM K2HPO4, 40 mM KH2PO4, 100 mM NaCl, 0.1% Triton X-100 [pH 7]), supplemented with 80 μg ml−1 of MUG substrate (4-methylumbelliferyl-β-d-galactopyranoside) (Sigma), was added to each well. Plates were shaken for 30 s and incubated at room temperature for 1 h in the dark. Following incubation, the optical density (600 nm) and the fluorescence intensity (excitation, 360 nm; emission, 460 nm) were measured using a Synergy HT Biotek plate reader. β-Galactosidase activity was normalized to the sample's OD (24, 25). The experiment was performed in triplicate and was repeated three times independently.
TEM.
Bacteria were grown as described above with and without vancomycin treatment. For negative staining, PBS-washed bacteria were adsorbed on Formvar carbon-coated grids and stained with 2% aqueous uranyl acetate. For transmission electron microscopy (TEM) sections, a bacterial pellet from 20 ml of culture was fixed in 2.5% glutaraldehyde in PBS at 4°C for 20 h, washed three times with PBS, and postfixed in 1% OsO4 in PBS at 4°C for 2 h. Dehydration was carried out in graded ethanol and embedding in glycid ether. Thin sections were mounted on Formvar carbon-coated grids and stained with uranyl acetate and lead citrate. All images were acquired using a Jeol 1200 EX transmission electron microscope (Jeol, Japan). Measurements of cell wall thickness were performed from three independent biological repeats; a total of 35 frames were taken for each strain and condition.
Mouse infection.
L. monocytogenes bacteria were grown in BHI medium at 30°C overnight. Six- to 8-week-old C57BL/6 female mice (Harlan Laboratories, Ltd., Israel) were infected via tail vein injections with 4 × 104 washed bacteria (5 mice in each group). Spleens and livers were harvested 72 h p.i. and homogenized in 0.2% saponin, and bacterial CFU were determined by plating. The experiment was repeated twice.
Measurement of peptidoglycan synthesis rate.
Overnight bacterial culture was diluted 1:100 into 10 ml of BHI, grown to an OD600 of 0.4, and supplemented with 20 μM N-acetylglucosamine and 10 μl of 1 μCi μl−1 of 14C-N-acetylglucosamine (American Radiolabeled Chemicals). The culture then was divided into two parts, and 0.8 μg ml−1 of vancomycin was added to one of them. One-hundred-microliter aliquots from cultures incubated without agitation at 37°C were withdrawn in triplicate every 30 min and added to 100 μl of boiling 8% SDS, and the mixture was incubated for 5 min at 95°C. Cell wall was collected on 0.45-μm-pore-size membrane filters (Millipore, catalog no. HAWP02500), washed with 15 ml of water, and counted using 5 ml of EcoLite(+) liquid scintillation cocktail with a PerkinElmer TriCarb 3110TR β-counter.
Peptidoglycan extraction and muropeptide analysis.
Cell wall and peptidoglycan were purified as described previously (26). Muropeptides were generated from highly purified cell wall and peptidoglycan samples by mutanolysin and then reduced using sodium borohydride. Muropeptide separation was performed by high-performance liquid chromatography (HPLC) as previously described for L. monocytogenes (27, 28). For activation of cytokines by cell wall samples, lyophilized cell wall extracts were resuspended at a concentration of 1.5 mg ml−1, and then the pH was adjusted to 7.5 with NaOH, and 20 μl was added to 2 × 106 BMD macrophages in 2.5 ml medium. After 6 h, macrophage RNA was harvested and analyzed for cytokine induction.
RESULTS
A functional MdrM transporter is required to trigger macrophages to elicit the IFN-β response.
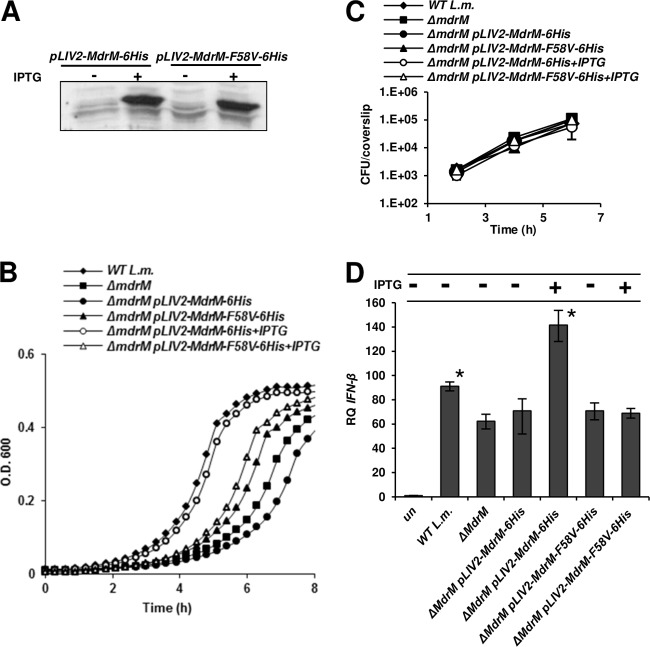
As mentioned, L. monocytogenes bacteria overexpressing the MdrM transporter have been shown to trigger infected macrophages to express enhanced IFN-β levels (7). Initially, we wanted to validate that this enhancement of the IFN-β response requires MdrM to be functional. To this end, an mdrM mutant was generated that harbored a mutation that inactivates function but preserves expression. In the S. aureus QacA transporter, substitution for tyrosine at position 63 with valine resulted in a nonfunctional transporter and enhanced sensitivity of the bacteria to a wide variety of drugs (29). Therefore, using site-directed mutagenesis, the corresponding phenylalanine in MdrM at position 58, F58, was substituted with valine. The resulting mdrM-F58V gene construct was tagged with histidine at the 3′ end and cloned into the integrative pLIV2 vector under an IPTG-inducible promoter to generate pLIV2-mdrM-F58V-6His. This plasmid or a control plasmid containing the His-tagged native mdrM gene (pLIV2-mdrM-6His) was conjugated to a ΔmdrM mutant, and the expression levels of the native and mutated MdrM were compared by Western blotting. Indeed, both the native MdrM and MdrM-F58V proteins were expressed and found in the membrane fraction at similar levels upon IPTG addition (Fig. 1A). Next, the ability of the MdrM and MdrM-F58V proteins to confer resistance to R6G was tested. As expected, the ΔmdrM mutant was more sensitive to R6G than the wild-type (WT) bacteria were, and introduction of the native mdrM gene (via pLIV2-mdrM-6His with IPTG induction) rescued the sensitivity. However, introduction of MdrM-F58V did not restore full growth, providing support that the F58V mutation does interfere with MdrM's transport function (Fig. 1B). Next, the capacity of this mutant to enhance the IFN-β response was tested. To this end, macrophages were infected with the ΔmdrM mutant harboring the pLIV2 plasmid expressing the native or the mutated MdrM. As shown in Fig. 1C, all strains grew to a similar extent intracellularly (Fig. 1C). Notably, only bacteria overexpressing the native MdrM induced an enhanced IFN-β response, while bacteria overexpressing the mutated MdrM did not (Fig. 1D). These results indicate that MdrM's function is required for induction of an IFN-β response during infection.
Fig 1.
MdrM transporter function is required for activation of the IFN-β response during macrophage infection. (A) Western blot analysis of bacterial membrane fraction probed for 6×His-tagged MdrM and MdrM-F58V (using HisProbe-HPR) expressed from the IPTG-inducible vector pLIV2, with and without IPTG. (B) Growth in the presence of R6G (3.5 μM) of WT L. monocytogenes (L.m.), the ΔmdrM mutant, and the ΔmdrM mutant harboring pLIV2 expressing MdrM-6×His or MdrM-F58V-6×His, with and without IPTG (1 mM) in BHI. The experiment was performed in a 96-well format in a Synergy HT Biotek plate reader. Growth curves from one representative experiment are shown. Error bars representing the standard deviation of a triplicate sample are hidden by the symbols. (C) Intracellular growth curves of WT L. monocytogenes, the ΔmdrM mutant, or the ΔmdrM mutant harboring pLIV2 expressing MdrM-6×His or MdrM-F58V-6×His, with and without IPTG, in RAW264 macrophages. Representative growth curves are shown. Error bars represent standard deviations of 3 biological repeats. (D) RT-qPCR analysis of IFN-β transcriptional levels in macrophages infected with WT L. monocytogenes, the ΔmdrM mutant, or the ΔmdrM mutant harboring pLIV2 expressing MdrM-6×His or MdrM-F58V-6×His, with and without IPTG, at 6 h postinfection (p.i.). Transcription levels are represented as relative quantity (RQ) compared to levels in uninfected cells (un). The data are an average of 3 independent experiments. Error bars represent 95% confidence intervals. *, P < 0.01 compared to the rest of the samples. The data in panels A to C are representative of at least 3 independent biological repeats.
