Abstract
Xylella fastidiosa, like related Xanthomonas species, employs an Rpf cell-cell communication system consisting of a diffusible signal factor (DSF) synthase, RpfF, and a DSF sensor, RpfC, to coordinate expression of virulence genes. While phenotypes of a ΔrpfF strain in Xanthomonas campestris could be complemented by its own DSF, the DSF produced by X. fastidiosa (XfDSF) did not restore expression of the XfDSF-dependent genes hxfA and hxfB to a ΔrpfF strain of X. fastidiosa, suggesting that RpfF is involved in XfDSF sensing or XfDSF-dependent signaling. To test this conjecture, rpfC and rpfF of X. campestris were replaced by those of X. fastidiosa, and the contribution of each gene to the induction of a X. campestris DSF-dependent gene was assessed. As in X. fastidiosa, XfDSF-dependent signaling required both X. fastidiosa proteins RpfF and RpfC. RpfF repressed RpfC signaling activity, which in turn was derepressed by XfDSF. A mutated X. fastidiosa RpfF protein with two substitutions of glutamate to alanine in its active site was incapable of XfDSF production yet enabled a response to XfDSF, indicating that XfDSF production and the response to XfDSF are two separate functions in which RpfF is involved. This mutant was also hypervirulent to grape, demonstrating the antivirulence effects of XfDSF itself in X. fastidiosa. The Rpf system of X. fastidiosa is thus a novel example of a quorum-sensing signal synthase that is also involved in the response to the signal molecule that it synthesizes.
INTRODUCTION
The xylem-limited plant pathogen Xylella fastidiosa causes serious diseases of several important agricultural crop plants, including Pierce's disease (PD) of grapevine and variegated chlorosis in citrus (CVC) (1, 2). X. fastidiosa is obligately transmitted from one plant to another by xylem-sap-feeding insects. Infected plants exhibit progressive leaf scorching consistent with water stress that is associated with the large numbers of xylem vessels that are occluded by bacterial cells (3). The virulence of X. fastidiosa is thus linked to its ability to migrate and proliferate within xylem vessels, and disease symptoms may largely be an inadvertent effect caused by successful colonization that interferes with xylem sap flow (4).
X. fastidiosa, like related Xanthomonas plant pathogens, utilizes one or more related signal molecules known as diffusible signaling factor (DSFs) to regulate its behavior in a cell density-dependent manner (5, 6). DSF molecules are typically cis-2-unsaturated fatty acids with chain lengths varying from 12 to 14 carbons (7), such as 2(Z)-11-methyldodecenoic acid (DSF) (8) and (2Z,5Z)-11-methyldodecadienoic acid (CDSF) (9), isolated from Xanthomonas species; 2(Z)-dodecenoic acid (BDSF), isolated from Burkholderia (10) and Xanthomonas (9) species; or 2(Z)-tetradecenoic acid, isolated from a grape strain of X. fastidiosa (XfDSF) (11). Another DSF-like molecule, 12-methyltetradecenoic acid, isolated from a X. fastidiosa CVC strain (12), has not been shown to be biologically active (11).
DSFs are synthesized by RpfF, which has 3-hydroxyacyl-acyl carrier protein (ACP) dehydratase and thioesterase activity (13). When DSF reaches a threshold concentration outside the cell, the bacteria activate their cognate receptor RpfC, a hybrid membrane sensor kinase that phosphorylates the intracellular response regulator RpfG. RpfG then converts the intercellular signal into an intracellular signal through its cyclic di-GMP phosphodiesterase (PDE) activity (14), which in turn alters the expression of target genes (15, 16). RpfF-dependent signaling involving DSF species accumulation has been shown to regulate motility, biofilm formation, and virulence in several Xanthomonas species and in X. fastidiosa (5, 6, 15, 17, 18).
Since a ΔrpfF strain of X. fastidiosa, blocked in the production of XfDSF, was hypervirulent to grapevine but unable to colonize and be transmitted by insect vectors, it was hypothesized that DSF signaling is used as a context-dependent lifestyle switch that enables a subset of its population of cells in a plant to become adhesive and thus able to be acquired by insects, a phenotype incompatible with movement through the plant (3, 4, 6). Consistent with this, RpfF suppresses the expression of genes involved in motility (e.g., pil genes, encoding type IV pili) and hydrolytic enzymes that disrupt pit membranes (e.g., pglA, which encodes a pectinase), enabling cell movement from one xylem vessel to another, but stimulates the expression of genes involved in cell aggregation and surface attachment (e.g., fimA, encoding type I pili, and hxfA and hxfB, encoding hemagglutinin-like proteins) and those that drive biofilm formation (e.g., gum genes, involved in exopolysaccharide [EPS] synthesis) (19, 20). The accumulation of XfDSF in X. fastidiosa thus favors transmission by insects but suppresses virulence to plants.
In a previous study, we showed that the promoters of hxfA and hxfB are induced by XfDSF in a wild-type (WT) strain (11), indicating that regulation of these genes by RpfF is mediated by the XfDSF that it synthesizes. hxfA′::phoA and hxfB′::phoA transcriptional fusions were thus transformed into a X. fastidiosa ΔphoA strain to generate XfDSF biosensors that were useful for the detection and isolation of XfDSF. While a strain incapable of production of XfDSF would make a better biosensor for this signal molecule, we noted that a ΔrpfF strain of X. fastidiosa was unresponsive to added XfDSF (11). This was surprising since in Xanthomonas campestris, the phenotypes of a ΔrpfF strain could be rescued by the addition of DSF to the growth medium (5, 15), and current models of DSF-mediated signaling involving physical interactions of RpfF and RpfC (16, 21) do not account for such an observation. In X. campestris, RpfF has been demonstrated to function only as a DSF synthase whose catalytic activity is repressed by physical interactions with RpfC (16, 21) and is apparently derepressed upon the interaction of DSF with RpfC (21). RpfF has not been suggested to aid RpfC in the signaling process. In addition, RpfC and RpfF of X. fastidiosa and X. campestris share only 58.4% and 64.5% amino acids identities, respectively (see Fig. S5 in the supplemental material). We thus reasoned that the signal perception mechanism of X. fastidiosa may be substantially different from those of other taxa such as X. campestris and that RpfF might itself also be involved in the signal transduction process. Since genetic studies of X. fastidiosa are challenging due to its slow growth and limited genetic tools, to test this model, we took advantage of the high level of homology between the Rpf systems of X. campestris and X. fastidiosa; we substituted the rpfF and rpfC genes of X. campestris for those of X. fastidiosa and assessed the expression of a DSF-dependent gene of X. campestris in response to XfDSF as a measure of signaling activity. We show that unlike the X. campestris Rpf system, sensing of XfDSF by X. fastidiosa requires the presence of RpfF. In support of this model, we also show that a mutant of X. fastidiosa blocked in DSF production due to a 2-amino-acid substitution in the active site of RpfF properly responds to exogenous XfDSF yet is hypervirulent to grape. The selectivity of the DSF-sensing systems used by X. campestris and X. fastidiosa for various DSF species is also examined using this hybrid sensing system.
MATERIALS AND METHODS
Growth media and growth conditions.
The bacterial strains and plasmids employed in this study are listed in Table 1. X. campestris pv. campestris 8004-derived strains were grown on King's B (KB) medium (22). X. fastidiosa cells were grown on Periwinkle Wilt Gelrite (PWG) medium (23) plates for 5 to 7 days prior to experiments and then transferred into PD3 broth (23). Antibiotics were added at the following concentrations: kanamycin at 50 μg ml−1, gentamicin at 15 μg ml−1, tetracycline at 10 μg ml−1, spectinomycin at 20 μg ml−1, and rifampin at 100 μg ml−1. All cultures were grown at 28°C in the dark.
Table 1.
Strains and plasmids used in this study
| Strain or plasmid | Genotype or description | Reference or source |
|---|---|---|
| Strains | ||
| X. campestris pv. campestris | ||
| 8004 | Wild type | |
| 8523 | X. campestris pv. campestris 8004 rpfF::Tn5lac (Rifr Kanr) | 5 |
| SC8 | X. campestris pv. campestris 8004 rpfC::Kanr | 19 |
| MIC1 | X. campestris pv. campestris 8004 ΔrpfCF (Genr) | This study |
| X. fastidiosa Temecula1 | Wild type | ATCC 700964 |
| Dif7 | X. fastidiosa Temecula1 ΔrpfF (markerless) | 19 |
| MIX2 | X. fastidiosa Temecula1 (Kanr between rpfC and rpfF) | This study |
| MIX2* | MIX2 rpfF* (E141A E161A) | This study |
| Plasmids | ||
| pCR4Blunt-TOPO | Invitrogen | |
| pENTR/D-MCS-Gent | attL1-MCS1-Genr-MCS2-attL2; Kanr | 42 |
| pLVC/D | attL1-attL2 gateway vector; Tetr | 24 |
| pMIC1 | pENTR-D X. campestris rpfGH-Genr-rpfB | This study |
| pMIC2 | pLVC-D X. campestris rpfGH-Genr-rpfB (suicide vector for rpfCF deletion) | This study |
| pBBR1MCS-2 | rep mob Kanr gene [aph(3′)-II] | 28 |
| pBBR1MCS-5 | rep mob Genr gene (aaaC1) | 28 |
| pXfHA | pBBR1MCS-5 hxfA′::phoA | 11 |
| pFXFkan | pUC19 Kanr [aph(3′)-II] | This study |
| pFXF3 | pFXFkan lacZ′::rpfF | This study |
| pFXF4 | pFXFkan lacZ′::rpfF* | This study |
| pFXF5 | pFXFkan rpfC-Kanr-rpfF | This study |
| pFXF6 | pFXFkan rpfC-Kanr-rpfF* (E141A E161A) | This study |
| pVSP61 | pVS1 and pACY184 Ori; Kanr | 43 |
| pKLN55 | pVSP61 X. campestris engXCA′::gfp | 6 |
| pF | pVSP61 X. fastidiosa rpfF | This study |
| pG | pBBR1MCS-2, X. fastidiosa rpfG, X. campestris engXCA′::gfp | This study |
| pC | pBBR1MCS-2, X. fastidiosa rpfC, X. campestris engXCA′::gfp | This study |
| pGC | pBBR1MCS-2, X. fastidiosa rpfGC, X. campestris engXCA′::gfp | This study |
| pGC gfp only | pBBR1MCS-2, X. fastidiosa rpfGC, promoterless gfp | This study |
| pCF | pBBR1MCS-2, X. fastidiosa rpfCF, X. campestris engXCA′::gfp | This study |
| pGCF | pBBR1MCS-2, X. fastidiosa rpfGCF, X. campestris engXCA′::gfp | This study |
| pGCF-FLAG | pBBR1MCS-2, X. fastidiosa rpfGCF (RpfC and RpfF are expressed with a C-terminal FLAG tag) | This study |
| pGCF* | pBBR1MCS-2, X. fastidiosa rpfGCF* (E141A E161A), X. campestris engXCA′::gfp | This study |
| pGCch | pBBR1MCS-2, X. fastidiosa rpfG, chimeric rpfC (bp 1–516 of X. fastidiosa and bp 514–2181 of X. campestris), X. campestris engXCA′::gfp | This study |
| pGCchgfp only | pBBR1MCS-2, X. fastidiosa rpfG, chimeric rpfC, promoterless gfp | This study |
| pGCchF | pBBR1MCS-2, X. fastidiosa rpfG, chimeric rpfC, X. fastidiosa rpfF, X. campestris engXCA′::gfp | This study |
Targeted disruption of rpfCF in X. campestris pv. campestris 8004.
