Abstract
Sll1951 is the surface layer (S-layer) protein of the cyanobacterium Synechocystis sp. strain PCC 6803. This large, hemolysin-like protein was found in the supernatant of a strain that was deficient in S-layer attachment. An sll1951 deletion mutation was introduced into Synechocystis and was easily segregated to homozygosity under laboratory conditions. By thin-section and negative-stain transmission electron microscopy, a ∼30-nm-wide S-layer lattice covering the cell surface was readily visible in wild-type cells but was absent in the Δsll1951 strain. Instead, the Δsll1951 strain displayed a smooth lipopolysaccharide surface as its most peripheral layer. In the presence of chaotropic agents, the wild type released a large (>150-kDa) protein into the medium that was identified as Sll1951 by mass spectrometry of trypsin fragments; this protein was missing in the Δsll1951 strain. In addition, Sll1951 was prominent in crude extracts of the wild type, indicating that it is an abundant protein. The carotenoid composition of the cell wall fraction of the Δsll1951 strain was similar to that of the wild type, suggesting that the S-layer does not contribute to carotenoid binding. Although the photoautotrophic growth rate of the Δsll1951 strain was similar to that of the wild-type strain, the viability of the Δsll1951 strain was reduced upon exposure to lysozyme treatment and hypo-osmotic stress, indicating a contribution of the S-layer to the integrity of the Synechocystis cell wall. This work identifies the S-layer protein in Synechocystis and shows that, at least under laboratory conditions, this very abundant, large protein has a supportive but not a critical role in the function of the cyanobacterium.
INTRODUCTION
More than half a century has passed since the first description of a bacterial protein surface layer by Houwink in 1953 (1). Over time it has become obvious that surface layer (S-layer) proteins are a very common feature among a wide variety of prokaryotes, including the archaeal and bacterial domains. S-layers are highly ordered, paracrystalline layers of protein on the surface of prokaryotes; S-layer proteins are often glycosylated and usually in noncovalent contact with an underlying membrane or peptidoglycan layer. The structural organization of S-layers is relatively simple, as most of them consist of only a single protein type.
Some S-layer proteins are members of the hemolysin protein family, as they possess copies of the RTX (repeat in toxin) nonapeptide motif. These proteins range in size from 40 to >600 kDa and appear to be widely distributed among bacterial genera (2). In certain Gram-negative strains, they can contribute significantly to pathogenicity. Hemolysins are commonly translocated from the cytoplasm to the cell surface through the type I secretion system (3, 4).
After export to the cell surface, an entropy-driven self-assembly of the S-layer polypeptides occurs and results in a lattice structure (5). Being exceptionally rigid and durable (6–8), archaeal S-layers assume primarily the functions of peptidoglycan in bacteria, thus stabilizing the cell shape, while in bacteria the roles of S-layers are more diverse. For example, the dense lattice with oblique, tetragonal, or hexagonal symmetry that is formed by the protein subunits may hinder the diffusion of molecules greater than 10 kDa, such as exoenzymes or antibodies, enabling certain strains to fend off predators and allowing pathogens to evade the immune systems of their hosts (9–11). In cyanobacteria, S-layers have also been reported to be involved in biomineralization processes, such as carbon sequestration (12, 13). Their remarkable physico-chemical properties, yielding the potential for a variety of biotechnological applications (14), and their role as an immunogen have led to extensive study of S-layer proteins in a variety of strains.
Even though cyanobacterial S-layers have been described in many instances since the early days of their discovery in 1973 (15, 16; for a review, see Smarda's report [17]), the only cyanobacterium for which the S-layer-encoding gene has been identified is Synechococcus sp. strain WH8102 (18), and in that case the protein, known as SwmA, may function in motility (18, 19). A related protein in Synechococcus sp. strain WH8102, SwmB, has also been associated with motility (20) and was recently described as conferring resistance to predation by dinoflagellates, possibly by upsetting attachment to cellular structures (21).
In silico analysis has shown that the Synechocystis genome contains multiple uncharacterized proteins with hemolysin-like features as well as porin-type proteins that resemble the S-layer proteins of selected Gram-positive bacteria (Table 1). We detected Sll1951, a large protein that resembles hemolysin and that has been characterized before (22, 23), in the growth medium of the Δsll1213 mutant strain of Synechocystis (data not shown); this mutant is unable to synthesize fucose and appears to lack the S-layer (24), thus laying the foundation for the present study.
Table 1.
ORFs in the Synechocystis genome that resemble sll1951 or whose product contains an SLH domaina
| Protein group and ORF | Length (no. of codons) | Gene product annotationb | No. of SLH domains | Reference(s) |
|---|---|---|---|---|
| Sll1951 and similar Synechocystis proteins | ||||
| sll1951 | 1,741 | Unknown protein; hemolysin-like S-layer protein (this study) | 0 | 22, 23 |
| sll1009 | 591 | FrpC, peptidase domains, iron-regulated protein | 0 | |
| sll0721 | 1,290 | Unknown protein; hemolysin-like | 0 | |
| slr1403 | 3,016 | Unknown protein; integrin domain homolog | 0 | |
| sll0723 | 1,771 | Unknown protein | 0 | |
| slr5005 | 3,797 | Unknown protein | 0 | |
| Synechocystis proteins with SLH domain(s) | ||||
| slr2000 | 321 | Hypothetical protein with SLH domains | 2 | 53, 54 |
| sll0772 | 546 | Hypothetical protein; carbohydrate-selective porin (OprB) domain | 1 | |
| sll1550 | 544 | Hypothetical protein; porin (OprB) and SLH domains | 1 | |
| slr1272 | 254 | Hypothetical protein with SLH domain | 1 | 55 |
| sll1271 | 572 | Hypothetical protein; porin (OprB) and SLH domains | 1 | |
| slr1841 | 630 | Hypothetical protein; porin (OprB) and SLH domains | 1 | 53, 55 |
| slr1908 | 591 | Hypothetical protein; porin (OprB) and SLH domains | 1 | 55 |
| slr0042 | 576 | Hypothetical protein; porin (OprB) and SLH domains | 1 |
SLH domain motifs were developed primarily based on S-layer sequences from Gram-positive bacteria. Sequence similarity determinations were performed by using BLAST, limiting matches to Synechocystis open reading frames and SLH domain motifs that are members of the PF00395 family (PFAM).