MdrM transporter and several MDR homologs are transcriptionally induced during intracellular growth.
MDR transporters are known to exhibit functional redundancy due to overlapping substrate specificity (9, 30). Since MdrM was shown to be responsible for a third of the IFN-β induction by infected macrophages (7), we examined if additional transporters are involved in mediation of the IFN-β response. A search of the L. monocytogenes strain 10403S genome for mdrM homologs revealed several genes encoding putative MDR transporters, among them the previously identified gene mdrT (Table 1). On this list, the LMRG_00200.6 gene (an ortholog of lmo0519 in EGD-e), named here mdrA, was highly similar to mdrM and mdrT, with 60% similarity and 45% identity in protein sequence, whereas the other protein genes exhibited only 33 to 44% sequence similarity (Table 1). To gain insight into the potential requirement for these transporters during L. monocytogenes infection, we analyzed their transcription levels during intracellular growth in macrophages. Our previous transcriptomic data from intracellularly grown bacteria indicated that all of these transporters are induced during infection of macrophages, except for LMRG_02080.6 (using microarray analysis [23]) (Table 1). We thus further compared the transcription levels of the induced MDR transporters by real-time quantitative PCR (RT-qPCR) analysis. As shown in Fig. 2, all of the transporters were transcriptionally upregulated during intracellular growth in macrophages, at least 4-fold, over their levels in BHI. These results suggest that each of the transporters might play an active role during L. monocytogenes infection.
Fig 2.
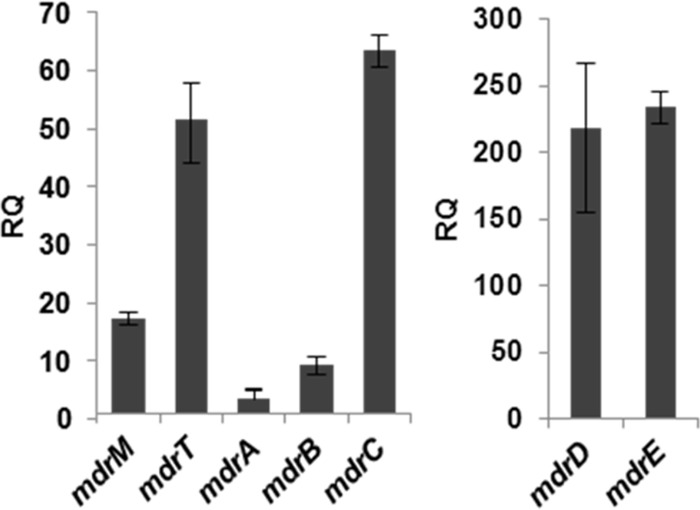
Transcription of MdrM transporter homologs is induced during L. monocytogenes intracellular growth. Transcription analysis of mdrM-like transporter genes during L. monocytogenes 10403S intracellular growth in BMD macrophages at 6 h p.i. in comparison to their levels during exponential growth in BHI medium using RT-qPCR analysis. Transcription levels are represented as relative quantity (RQ) compared to the transcription levels during growth in BHI. If the transcript levels are equal under both conditions, RQ = 1. Transcription levels were normalized to the levels of 16S rRNA as a reference gene. The data are representative of 3 independent biological repeats (n = 3). Error bars represent 95% confidence intervals (as described in Materials and Methods).
A set of MdrM-like transporters control most of the type I interferon response to L. monocytogenes infection and virulence.
To examine whether MdrM homologs contribute to IFN-β induction during infection, we generated a series of in-frame deletion mutants harboring single or multiple (double, triple, quadruple, and quintuple) MDR gene deletions (Table 2). All of the MDR mutants grew similarly to WT bacteria both in BHI broth and intracellularly in macrophages, except for the mdrMTAD mutant, which exhibited a moderate intracellular growth defect (see Fig. S1A and B in the supplemental material). The IFN-β response elicited by macrophages after infection with each of the mutants was evaluated using RT-qPCR analysis of IFN-β transcript levels. Overall, we observed that the greater the number of transporters that were deleted, the lower the IFN-β levels that were expressed by infected cells (Fig. 3A). Notably, macrophages infected with the ΔmdrMTAC quadruple mutant (with the mdrM, mdrT, mdrA, and mdrC genes deleted) exhibited the lowest IFN-β level among the tested mutants, approximately 15% the amount of IFN-β relative to macrophages infected with WT bacteria. Infection with the ΔmdrMTAC mutant was also observed to induce macrophages to transcribe low levels of IL-6 but normal levels IL-1α, indicating that the action of these transporters primarily affects the induction of the type I interferon response (in which both IL-6 and IFN-β are included) (Fig. 3B). The latter observation corroborated the previous characterization of the ΔmdrM mutant showing it to particularly modulate the type I interferon response rather than general proinflammatory responses (7). In summary, this analysis revealed that several MDR transporters, homologs of MdrM, are functionally involved in the activation of the type I interferon response during L. monocytogenes infection.
Table 2.
Bacterial strains used in this study
| Strain | Genotype | Source or reference |
|---|---|---|
| Listeria monocytogenes | ||
| 10403S (WT) | Strr | D. A. Portnoy, lab stock |
| ΔmdrM mutant | ΔmdrM | 7 |
| ΔmdrA mutant | ΔmdrA | This study |
| ΔmdrC mutant | ΔmdrC | This study |
| ΔmdrB mutant | ΔmdrB | This study |
| ΔmdrD mutant | ΔmdrD | This study |
| ΔmdrE mutant | ΔmdrE | This study |
| ΔmdrMC mutant | ΔmdrM ΔmdrC | This study |
| ΔmdrMTA mutant | ΔmdrM ΔmdrT ΔmdrA | This study |
| ΔmdrMTAC mutant | ΔmdrM ΔmdrT ΔmdrA ΔmdrC | This study |
| ΔmdrMTAB mutant | ΔmdrM ΔmdrT ΔmdrA ΔmdrB | This study |
| ΔmdrMTAD mutant | ΔmdrM ΔmdrT ΔmdrA ΔmdrD | This study |
| ΔmdrMTAE mutant | ΔmdrM ΔmdrT ΔmdrA ΔmdrE | This study |
| ΔmdrMTABC mutant | ΔmdrM ΔmdrT ΔmdrA ΔmdrB ΔmdrC | This study |
| ΔmdrM mutant (pLIV2-mdrM-6His) | ΔmdrM pLIV2 expressing MdrM-6×His | 7 |
| ΔmdrM mutant (pLIV2-mdrM-F58V-6His) | ΔmdrM pLIV2 expressing MdrM-F58V-6×His | This study |
| ΔmdrMTAC mutant (pLIV2-mdrM-6His) | ΔmdrMTAC pLIV2 expressing MdrM-6×His | This study |
| ΔmdrMTAC mutant (pLIV2-mdrT) | ΔmdrMTAC pLIV2-mdrT | This study |
| ΔmdrMTAC mutant (pLIV2-mdrA) | ΔmdrMTAC pLIV2-mdrA | This study |
| ΔmdrMTAC mutant (pLIV2-mdrC) | ΔmdrMTAC pLIV2-mdrC | This study |
| WT (pLIV2-pdeA) | pLIV2-pdeA | This study |
| ΔmdrMTAC mutant (pLIV2-pdeA) | ΔmdrM ΔmdrT ΔmdrA ΔmdrC pLIV2-pdeA | This study |
| WT (pLIV2-dacA) | pLIV2-dacA | This study |
| ΔmdrMTAC mutant (pLIV2-dacA) | ΔmdrM ΔmdrT ΔmdrA ΔmdrC pLIV2-dacA | This study |
| ΔmarR mutant | ΔmarR (LMRG_01348.6 [lmo1618]) | 7 |
| WT (pPL2-PmdrClacZ) | 10403S harboring integrative plasmid pPL2 containing lacZ gene under mdrC promoter | This study |
| ΔmdrMTA mutant (pPL2-PmdrClacZ) | 10403S ΔmdrMTA harboring integrative plasmid pPL2 containing lacZ gene under mdrC promoter | This study |
| Escherichia coli | ||
| DH12s | ϕ80dlacZΔM15 mcrA Δ(mrr-hsdRMS-mcrBC) araD139 Δ(ara leu)7697 Δ(lacX74) galU galK rpsL (Strr) nupG recA1/[F′ proAB+ lacIqZΔM15] | |
| XL-1b | recA1 endA1 gyrA96 thi-1 hsdR17 supE44 relA1 lac [F′ proAB lacIqZΔM15 Tn10 (Tetr)] | Stratagene |
| SM-10 | Conjugation donor; F− thi-1 thr-1 leuB6 recA tonA21 lacY1 supE44 (Muc+) λ− [RP4-2(Tc::Mu)] Kmr Tra+ | 54 |
Fig 3.