In X. campestris, rpfC and rpfF are a part of two adjacent but convergent operons, rpfGHC and rpfBF (see Fig. S1 in the supplemental material). A X. campestris ΔrpfCF double mutant was constructed by double-crossover recombination between the two genomic segments that flank the rpfCF locus, using a suicide vector harboring a gentamicin resistance gene flanked by the same DNA segments. The flanking regions were amplified from the genome of WT X. campestris 8004 by using the primers listed in Table S1 in the supplemental material, with Phusion High-Fidelity DNA polymerase (Finnzymes, Finland). The PCR products were then subcloned into the pENTR/D entry vector (Invitrogen, USA) already harboring a gentamicin resistance gene flanked by multicloning sites and attL1-attL2 bacteriophage lambda recombination sites. The fragment introduced between the attL sites was then cloned into the suicide vector pLVC-D (24) by site-specific recombination at the attL sites using Gateway LR Clonase II enzyme mix (Invitrogen, USA). Plasmid pLVC-D was conjugated from Escherichia coli S17-1 into strain 8004. Since pLVC-D is unable to replicate in X. campestris, tetracycline- and gentamicin-resistant colonies were expected to have undergone a single-crossover event between sequences contained in pLVC-D in the strain 8004 genome. Such colonies were isolated and grown overnight on KB medium supplemented with gentamicin. Colonies that had undergone a second crossover event were isolated based upon their loss of tetracycline resistance. The resultant ΔrpfCF strain had a clear, dry phenotype (EPS deficient) (see Fig. S2 in the supplemental material), in contrast to the mucoid appearance of the WT. EPS abundance was estimated as the total carbohydrate content extracted from colonies by using anthrone reagent (25).
Construction of complementation vectors.
In X. fastidiosa, rpfC and rpfF are organized in the same manner as in X. campestris except that rpfB, involved in DSF processing (26), is located elsewhere and rpfH is absent (see Fig. S1 in the supplemental material). Genomic segments containing rpf genes were amplified from the X. fastidiosa genome, the promoter region of engXCA was amplified from the X. campestris 8004 genome, and gfp was amplified from pGFP_89 (27), using the primers listed in Table S1 in the supplemental material. All plasmids were constructed in three steps: first, the X. fastidiosa component was cloned into pBBR1MCS-2 (28) at the HindIII/SpeI restrictions sites (NEB, USA); second, promoterless gfp harboring an additional AvrII restriction site on its 5′ end was cloned into the same vector at the HindIII/ApaI restriction sites; and third, the engXCA promoter was then cloned at the HindIII/AvrII restriction sites to produce an engXCA′::gfp transcriptional fusion. The promoterless subvectors served to verify that there was no expression of the gfp reporter gene due to readthrough from the upstream region which carries the rpf components. All vectors (see Fig. S3 and S4 in the supplemental material for schematic representations) were introduced into the X. campestris ΔrpfCF strain by electroporation (2 V, 25 μF, and 200 Ω) (Gene Pulser; Bio-Rad, USA).
X. fastidiosa rpfF and its native promoter were cloned into pVSP61 at the EcoRI restriction site, after amplification using the primers listed in Table S1 in the supplemental material, to produce pF. pF and pG (in which rpfG was cloned into pBBR1MCS-2) have compatible origins of replication and thus could be cotransformed into, and comaintained by, the X. campestris host.
To construct a chimeric rpfC gene, the trans-membrane domain (TMD)-encoding region of X. campestris rpfC (513 bp) was replaced with that of X. fastidiosa (516 bp) by using in-frame overlap PCR with the primers listed in Table S1 in the supplemental material. Each region was first amplified from the genomic DNA of the respective organism, and 20 ng of each was mixed and subjected to a PCR of 15 cycles in the absence of primers. The filled-in full-length PCR product was then primed with the edge primers and subjected to an additional 20 cycles of amplification. The end product was subcloned into pCR4Blunt-TOPO by using the Zero Blunt TOPO PCR cloning kit (Invitrogen, USA), digested by HindIII and SpeI, and finally cloned into pBBR1MCS-2. Overlap PCR was also employed in a similar manner to fuse the rpfF gene to the construct harboring chimeric rpfC (rpfCch) (to generate pGCchF) (see Fig. S4 in the supplemental material) and to fuse the rpfG promoter to the rpfC promoter (to generate pC) (see Fig. S3 in the supplemental material).
Extraction of DSF from bacterial cultures.
DSF was extracted from X. campestris strains grown for 72 h in 1 liter of KB broth that was shaken at 200 rpm at 28°C. The pH of the medium was then adjusted to pH 4.0 (9), an equal volume of ethyl acetate was added to the medium, the mixture was mixed for 10 min, the ethyl acetate was then separated from the medium, and the ethyl acetate fraction was concentrated by evaporation using a Rotavapor R evaporator (Buchi, Switzerland). The dried residue was dissolved in 0.001 of the original volume of methanol (MeOH). As a control, synthetic DSFs were added and extracted from sterile KB broth for estimating extraction efficiency (50 to 100%). All extracts were stored at −20°C.
Measurement of engXCA′::gfp expression.
DSF, BDSF, CVC-DSF, and myristic acid were purchased from Sigma-Aldrich (catalog no. 42052, 49619, M3664, and M3128, respectively), dissolved in MeOH to a concentration of 100 mM, and stored at −20°C. Assay plates were prepared by adding DSF-containing culture extracts or purified molecules in MeOH at various concentrations into empty wells of Falcon 48-well tissue culture plates (Becton Dickinson, USA), and MeOH was allowed to escape. An equal volume of MeOH only was added to wells as a negative control. Hot (60°C) KB agar medium containing kanamycin (1 ml) was then added to each well. Inoculums of all X. campestris biosensor strains were grown for 3 days on KB agar containing kanamycin and suspended in 10 mM phosphate buffer (pH 7.4), the cell density was adjusted to an optical density at 600 nm (OD600) of 0.1 (Spectronic 21D spectrophotometer; Milton Roy, USA), and 3-μl drops were spotted onto each well. Colonies were examined with a Stereo Lumar epifluorescence dissecting microscope (Zeiss, Germany), and cells were then collected with a sterile cotton swab and suspended into 0.4 ml 10 mM phosphate buffer (OD600 = 0.2 to 0.3) in Falcon 48-well tissue culture plates (Becton Dickinson). For each tube, relative fluorescence units (RFU) and OD600 were recorded by using a Synergy 2 plate reader (BioTek, USA), and green fluorescent protein (GFP) fluorescence was normalized as RFU OD600−1.
Construction of enzymatically blocked RpfF protein.
Replacement of the native rpfF allele (rpfFN) with the mutant allele (E141A E161A) (rpfF*) was carried out by transforming X. fastidiosa with a pUC19-based suicide vector harboring a kanamycin [aph(3′)-II] resistance gene, flanked by two 874-bp DNA fragments with the rpfF* allele on one side and the 3′ end of the rpfC gene (1,000 bp out of 1,992 bp) on the other side. The kanamycin resistance gene was designed to replace the 22-bp intergenic region between rpfC and rpfF. As a control, an isogenic strain containing the native rpfF gene and the intergenic replacement was constructed as well. The kanamycin resistance gene was amplified from pBBR1MCS-2 (28), using Phusion DNA polymerase (Finnzymes, Finland) and the primers listed in Table S1 in the supplemental material. The PCR products and pUC19 were digested with BamHI (NEB, USA) and ligated with T4 DNA ligase (Roche) to generate pFXFkan. The orientation of the resistance gene was determined by sequencing. The DNA fragment carrying the rpfF* allele was constructed by overlap PCR (see above), using two ∼70-bp complementary primers (E141/161A-F and E141/161A-R) (see Table S1 in the supplemental material) harboring two GAA-to-GCA substitutions and cloned into pCR4Blunt-TOPO (Invitrogen, USA). The mutated allele and the native rpfF allele were amplified by using the edge primers only [rpfF-F(XbaI) and rpfF-R(HindIII)] from pCR4Blunt-TOPO and the X. fastidiosa genome, respectively. The rpfF alleles were initially cloned into pFXFkan in an orientation that allows their expression from the lacZ promoter located on the plasmid backbone, using XbaI and HindIII restriction sites to yield pFXF3 and pFXF4. Both alleles were then reamplified by using a nested primer [rpfF-F(SacI)] and a new reverse primer [rpfF-R(KpnI)] and cloned into pFXFkan in an orientation allowing proper integration into the X. fastidiosa genome, using SacI and KpnI restriction sites. The second flanking region harboring part of rpfC was amplified from the X. fastidiosa genome by using primers rpfC-F(HindIII) and rpfC-R(XbaI) and cloned into the other multicloning site of pFXFkan in an orientation allowing proper integration into the X. fastidiosa genome, using the HindIII and XbaI restriction sites. The resulting suicide vectors (pFXF5 and pFXF6) were transformed into X. fastidiosa by exploiting its natural competence (29). Transformed cells were cultured on PWG medium supplemented with kanamycin and incubated for 2 to 3 weeks at 28°C. Colonies were screened for replacement of the rpfFN allele with the mutated rpfF* allele by PCR using the edge primers and sequencing.
Quantitative reverse transcription-PCR.