The gene product annotations reflect annotations in the NCBI database.
Sll1951 shares local similarity with proteins from other prokaryotes because of the hemolysin motifs present, but neither orthologs nor paralogs covering the entire coding region were found. However, the features of Sll1951, encoded by a 5,226-bp open reading frame (ORF) in the Synechocystis strain we studied, support a cell wall function, as it appears to lack transmembrane regions, and sulfur-containing amino acids are greatly underrepresented in its primary structure (7 Met [0.4%] and 0 Cys). Microarray data show high levels of transcription of sll1951 under standard growth conditions (25), and its codon usage is optimized for rapid translation (26). A possible ribosome-binding site (AGGAG) is located at bp −19 from the start codon, whereas the most probable −10 and −35 transcriptional signals, as predicted via BPROM (Softberry Inc., Mount Kisco, NY), appear to be distant at positions −95 (TGCTATGAT) and −114 (TTGACA), respectively; this is not uncommon for S-layer protein genes (27, 28). We examined Sll1951 as a candidate for the Synechocystis S-layer protein, confirmed the assignment, and provide a phenotypical and biochemical analysis of a mutant lacking this gene.
MATERIALS AND METHODS
Cultivation of strains.
Synechocystis sp. strain PCC 6803 wild type and the Δsll1951 strain, carrying a deletion of sll1951, were grown axenically on a rotary shaker at 30°C in BG-11 medium (29) supplemented with 10 mM TES [(N-Tris-hydroxymethylmethyl-2-aminoethanesulfonic acid)-NaOH; pH 8.2]. Cultures were illuminated with continuous white fluorescent light at an intensity between 25 and 60 μmol photons m−2 s−1. For transmission electron microscopy, cultures were continuously bubbled with air under otherwise-identical growth conditions. Plate cultures were maintained on BG-11 with 1.5% (wt/vol) Difco agar supplemented with 0.3% (wt/vol) sodium thiosulfate. For photomixotrophic growth, glucose was added to a final concentration of 5 mM. All liquid cultures of the Δsll1951 mutant were started with a 25-μg/ml chloramphenicol supplement, which did not alter growth or characteristics of the mutant strain. Culture densities were measured with a Shimadzu UV-160 spectrophotometer at 730 nm. The Escherichia coli strain DH10B, carrying the psll1951 deletion construct, was maintained at 37°C on Luria-Bertani plates in the presence of 25 μg/ml chloramphenicol.
Construction of the Δsll1951 deletion strain.
A 398-bp upstream region (Synechocystis genome positions 1427604 to 1428001 according to CyanoBase [30; http://genome.kazusa.or.jp/cyanobase]), including 50 bp of the sll1951 ORF, was amplified by PCR using the forward primer 5′-CGGATACACCACTGCAGGTGTAC-3′, with engineered PstI and removed EagI sites, and the reverse primer 5′-GAAAGTAAGGGAGGTCGACAAAGGTG-3′, which includes an engineered SalI site. (Underlined nucleotides constitute the noted restriction site, and italicized residues are mutated relative to the genomic Synechocystis sequence.) A 569-bp downstream region (positions 1422150 to 1422716) including 293 bp of the sll1951 ORF was amplified by PCR using the forward primer 5′-GGAATCGGCCGGCTTTAGC-3′, with an engineered EagI site, and the reverse primer 5′-CGATCGGATCCTTAATGCCTCTGC-3′, with an engineered BamHI site. Note that the sll1951 ORF runs counterclockwise on the genome, and therefore forward and reverse primers actually are in the opposite and same direction, respectively, relative to the sll1951 ORF.
The chloramphenicol resistance cassette originating from pBR325 and cloned into pUC19 was amplified by PCR using primers to create termini complementary to both the 5′-region upstream and the 3′-region downstream of the previously created PCR products (indicated in bold letters): 5′-GATGTTATTAGCAGAGGCATTAAGGATCCTCTAGAGTCACTTCAC-3′ and 5′-CTACAGATCATGTACACCTGCAGGTCGGCATTTGAGAAGC-3′. In another PCR mixture, all three products were combined, using a primer recognizing the end of the amplified upstream region of sll1951 (5′-GAAAGTAAGGGAGGTCGACAAAGGTG-3′) and one hybridizing to the end of the amplified downstream region (5′-GGAATCGGCCGGCTTTAGC-3′), resulting in a linear 2.3-kbp fragment carrying the chloramphenicol resistance cassette flanked by up- and downstream sll1951 regions; this PCR product was used to transform the Synechocystis wild-type strain. The transformation procedure was described previously (31). For future reference, the linear fragment was also cloned into pBluescript II KS− at its polylinker sites for SalI and EagI. Transformed Synechocystis cells were initially grown on 0.4-μm-pore-size Nuclepore Track-Etch polycarbonate membranes (Whatman Inc., Piscataway, NJ) on BG-11 supplied with 5 mM glucose and 5 μg/ml chloramphenicol. Five colonies were picked, and the transformants were replated on BG-11, gradually increasing the chloramphenicol concentration to 62.5 μg/ml, the concentration at which complete segregation of the Δsll1951 strain was achieved, as confirmed by PCR. The ease with which complete segregation was achieved indicated that loss of Sll1951 does not place cells at a major disadvantage under the laboratory conditions used.
Transmission electron microscopy.