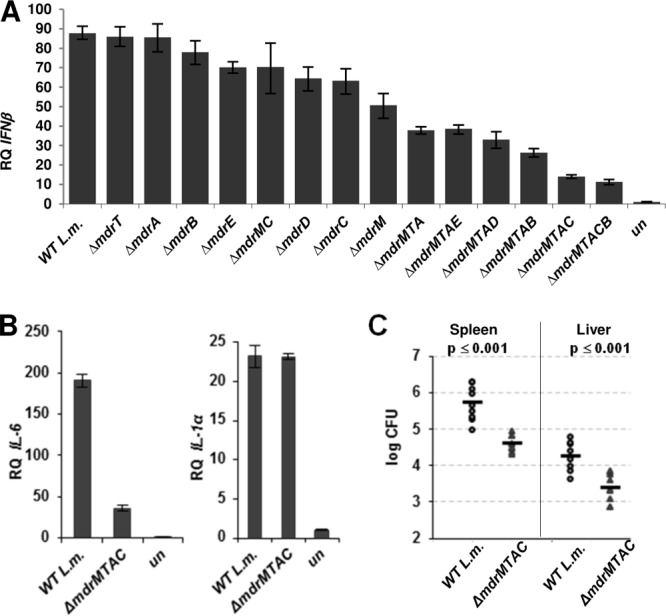
MdrM-like transporters are responsible for most of the type I interferon induction upon L. monocytogenes infection. (A) RT-qPCR analysis of IFN-β transcriptional levels in BMD macrophages infected with WT L. monocytogenes or MDR deletion mutants. (B) RT-qPCR analysis of IL-6 and IL-1α induction in BMD macrophages infected with WT L. monocytogenes in comparison to the ΔmdrMTAC mutant. Transcription levels are represented as relative quantity (RQ) compared to uninfected cells (un). The data in panels A and B represent at least 3 biological repeats. Error bars represent 95% confidence intervals (as described in Materials and Methods). (C) Intravenous infection of C57BL/6 mice with WT L. monocytogenes and the ΔmdrMTAC mutant. Bacterial CFU were numerated at 72 h p.i. from livers and spleens taken from 10 infected mice for each strain. The results are means of 2 independent experiments in which 5 mice were infected in each group. Horizontal bars represent the mean. The P value was calculated using Student's t test.
Since the ΔmdrMTAC mutant grew like WT bacteria in macrophages (see Fig. S1B in the supplemental material) yet triggered a reduced type I interferon response, we examined if this phenotype influenced virulence in mice. Young female C57BL/6 mice were injected intravenously with ΔmdrMTAC mutant or WT bacteria (total of 10 mice for each strain). Seventy-two hours postinfection (p.i.), a log decrease in the number of bacterial CFU was observed in the livers and spleens of the ΔmdrMTAC strain-infected mice compared to that observed in mice infected with WT bacteria (Fig. 3C). These results further support the premise that the MdrM-like transporters are active in vivo and play a role in promoting L. monocytogenes virulence.
MdrM-like transporters are expressed and required during cell wall stress.
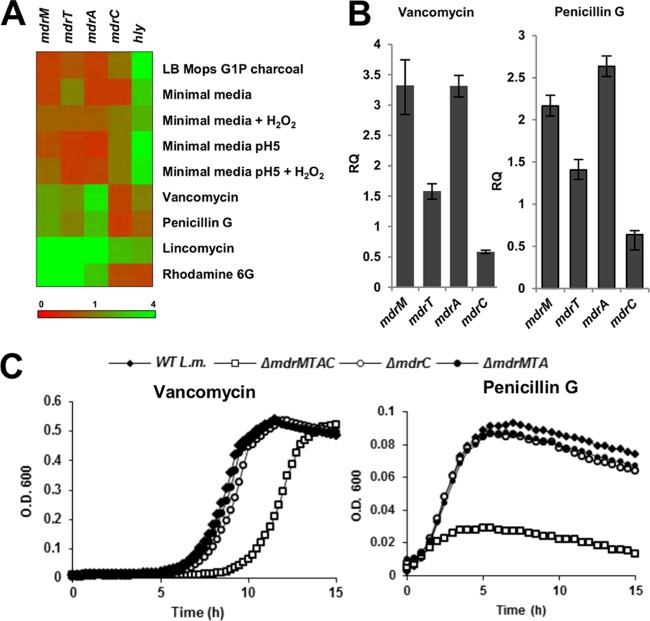
It was still not clear to us what was activating the MdrM-like transporters during intracellular growth. We reasoned that some physiological process was inducing the transporters' function in vivo. Therefore, we searched for physiological conditions that might require the MdrM-like transporters' activity. To this end, the transcription profile of four transporter genes was measured using RT-qPCR analysis under a set of in vitro conditions that mimic different physiological environments. In these studies, we focused on the four transporters MdrM, MdrT, MdrA, and MdrC (MTAC transporters), as together these were responsible for most of the IFN-β induction during infection of macrophages. The conditions involved cell wall stresses (growth in the presence of vancomycin or penicillin G), acidic pH (near 5), oxidative stress (using hydrogen peroxide), and growth in minimal media, all representing conditions that likely exist within the phagosome compartment. In addition, growth in the presence of glucose-1P and charcoal was tested as these conditions are known to activate PrfA, the master regulator of L. monocytogenes virulence (31). Lincomycin and R6G served as positive controls for MDR substrates known to induce expression of MDR transporters (7, 8). The hly gene (encoding LLO toxin) was used as a reporter for the induction of virulence genes.
To summarize the RT-qPCR results, the data are presented as a heat map (Fig. 4A). In general, we observed that while the transporter genes were largely induced by lincomycin and R6G, they were downregulated under all conditions that triggered hly expression (Fig. 4A). These findings indicate that the transporters and the virulence genes are differentially regulated, suggesting that different signals may induce the MDR transporters in vivo. Notably, among the tested conditions, growth in the presence of vancomycin and penicillin G resulted in upregulation of most transporter genes, with the exception of mdrC, which was downregulated under these conditions (Fig. 4A and B). Vancomycin and penicillin G are both inhibitors of peptidoglycan (PGN) synthesis and operate extracellularly on the expanding PGN polymer by blocking PGN peptides from cross-linking. Vancomycin is a branched tricyclic glycosylated heptapeptide that targets the terminal d-alanyl-d-alanine moiety of PGN peptides, while penicillin G, a β-lactam antibiotic, is a structural analogue of d-alanyl-d-alanine that inhibits transpeptidation.
Fig 4.
MdrM-like transporters are required for cell wall stress responses. (A) RT-qPCR analysis, presented as a heat map, of transcriptional levels of the mdrM, mdrT, mdrA, mdrC, and hly genes in WT L. monocytogenes grown under different in vitro conditions: BHI supplemented with vancomycin (1 μg ml−1), penicillin G (0.08 μg ml−1), lincomycin (3 μg ml-1), or rhodamine 6G (50 μM); LB with MOPS (morpholinepropanesulfonic acid) and G1P charcoal; or minimal medium at pH 7, minimal medium at pH 5, or minimal medium with H2O2 at pH 5. Transcription levels are represented as relative quantity (RQ) compared to the levels in BHI or in minimal medium at pH 7, respectively. Data represent 3 biological repeats. In all samples, statistical deviation did not exceed 15% with a 95% confidence level. (B) RT-qPCR analysis of transcriptional levels of the mdrM, mdrT, mdrA, and mdrC genes in WT L. monocytogenes grown in BHI supplemented with vancomycin (1 μg ml−1) or penicillin G (0.08 μg ml−1) for 2 h. Transcription levels (RQ) are relative to the levels in BHI without drugs. The data represent 3 biological repeats. Error bars represent 95% confidence intervals. (C) Growth curve of WT L. monocytogenes and MDR mutants in BHI medium supplemented with vancomycin (1 μg ml−1) or penicillin G (0.08 μg ml−1). The data represent 3 biological repeats. The experiment was performed in a 96-well format in a Synergy HT Biotek plate reader. Representative growth curves are shown. Error bars representing the standard deviation of the triplicate sample are hidden by the symbols. Growth curve experiments were performed in at least 3 independent biological repeats.