Gene expression analysis was performed on X. fastidiosa or X. campestris strains subjected to an experimental setup identical to that employed for transcriptional fusion analysis. Total RNA was isolated by using an RNeasy RNA extraction kit (Qiagen, Germany); DNA was eliminated by using an on-column RNase-Free DNase apparatus (Qiagen, Germany). RNA samples were stored at −80°C. One microgram total RNA was used for cDNA synthesis before each analysis, using 3 μg of random hexamers and Superscript II reverse transcriptase (Life Technologies, USA) according to the manufacturer's instructions. Quantitative PCR was performed with an ABI Prism 7100 sequence detection system (Applied Biosystems, USA). Detection of PCR products was done by measuring the increase in fluorescence produced upon binding of SYBR green dye (Qiagen, Germany) to double-stranded DNA. Both rpoD and rpsO were used as endogenous control genes to normalize gene expression. To ensure that the threshold cycle (CT) values obtained were from a single PCR product, melting-curve analysis was run at the end of each expression analysis. Relative quantity (RQ) of target mRNA was calculated from the CT (30) as follows: ΔCT = CT (target gene) − CT (endogenous control), ΔΔCT = ΔCT (treatment) − ΔCT (reference), and RQ = 2(−ΔΔCT). To calculate the ratio between rpfF and rpfC transcripts, primer pairs with similar amplification efficiencies were employed (determined as described in the instrument handbook). RQ values (ratios between two compared samples) are presented as means ± standard deviations from three biological replicates of quantitative reverse transcription-PCR (qRT-PCR) assays performed in triplicates.
Transformation of pXfHA into X. fastidiosa.
Plasmid pXfHA carrying the hxfA′::phoA transcription fusion (11) was transformed into the X. fastidiosa WT, ΔrpfF, rpfFN, and rpfF* strains by using transformation protocols developed previously (31, 32). In this DSF-responsive fusion cloned into pBBR1MCS-5 (28), the endogenous phoA gene of X. fastidiosa is transcribed from the promoter of hxfA and translated from the ribosome binding site (RBS) of rpsU (AAAGGAAGGAATGGTC [the predicted RBS is underlined]). Prior to transformation, pXfHA was maintained and extracted from E. coli EAM1 (expressing X. fastidiosa methylase PD1607 [32]). pXfHA was coelectroporated into X. fastidiosa electrocompetent cells together with TypeOne restriction inhibitor (TRI) (Epicentre Biotechnologies, USA), and the resulting transformants were selected on PWG plates containing 10 μg ml−1 gentamicin.
Alkaline phosphatase assays.
Alkaline phosphatase (AP) activity was quantified after cells were permeabilized and disrupted (33). Inoculums of WT, ΔrpfF, rpfFN, or rpfF* strain cultures harboring pXfHA were grown for 5 to 6 days at 28°C on PWG plates containing gentamicin prior to suspension in PD3 broth containing gentamicin (final OD600 = 0.05) and application onto Falcon 48-well tissue culture plates (Becton Dickinson) (400 μl per well) containing various amounts of XfDSF. At various time intervals, the alkaline phosphatase activity of the cultures in the wells was determined. The plate was centrifuged for 10 min at 2,254 × g in an Eppendorf model 5804 centrifuge (Eppendorf, Germany), the growth medium was removed by aspiration, and the cells were resuspended in 0.4 ml 10 mM Tris base (pH 8.0) containing 10 mM MgSO4. The cells were pelleted again by centrifuging for 10 min at 2,254 × g and resuspended in 0.4 ml 1 M Tris base (pH 8.0) containing 0.4 mM ZnCl2, and the cell density (OD600) in each well was measured. The cells were then disrupted by adding 7 μl 0.1% SDS and 7 μl chloroform to each well, followed by 5 min of shaking (200 rpm) at room temperature. Sixty microliters of 1 M Tris base (pH 8.0)–0.4 mM ZnCl2 supplemented with 100 μM fluorescein diphosphate (Anaspec, USA) stock solution was then added to each well, and fluorescence measurements (excitation at 485 nm and emission at 515 nm) were taken at 2-min intervals for 30 min. The OD600 and fluorescence were measured by using a Synergy 2 plate reader (BioTek, USA). Enzyme activity was calculated as the rate of increase of fluorescence with time divided by the cell density.
Reverse-phase high-performance liquid chromatography analysis.
DSFs were detected and quantified by reverse-phase high-performance liquid chromatography (HPLC) using the Agilent Technologies 1200 Series High-Performance Liquid 150 chromatography system as follows: 5 μl of the sample was injected into an HPLC column (150-mm length and 4.6-mm internal diameter) (Ascentis Express C18 column; Supelco, USA), which was eluted at a flow rate of 1 ml min−1 with two solvent gradients of 0.1% trifluoroacetic acid (eluent A) and methanol plus 0.1% trifluoroacetic acid (eluent B) at 50°C. The gradient conditions were as follows, starting at 20% eluent A and 80% eluent B. Eluent A was linearly decreased to 10% over 20 min, while eluent B was increased to 90%. The elution pattern was monitored at 210 nm. The DSF concentration in crude extracts was determined based upon on their peak area and calculated from standard curves generated for each of the DSF species by using synthetic forms.
Analysis of XfRpfC and XfRpfF protein expression.
To verify that X. fastidiosa RpfC and RpfF are expressed in the heterologous Xanthomonas expression system, plasmid pGCF was reconstructed with rpfC and rpfF alleles each carrying a C-terminal FLAG tag. The rpfGCF-FLAG DNA fragment was generated by amplifying the DNA fragment consisting of rpfG and rpfC using primers lysS-F(HindIII) and rpfC-FLAG and by amplifying the fragment consisting of rpfF using primers rpfF-FLAG and rpfF-R(SpeI) (see Table S1 in the supplemental material) from the X. fastidiosa Temecula1 genome. Primers rpfC-FLAG and rpfF-FLAG harbor the 22-bp rpfC-rpfF intergenic region as a tail. The two PCR products were assembled into the complete rpfGCF fragment by overlap PCR (see above) and cloned into pBBR1MCS-2 through the HindIII/SpeI sites to generate pGCF-FLAG. pGCF-FLAG was introduced into the X. campestris rpfCF deletion strain. The strain harboring pGCF (untagged alleles) was employed as a negative control. Both strains were grown for 3 days in KB broth supplemented with kanamycin (28°C at 200 rpm in the dark) prior to protein extraction and separation by using SDS-PAGE. The relative abundance of RpfC and RpfF was assessed by Western blotting with rabbit anti-FLAG monoclonal serum (catalog no. F7245; Sigma-Aldrich) and horseradish peroxidase (HRP)-conjugated anti-rabbit IgG (catalog no. W401B; Promega).
Pathogenicity assays.
Cell suspensions (OD600 = 1.0) of various strains recovered after growth for 7 days on PWG medium were inoculated into 8 Thompson seedless or 12 Cabernet Sauvignon grapevines (ca. 50 cm in height), using a droplet puncture method as described previously (34). The number of symptomatic leaves was counted weekly starting at 7 weeks after inoculation. Symptomatic leaves were scored for the presence of typical Pierce's disease symptoms (loss of chlorophyll and death of the leaf margins). Noninoculated plants were used to distinguish Pierce's disease symptoms from leaf abnormalities not associated with X. fastidiosa infection. The population size of X. fastidiosa strains was determined 12 weeks after inoculation in petioles collected 50 cm from the inoculation point. The petioles were surface sterilized, weighed, and macerated; serial dilutions of tissue macerates were applied onto PWG plates; colonies were enumerated after 14 days of incubation at 28°C; and the population size was normalized by the weight of the plant tissue, as described previously (35).
RESULTS
Response of X. fastidiosa to XfDSF requires the XfDSF synthase RpfF.
Perception of XfDSF in X. fastidiosa was assessed by monitoring the expression of the hxfA′::phoA transcriptional fusion, previously shown to be induced by XfDSF (11), in the WT and ΔrpfF strains grown in the presence or absence of 10 μM synthetic XfDSF. The WT strain is expected to produce XfDSF that might mask its response to exogenous XfDSF, while the ΔrpfF strain is blocked in XfDSF production. The basal expression levels (no addition of XfDSF) of the hxfA′::phoA transcriptional fusion were similar in both strains until 96 h of growth, when XfDSF production by the WT strain increased the hxfA expression level by 1.7-fold in comparison to the ΔrpfF strain (Fig. 1A). Addition of exogenous XfDSF resulted in a 3-fold-higher expression level of the hxfA promoter at earlier times (72 h) in the WT strain but surprisingly did not induce hxfA in the ΔrpfF strain. This was surprising since phenotypes such as endoglucanase (engXCA) promoter activity could be restored in a ΔrpfF mutant of X. campestris by the addition of synthetic DSF to the growth medium (8). qRT-PCR analysis revealed that in addition to hxfA, the expression level of another hemagglutinin-encoding gene, hxfB (36), which is located elsewhere in the genome but is similarly downregulated in the ΔrpfF strain (19) (Fig. 1B), was also elevated by exposure to 10 μM XfDSF (3.08-fold ± 0.05-fold) in the WT strain but not in the ΔrpfF mutant (0.98-fold ± 0.68-fold) (Fig. 1B). Taken together, these observations suggested that perception of XfDSF in X. fastidiosa was impaired in the ΔrpfF strain and that RpfF is involved not only in XfDSF synthesis but also in the response to XfDSF.
Fig 1.
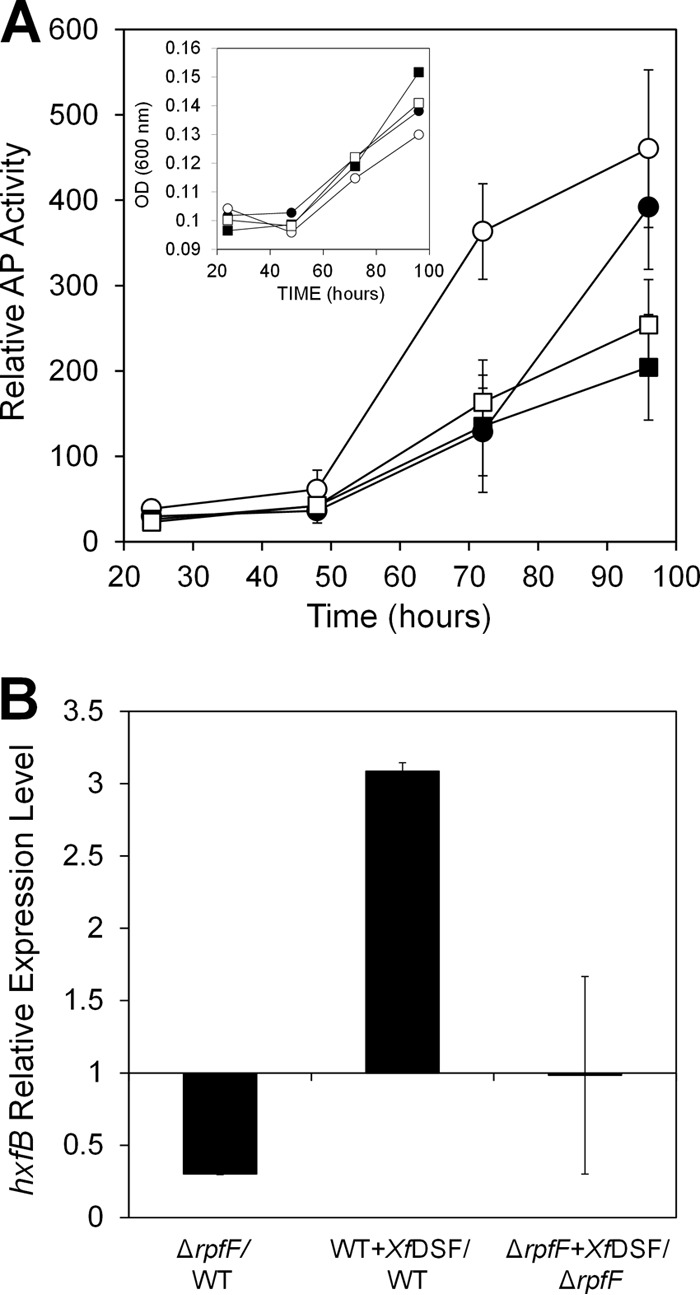
(A) Alkaline phosphatase activity indicative of induction of the hxfA promoter in cells of WT X. fastidiosa (circles) or a ΔrpfF strain (squares) harboring an hxfA′::phoA transcriptional fusion during 4 days of growth in PD3 broth in the presence (open symbols) or absence (filled symbols) of 10 μM 2(Z)-tetradecenoic acid (XfDSF) (n = 3). Growth of the bacterial strains is shown in the inset. (B) qRT-PCR analysis of hxfB expression in X. fastidiosa WT and ΔrpfF strains after 3 days of growth in PD3 broth in the presence or absence of 10 μM XfDSF (n = 3). Expression is expressed as the ratio between mRNA levels in the mutant and those in the WT strain or as the ratio between mRNA levels in the presence and those in the absence of XfDSF in each of the strains. Vertical bars are standard deviations of the means.