Liquid cultures of Synechocystis wild type and the Δsll1951 mutant were grown in BG-11 at 50 μmol photons m−2 s−1 under continuous active aeration to an optical density at 730 nm (OD730) between 0.5 and 0.6 (exponential phase) and then filtered onto 0.4-μm-pore size Nuclepore Track-Etch membranes with very mild suction. The membranes were placed on fresh BG-11 plates to prevent drying of the cells. Prior to high-pressure-freezing (HPF) fixation, the cells were scraped off the membranes with a toothpick and smeared into a slot grid that was sandwiched between two halves of inverted copper planchets. HPF was performed at 210,000 kPa and −196°C using a Bal-Tec HPM 010 high-pressure freezer (Bal-Tec Corporation, Middlebury, CT). While keeping the temperature at −80°C, the planchets were transferred into 10 ml of anhydrous acetone and postfixed for 2 h in fresh 1% (wt/vol) OsO4 in acetone. The planchets were warmed to −20°C for 2 h, then to 4°C for 1 h, and washed three times with 10 ml of acetone to further dehydrate the cells. Chunks of fixed cells were isolated from the planchets and infiltrated with increasing concentrations (25, 50, 75, and three times with 100%) of Spurr's resin (32) in acetone for 12 h each. The specimens were transferred into plastic molds and polymerized in a convection oven at 60°C overnight. Thin 70-nm sections were cut on a Leica Ultracut R microtome (Leica, Vienna, Austria), attached to Formvar-coated copper slot grids, and then poststained either with only 1% uranyl acetate in 50% ethanol for 6 min or with uranyl acetate for 6 min and Sato's lead citrate (33, 34) for 3 min. Electron micrographs were taken on a CM12S transmission electron microscope (Philips Electronic Instruments, Co., Mahwah, NJ) equipped with a Gatan 791 charge-coupled-device (CCD) 1,024- by 1,024-pixel-area digital camera (Gatan, Inc., Pleasanton, CA) with Gatan Digital Micrograph version 3.9.1 software, at 80 kV.
Discontinuous sucrose gradient centrifugation of cell extracts.
The density distribution of membrane/protein fractions was initially determined from 100-ml cultures that were grown photoautotrophically at 50 μmol photons m−2 s−1 in BG-11 with continuous active aeration to an OD730 of approximately 1. Cells were then harvested and processed to analyze carotenoids and proteins: 100-ml precultures of wild-type and mutant strains were grown mixotrophically at 50 μmol photons m−2 s−1 in BG-11 supplemented with 5 mM glucose (and 25 μg/ml chloramphenicol for the mutant) to an OD730 of at least 1 and then used to inoculate 3.5 liters of BG-11 medium in Erlenmeyer flasks. These subcultures were grown photoautotrophically at 120 μmol photons m−2 s−1 with continuous active aeration until an OD730 of ∼0.7 was reached. Cells were harvested by a 10-min centrifugation at 10,800 × g at room temperature. The pellets were washed in 50 mM MES (2-N-morpholinoethanesulfonic acid)–NaOH (pH 6.5), sharply decanted, and then frozen at −80°C.
Pellets were thawed and then resuspended with a brush in 5 ml of 20% (wt/vol) sucrose in 40 mM MES-NaOH (pH 6.5), 4 mM EDTA, and a protease inhibitor cocktail (1 mM phenylmethanesulfonyl fluoride, 1 mM benzamidine, 1 mM ε-amino caproic acid). After adjusting the volume to 25 ml with additional sucrose/MES-NaOH/EDTA, the cells were cooled on ice and then broken by three cycles of French pressing at 13.8 MPa. Unbroken cells were removed by a 10-min centrifugation at 4,400 × g at 4°C. Ten-milliliter aliquots of protein extract were used as the 20% sucrose step in a 38-ml discontinuous gradient consisting of the following sucrose concentrations (as wt/vol)/volumes (in ml): 80/3, 60/10, 48/10, 20/10, and 10/5. The samples were subjected to ultracentrifugation for 18 h at 112,000 × g at 4°C in an SW 28 rotor (Beckman Coulter Inc., Brea, CA). The colored fractions were removed and stored at −80°C.
Analysis of proteins by SDS-PAGE and mass spectroscopy (MS).
Proteins were separated by SDS-PAGE by using gels prepared according to methods described in reference 35, either with 6% stacking and 10% or 12% acrylamide running gels or with 6% stacking and 12 to 20% continuous gradient running gels. All acrylamide percentages are expressed as the weight per volume. To check for glycosylation, gels were stained using a Pierce glycosylation staining kit following the manufacturer's recommendations. For mass spectrometry, protein bands were cut from gels with a scalpel, and the pieces were trimmed to small cubes of about 1 mm3. The cubes were incubated in ∼150 μl water for 1 h at room temperature. To dehydrate the sample, 200 μl of 35% (vol/vol) acetonitrile in 20 mM freshly prepared ammonium bicarbonate was added, and the incubation continued at room temperature for another 75 min. This dehydration step was repeated once. The liquid was removed, and the gel pieces were dried in a Speedvac concentrator (Thermo Scientific) for 20 min. Protein samples were put on ice and rehydrated for 90 min with a solution of 20 mM ammonium bicarbonate containing 5 μg/ml sequencing-grade modified trypsin (Promega Corporation, Madison, WI), followed by in-gel digestion overnight at 37°C. Samples were spun down, and 3 μl of the supernatant was loaded onto a μ-precolumn cartridge (Acclaim PepMap 100 C18, 5 μm; Dionex, Sunnyvale, CA) that was connected in line with the main column (Dionex Acclaim PepMap 100 C18, 75-μm inner diameter [ID], 15 cm), with flow rates of 5 μl/min and 300 nl/min, respectively. Elution of protein fragments was carried out on a Dionex UltiMate 3000 LC system (Dionex). While the solvent mixture (5% acetonitrile and 0.1% formic acid in water) was kept constant in the precolumn, the concentration of acetonitrile in the main column was increased, using a linear gradient, starting with 5% concentation/4 min (5/4), then increased over 7.5 min to 20/11.5, and then further to 50/51.5, 65/59, and finally 95/69. Eluted fragments were subjected to electrospray ionization, and their masses were determined on a microTOF-Q mass spectrometer (Bruker Daltonics Inc., Billerica, MA).