To examine more directly if the transporters play a role in the response to vancomycin and penicillin G treatments, transporter mutants and WT bacteria were grown in the presence of sublethal concentrations of these drugs (1 μg ml−1 of vancomycin and 0.08 μg ml−1 of penicillin G). Interestingly, the quadruple ΔmdrMTAC mutant was more susceptible to these drugs, whereas WT, ΔmdrMTA, and ΔmdrC bacteria grew similarly (Fig. 4C). The MICs of penicillin and vancomycin were determined as 0.08 μg ml−1 and 1.5 μg ml−1 for ΔmdrMTAC bacteria and 0.15 μg ml−1 and 2 μg ml−1 for WT bacteria, respectively. To assess the contribution of MdrC in the background of the ΔmdrMTA mutant, we analyzed its transcription level in ΔmdrMTA and WT bacteria using a translational fusion of the lacZ reporter gene to the mdrC promoter region. Notably, we observed that the transcription level of the mdrC gene in the ΔmdrMTA mutant was upregulated (3-fold) in comparison to its level in WT bacteria (see Fig. S2 in the supplemental material). These observations suggest that the Mdr transporters exhibit redundant activities and that they are, respectively, regulated in order to compensate for each other. Indeed, introduction in trans of a copy of each of the transporter genes into the ΔmdrMTAC mutant (using plasmid pLIV2 with the IPTG-inducible promoter) only partially complemented its growth ability under vancomycin treatment (see Fig. S3 in the supplemental material). Overall, these results indicate that the Mdr transporters play active and overlapping roles in the response to vancomycin and penicillin G. Importantly, since vancomycin and penicillin G operate extracellularly on the PGN polymer and are not expected to cross the cytoplasmic membrane to the bacterial cytosol (particularly vancomycin), a simple drug efflux mechanism cannot explain the increased sensitivity of the ΔmdrMTAC mutant to these drugs. In our subsequent studies, we used only vancomycin, since active efflux has never been reported as a mechanism of resistance for this drug.
The ΔmdrMTAC mutant responds aberrantly to cell wall stress.
To gain insight into the functional role of the MDR transporters during vancomycin stress, we examined bacteria using transmission electron microscopy (TEM). Changes in cell wall morphology are expected upon inhibition of PGN synthesis, and therefore, we anticipated visual differences between ΔmdrMTAC and WT bacteria upon vancomycin treatment. Inspection of TEM images confirmed that when grown without vancomycin treatment, both bacterial strains look similar (Fig. 5A). However, 2 h subsequent to addition of a sublethal concentration of vancomycin, WT bacteria were surrounded by massive extracellular material that was largely lacking around the ΔmdrMTAC mutant (Fig. 5A). Further analysis of TEM sections revealed that, without vancomycin treatment, WT bacteria and the ΔmdrMTAC mutant exhibit a similar defined cell wall structure with an average thickness of 21 nm (P = 0.1, based on 50 measurements) (Fig. 5B). In contrast, under vancomycin treatment, WT bacteria and the ΔmdrMTAC mutant were found to be significantly different (P < 0.001, based on 100 measurements). Under these conditions, a large population of the WT bacteria exhibited a very thick cell wall layer of up to 63 nm (Fig. 5B). The cell wall thickness ranged from 25 to 63 nm (35-nm average), as opposed to the range of 18 to 26 nm (24-nm average) associated with ΔmdrMTAC mutant bacteria (Fig. 5B). Cell wall thickening in response to vancomycin stress was reported previously for S. aureus bacteria, which were shown to respond to vancomycin treatment by accumulating peptidoglycan to facilitate vancomycin trapping (drug titration) (32, 33). In accordance with this mechanism, WT L. monocytogenes cells that were observed to undergo cell wall thickening in the presence of vancomycin grew better than the ΔmdrMTAC mutant cells (Fig. 5C and 4C). These observations suggest that the ΔmdrMTAC mutant might be defective in the ability to produce peptidoglycan upon vancomycin stress.
Fig 5.
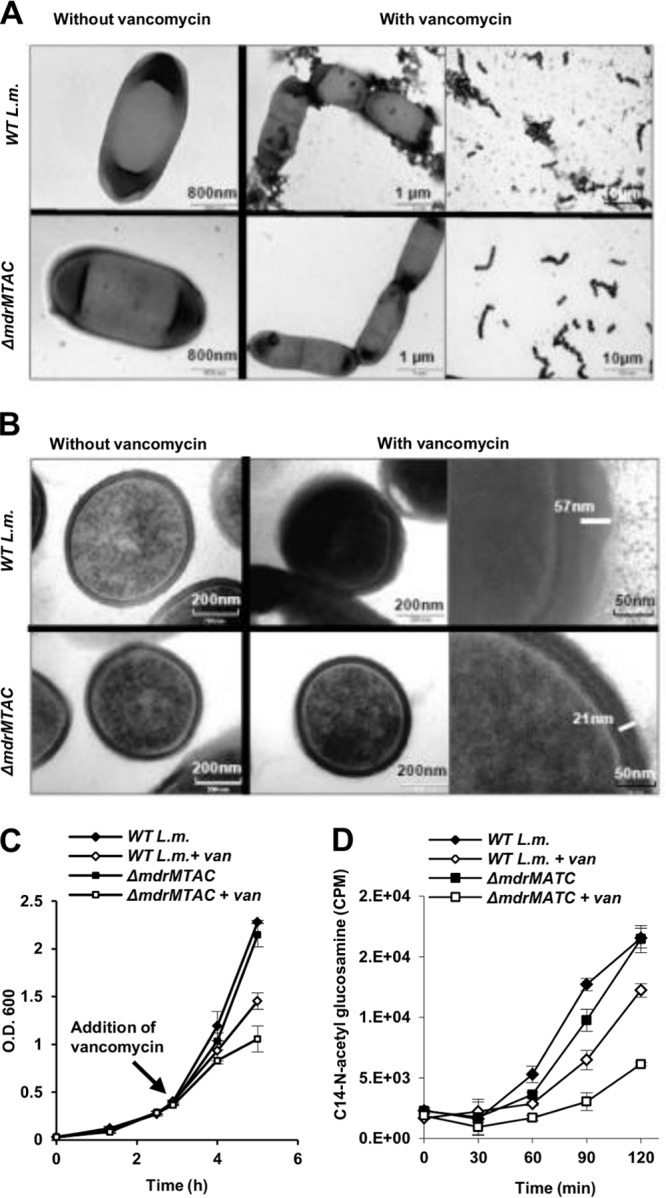
The ΔmdrMTAC mutant does not overproduce peptidoglycan in response to vancomycin stress. (A) Negative-staining TEM images of WT L. monocytogenes and the ΔmdrMTAC mutant grown with and without vancomycin treatment. Growth curves are presented in panel C. (B) TEM section images of WT L. monocytogenes and the ΔmdrMTAC mutant grown with and without vancomycin treatment. Growth curves are presented in panel C. Images in panels A and B represent 3 independent biological repeats; a total of 35 frames were taken for each strain and condition. (C) Growth curves of bacteria taken for TEM analysis (A and B). Vancomycin (1 μg ml−1) was added at an OD600 of 0.4, and bacteria were harvested 2 h later for fixation and staining. The data are means of 3 independent biological experiments. Error bars represent standard deviations. (D) Analysis of the peptidoglycan synthesis rate in WT L. monocytogenes and the ΔmdrMTAC mutant, grown with and without vancomycin (0.8 μg ml−1) treatment, as measured by incorporation of 14C-N-acetylglucosamine. Vancomycin and N-acetylglucosamine were added during bacterial growth at an OD600 of 0.4, and incorporation of 14C-N-acetylglucosamine was analyzed at 30-min intervals upon addition (see the growth curves presented in Fig. S4 in the supplemental material). Error bars represent standard deviations of triplicate samples. The data represent 2 biological repeats.