The RpfGCF signaling system of X. fastidiosa (here referred to as XfRpfGCF) should function autonomously in a given genetic background since it consists of all of the components needed for intercellular signaling: XfRpfF confers synthesis of XfDSF, while XfRpfC senses XfDSF, and XfRpfG hydrolyzes the universal secondary messenger cyclic di-GMP to confer intracellular signaling (14). Therefore, it was deemed possible to reconstitute the XfRpf system in heterologous taxa that employ cyclic di-GMP-mediated intracellular signaling and assess the expression of genes that are regulated by cyclic di-GMP. Since a detailed study of the XfRpf system in X. fastidiosa, a fastidious microorganism, was extremely challenging, we took advantage of the homology between the Rpf systems of X. fastidiosa and X. campestris to study the role of XfRpfF in the response to XfDSF in this more tractable host. We therefore established a heterologous expression system consisting of a X. campestris ΔrpfFC double mutant harboring a native DSF-dependent engXCA′::gfp transcriptional fusion as a reporter for RpfG activity that reduces cyclic di-GMP levels, thus enabling Clp to induce the engXCA promoter (37, 38). This surrogate host retained its own rpfG gene. X. fastidiosa rpfC and rpfF were thus introduced into this surrogate background along with X. fastidiosa rpfG under the control of their native promoters on plasmids to ensure proper signaling output (see Fig. S3 in the supplemental material).
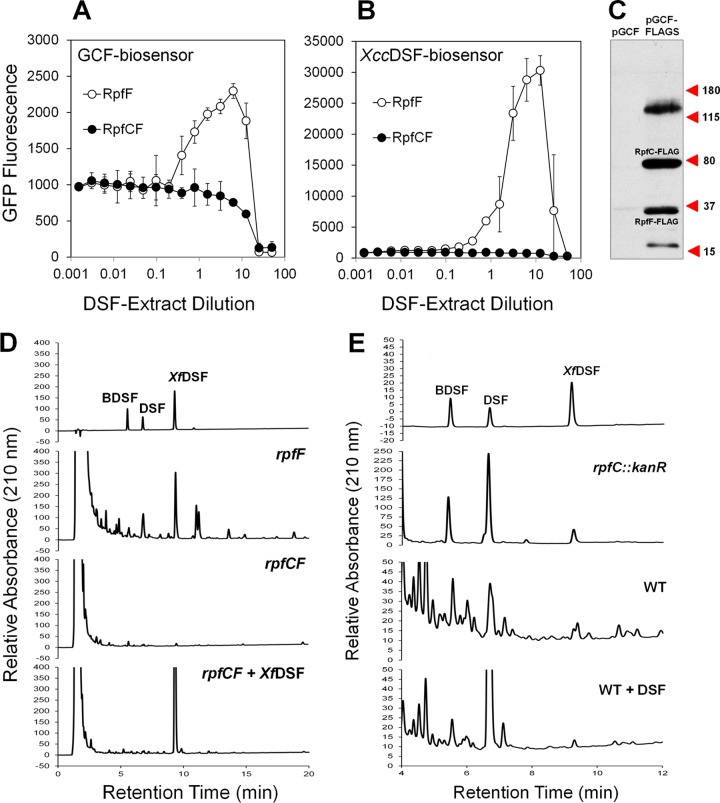
As evident from the relative GFP fluorescence of each strain measured after 3 days of growth on KB plates lacking XfDSF, the engXCA′::gfp transcriptional fusion was induced about 2.2-fold more in the presence XfRpfGC than when only XfRpfG was present (Fig. 2A). Importantly, when XfRpfF was expressed in the X. campestris ΔrpfCF strain together with XfRpfC and XfRpfG, GFP fluorescence, indicative of engXCA expression, was strongly reduced (Fig. 2A). Since in X. campestris, RpfC and RpfF physically interact (21), XfRpfF appeared to repress XfRpfC signaling activity through a physical interaction.
Fig 2.
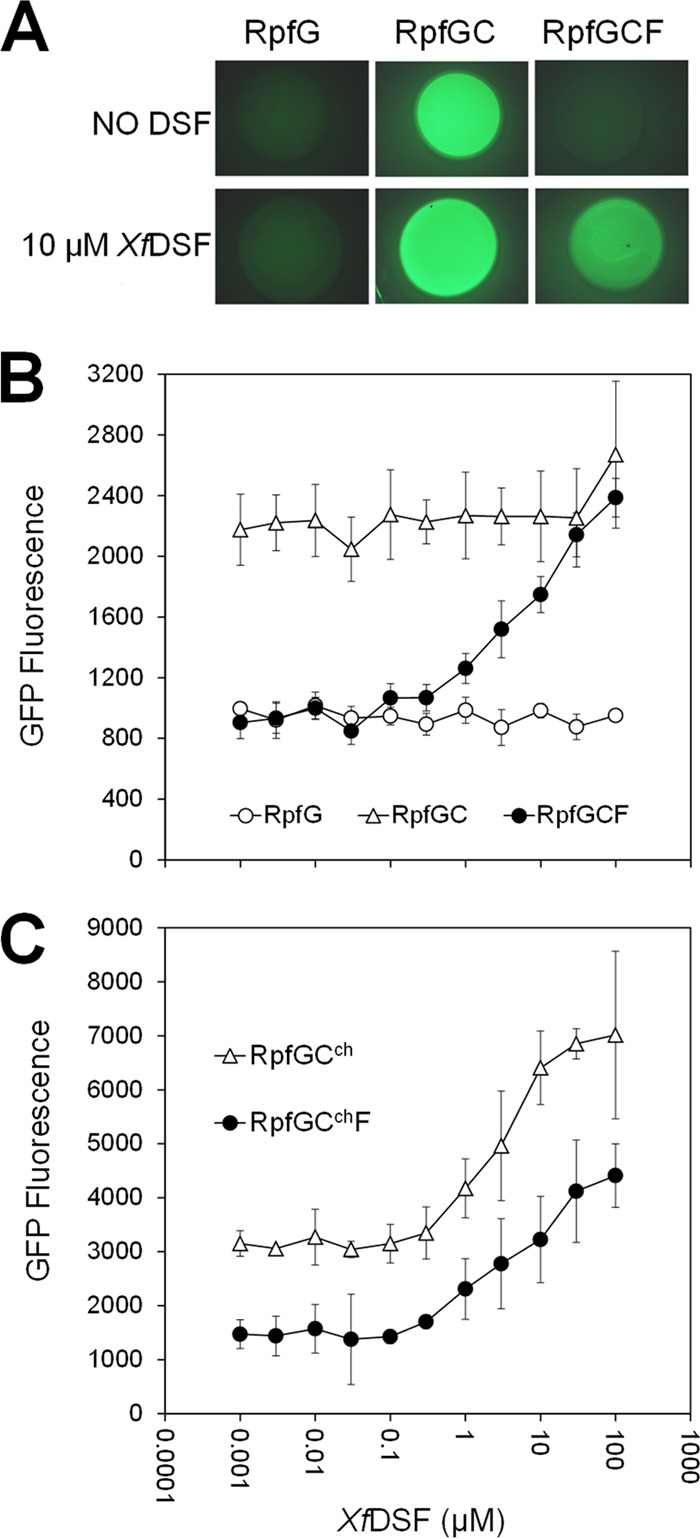
GFP fluorescence of an X. campestris rpfCF deletion mutant strain harboring an engXCA′::gfp transcriptional fusion as well as various X. fastidiosa Rpf components. (A) Fluorescence images (magnification, ×10) of colonies grown for 3 days on KB agar in 48-well tissue culture plates and grown without (top row) or with (bottom row) the addition of 10 μM XfDSF. (B) Cell-normalized fluorescence of a X. campestris rpfCF deletion mutant harboring X. fastidiosa rpfG, rpfGC, or rpfGCF when grown on KB agar containing various concentrations of 2(Z)-tetradecenoic acid (XfDSF) (n = 6). (C) Cell-normalized fluorescence of a X. campestris rpfCF deletion mutant harboring X. fastidiosa rpfG and rpfCch or X. fastidiosa rpfG, rpfF, and rpfCch when grown on KB agar containing various concentrations of 2(Z)-tetradecenoic acid (XfDSF) (n = 3).
To test the role of XfDSF in the signaling process, the GFP fluorescence of these strains was tested in the presence of 10 μM XfDSF [2(Z)-tetradecenoic acid] (11). No increase in the expression level of engXCA was observed in strains harboring XfRpfGC compared to cultures grown in the absence of XfDSF (Fig. 2A), suggesting that XfRpfC and XfRpfG are not sufficient to restore DSF sensing to the ΔrpfCF strain of X. campestris. However, the GFP fluorescence of the strain also bearing XfRpfF was 1.8-fold higher in the presence of 10 μM XfDSF than that of cells grown in the absence of this signal molecule (Fig. 2A), suggesting that XfDSF derepressed the inhibitory effect of XfRpfF, allowing the engXCA′::gfp transcriptional fusion to be induced. Measurements of transcript abundance using qRT-PCR revealed that rpfC was expressed at similar levels (ratio of 1.33 ± 0.56) in the X. campestris strains harboring X. fastidiosa rpfGC and rpfGCF, indicating that the decrease in reporter gene product abundance, indicative of XfRpfC signaling, was not due to lower levels of expression of XfRpfC in the presence of XfRpfF.