Mass data were deconvoluted by using Bruker's DataAnalysis v4.0 software and then imported into BioTools v3.1 (Bruker Daltonics Inc., Billerica, MA). The data were then submitted to one of the MASCOT databases (MSDB, Swiss-Prot, or NCBIlnr) as semi-trypsin-digested tandem MS (MS/MS) fragments with the variable modifications of oxidation and propionamide.
Analysis of carotenoids.
For analysis by high-performance liquid chromatography (HPLC), pigments were extracted from cell pellets with 100% methanol, and aliquots were loaded onto a semipreparative column (4.0 by 250 mm, C18; Waters Spherisorb ODS2).
Pigment extraction from membrane/cell wall fractions depended on their sucrose content. For the high-density membrane/cell wall fraction (80% sucrose), 100-μl aliquots were washed twice with 1 ml of 50 mM MES-NaOH (pH 6.5) to minimize the sucrose content, and the pellets were extracted twice with 300 μl of 100% methanol. Subsequently, 200 μl of the extract was loaded onto the semipreparative column. For the low-density cell membrane/cell wall fraction (10% sucrose), 100-μl aliquots were diluted with 900 μl of 50 mM MES-NaOH (pH 6.5) and, since the cell membrane fraction could not be pelleted, the resulting suspension was extracted with 500 μl of chloroform. The solvent was evaporated under a stream of nitrogen, and the pigments were redissolved in 500 μl of 100% methanol. Aliquots (200 μl) were loaded onto the semipreparative column.
The pigments were separated on an HP-1100 Chemstation with a 24-min gradient of ethyl acetate in water-methanol, with an initial water content of 10% decreasing linearly to 0% at the third minute. Ethyl acetate was added increasing linearly from 0 to 70% from minutes 3 to 11, and then from 70% to 100% from minute 11 to 21. The elution of carotenoids was tracked based on the absorbance at 452 and 475 nm.
Light microscopy.
Cells were grown in BG-11 supplemented with 5 mM glucose at 40 μmol photons m−2 s−1 and then harvested in a clinical centrifuge operated at 1,200 rpm for 30 min. The cell density was adjusted to an OD730 of 1, and cells were resuspended in 50 mM HEPES-NaOH (pH 7.2) with either 0.5% (wt/vol) lauryl sarcosine or 0.5% Triton X-100. Light microscopy was carried out after a 1-h exposure to the detergents. To assess osmotic effects on wild-type and Δsll1951 cells, 3 × 108 cells of equally cultivated strains were exposed for 2 h to 10 mg/ml lysozyme in 50 mM HEPES-NaOH (pH 7.2) and 2.5 mM EDTA, harvested by centrifugation, and then resuspended in 50 mM HEPES-NaOH (pH 7.2) containing 5 mM CaCl2. The cells were then incubated at room temperature for 30 min. After that, half of the volume of each sample was transferred to a new tube and pelleted by centrifugation. Immediately before light microscopy, the cell pellets were resuspended in either a 1 M aqueous glucose solution or water.
For differential interference contrast (DIC) microscopy, one drop of cell suspension was pipetted onto a glass slide and covered with a 22- by 22-mm coverslip. The cells were inspected with an Axioscop microscope (Carl Zeiss, Thornwood, NY) with a Plan-Neofluar 100× oil immersion objective with a 1.3-numerical aperture and a 1.4-numerical aperture oil immersion condenser lens. Images were taken with a Hamamatsu C3200-07 CCD camera.
RESULTS
The Δsll1951 deletion mutant.
Cells of Synechocystis wild type that had been transformed with a PCR fragment introducing a Δsll1951 deletion mutation segregated their wild type and mutant chromosomes (Synechocystis has multiple chromosome copies per cell) after only six passages on BG-11 plates as well as upon continuous photomixotrophic growth in liquid cultures supplemented with 50 μg/ml of chloramphenicol. Compared to the Synechocystis wild type, the colonies of the Δsll1951 strain appeared to have similar pigmentation but a more flat, spread morphology and a shiny surface. The photoautotrophic doubling time of the Δsll1951 strain at a light intensity of 45 μmol photons m−2 s−1 was similar to that of the wild type (9 versus 10 h), but the Δsll1951 strain had a significant lag phase of up to close to a day under some conditions (photoautotrophic growth, as well as photomixotrophic growth at low light intensity [1 to 2 μmol photons m−2 s−1]). However, after the lag phase, the photomixotrophic, low-intensity growth rate was the same as that of the control, even though the cultures developed a brown-green color. In contrast, we were unable to grow the Δsll1951 strain mixotrophically at very low light intensity (0.15 μmol photons m−2 s−1) or under light-activated heterotrophic growth (LAHG; 15 min of 50 μmol photons m−2 s−1 per 24 h) conditions.
Regardless of the growth conditions, liquid cultures of the Δsll1951 strain always assumed a slightly yellow tint due to increased loss and export of compounds into the medium, and cultures of the Δsll1951 strain with an OD730 above 0.5 exhibited an increased tendency to foam. Assessment of sensitivity of wild-type and mutant strains to the antibiotics polymyxin B, carbenicillin (data not shown), and colistin via an antibiotic sensitivity test showed essentially equal zones of inhibition (see Fig. S1 in the supplemental material), suggesting that cell wall permeability to these antibiotics was not altered.
Light and electron microscopy.