To further corroborate this hypothesis, we performed 14C-N-acetylglucosamine incorporation measurements to assess the rate of PGN synthesis during growth with and without vancomycin treatment. Bacteria were grown to the mid-log phase before vancomycin and 14C-N-acetylglucosamine were added to the cultures. In this experiment, an even lower concentration of vancomycin was used (0.8 μg ml−1) to reduce the growth inhibition of the ΔmdrMTAC mutant (see Fig. S4 in the supplemental material). Every 30 min, samples of bacteria were filtrated, washed, and counted for 14C labeling. In line with our hypothesis, this analysis demonstrated that, upon vancomycin treatment, the rate of N-acetylglucosamine incorporation was significantly slower in the ΔmdrMTAC mutant than in the WT bacteria (Fig. 5D). These differences in PGN synthesis were detectable even before inhibition of ΔmdrMTAC cell growth by vancomycin was observed (see Fig. S4). Taken together, these results suggest that the MTAC transporters play a role in enhancing PGN synthesis upon vancomycin stress.
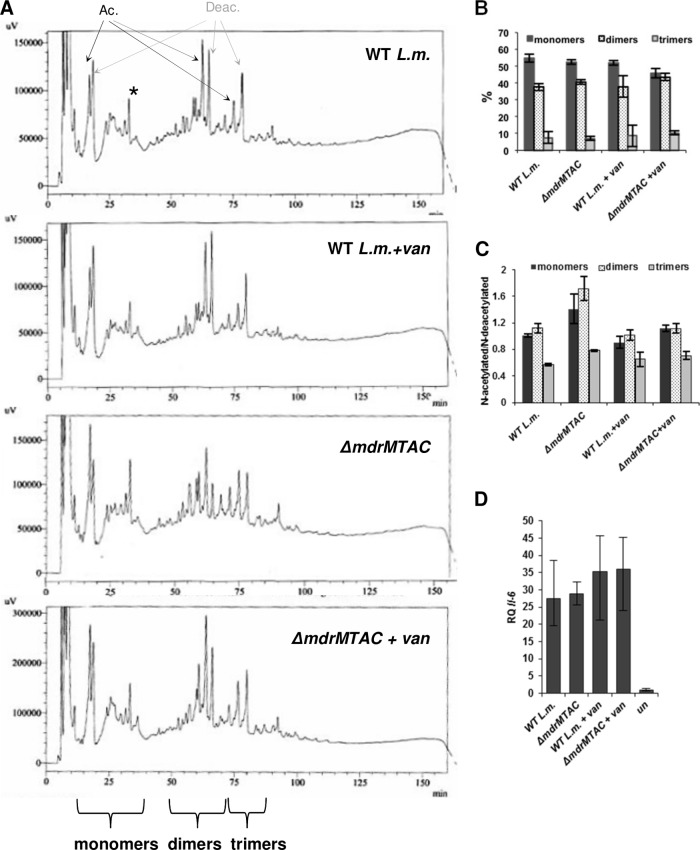
MdrM-like transporters are not involved in PGN′s assembly, structure, or immunostimulatory activity.
To examine if the MTAC transporters are involved in PGN polymer assembly, we compared the PGN structures of the ΔmdrMTAC mutant and WT bacteria with and without vancomycin treatment. Cell wall was extracted from bacteria and digested with mutanolysin to generate a soluble mixture of PGN muropeptides. Muropeptides were separated by reversed-phase high-pressure liquid chromatography (RP-HPLC) and analyzed. Notably, no difference was observed in the muropeptide profiles or their cross-linking levels between WT and ΔmdrMTAC bacteria (as evident from the detected peaks in the HPLC profile in Fig. 6A and B). Of note, a moderate difference of ∼30% in the peptidoglycan N-acetylation level was observed, with the ΔmdrMTAC mutant displaying more N-acetylated muropeptides than WT bacteria (Fig. 6A and C). Next, the immunostimulatory properties of cell walls derived from each strain were compared. Cell wall extracts from ΔmdrMTAC and WT bacteria grown with and without vancomycin were added to BMD macrophages, and interleukin-6 (IL-6) induction was measured using RT-qPCR analysis. IL-6 was chosen as it is induced by both type I interferon and proinflammatory responses. As shown in Fig. 6D, all extracts activated the same level of IL-6, indicating that the immunostimulatory potency of the ΔmdrMTAC cell wall is unchanged, in accordance with the overall similar structure of ΔmdrMTAC PGN to wild-type PGN. Taken together, these results indicate that the MTAC transporters are probably not involved in PGN polymer assembly but play a role in the regulation of PGN synthesis during vancomycin stress.
Fig 6.
MdrM-like transporters do not impact peptidoglycan composition. (A) HPLC analysis of cell-wall-derived muropeptides of WT L. monocytogenes and ΔmdrMTAC bacteria, grown with and without vancomycin (van) treatment. HPLC peaks associated with N-acetylated muropeptides are marked as “Ac,” and peaks associated with N-deacetylated muropeptides are marked as “Deac.” The peak highlighted with an asterisk corresponds to O-acetylated monomer. The data represent 5 biological repeats. (B) Degree of muropeptide cross-linking, presented as the percentage of monomer, dimer, and trimer muropeptides (based on the integrated area of the corresponding peaks in the HPLC analysis) of WT L. monocytogenes or the ΔmdrMTAC mutant grown with and without vancomycin treatment. The data are means of 5 biological repeats. Error bars represents standard deviations of the independent samples. (C) The degree of peptidoglycan N-acetylation in muropeptides derived from WT L. monocytogenes or the ΔmdrMTAC mutant grown with and without vancomycin treatment, presented as the ratio of the integrated area of peaks corresponding to N-acetylated/N-deacetylated muropeptides for monomeric, dimeric, and trimeric units in the HPLC analysis. The data are means of 5 biological repeats. Error bars represent standard deviations of the independent samples. (D) RT-qPCR analysis of IL-6 transcriptional levels in BMD macrophages treated for 6 h with cell wall extracts derived from WT L. monocytogenes and ΔmdrMTAC bacteria, grown with and without vancomycin (van; 1 μg ml−1). Transcription levels are represented as relative quantity (RQ) compared to levels in untreated cells (un). The data represent 3 biological repeats. Error bars represent 95% confidence intervals (as described in Materials and Methods).
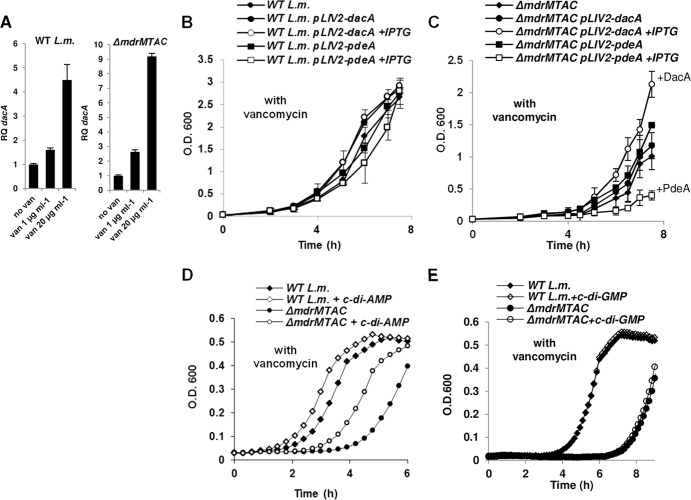
c-di-AMP and Mdr-MTAC transporters regulate the response to cell wall stress.