The X. campestris ΔrpfCF mutants bearing XfRpfG, XfRpfGC, and XfRpfGCF were further tested for their responsiveness to XfDSF. While the strains bearing XfRpfG or XfRpfGC did not respond to XfDSF, the strain harboring XfRpfGCF exhibited progressive, dose-dependent GFP fluorescence in response to the addition of >0.5 μM XfDSF, exhibiting up to a 2.5-fold increase in GFP fluorescence in response to 100 μM XfDSF (Fig. 2B). We previously reported that a similar threshold concentration of XfDSF was also detected by a X. fastidiosa-based XfDSF biosensor harboring an hxfA′::phoA reporter gene fusion (11). Because of the utility of this strain to assess the concentration of XfDSF, we refer to it as the GCF biosensor. Taken together, it can be concluded that in the absence of XfDSF, XfRpfC can mediate signal transduction in X. campestris to induce the engXCA promoter, a function that is repressed by XfRpfF (Fig. 2A and B). However, in the presence of XfRpfF, XfRpfC signaling activity is derepressed upon acquisition of XfDSF, suggesting that in the XfRpf system, XfRpfF is involved in both XfDSF synthesis and XfDSF-dependent signaling.
It is clear that while RpfC is sufficient for initiating signaling in response to DSF in X. campestris, this is not true for X. fastidiosa, and therefore, XfRpfC must differ functionally from that of X. campestris. A cytoplasmic REC domain of RpfC has been shown to interact physically with RpfF in X. campestris, and this interaction has been proposed to be abolished in the presence of DSF (16, 21). The trans-membrane domain presumably has a role in binding DSF. To determine if the cytoplasmic domain of XfRpfC was responsible for the XfRpfF-dependent responsiveness to XfDSF, we replaced the TMD (see Fig. S4 and S5 in the supplemental material) of RpfC of X. campestris with that of X. fastidiosa to generate chimeric RpfC (termed RpfCch), which is expected to function like XfRpfC in sensing DSF species but to lack an XfRpfF dependency for signaling like that of X. campestris RpfC itself. For consistency, the rpfCch allele was introduced into the X. campestris ΔrpfCF strain along with X. fastidiosa rpfG on the vector pGCch. As before, the GFP fluorescence of the engXCA′::gfp transcriptional fusion was assessed in response to added XfDSF. When grown in the presence of 10 μM XfDSF, its expression level increased by ca. 2-fold (Fig. 2C), indicating that RpfF was not essential for the signaling activity of the cytoplasmic domain of RpfCch consisting of X. campestris RpfC (see Fig. S4 in the supplemental material). However, when XfRpfF was expressed in this strain in addition to XfRpfG and RpfCch on plasmid pGCchF, we again observed a lower basal level of GFP fluorescence (Fig. 2C). Thus, XfRpfF could suppress the signaling activity of RpfC of X. campestris but was not required for the apparent XfDSF-mediated derepression. The abundance of rpfCch transcript in strains harboring pGCchF and pGCch was similar (ratio, 1.78 ± 1.45), indicating again that repression of RpfCch signaling by XfRpfF was not due to repression of rpfCch transcription. The X. campestris ΔrpfCF strain harboring pGCch is useful for assessing the concentration of XfDSF and is referred to as the GCch biosensor.
Exposure of the GCF biosensor and the GCch biosensor to as little as 0.5 to 1 μM the DSF species DSF, BDSF, or XfDSF conferred elevated levels of GFP fluorescence. In contrast, the X. campestris DSF biosensor specific for X. campestris DSF consisting of a X. campestris rpfF::Tn5lac mutant harboring an engXCA′::gfp transcriptional fusion (6) (here called the XccDSF biosensor) more selectively responded to DSF species, sensing as little as ca. 0.2 μM DSF and 1 μM BDSF but only >10 μM XfDSF (Table 2; see also Fig. S6 in the supplemental material). It is noteworthy that the minimal concentration of XfDSF detectable with the GCF biosensor and the GCch biosensor (each ca. 0.5 μM) was similar to that detectable with an hxfA′::phoA-based biosensor in X. fastidiosa itself (11). None of the biosensors responded to CVC-DSF (12-methyltetradecanoic acid) (12) or to myristic acid (Table 2; see also Fig. S6 in the supplemental material). The results suggest that the XfRpf system is more promiscuous than that of X. campestris.
Table 2.
Minimum concentrations of various DSF molecules that induce the engXCA′::gfp reporter gene fusion in X. campestris harboring various RpfF and RpfC components
| Molecule | Minimum concn (μM) (range) of molecule sensed by: |
||
|---|---|---|---|
| XccDSF biosensor | GCF biosensor | GCch biosensor | |
| DSF | 0.1–0.3 | 0.3–1 | 0.3–1 |
| BDSF | 0.3–1 | 0.3–1 | 0.3–1 |
| XfDSF | 6–10 | 0.3–1 | 0.3–1 |
| CVC-DSF | No activity | No activity | No activity |
| Myristic acid | No activity | No activity | No activity |
RpfC represses RpfF XfDSF synthesis activity.
Since the GCF biosensor harbors rpfF from X. fastidiosa, it should be able to produce DSF. However, it was reported that in X. campestris, RpfC inhibits RpfF DSF synthase activity (8, 39). We thus tested if XfRpfC similarly regulates XfRpfF by assessing DSF levels in culture extracts of a X. campestris ΔrpfCF strain harboring either X. fastidiosa rpfF, both rpfF and rpfC, or a combination of rpfF, rpfC, and rpfG when cultured for 3 days. Culture extracts were tested for the presence of DSF with both the GCF biosensor, which is highly responsive to XfDSF, and the XccDSF biosensor, which is most responsive to DSF, as well as by reverse-phase HPLC. Both DSF biosensors were activated by the DSF-containing culture extracts of cells harboring X. fastidiosa rpfF, while no DSF species were detected in extracts of cells harboring either both rpfF and rpfC (Fig. 3A and B) or a combination of rpfF, rpfC, and rpfG (not shown). HPLC analysis revealed the presence of DSF, BDSF, and XfDSF in extracts of a ΔrpfFC mutant of X. campestris harboring XfRpfF (Fig. 3D and Table 3) and, surprisingly, in extracts of WT X. campestris and an rpfC::Kanr mutant strain as well (Fig. 3E and Table 3). None of these species were found in cultures of the ΔrpfCF mutant itself or this strain harboring both XfRpfF and XfRpfC (Fig. 3C and Table 3). These results clearly show that XfRpfC suppresses the DSF synthase activity of XfRpfF. While XfDSF constituted only 4 to 9% of the DSF in extracts of the native X. campestris strains, it represented 35% of the DSF in the extract of the X. campestris ΔrpfFC mutant expressing X. fastidiosa rpfF. The increased proportional abundance of XfDSF apparently reflects a difference in the specificity of the RpfF proteins from X. fastidiosa and X. campestris, given that a similar pool of substrates should have been present in the X. campestris host.
Fig 3.
Evaluation of DSF production in DSF-containing culture extracts of X. campestris ΔrpfCF strains bearing either X. fastidiosa RpfF alone or both RpfC and RpfF. (A and B) Cell-normalized fluorescence indicative of engXCA′::gfp transcriptional activity is shown for two different DSF biosensors, the GCF biosensor harboring rpf genes (A) and the XccDSF biosensor that harbors X. campestris native rpf genes (B). Vertical bars are standard deviations of the means (n = 3). (C) Western blot analysis of the relative abundance of X. fastidiosa RpfC and RpfF in X. campestris ΔrpfCF strains bearing pGCF-FLAG in which both proteins carry a C-terminal FLAG tag. (D and E) HPLC chromatograms of DSF species produced by the X. campestris ΔrpfCF mutant expressing X. fastidiosa rpfF or rpfF and rpfC in the presence or absence of 50 μM XfDSF (D) or by the X. campestris rpfC mutant and WT strains in the presence or absence of 50 μM DSF (E) (time of extraction = 3 days). The top HPLC chromatograms show retention times of 250 μM (D) or 50 μM (E) synthetic standards.
Table 3.
Concentrations of BDSF, DSF, and XfDSF in culture extracts of various X. campestris strains after 3 days of growth
| Strain | Mean concn (nM) ± SDa |
||
|---|---|---|---|
| BDSF | DSF | XfDSF | |
| WT | 114 ± 102 | 119 ± 31 | 25 ± 24 |
| rpfC::Kanr | 357 ± 42 | 986 ± 57 | 57 ± 14 |
| rpfF::Tn5lac | Undetected | Undetected | Undetected |
| ΔrpfCF | Undetected | Undetected | Undetected |
| ΔrpfCF pF | 209 ± 87 | 558 ± 131 | 423 ± 166 |
| ΔrpfCF pCF | Undetected | Undetected | Undetected |
| ΔrpfCF pGCF | Undetected | Undetected | Undetected |
| ΔrpfCF pGCchF | Undetected | Undetected | Undetected |
| ΔrpfCF pCF + 5 μM XfDSF | Undetected | Undetected | Not shownb |
| ΔrpfCF pCF + 50 μM XfDSF | Undetected | Undetected | Not shownb |
| WT + 5 μM DSF | 100 | Not shownb | 27 |
| WT + 50 μM DSF | 122 ± 93 | Not shownb | 31 ± 17 |
Values presented are the means of 3 biological replicates ± standard deviations of the means.
See Table S2 in the supplemental material.