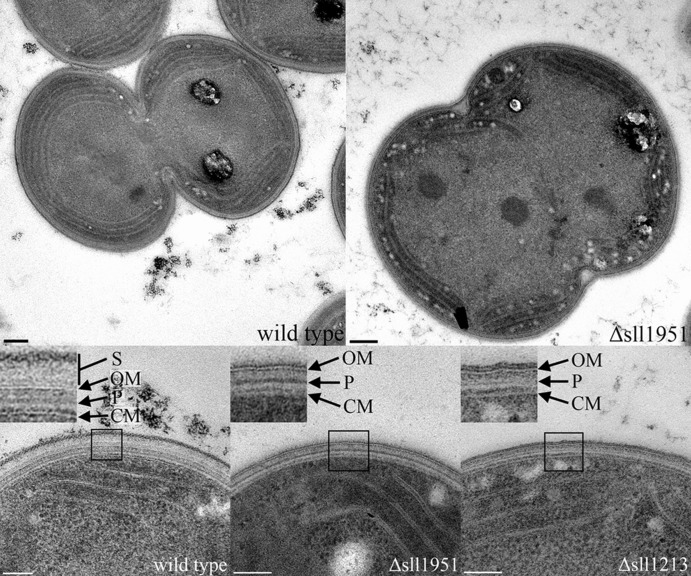
Transmission electron microscopy of cells fixed by high-pressure freezing was performed on photoautotrophically grown strains of Synechocystis wild type and the Δsll1951 strain, as well as a Δsll1213 strain, lacking myxoxanthophyll due to deletion of the fucose synthetase gene (24). Both the Δsll1951 and Δsll1213 strains showed an altered cell wall relative to the wild type: wild-type cells were surrounded by a surface layer spanning a distance of approximately 30 nm from the outer membrane (OM), whereas this layer was absent in both deletion strains (Fig. 1). This observation was further supported by negative stain images of wild-type and Δsll1951 cells, which showed the presence and absence, respectively, of an S-layer that is apparent as a honeycomb-like surface structure (Fig. 2).
Fig 1.
Whole-cell image of wild-type (top left) and Δsll1951 (top right) cells fixed by high-pressure freezing. Bar (top images), 0.2 μm. High-magnification images of the cell periphery are shown for the wild-type (bottom left), Δsll1951 (bottom middle), and Δsll1213 (bottom right) strains. Bar (bottom images), 100 nm. Insets are ca. 100 by 100 nm. S, surface layer; OM, outer membrane; P, peptidoglycan; CM, cytoplasmic membrane.
Fig 2.
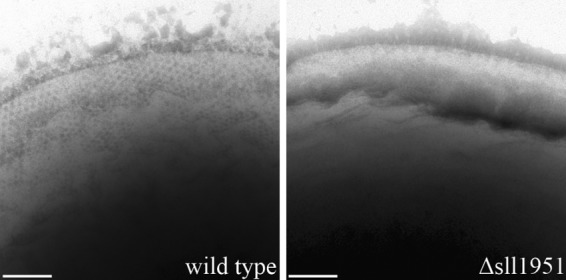
Phosphotungstic acid negative-stained images of cells of the wild-type (left) and Δsll1951 (right) strains. A honeycomb-like structure of the surface is visible at the periphery of the wild-type strain; it shifts out of the focal plane toward the bottom of the image. The S-layer is absent in the Δsll1951 cell. Bar, 100 nm.
In cells of the Δsll1951 strain, the outer membrane appeared to be covered with a diffuse, weakly electron dense layer of about 5 to 10 nm thickness, protruding from the outer membrane. This layer likely represents the lipopolysaccharide layer that, in the wild type, is in contact with glycosylated moieties of the S-layer protein (Fig. 1). Even though the wild-type surface layer was absent in the Δsll1213 strain as well, its outer membrane seemed to be covered by a rather electron-dense layer that was similar to a membrane bilayer in appearance and thickness.
The peptidoglycan layer of the wild-type strain was located centrally in the periplasmic space between the cytoplasmic membrane and outer membrane. Its electron density was similar to that of the peptidoglycan layer of the Δsll1951 strain but higher than the corresponding layer in the Δsll1213 strain. Consequently, both the Synechocystis wild-type and the Δsll1951 cells were less prone to cell wall deformation during the fixation of specimens for electron microscopy.
The cytoplasmic membrane bilayer was much less pronounced in the mutants than it was in the wild type, even on micrographs that clearly displayed the details of the outer membrane. This phenomenon has been observed before (36) and is probably due to compression of the cytoplasmic membrane by the cytoplasm.
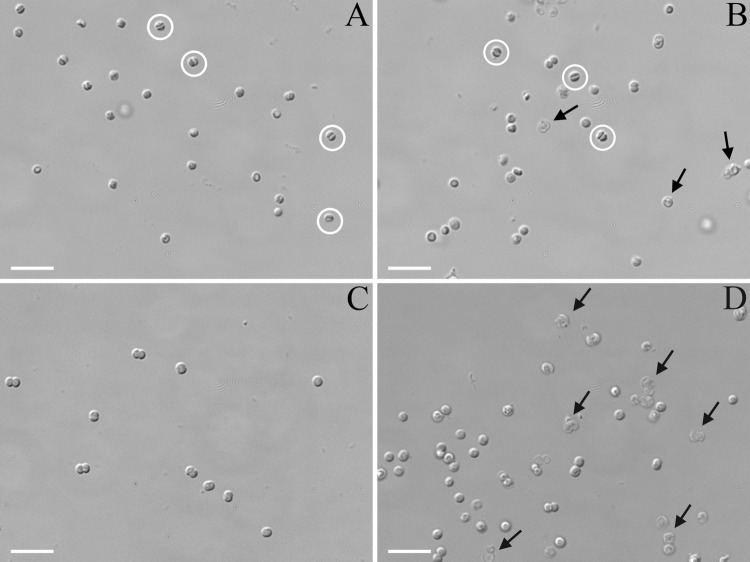
DIC light microscopy was performed on cells of Synechocystis wild type and the Δsll1951 strain (Fig. 3). Without pretreatment, both strains looked identical. Similarly, a 20-min exposure of both strains to lysozyme (10 mg/ml) did not seem to have an effect on the appearance of the cells. Cyanobacteria are generally less susceptible to lysozyme treatment due to a peptidoglycan layer that is thicker and has a greater degree of cross-linking than most other Gram-negative bacteria (37). Hyperosmotic stress caused by 1 M glucose led to spheroplast formation in both strains, particularly in dividing cells, and some cell lysis was observed in the Δsll1951 strain. However, a combination of lysozyme treatment followed by exposure to hypo-osmotic stress had a much more severe effect on Δsll1951 cells than on the wild type (Fig. 3). Washing cells with deionized water after lysozyme treatment resulted in a significant degree of lysis and the appearance of ghost-like Δsll1951 cells, whereas only very few wild-type cells were destroyed under hypo-osmotic conditions.