It was recently shown that L. monocytogenes MdrM and MdrT facilitate c-di-AMP secretion (11, 12). We speculated that the MDR transporters might regulate the enhancement of PGN synthesis in response to vancomycin by controlling c-di-AMP secretion. To provide evidence for a physiological association between c-di-AMP and the MTAC transporters, we first asked whether c-di-AMP is produced under vancomycin stress. Since c-di-AMP production was shown to correlate with dacA (diadenylate cyclase) gene expression (18), dacA transcription levels were measured upon various vancomycin treatments using RT-qPCR analysis. WT bacteria and ΔmdrMTAC mutant bacteria grown in the presence of a sublethal concentration of vancomycin (1 μg ml−1) or exposed briefly to high concentrations of vancomycin (20 μg ml−1 for 10 min) were both observed to induce dacA gene transcription in comparison to nontreated bacteria. WT bacteria induced 1.5- and 4.5-fold higher transcription levels of dacA under the respective conditions, while the ΔmdrMTAC mutant induced 3- and 9-fold higher levels, respectively (Fig. 7A). To evaluate the influence of c-di-AMP production on L. monocytogenes growth under vancomycin stress, the dacA gene and pdeA (phosphodiesterase) gene were overexpressed in ΔmdrMTAC and WT bacteria from an IPTG-inducible promoter (using the integrative plasmid pLIV2) (Table 2). The overexpression of dacA and pdeA genes had only a moderate effect on the growth of WT bacteria in the presence of a sublethal concentration of vancomycin (1 μg ml−1) (Fig. 7B); however, the growth of the ΔmdrMTAC mutant under the same condition was seemingly altered upon overexpression of these genes (Fig. 7C). Overexpression of the dacA gene suppressed the growth inhibition of the ΔmdrMTAC mutant by vancomycin, whereas overexpression of pdeA rendered the mutant more susceptible to this drug (Fig. 7C). In accordance with these observations, we further detected an increase in cell wall thickness (by 17%) in the ΔmdrMTAC mutant overexpressing dacA using TEM section analysis (P < 0.001, based on 30 measurements). Notably, the effect of dacA and pdeA overexpression on the growth of WT bacteria was still moderate, even when the concentration of vancomycin was increased to further inhibit growth (see Fig. S5A and B in the supplemental material). Furthermore, since overexpression of the dacA and pdeA genes had no effect on the growth of WT and ΔmdrMTAC bacteria in the absence of vancomycin stress (see Fig. S5C and D), we surmise that c-di-AMP and the MTAC transporters are both involved in the response to the vancomycin stress.
Fig 7.
Mdr-MTAC transporters and c-di-AMP are functionally associated in the response to cell wall stress. (A) RT-qPCR analysis of transcriptional levels of the dacA gene in WT L. monocytogenes and ΔmdrMTAC bacteria grown in BHI or supplemented with vancomycin (van; 1 μg ml−1 for 2 h or 20 μg ml−1 for 10 min). Transcription levels are represented as relative quantity (RQ) compared to the levels in BHI alone. The data represent 3 biological independent repeats. Error bars represent 95% confidence intervals (as described in Materials and Methods). (B) Growth curves of WT L. monocytogenes strains harboring the pLIV2 plasmid with an IPTG-inducible promoter, expressing dacA or pdeA genes in BHI supplemented with vancomycin (1 μg ml−1) with or without IPTG. The experiment was performed in flasks. The data are means of 3 independent biological experiments. Error bars represent standard deviations. (C) Growth curves of ΔmdrMTAC bacteria harboring the pLIV2 plasmid with an IPTG-inducible promoter expressing the dacA or pdeA genes in BHI supplemented with vancomycin with or without IPTG. The experiment was performed in flasks. The data are means of 3 independent biological experiments. Error bars represent standard deviations. (D) Growth curve of WT L. monocytogenes or the ΔmdrMTAC mutant in BHI supplemented with vancomycin with and without addition of 3 μg ml−1 c-di-AMP. This experiment was performed in 3 biological repeats in a 96-well format in a Synergy HT Biotek plate reader. Growth curves from one representative experiment are shown. Error bars representing standard deviations of a triplicate sample are hidden by the symbols. (E) Growth curve of WT L. monocytogenes or the ΔmdrMTAC mutant in BHI supplemented with vancomycin with and without addition of 3 μg ml−1 c-di-GMP. The experiment was performed in 3 biological repeats in a 96-well format in a Synergy HT Biotek plate reader. Growth curves from one representative experiment are shown. Error bars representing standard deviations of a triplicate sample are hidden by the symbols.
Finally, we studied whether exogenous addition of purified c-di-AMP to bacterial cultures could recapitulate the phenotype observed with the dacA-overexpressing bacteria. To this end, WT and ΔmdrMTAC bacteria were grown in the presence of vancomycin (1 μg ml−1), and purified c-di-AMP or c-di-GMP was added to the bacterial cultures. Notably, both ΔmdrMTAC and WT bacteria exhibited a shorter lag phase when c-di-AMP was added, whereas c-di-GMP addition had no effect (Fig. 7D and E). Similarly to the dacA and pdeA overexpression experiments, c-di-AMP or c-di-GMP addition had no effect on the growth of the bacteria in the absence of vancomycin stress (see Fig. S5E and F in the supplemental material). Together, these results indicate a possible role for c-di-AMP in the response to vancomycin and, furthermore, hint at a physiological association between DacA, PdeA, and the MDR transporters in mediating a response to this stress.
DISCUSSION
In this study, we investigated the physiological functions of L. monocytogenes MDR transporters and c-di-AMP with the goal of understanding better the mechanism that leads infected macrophages to induce IFN-β. We discovered that not only MdrM but also a set of related putative MDR transporters together mediate IFN-β induction in infected macrophages. A screen for physiological conditions that demand the activity of these transporters revealed that they are involved in regulation of peptidoglycan synthesis in response to cell wall stress. The phenotypes observed upon disturbance of peptidoglycan synthesis pointed out a possible link between the function of MDR transporters and c-di-AMP production. Although we demonstrate a physiological association between MDRs and c-di-AMP in this study, it remains unclear if the MDRs play a role in regulation of c-di-AMP intracellular concentration (via c-di-AMP efflux) or translocate c-di-AMP outside the bacterial cell to bind an extracellular target or targets, or alternatively, if the MDRs simply bind c-di-AMP to regulate various cellular processes, as was recently shown with potassium ion transporters (34). Further studies are required to discriminate between these various possibilities.
Very little is known about the physiological functions of MDR transporters in bacteria, aside from drug efflux (35, 36). Bacterial genomes contain a large arsenal of MDR transporter genes, many of which are highly similar and possess overlapping substrate specificity (9, 37, 38). The generally accepted hypothesis is that MDR transporters evolved independently during evolution to cope with a wide array of physiological substrates, allowing bacteria to survive diverse ecological niches (39). Studies in the last decade have revealed diverse functions of MDR transporters that are not related to drug efflux (36, 40). For example, involvement of MDR transporters in lipid transport, pH homeostasis (41), virulence (42, 43), and quorum sensing (44) has been documented, with fatty acids, ions, bile salts, antibacterial peptides, and precursors of quorum-sensing molecules suggested as natural substrates (45–51). Despite the advance in our understanding of MDR transporters, in silico prediction of MDR transporter functions remains challenging. The initial discovery that the L. monocytogenes MdrM transporter modulates the type I interferon response raised many questions regarding the mechanism of this function. Primarily, it was not clear whether this function evolved specifically to subvert the host immune system or, alternatively, represents the inadvertent consequence of a more basic bacterial physiological function. Later the report that c-di-AMP is secreted by MdrM and leads to IFN-β induction (11) further highlighted the need to better understand the natural biological process that involves MdrM and c-di-AMP. Here we show that four homologous L. monocytogenes MDR transporters not only are triggering IFN-β induction during infection but also are novel players in the response to cell wall stress. Remarkably, the ΔmdrMTAC mutant lacking the four transporters failed to trigger enhanced production of PGN in response to vancomycin stress, a mechanism known to facilitate vancomycin resistance (32). In light of the observation that the growth defect of the ΔmdrMTAC mutant under vancomycin stress was not accompanied by production of aberrant PGN, we propose that the MDR transporters play a regulatory role in PGN synthesis rather than a direct role in PGN biogenesis. The findings that increased production of c-di-AMP (via dacA overexpression) or addition of purified c-di-AMP rescued the growth inhibition of the ΔmdrMTAC mutant under vancomycin stress support that c-di-AMP is involved downstream of the transporters' function. However, the possibility that c-di-AMP affects the observed phenotypes indirectly cannot be excluded (14, 34, 52, 53).
Cyclic di-AMP has been recently demonstrated to play a role in the response to cell wall stresses in both S. aureus and Bacillus subtilis. Two independent genetic screens highlighted c-di-AMP as a key regulator of lipoteichoic acid (LTA)- and PGN-related stress. In S. aureus, a genetic screen designed to identify suppressor mutations that restore the ability of LTA-deficient mutants to grow identified mutations in pde gene and revealed a global role for c-di-AMP in cell wall regulation (15). In B. subtilis, a search for genes that facilitate intrinsic resistance to β-lactam antibiotics also identified a pde gene and c-di-AMP as key players (16). Notably, increased production of c-di-AMP resulted in enhanced resistance of B. subtilis bacteria to β-lactams (16), a phenotype that is similar to our observation that overexpression of the dacA gene suppressed the growth defect of the ΔmdrMTAC mutant under vancomycin stress. Interestingly, a connection between c-di-AMP, MDRs, and cell wall stress was pointed out previously in a study that examined global genomic changes in a β-lactam-resistant methicillin-resistant S. aureus (MRSA) strain. In this study, mutations were identified in three genes encoding a penicillin binding protein, PDE, and an MDR transporter (54). While it was not clear to the authors how these genes associate, we now surmise that c-di-AMP and MDR transporters function together to regulate cell wall synthesis. Notably, this transporter's function might be part of an intrinsic mechanism of bacteria to overcome cell wall stress. Such a function is most likely required during infection of mammalian cells, where bacteria are subjected to various host antimicrobial mechanisms targeting the bacterial cell wall. In this regard, the in vivo virulence defect of the ΔmdrMTAC mutant may be linked to the transporters' function in resistance to cell wall stress; however, since the transporter's function poses additional effects on the innate immune system, the observed in vivo phenotype is complex to decipher.