To ensure that rpfF is expressed at similar levels in the X. campestris strain harboring X. fastidiosa rpfGCF (cloned into pBBR1MCS-2) as in the strain harboring rpfF only (cloned into pVSP61), the abundance of rpfF transcript was tested by qRT-PCR. The expression levels of rpfF were 2.8-fold ± 0.69-fold higher in the strain harboring rpfGCF and 2.58-fold ± 0.26-fold higher in the strain harboring rpfGCchF than in the strain harboring only rpfF. This indicates that the lower apparent DSF synthesis level seen in X. campestris harboring either XfRpfC or RpfCch is not due to an underexpression of the XfDSF synthase XfRpfF. However, since XfRpfC represses XfRpfF-mediated XfDSF synthesis activity, it is possible that in the X. campestris strains, the stoichiometry of XfRpfC to XfRpfF favors repression of XfDSF synthesis. Therefore, we calculated the ratio of rpfF and rpfC transcripts in both the X. campestris strain expressing X. fastidiosa rpfCF genes and the WT X. fastidiosa strain by using primer pairs with similar amplification efficiencies. The ratios of rpfF to rpfC transcript levels were 0.83 ± 0.05 and 2.19 ± 0.82 in the X. campestris strain and WT X. fastidiosa, respectively, suggesting that, based on the assumption that transcript levels reflect protein abundance, the blockage of DSF synthesis that occurred in the X. campestris strain expressing X. fastidiosa rpfCF is due to a lack of free RpfF. To further demonstrate this conclusion, the pGCF vector was reconstructed with rpfC and rpfF variants both harboring a C-terminal FLAG tag-encoding sequence. Introduction of pGCF-FLAG into the X. campestris rpfCF mutant followed by expression analysis of both proteins indicated that XfRpfF is indeed expressed at a somewhat lower level than XfRpfC (Fig. 3C). Although denaturation conditions were employed, we also observed a high-molecular-mass (>115-kDa) complex, apparently of RpfC and RpfF, that could be resolved partially into the two monomers by extended boiling of the sample (not shown), suggesting that the two proteins physically interact as in X. campestris.
It was previously suggested that RpfC dissociates from RpfF upon acquisition of DSF, thereby accelerating DSF production (7, 16), but there has been no demonstration of the expected autoinduction of DSF production. To test if such autoinduction occurs, the WT X. campestris strain or the ΔrpfCF mutant bearing X. fastidiosa rpfF and rpfC were grown in the presence of 0, 5, or 50 μM DSF or XfDSF, respectively, and the production of the DSF species that were not supplemented in the growth medium was assessed by reverse-phase HPLC. We did not observe an increase in the abundance of BDSF and XfDSF in cultures of the WT strain (Table 3) or apparent production of BDSF or DSF in the ΔrpfCF strain harboring X. fastidiosa rpfF and rpfC (Table 3) in cultures supplemented with DSF or XfDSF, respectively. In contrast, we did observe a reduction in the abundance of the supplemented DSF species (see Table S2 in the supplemental material), indicating that it was degraded. These results suggest that inhibition of DSF synthesis activity by RpfC might not be derepressed by DSF, and therefore, the proposed positive-feedback model of DSF production should be readdressed, taking into account DSF degradation and the stoichiometric ratio of RpfF to RpfC.
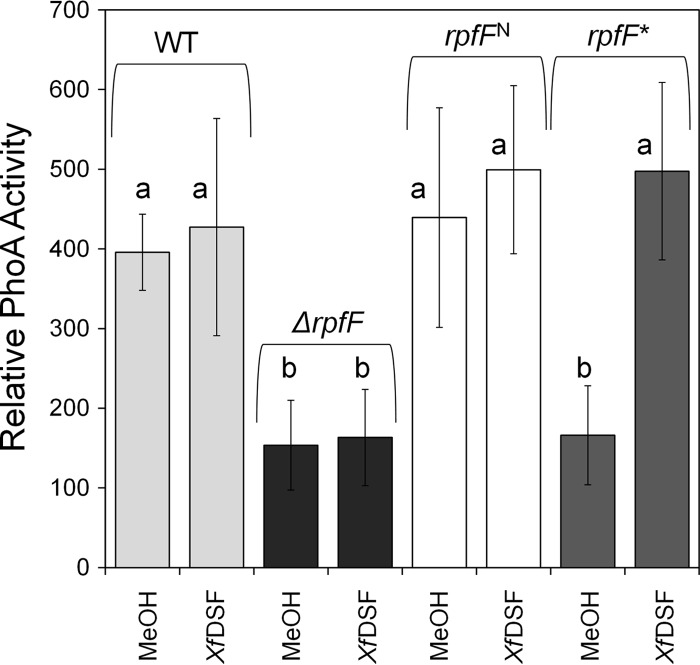
The XfRpfF(E141A E161A) mutant is blocked in XfDSF production but enables a response to XfDSF.
All RpfF proteins are structurally similar to members of the crotonase superfamily, with two highly conserved catalytic glutamate residues located at their active pockets (7, 13, 21). To differentiate between the two functions of XfRpfF, namely, XfDSF synthesis and XfDSF-dependent signaling, its XfDSF synthesis activity was blocked by substituting alanine for these glutamate residues (E141A and E161A) to yield the variant designated rpfF*. E. coli strains expressing native rpfF or the mutated allele were employed to verify loss of XfDSF synthase activity (see Fig. S7 in the supplemental material), and the mutated allele was also substituted for the native rpfF allele in X. fastidiosa. As a control, we also constructed a near-isogenic strain, designated rpfFN, that, like the rpfF* mutant, carried a kanamycin resistance cassette in the rpfC-rpfF intergenic region but harbored a native rpfF allele. As expected, XfDSF production was abolished in the rpfF* mutant of X. fastidiosa (see Fig. S8 in the supplemental material), while the rpfFN strain exhibited normal XfDSF accumulation. Both the rpfFN and rpfF* strains, however, exhibited a somewhat reduced growth rate in culture (see Fig. S9A in the supplemental material). We suspect that the 22-bp rpfC-rpfF intergenic region that was replaced by site-directed recombination with a kanamycin resistance cassette confers, unexpectedly, an important trait. The high level of conservation of this region among all sequenced X. fastidiosa strains supports this conjecture (see Fig. S9B in the supplemental material).
The responsiveness of these strains to exogenous XfDSF, in comparison to the ΔrpfF strain and its corresponding WT strain, was then assessed by introducing plasmid pXfHA carrying a XfDSF-responsive hxfA′::phoA transcriptional fusion (11) and determining alkaline phosphatase activity. As described above (Fig. 1A), the expression of hxfA in WT cells was enhanced in response to exogenous XfDSF, while no such response was seen in the ΔrpfF strain. Alkaline phosphatase activity in the absence of added XfDSF increased slowly with time in the WT strain due to the production of endogenous XfDSF. Importantly, at 96 h, while activity in the WT was no longer evident regardless of XfDSF addition, the rpfF* mutant exhibited similarly low alkaline phosphatase activity compared to the ΔrpfF strain in the absence of added XfDSF, yet its response to exogenous XfDSF was similar to that of the WT strain (Fig. 4). This indicated that the XfDSF synthesis activity of XfRpfF is not required to enable its role in XfDSF-dependent signaling, a function that is expected to be mediated through protein-protein interactions with XfRpfC.
Fig 4.
Relative alkaline phosphatase activity reflecting the induction of the hxfA promoter in a X. fastidiosa WT strain and its ΔrpfF derivative as well as the rpfFN strain (kanamycin resistant) and its rpfF* mutant derivative with changes in the DSF catalytic site, each harboring an hxfA′::phoA transcriptional fusion, after 96 h of growth in PD3 broth in the presence or absence of 10 μM 2(Z)-tetradecenoic acid (XfDSF). Error bars represent the standard deviations of the means (n = 6). Statistically significant differences (P < 0.05) between samples are represented by the letters “a” and “b.”
To demonstrate the functionality of XfRpfF* in XfDSF sensing, the GCF biosensor was reconstructed with the rpfF* allele replacing native rpfF. One can posit that the suppression of signaling activity of XfRpfC by XfRpfF (as observed for the GCF biosensor and the GCchF biosensor) could be due to XfRpfC-dependent production of variant DSF species that repress XfRpfC (e.g., so-called antagonistic DSFs). However, the GCF and GCF* biosensors exhibited similar GFP fluorescence levels when a given concentration of XfDSF was added to cell suspensions, thus indicating that substitution of the rpfF* allele did not influence either the intensity or the sensitivity of the response to XfDSF (see Fig. S10 in the supplemental material). This strongly suggests that the suppressive effect of XfRpfF on XfRpfC signaling activity is not mediated by antagonistic DSF species that might be produced by XfRpfF when XfRpfC is present.
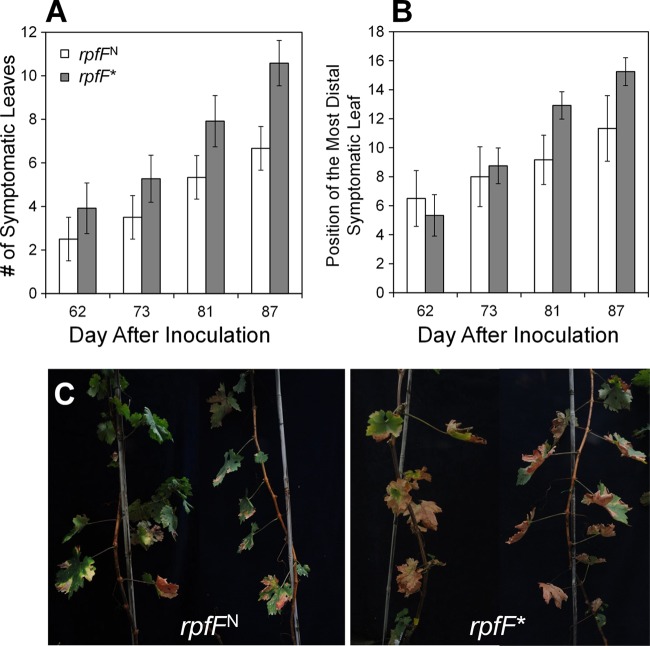
XfDSF is itself an antivirulence factor.
While rpfF mutants of X. fastidiosa had been shown to be hypervirulent to grape, it was unclear whether this was due to an inappropriate expression of a variety of traits dependent on RpfF for expression or due to the lack of XfDSF itself in such mutants. We thus compared the abilities of the rpfF* mutant, which is apparently capable of proper interactions with XfRpfC but lacks the ability to produce XfDSF, and the near-isogenic rpfFN strain to cause Pierce's disease in grapevine after mechanical inoculation. Although both strains exhibited somewhat reduced virulence compared to the WT strain, apparently due to their lower growth rate (see Fig. S9 in the supplemental material), both strains caused symptoms typical of Pierce's disease. Importantly, the rpfF* mutant was consistently more virulent than its isogenic rpfFN strain, as measured by the number of symptomatic leaves per plant on both Cabernet Sauvignon and Thompson seedless grapes (Fig. 5A and C; see also Fig. S11 and S12 in the supplemental material). Likewise, the rpfF* mutant moved more extensively in the plant, as documented by the location of the most distal leaf exhibiting disease symptoms (Fig. 5B). The severity of disease incited by the rpfF* mutant was significantly greater than that induced by the rpfFN strain, especially when measured 12 weeks or more after inoculation (P < 0.005). The rpfF* mutant also attained a larger population size (6.4 × 107 ± 5.2 × 106 cells g−1) in samples of Thompson seedless grape taken from petioles located 50 cm from the point of inoculation than the isogenic rpfFN strain (1.9 × 107 ± 1.1 × 106 cells g−1). Similarly, the rpfF* mutant was about 3.3-fold more abundant than the rpfFN strain in petioles collected at this distance from the point of inoculation of Cabernet Sauvignon plants (1.9 × 107 ± 5 × 106 cells g−1 and 6.5 × 106 ± 4.8 × 106 cells g−1, respectively). XfDSF is thus itself a trait that acts to suppress growth and movement of the pathogen, thereby suppressing virulence to grape.