Fig 3.
DIC light microscopy images of Synechocystis wild-type (A and C) or Δsll1951 (B and D) cells after lysozyme treatment, followed by either hyperosmotic shock (A and B) or hypo-osmotic shock (C and D). Control cells (not lysozyme treated) resembled the cells shown in panel C. Water efflux caused significant cell shrinkage (exemplified by cells with white circles) in many cells of both strains and some cell lysis in the Δsll1951 strain (exemplified in cells indicated by arrows). The Δsll1951 cells were significantly more susceptible to the hypo-osmotic effects than the wild type; this shock led to significant lysis and the appearance of ghost-like cells (exemplified in cells indicated by arrows). Bar, 5 μm.
Analysis of Δsll1951 cell wall components.
To confirm the absence of Sll1951 in the Δsll1951 strain and to determine whether another protein may partially replace Sll1951, crude protein extracts were isolated from the wild-type and Δsll1951 strains. As would be expected from the wild-type growth rate of the Δsll1951 strain at a light intensity of 45 μmol photons m−2 s−1, protein profiles of both strains were similar except for the absence of two high-molecular-mass bands (>160 kDa) in the Δsll1951 strain (Fig. 4). MASCOT analysis of the upper dominant bands after trypsin digestion revealed the presence of two proteins in the wild type: Sll1951, with a coverage of 16%, and Slr0335, the phycobilisome core-membrane linker protein, ApcE, with a coverage of 53% in the higher-molecular-mass band. The lower band also contained Sll1951 and Slr0335 (19% and 18% coverage, respectively), with Sll1951 more highly represented. As Sll1951 is glycosylated, the percentage of MASCOT matches was relatively limited. The comigration of Sll1951 and Slr0335 (178 and 100 kDa, respectively, based on their amino acid sequences) in the upper band most likely was due to their strongly opposing predicted pIs of 3.5 (Sll1951) and 9.25 (Slr0335), and so an electrostatic interaction between the denatured strands of both proteins is likely, resulting in comigrating bands. Indeed, the bands ran at apparent molecular masses much higher than those of the individual polypeptides. Glycosylation of Sll1951 contributes to relatively slow migration on the gel and may also be the reason for the relatively low overall coverage of the trypsinized protein in the mass spectrometer; coverage was highest toward the C-terminal region.
Fig 4.
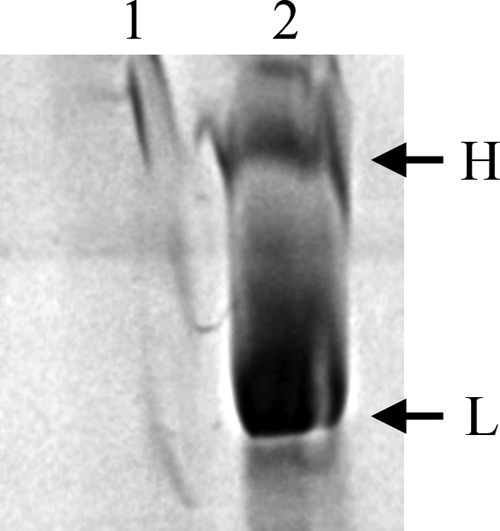
High-molecular-mass bands (>160 kDa) of crude protein extracts from the Δsll1951 strain (lane 1) and the wild type (lane 2) on a 12 to 20% SDS-PAGE gradient gel. Bands at the indicated positions (H and L) were cut, processed, and analyzed by mass spectrometry. Although Sll1951 and Slr0335 were detected in both bands, Sll1951 was highly abundant in band L, whereas Slr0335, the phycobilisome core-membrane linker protein, was mainly present in band H. The molecular mass marker lane was omitted, as the largest marker protein (160 kDa) migrated faster than the L band.
Supernatants of Δsll1951 mutant cultures frequently had a dark yellow/cerise tint, whereas the wild-type supernatants remained mostly colorless, even when cultures were grown to high density. The absorption of the supernatants of all cultures was markedly increased below 400 nm, with maxima at 312 and 266 nm (Fig. 5), and with the Δsll1951 supernatant about twice as intensely colored than the wild type (note the dilution of the supernatants of the mutant cultures in Fig. 5). However, release of compounds into the medium was yet about 3-fold more pronounced in cultures of the Δsll1213 fucose synthase mutant, lacking the S-layer and the carotenoid myxoxanthophyll, than in the Δsll1951 mutant strain.
Fig 5.
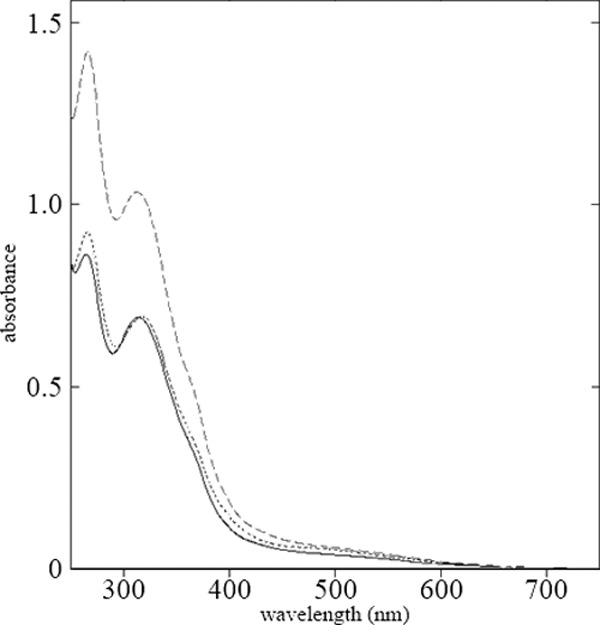
Absorbance spectra for supernatants of wild-type (solid line), Δsll1951 (dotted line), and Δsll1213 (dashed line) cultures that had been grown photomixotrophically to high density (OD730, >4). Supernatants of Δsll1213 and Δsll1951 cultures were diluted 1:5 and 1:2, respectively. Spectra were taken after filtration through a 0.2-μm-pore-size filter.