Finally, in light of the growing notion that bacteria use diverse cyclic nucleotide (or dinucleotide) messengers (such as c-di-GMP and c-di-AMP) to regulate basic processes (e.g., adhesion, biofilm, virulence, DNA damage, and cell wall stress) (55, 56), it is not surprising that the innate immune system developed mechanisms to detect these vital molecules. The study presented here further supports the premise that c-di-AMP is a vital molecule that exhibits multiple functions during L. monocytogenes infection of mammalian cells.
Supplementary Material
ACKNOWLEDGMENTS
We thank Eitan Bibi, Ilya Borovok, and the Herskovits lab members for critical review of this work. We thank Lev Rabinovich for help with experiments.
This work was partially supported by ERA-NET Pathogenomics (Israel Ministry of Health [MOA]), IRG-FP7 and Israel Science Foundation grants to A.A.H., by the Legacy Heritage Grant 1640/08 of the Israeli Science Foundation to R.N.-P., and by ERC starting grant 202283 (PGN from SHAPE to VIR) to I.G.B. The Rina and Yoel Saraf Family funded M.K.Z.'s scholarship.
Footnotes
Published ahead of print 20 September 2013
Supplemental material for this article may be found at http://dx.doi.org/10.1128/JB.00794-13.
REFERENCES
- 1.Dussurget O, Pizarro-Cerda J, Cossart P. 2004. Molecular determinants of Listeria monocytogenes virulence. Annu. Rev. Microbiol. 58:587–610 [DOI] [PubMed] [Google Scholar]
- 2.O'Riordan M, Yi CH, Gonzales R, Lee KD, Portnoy DA. 2002. Innate recognition of bacteria by a macrophage cytosolic surveillance pathway. Proc. Natl. Acad. Sci. U. S. A. 99:13861–13866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ishikawa H, Ma Z, Barber GN. 2009. STING regulates intracellular DNA-mediated, type I interferon-dependent innate immunity. Nature 461:788–792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Perry AK, Chen G, Zheng D, Tang H, Cheng G. 2005. The host type I interferon response to viral and bacterial infections. Cell Res. 15:407–422 [DOI] [PubMed] [Google Scholar]
- 5.Stockinger S, Reutterer B, Schaljo B, Schellack C, Brunner S, Materna T, Yamamoto M, Akira S, Taniguchi T, Murray PJ, Muller M, Decker T. 2004. IFN regulatory factor 3-dependent induction of type I IFNs by intracellular bacteria is mediated by a TLR- and Nod2-independent mechanism. J. Immunol. 173:7416–7425 [DOI] [PubMed] [Google Scholar]
- 6.O'Connell RM, Vaidya SA, Perry AK, Saha SK, Dempsey PW, Cheng G. 2005. Immune activation of type I IFNs by Listeria monocytogenes occurs independently of TLR4, TLR2, and receptor interacting protein 2 but involves TNFR-associated NF kappa B kinase-binding kinase 1. J. Immunol. 174:1602–1607 [DOI] [PubMed] [Google Scholar]
- 7.Crimmins GT, Herskovits AA, Rehder K, Sivick KE, Lauer P, Dubensky TW, Jr, Portnoy DA. 2008. Listeria monocytogenes multidrug resistance transporters activate a cytosolic surveillance pathway of innate immunity. Proc. Natl. Acad. Sci. U. S. A. 105:10191–10196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brown MH, Skurray RA. 2001. Staphylococcal multidrug efflux protein QacA. J. Mol. Microbiol. Biotechnol. 3:163–170 [PubMed] [Google Scholar]
- 9.Nikaido H. 2009. Multidrug resistance in bacteria. Annu. Rev. Biochem. 78:119–146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Quillin SJ, Schwartz KT, Leber JH. 2011. The novel Listeria monocytogenes bile sensor BrtA controls expression of the cholic acid efflux pump MdrT. Mol. Microbiol. 81:129–142 [DOI] [PubMed] [Google Scholar]
- 11.Woodward JJ, Iavarone AT, Portnoy DA. 2010. c-di-AMP secreted by intracellular Listeria monocytogenes activates a host type I interferon response. Science 328:1703–1705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yamamoto T, Hara H, Tsuchiya K, Sakai S, Fang R, Matsuura M, Nomura T, Sato F, Mitsuyama M, Kawamura I. 2012. Listeria monocytogenes strain-specific impairment of the TetR regulator underlies the drastic increase in cyclic di-AMP secretion and beta interferon-inducing ability. Infect. Immun. 80:2323–2332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Romling U. 2008. Great times for small molecules: c-di-AMP, a second messenger candidate in Bacteria and Archaea. Sci. Signal. 1:pe39. 10.1126/scisignal.133pe39 [DOI] [PubMed] [Google Scholar]
- 14.Oppenheimer-Shaanan Y, Wexselblatt E, Katzhendler J, Yavin E, Ben-Yehuda S. 2011. c-di-AMP reports DNA integrity during sporulation in Bacillus subtilis. EMBO Rep. 12:594–601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Corrigan RM, Abbott JC, Burhenne H, Kaever V, Grundling A. 2011. c-di-AMP is a new second messenger in Staphylococcus aureus with a role in controlling cell size and envelope stress. PLoS Pathog. 7:e1002217. 10.1371/journal.ppat.1002217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Luo Y, Helmann JD. 2012. Analysis of the role of Bacillus subtilis sigma(M) in beta-lactam resistance reveals an essential role for c-di-AMP in peptidoglycan homeostasis. Mol. Microbiol. 83:623–639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Witte G, Hartung S, Buttner K, Hopfner KP. 2008. Structural biochemistry of a bacterial checkpoint protein reveals diadenylate cyclase activity regulated by DNA recombination intermediates. Mol. Cell 30:167–178 [DOI] [PubMed] [Google Scholar]
- 18.Witte CE, Whiteley AT, Burke TP, Sauer JD, Portnoy DA, Woodward JJ. 2013. Cyclic di-AMP is critical for Listeria monocytogenes growth, cell wall homeostasis, and establishment of infection. mBio 4(3):e00282–13. 10.1128/mBio.00282-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Simon R, Priefer U, Puhler A. 1983. A broad host range mobilization system for in vivo genetic engineering: transposon mutagenesis in Gram negative bacteria. Nat. Biotechnol. 1:784–791 [Google Scholar]
- 20.Phan-Thanh L, Gormon T. 1997. A chemically defined minimal medium for the optimal culture of Listeria. Int. J. Food Microbiol. 35:91–95 [DOI] [PubMed] [Google Scholar]
- 21.Portnoy DA, Schreiber RD, Connelly P, Tilney LG. 1989. Gamma interferon limits access of Listeria monocytogenes to the macrophage cytoplasm. J. Exp. Med. 170:2141–2146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Herskovits AA, Auerbuch V, Portnoy DA. 2007. Bacterial ligands generated in a phagosome are targets of the cytosolic innate immune system. PLoS Pathog. 3:e51. 10.1371/journal.ppat.0030051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lobel L, Sigal N, Borovok I, Ruppin E, Herskovits AA. 2012. Integrative genomic analysis identifies isoleucine and CodY as regulators of Listeria monocytogenes virulence. PLoS Genet. 8:e1002887. 10.1371/journal.pgen.1002887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vidal-Aroca F, Giannattasio M, Brunelli E, Vezzoli A, Plevani P, Muzi-Falconi M, Bertoni G. 2006. One-step high-throughput assay for quantitative detection of beta-galactosidase activity in intact gram-negative bacteria, yeast, and mammalian cells. Biotechniques 40:433–434, 436, 438 [DOI] [PubMed] [Google Scholar]
- 25.Chanda PK, Ganguly T, Das M, Lee CY, Luong TT, Sau S. 2007. Detection of antistaphylococcal and toxic compounds by biological assay systems developed with a reporter Staphylococcus aureus strain harboring a heat inducible promoter-lacZ transcriptional fusion. J. Biochem. Mol. Biol. 40:936–943 [DOI] [PubMed] [Google Scholar]