Fig 5.
Virulence of the X. fastidiosa rpfFN strain, harboring a kanamycin marker gene in the intergenic region between rpfF and rpfC, and the rpfF* mutant strain, blocked in DSF production due to changes in the DSF catalytic site, to Cabernet Sauvignon grapevines. (A) Severity of Pierce's disease symptoms incited by these strains, expressed as the mean number of symptomatic leaves per vine (n = 12). (B) Position of the leaf most distal to the point of inoculation that exhibited symptoms of Pierce's disease when assessed at the times shown on the abscissa. The vertical bars represent the standard errors of the means. (C) Representative images of vines infected with the rpfFN strain and the rpfF* mutant.
DISCUSSION
Several bacterial pathogens produce DSF-type quorum-sensing signals which they then perceive and integrate into the global regulatory network of the cell to modulate virulence and biofilm formation. This study and other recent works, however, suggest that although common regulatory components may be involved, the signal transduction processes may differ substantially in these taxa. A recent study revealed that the Burkholderia cenocepacia DSF signaling system differs from that of X. campestris in its mechanisms of signal perception (40). While cyclic di-GMP degradation is stimulated by the binding of DSF to its trans-membrane receptor RpfC, which in turn phosphorylates the cyclic di-GMP PDE RpfG in X. campestris (14), in B. cenocepacia, a decrease in the intracellular cyclic di-GMP level is mediated by binding of BDSF to RpfR, an intracellular BDSF receptor that contains Per-Arnt-Sim (PAS)-GGDEF-EAL domains (40). In this study, we show that although X. fastidiosa and X. campestris employ similar Rpf components to perceive DSF, the signaling mechanism of each taxon is substantially different. We assign here a novel additional role for XfRpfF, a quorum-sensing signal molecule synthase, in the signaling reaction stimulated by the signal molecule that it produces. Our data indicate that XfDSF-dependent signaling by XfRpfC requires the XfDSF synthase XfRpfF and that there is a distinction between its XfDSF synthetase activity and its role in XfDSF-dependent signaling. Since RpfF and RpfC were shown to physically interact in X. campestris (16, 21), we assume that a similar interaction mediating the regulatory role attributed to RpfF also occurs in X. fastidiosa. Therefore, in X. fastidiosa, the XfDSF-sensing apparatus is apparently a protein complex of XfRpfC and XfRpfF, which impose a bidirectional regulatory effect on each other: XfRpfC inhibits the XfRpfF XfDSF synthesis activity, while XfRpfF inhibits XfRpfC signaling activity in the absence of XfDSF. It is noteworthy that additional proteins might be involved in DSF signaling; Chatterjee et al. (19) suggested that a second XfDSF sensor protein might function in parallel with XfRpfC in X. fastidiosa, and recently, An et al. (41) presented evidence for a second mechanism of DSF sensing in X. campestris.
XfDSF production and accumulation during X. fastidiosa growth in vitro are apparent by 3 days (Fig. 1), as evident from the increase in hxfA′::phoA activity in the WT strain without added XfDSF. Therefore, it is surprising that we did not observe production of DSF species in the GCF biosensor after 3 days of growth. This is likely to be due to a stoichiometry of XfRpfC to XfRpfF that favors complex formation and thus persistent suppression of XfRpfF XfDSF synthesis activity by XfRpfC. In the heterologous Xanthomonas system, XfRpfC and XfRpfF were expressed from their native promoters on a moderate-copy-number plasmid, and their expression levels do not necessarily reflect their actual expression levels and relative abundance in X. fastidiosa. It thus can be hypothesized that production of XfDSF in X. fastidiosa is regulated by altering the level of each of these proteins. Production would be achieved when the abundance of XfRpfF was greater than that of XfRpfC, while suppression would occur when the abundance of XfRpfF was less than that of XfRpfC. Such a model has yet to be tested.
The XfRpf/XfDSF system of X. fastidiosa also differs from that of X. campestris in the hierarchical arrangement of DSF signaling within the regulatory cascade that leads to the expression of DSF-dependent traits. Both DSF accumulation and expression of the DSF-dependent protease encoded by ptrA occurred at the early stationary growth phase in X. campestris. The addition of a DSF-containing culture extract to a WT culture, however, did not immediately induce ptrA (5), suggesting that DSF is not the sole determinant of the expression of such DSF-dependent traits. Such multiple-context-dependent regulation was not seen in X. fastidiosa, since the addition of synthetic XfDSF at early stages of growth resulted in an early expression of the XfDSF-dependent adhesin encoded by hxfA (Fig. 1). In addition, XfRpfC and RpfC of X. campestris differ in their responsiveness toward various DSF species, as evident from the different minimal concentrations needed to activate signaling in the Xanthomonas-based DSF biosensors harboring different RpfC components: X. campestris RpfC interacts much more efficiently with DSF and BDSF than with XfDSF, while XfRpfC apparently interacts with these molecules at similarly low concentrations (see Fig. S6 in the supplemental material).
It was previously shown that the identity and relative proportion of different DSF species produced by X. campestris were influenced by the composition of the growth medium (9). Therefore, it was perhaps not surprising that X. campestris produced XfDSF in addition to DSF and BDSF when grown in KB broth. XfDSF was not previously found in X. campestris cultures. It thus appears that RpfF is a very promiscuous enzyme, with its products being dependent largely upon available intracellular substrates, which in turn are strongly influenced by the growth medium and environment. Therefore, it seems reasonable that RpfF has evolved in each of the species X. fastidiosa and X. campestris to accommodate substrates present in the host cells, which in turn inhabit quite different niches, the xylem and the apoplast, in their host plants. It is reasonable to expect that their cognate RpfC has evolved to interact with those DSF species most prominent in cells in their natural habitat. In any event, the environmental-context-dependent production of different DSF species observed here implies that these species could produce novel DSF species under natural conditions. Elucidation of the signaling molecules made in such settings should enable a better understanding of the role of quorum sensing in relation to plant disease in these taxa.
Models of DSF signaling in X. campestris suggested that positive feedback would occur, yet this had not been tested. Detailed genetic, biochemical, and structural analyses of X. campestris have demonstrated that the regulation of DSF production by RpfC is independent of its phosphorelay activity and that RpfC negatively controls DSF production via a posttranslational mechanism involving domain-specific protein-protein interactions (16, 21). While rpfC deletion mutants as well as REC domain deletion variants overproduce DSF, overexpression of the REC domain, with which RpfF apparently interacts, abolished DSF production. According to this model, at low cell densities, the unphosphorylated RpfC maintains a conformation that facilitates formation of a complex between RpfF and RpfC, limiting DSF production to a basal level. At high cell densities, the accumulated DSF molecules trigger autophosphorylation of RpfC and release of RpfF, resulting in increased DSF signal production. However, DSF-dependent release of RpfF from RpfC has not yet been demonstrated, and our attempts to do so by spiking a culture of X. campestris harboring XfRpf components with XfDSF or DSF did not result in the accumulation of other DSF species, as assessed by chemical analysis (Table 3), leading us to the conclusion that this mechanism does not occur in X. fastidiosa. Similarly, no such positive feedback was seen when a X. campestris WT strain was spiked with DSF (Table 3). Thus, the current model for RpfF/RpfC interactions developed for X. campestris needs to be readdressed.
RpfF is a surprisingly multifunctional protein that plays a central role in the biology of X. fastidiosa. DSF synthesis in B. cenocepacia by RpfF was reported to involve dual processes of dehydration of a 3-hydroxydodecanoyl-ACP to 2(Z)-dodecenoyl-ACP and the cleavage of the thioester bond to yield a free acid (13). We show here that XfRpfF has yet another role in XfDSF-dependent signaling in X. fastidiosa. Because of the proposed multifunctionality of XfRpfF, we separated its components that contribute to its various roles by site-directed changes which blocked its XfDSF synthase activity yet retained its ability to participate in XfDSF signaling. Given that the virulence of the rpfF* mutant, which is blocked in XfDSF production but can still participate in XfDSF signaling, was significantly greater than that of the WT strain (Fig. 5; see also Fig. S11 and S12 in the supplemental material), XfDSF itself is clearly a trait that acts to suppress growth and movement of the pathogen while promoting insect transmission. The accumulation of XfDSF thus appears to enable cells to prevent overgrowth in the xylem, a process that leads eventually to their death (4). Likewise, the accumulation of signal molecules can increase the adhesiveness of the cells to better enable their acquisition by insect vectors and thus subsequent transmission to new plants.
Supplementary Material
ACKNOWLEDGMENTS
M.I. was supported by Vaadia-BARD postdoctoral fellowship award no. FI-427-09 from BARD, the United States-Israel Binational Agricultural Research and Development Fund. Research was funded by the Pierce's Disease Control Program (PDCP), California Department of Food and Agriculture (CDFA). A.M.D.S. was supported by research fellowship award 2010/16409-7 from the Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP).
We thank Stephanie Kung for her assistance with transformation of X. fastidiosa and Sophia Tran for helping with greenhouse experiments.
We declare that we have no conflicts of interest.
Footnotes
Published ahead of print 20 September 2013
Supplemental material for this article may be found at http://dx.doi.org/10.1128/JB.00713-13.