The results presented thus far suggest that the Δsll1951 strain leaks or excretes more UV-absorbing metabolites than the wild type. To determine whether proteins are also more easily lost from the cells, the supernatant protein profiles of Δsll1951 and wild-type cultures that had been exposed to a mild detergent (0.5% Triton X-100 or N-lauryl sarcosine) or denaturing conditions, such as high temperature (60°C) with 5 mM EDTA or 6 M urea with 5 mM EDTA, were analyzed (Fig. 6). While these treatments had a negligible effect on the wild-type strain, resulting in only one dominating high-molecular-mass band on the gel that was identified as Sll1951 by mass spectrometry of trypsinized fragments, Δsll1951 cells were affected significantly by treatment with 6 M urea–5 mM EDTA, and a number of proteins were observed in the supernatant, possibly resulting from lysed cells. As the Sll1951 wild-type band stained positive for glycosylation (Fig. 6) and as this glycosylated band was absent in the Δsll1951 strain, our results indicated that Sll1951 indeed is glycosylated.
Fig 6.
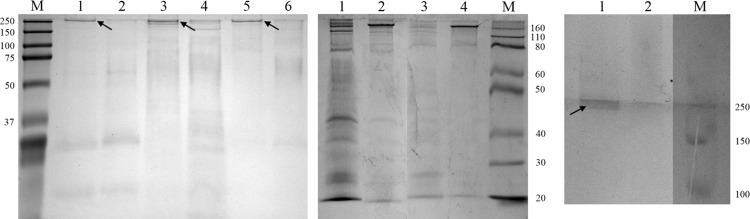
(Left) SDS-PAGE (12%) of supernatants from cultures of the wild type (odd-numbered lanes) and the Δsll1951 strain (even-numbered lanes) that were harvested during exponential growth (OD730, ∼0.6), after 2-h treatments with 0.5% N-lauryl sarcosine (lanes 3 and 4) or 0.5% Triton X-100 (lanes 5 and 6). Sll1951 was present in the wild-type samples, as indicated by the arrows. Untreated controls are shown in lanes 1 and 2. (Middle) SDS-PAGE (10%) of supernatants of Δsll1951 (odd-numbered lanes) and wild-type (even-numbered lanes) cultures after treatment with 6 M urea–5 mM EDTA (lanes 1 and 2) or 5 mM EDTA at (lanes 3 and 4) at 60°C for 1 h. Empty lanes between lanes 1 and 2 and between 2 and 3 were cropped. (Right) Glycosylation stain of SDS-PAGE (10%) of supernatants of wild-type (lane 1) and Δsll1951 (lane 2) cultures after treatment with 6 M urea–5 mM EDTA for 1 h. Lanes between lanes 1 and 2 and between 2 and the marker (M) were cropped.
However, when cultures were grown to higher density (OD730 of >2), additional, mainly high-molecular-mass proteins were isolated from supernatants of the wild type with or without addition of mild detergents, whereas no such proteins were present in the Δsll1951 mutant (Fig. 7). The most intense protein bands in this high-molecular-mass region of the gel were extracted, trypsinized, and analyzed by mass spectrometry. In the wild type, among the higher-molecular-mass proteins, Sll1951 was present in bands A, B, D, E, and F; a few other large proteins appeared more sporadically (Table 2). The variety of Sll1951 bands with different mobilities may indicate different aggregation or glycosylation states and/or degradation. No high-molecular-mass bands appeared for the Δsll1951 strain, suggesting that another protein (such as one of the other RTX proteins) does not act as a partial substitute if Sll1951 is missing.
Fig 7.
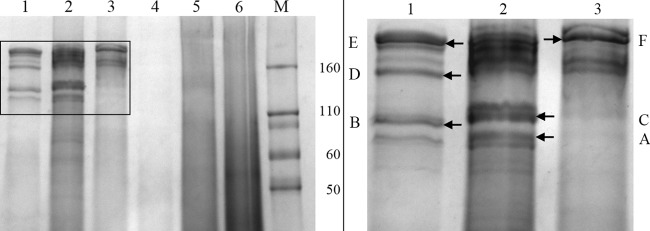
SDS-PAGE gradient gel (12 to 20%) of supernatants of wild-type (lanes 1 to 3) and the Δsll1951 (lanes 4 to 6) cultures that were grown to high density (OD730, >2). Immediately after harvesting, the cell pellets were treated with either 0.5% N-lauryl sarcosine (lanes 2 and 5) or 0.5% Triton X-100 (lanes 3 and 6) for 2 h and were centrifuged again, resulting in the supernatant that was run on the gel. Untreated controls were loaded onto lanes 1 and 4. The panel to the right is a magnification of the area in the left panel outlined by a rectangle. Protein bands were isolated from the gel at the positions indicated by arrows and letters and subjected to mass spectrometry as indicated in Table 2.
Table 2.