- 26.Girardin SE, Boneca IG, Viala J, Chamaillard M, Labigne A, Thomas G, Philpott DJ, Sansonetti PJ. 2003. Nod2 is a general sensor of peptidoglycan through muramyl dipeptide (MDP) detection. J. Biol. Chem. 278:8869–8872 [DOI] [PubMed] [Google Scholar]
- 27.Boneca IG, Dussurget O, Cabanes D, Nahori MA, Sousa S, Lecuit M, Psylinakis E, Bouriotis V, Hugot JP, Giovannini M, Coyle A, Bertin J, Namane A, Rousselle JC, Cayet N, Prevost MC, Balloy V, Chignard M, Philpott DJ, Cossart P, Girardin SE. 2007. A critical role for peptidoglycan N-deacetylation in Listeria evasion from the host innate immune system. Proc. Natl. Acad. Sci. U. S. A. 104:997–1002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Aubry C, Goulard C, Nahori MA, Cayet N, Decalf J, Sachse M, Boneca IG, Cossart P, Dussurget O. 2011. OatA, a peptidoglycan O-acetyltransferase involved in Listeria monocytogenes immune escape, is critical for virulence. J. Infect. Dis. 204:731–740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wu J, Hassan KA, Skurray RA, Brown MH. 2008. Functional analyses reveal an important role for tyrosine residues in the staphylococcal multidrug efflux protein QacA. BMC Microbiol. 8:147. 10.1186/1471-2180-8-147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nishino K, Yamaguchi A. 2001. Analysis of a complete library of putative drug transporter genes in Escherichia coli. J. Bacteriol. 183:5803–5812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ripio MT, Brehm K, Lara M, Suarez M, Vazquez-Boland JA. 1997. Glucose-1-phosphate utilization by Listeria monocytogenes is PrfA dependent and coordinately expressed with virulence factors. J. Bacteriol. 179:7174–7180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cui L, Ma X, Sato K, Okuma K, Tenover FC, Mamizuka EM, Gemmell CG, Kim MN, Ploy MC, El-Solh N, Ferraz V, Hiramatsu K. 2003. Cell wall thickening is a common feature of vancomycin resistance in Staphylococcus aureus. J. Clin. Microbiol. 41:5–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kawai M, Yamada S, Ishidoshiro A, Oyamada Y, Ito H, Yamagishi J. 2009. Cell-wall thickness: possible mechanism of acriflavine resistance in meticillin-resistant Staphylococcus aureus. J. Med. Microbiol. 58:331–336 [DOI] [PubMed] [Google Scholar]
- 34.Corrigan RM, Campeotto I, Jeganathan T, Roelofs KG, Lee VT, Grundling A. 2013. Systematic identification of conserved bacterial c-di-AMP receptor proteins. Proc. Natl. Acad. Sci. U. S. A. 110:9084–9089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Neyfakh AA. 1997. Natural functions of bacterial multidrug transporters. Trends Microbiol. 5:309–313 [DOI] [PubMed] [Google Scholar]
- 36.Piddock LJ. 2006. Multidrug-resistance efflux pumps—not just for resistance. Nat. Rev. Microbiol. 4:629–636 [DOI] [PubMed] [Google Scholar]
- 37.Ren Q, Paulsen IT. 2007. Large-scale comparative genomic analyses of cytoplasmic membrane transport systems in prokaryotes. J. Mol. Microbiol. Biotechnol. 12:165–179 [DOI] [PubMed] [Google Scholar]
- 38.Lewinson O, Adler J, Sigal N, Bibi E. 2006. Promiscuity in multidrug recognition and transport: the bacterial MFS Mdr transporters. Mol. Microbiol. 61:277–284 [DOI] [PubMed] [Google Scholar]
- 39.Paulsen IT. 2003. Multidrug efflux pumps and resistance: regulation and evolution. Curr. Opin. Microbiol. 6:446–451 [DOI] [PubMed] [Google Scholar]
- 40.Martinez JL, Sanchez MB, Martinez-Solano L, Hernandez A, Garmendia L, Fajardo A, Alvarez-Ortega C. 2009. Functional role of bacterial multidrug efflux pumps in microbial natural ecosystems. FEMS Microbiol. Rev. 33:430–449 [DOI] [PubMed] [Google Scholar]
- 41.Krulwich TA, Lewinson O, Padan E, Bibi E. 2005. Do physiological roles foster persistence of drug/multidrug-efflux transporters? A case study. Nat. Rev. Microbiol. 3:566–572 [DOI] [PubMed] [Google Scholar]
- 42.Hirakata Y, Srikumar R, Poole K, Gotoh N, Suematsu T, Kohno S, Kamihira S, Hancock RE, Speert DP. 2002. Multidrug efflux systems play an important role in the invasiveness of Pseudomonas aeruginosa. J. Exp. Med. 196:109–118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nishino K, Latifi T, Groisman EA. 2006. Virulence and drug resistance roles of multidrug efflux systems of Salmonella enterica serovar Typhimurium. Mol. Microbiol. 59:126–141 [DOI] [PubMed] [Google Scholar]
- 44.Evans K, Passador L, Srikumar R, Tsang E, Nezezon J, Poole K. 1998. Influence of the MexAB-OprM multidrug efflux system on quorum sensing in Pseudomonas aeruginosa. J. Bacteriol. 180:5443–5447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lee EH, Shafer WM. 1999. The farAB-encoded efflux pump mediates resistance of gonococci to long-chained antibacterial fatty acids. Mol. Microbiol. 33:839–845 [DOI] [PubMed] [Google Scholar]
- 46.Lewinson O, Padan E, Bibi E. 2004. Alkalitolerance: a biological function for a multidrug transporter in pH homeostasis. Proc. Natl. Acad. Sci. U. S. A. 101:14073–14078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lacroix FJ, Cloeckaert A, Grepinet O, Pinault C, Popoff MY, Waxin H, Pardon P. 1996. Salmonella typhimurium acrB-like gene: identification and role in resistance to biliary salts and detergents and in murine infection. FEMS Microbiol. Lett. 135:161–167 [DOI] [PubMed] [Google Scholar]
- 48.Thanassi DG, Cheng LW, Nikaido H. 1997. Active efflux of bile salts by Escherichia coli. J. Bacteriol. 179:2512–2518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bengoechea JA, Skurnik M. 2000. Temperature-regulated efflux pump/potassium antiporter system mediates resistance to cationic antimicrobial peptides in Yersinia. Mol. Microbiol. 37:67–80 [DOI] [PubMed] [Google Scholar]
- 50.Pearson JP, Van Delden C, Iglewski BH. 1999. Active efflux and diffusion are involved in transport of Pseudomonas aeruginosa cell-to-cell signals. J. Bacteriol. 181:1203–1210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Aendekerk S, Diggle SP, Song Z, Hoiby N, Cornelis P, Williams P, Camara M. 2005. The MexGHI-OpmD multidrug efflux pump controls growth, antibiotic susceptibility and virulence in Pseudomonas aeruginosa via 4-quinolone-dependent cell-to-cell communication. Microbiology 151:1113–1125 [DOI] [PubMed] [Google Scholar]
- 52.Rao F, See RY, Zhang D, Toh DC, Ji Q, Liang ZX. 2010. YybT is a signaling protein that contains a cyclic dinucleotide phosphodiesterase domain and a GGDEF domain with ATPase activity. J. Biol. Chem. 285:473–482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhang L, Li W, He ZG. 2013. DarR, a TetR-like transcriptional factor, is a cyclic di-AMP-responsive repressor in Mycobacterium smegmatis. J. Biol. Chem. 288:3085–3096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Banerjee R, Gretes M, Harlem C, Basuino L, Chambers HF. 2010. A mecA-negative strain of methicillin-resistant Staphylococcus aureus with high-level beta-lactam resistance contains mutations in three genes. Antimicrob. Agents Chemother. 54:4900–4902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gomelsky M. 2011. cAMP, c-di-GMP, c-di-AMP and now cGMP: bacteria use them all! Mol. Microbiol. 79:562–565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hengge R. 2009. Principles of c-di-GMP signalling in bacteria. Nat. Rev. Microbiol. 7:263–273 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.