REFERENCES
- 1.Purcell AH, Hopkins DL. 1996. Fastidious xylem-limited bacterial plant pathogens. Annu. Rev. Phytopathol. 34:131–151 [DOI] [PubMed] [Google Scholar]
- 2.Hopkins DL, Purcell AH. 2002. Xylella fastidiosa: cause of Pierce's disease of grapevine and other emergent diseases. Plant Dis. 86:1056–1066 [DOI] [PubMed] [Google Scholar]
- 3.Chatterjee S, Newman KL, Lindow SE. 2008. Cell-cell signaling in Xylella fastidiosa suppresses movement and xylem vessel colonization in grape. Mol. Plant Microbe Interact. 21:1309–1315 [DOI] [PubMed] [Google Scholar]
- 4.Chatterjee S, Almeida RPP, Lindow SE. 2008. Living in two worlds: the plant and insect lifestyles of Xylella fastidiosa. Annu. Rev. Phytopathol. 46:243–271 [DOI] [PubMed] [Google Scholar]
- 5.Barber CE, Tang JL, Feng JX, Pan MQ, Wilson TJG, Slater H, Dow JM, Williams P, Daniels MJ. 1997. A novel regulatory system required for pathogenicity of Xanthomonas campestris is mediated by a small diffusible signal molecule. Mol. Microbiol. 24:555–566 [DOI] [PubMed] [Google Scholar]
- 6.Newman KL, Almeida RPP, Purcell A, Lindow SE. 2004. Cell-cell signaling controls Xylella fastidiosa interactions with both insects and plants. Proc. Natl. Acad. Sci. U. S. A. 101:1737–1742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Deng Y, Wu J, Tao F, Zhang LH. 2011. Listening to a new language: DSF-based quorum sensing in Gram-negative bacteria. Chem. Rev. 111:160–173 [DOI] [PubMed] [Google Scholar]
- 8.Wang LH, He Y, Gao Y, Wu JE, Dong YH, He C, Wang SX, Weng LX, Xu JL, Tay L, Fang RX, Zhang LH. 2004. A bacterial cell-cell communication signal with cross-kingdom structural analogues. Mol. Microbiol. 51:903–912 [DOI] [PubMed] [Google Scholar]
- 9.He YW, Wu J, Cha JS, Zhang LH. 2010. Rice bacterial blight pathogen Xanthomonas oryzae pv. oryzae produces multiple DSF-family signals in regulation of virulence factor production. BMC Microbiol. 10:187. 10.1186/1471-2180-10-187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boon C, Deng Y, Wang LH, He Y, Xu JL, Fan Y, Pan SQ, Zhang LH. 2008. A novel DSF-like signal from Burkholderia cenocepacia interferes with Candida albicans morphological transition. ISME J. 2:27–36 [DOI] [PubMed] [Google Scholar]
- 11.Beaulieu ED, Ionescu M, Chatterjee S, Yokota K, Trauner D, Lindow S. 2013. Characterization of a diffusible signaling factor (DSF) from Xylella fastidiosa. mBio 4(1):e00539–12. 10.1128/mBio.00539-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Colnaghi Simionato AV, da Silva DD, Lambais MR, Carrilho E. 2007. Characterization of a putative Xylella fastidiosa diffusible signal factor by HRGC-EI-MS. J. Mass Spectrom. 42:1375–1381 [DOI] [PubMed] [Google Scholar]
- 13.Bi H, Christensen QH, Feng Y, Wang H, Cronan JE. 2012. The Burkholderia cenocepacia BDSF quorum sensing fatty acid is synthesized by a bifunctional crotonase homologue having both dehydratase and thioesterase activities. Mol. Microbiol. 83:840–855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ryan RP, Fouhy Y, Lucey JF, Crossman LC, Spiro S, He YW, Zhang LH, Heeb S, Camara M, Williams P, Dow JM. 2006. Cell-cell signaling in Xanthomonas campestris involves an HD-GYP domain protein that functions in cyclic di-GMP turnover. Proc. Natl. Acad. Sci. U. S. A. 103:6712–6717 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 15.He YW, Wang C, Zhou L, Song H, Dow JM, Zhang LH. 2006. Genome scale analysis of diffusible signal factor regulon in Xanthomonas campestris pv. campestris: identification of novel cell-cell communication-dependent genes and functions. Mol. Microbiol. 59:610–622 [DOI] [PubMed] [Google Scholar]
- 16.He YW, Wang C, Zhou L, Song H, Dow JM, Zhang LH. 2006. Dual signaling functions of the hybrid sensor kinase RpfC of Xanthomonas campestris involve either phosphorelay or receiver domain-protein interaction. J. Biol. Chem. 281:33414–33421 [DOI] [PubMed] [Google Scholar]
- 17.Guo Y, Zhang Y, Li JL, Wang N. 2012. Diffusible signal factor-mediated quorum sensing plays a central role in coordinating gene expression of Xanthomonas citri subsp. citri. Mol. Plant Microbe Interact. 25:165–179 [DOI] [PubMed] [Google Scholar]
- 18.Rai R, Ranjan M, Pradhan B, Chatterjee S. 2012. Atypical regulation of virulence-associated functions by a diffusible signal factor in Xanthomonas oryzae pv. oryzae. Mol. Plant Microbe Interact. 25:789–801 [DOI] [PubMed] [Google Scholar]
- 19.Chatterjee S, Wistrom C, Lindow SE. 2008. A cell-cell signaling sensor is required for virulence and insect transmission of Xylella fastidiosa. Proc. Natl. Acad. Sci. U. S. A. 105:2670–2675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang N, Li JL, Lindow SE. 2012. RpfF-dependent regulon of Xylella fastidiosa. Phytopathology 102:1045–1053 [DOI] [PubMed] [Google Scholar]
- 21.Cheng Z, He YW, Lim SC, Qamra R, Walsh MA, Zhang LH, Song H. 2010. Structural basis of the sensor-synthase interaction in autoinduction of the quorum sensing signal DSF biosynthesis. Structure 18:1199–1209 [DOI] [PubMed] [Google Scholar]
- 22.King E, Ward M, Raney D. 1954. Two simple media for the demonstration of pyocyanin and fluorescin. J. Lab. Clin. Med. 44:301–307 [PubMed] [Google Scholar]
- 23.Davis MJ, Whitcomb RF, Gillaspie AG., Jr 1981. Fastidious bacteria of plant vascular tissue and invertebrates (including so called Rickettsia-like bacteria), p 2172–2188 In Starr MP, Stolp H, Truper HG, Balows A, Schlegel HG. (ed), The prokaryotes: a handbook on habitats, isolation, and identification of bacteria. Springer-Verlag, Heidelberg, Germany [Google Scholar]
- 24.Marco ML, Legac J, Lindow SE. 2005. Pseudomonas syringae genes induced during colonization of leaf surfaces. Environ. Microbiol. 7:1379–1391 [DOI] [PubMed] [Google Scholar]
- 25.Ionescu M, Belkin S. 2009. Overproduction of exopolysaccharides by an Escherichia coli K-12 rpoS mutant in response to osmotic stress. Appl. Environ. Microbiol. 75:483–492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Almeida RP, Killiny N, Newman KL, Chatterjee S, Ionescu M, Lindow SE. 2012. Contribution of rpfB to cell-to-cell signal synthesis, virulence, and vector transmission of Xylella fastidiosa. Mol. Plant Microbe Interact. 25:453–462 [DOI] [PubMed] [Google Scholar]
- 27.Kudla G, Murray AW, Tollervey D, Plotkin JB. 2009. Coding-sequence determinants of gene expression in Escherichia coli. Science 324:255–258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kovach ME, Elzer PH, Hill DS, Robertson GT, Farris MA, Roop RM, II, Peterson KM. 1995. Four new derivatives of the broad-host-range cloning vector pBBR1MCS, carrying different antibiotic-resistance cassettes. Gene 166:175–176 [DOI] [PubMed] [Google Scholar]
- 29.Kung SH, Almeida RP. 2011. Natural competence and recombination in the plant pathogen Xylella fastidiosa. Appl. Environ. Microbiol. 77:5278–5284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Livak KJ, Schmittgen TD. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2-Delta Delta C(T) method. Methods 25:402–408 [DOI] [PubMed] [Google Scholar]
- 31.Matsumoto A, Young GM, Igo MM. 2009. Chromosome-based genetic complementation system for Xylella fastidiosa. Appl. Environ. Microbiol. 75:1679–1687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Matsumoto A, Igo MM. 2010. Species-specific type II restriction-modification system of Xylella fastidiosa temecula1. Appl. Environ. Microbiol. 76:4092–4095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Torriani A. 1968. Alkaline phosphatase of Escherichia coli, p 212–218 In Grossman L, Moldave K. (ed), Methods in enzymology, vol 12B Academic Press, New York, NY [Google Scholar]
- 34.Hill BL, Purcell AH. 1995. Multiplication and movement of Xylella fastidiosa within grapevine and four other plants. Phytopathology 85:1368–1372 [Google Scholar]
- 35.Baccari C, Lindow SE. 2011. Assessment of the process of movement of Xylella fastidiosa within susceptible and resistant grape cultivars. Phytopathology 101:77–84 [DOI] [PubMed] [Google Scholar]
- 36.Guilhabert MR, Kirkpatrick BC. 2005. Identification of Xylella fastidiosa antivirulence genes: hemagglutinin adhesins contribute to biofilm maturation to X. fastidiosa and colonization and attenuate virulence. Mol. Plant Microbe Interact. 18:856–868 [DOI] [PubMed] [Google Scholar]
- 37.Hsiao YM, Liao HY, Lee MC, Yang TC, Tseng YH. 2005. Clp upregulates transcription of engA gene encoding a virulence factor in Xanthomonas campestris by direct binding to the upstream tandem Clp sites. FEBS Lett. 579:3525–3533 [DOI] [PubMed] [Google Scholar]
- 38.He YW, Ng AY, Xu M, Lin K, Wang LH, Dong YH, Zhang LH. 2007. Xanthomonas campestris cell-cell communication involves a putative nucleotide receptor protein Clp and a hierarchical signaling network. Mol. Microbiol. 64:281–292 [DOI] [PubMed] [Google Scholar]
- 39.Slater H, Alvarez-Morales A, Barber CE, Daniels MJ, Dow JM. 2000. A two-component system involving an HD-GYP domain protein links cell-cell signalling to pathogenicity gene expression in Xanthomonas campestris. Mol. Microbiol. 38:986–1003 [DOI] [PubMed] [Google Scholar]
- 40.Deng Y, Schmid N, Wang C, Wang J, Pessi G, Wu D, Lee J, Aguilar C, Ahrens CH, Chang C, Song H, Eberl L, Zhang LH. 2012. Cis-2-dodecenoic acid receptor RpfR links quorum-sensing signal perception with regulation of virulence through cyclic dimeric guanosine monophosphate turnover. Proc. Natl. Acad. Sci. U. S. A. 109:15479–15484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.An SQ, Febrer M, McCarthy Y, Tang DJ, Clissold L, Kaithakottil G, Swarbreck D, Tang JL, Rogers J, Dow JM, Ryan RP. 2013. High-resolution transcriptional analysis of the regulatory influence of cell-to-cell signalling reveals novel genes that contribute to Xanthomonas phytopathogenesis. Mol. Microbiol. 88:1058–1069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shepherd RW, Lindow SE. 2009. Two dissimilar N-acyl-homoserine lactone acylases of Pseudomonas syringae influence colony and biofilm morphology. Appl. Environ. Microbiol. 75:45–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Loper JE, Lindow SE. 1994. A biological sensor for iron available to bacteria in their habitats on plant surfaces. Appl. Environ. Microbiol. 60:1934–1941 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.