Mass spectrometry-identified proteins in the supernatants of stationary-phase wild-type Synechocystis cultures
| Band | MASCOT score | Coverage (%) | Mass (Da) | Protein | Assignment |
|---|---|---|---|---|---|
| A | 561 | 14 | 178,153 | Sll1951 | S-layer |
| A | 170 | 15 | 112,064 | Slr0897 | Endo-1,4-beta-glucanase |
| A | 3,349 | 60 | 149,299 | Sll0654 | Alkaline phosphatase |
| B | 992 | 13 | 178,153 | Sll1951 | S-layer |
| C | 1,658 | 29 | 196,415 | Sll0656 | Extracellular nuclease |
| D | 742 | 17 | 178,153 | Sll1951 | S-layer |
| E | 664 | 17 | 178,153 | Sll1951 | S-layer |
| F | 626 | 16 | 178,153 | Sll1951 | S-layer |
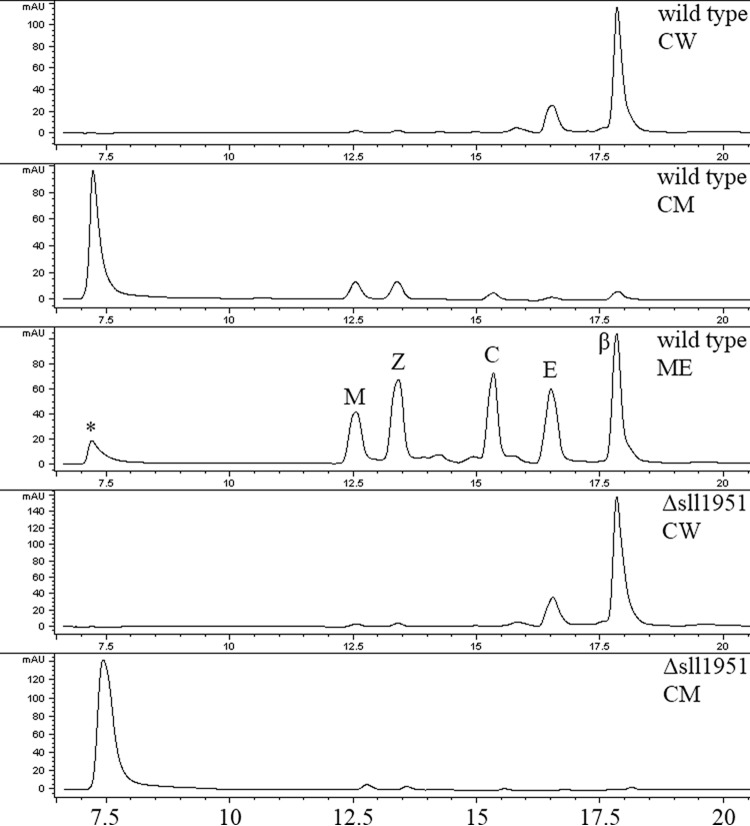
As the cell wall may contain carotenoids (24, 38), the carotenoid content of both strains was analyzed with a focus on the cell periphery. The distribution and concentration of carotenoids in the mutant remained largely unchanged (Fig. 8), suggesting that carotenoids are not directly associated with the S-layer. The cell wall fractions that included the peptidoglycan layer and outer membrane were of highest density and accumulated at the bottom of the centrifugation tubes. This fraction was orange/light brown in color in both strains and accumulated a carotenoid that eluted early in the hydrophilic range of the methanol-ethylacetate gradient, indicating possible hydroxylation and/or glycosylation (Fig. 8). This carotenoid may be myxol (24), but it was not characterized further, as it was not affected by the lack of Sll1951. In contrast to the cell wall fraction, the yellow cytoplasmic membrane fraction was of lowest density and barely penetrated the sucrose on top of the gradient. The cytoplasmic membrane contained mostly β-carotene and echinenone. As expected, all isolated cell wall fractions were virtually chlorophyll-free.
Fig 8.
Carotenoid HPLC profiles of Synechocystis wild-type and Δsll1951 membrane fractions. Predominant carotenoids of the cytoplasmic membrane (CM) fraction (wild type and Δsll1951) were echinenone (E; 16.5 min) and β-carotene (β; 17.8 min), as demonstrated with the whole-cell pigment methanol extract (ME) from wild-type cells. Carotenoids were identified by their online spectra. The cell wall (CW) fractions were mostly devoid of carotenoids, with the exception of an unidentified carotenoid (*), which eluted at 7.0 to 7.5 min. Other pigments detected were myxoxanthophyll (M) and chlorophyll (C). The x axis of the HPLC spectrum is the elution time (in minutes); the y axis is the absorbance.
Since the Synechocystis cell wall plays a role in DNA uptake and thus in the natural transformability of this strain, we compared the Δsll1951 mutant to the wild type with respect to its transformability. Upon transformation with a plasmid conferring antibiotic resistance (31), both strains formed similar numbers of colonies on antibiotic-containing plates (data not shown). Therefore, absence of the S-layer has no influence on the transformability of Synechocystis.
Transcript levels of S-layer mRNA in the Synechocystis wild type.
By using a tiling array approach, the abundances of mRNAs of sll1951, ORFs related to sll1951, and also a variety of Synechocystis proteins containing at least one putative surface layer homologous domain (SLH domain) (Table 1) were analyzed in transcript preparations from wild-type cultures in the exponential growth phase (OD730, 0.5) and late linear phase (OD730, 4). SLH domains may anchor proteins to the peptidoglycan layer, and SLH domains are not limited to S-layer proteins; conversely, not all S-layer proteins have SLH domains (39). Of the ORFs listed in Table 1, slr1841 was the most highly expressed. During exponential growth, transcript levels of slr1841 were about three times as high as levels for sll1951 (see Fig. S2 in the supplemental material). While transcript levels of sll1951 remained high even in late stationary phase, levels for slr1841 dropped markedly. However, Slr1841, a protein with a single SLH, was never detected in S-layer preparations, and the presence of a putative N-terminal transmembrane helix makes Slr1841 a highly unlikely candidate as a contributor to the S-layer of Synechocystis. Transcript levels of all other ORFs carrying putative hemolysin Ca2+-binding domains were low compared to that of sll1951, particularly during exponential growth (see Fig. S2).
DISCUSSION
The cyanobacterium Synechocystis sp. strain PCC 6803 has become a very important model organism over the last few decades, with a majority of research devoted to studying its plant-like photosynthesis. However, our knowledge about the Synechocystis cell wall, and especially its surface layer protein, is still limited. S-layer proteins are frequently found as parts of the cell wall in prokaryotes, including cyanobacteria (17). The limited characterization of most cyanobacterial strains and the highly diverse nature of S-layer proteins have made the identification of genes encoding these proteins a challenging task. The first ultrastructural report of a cyanobacterial S-layer protein involved a Synechocystis strain isolated in Helsinki (16), but no data were provided on the biochemical composition of the S-layer. The first and, to our knowledge, only study to date in which the identity of a cyanobacterial S-layer protein was determined was in Synechococcus sp. strain WH8102 (18). This protein, SwmA, is an 81- to 83-kDa protein according to its sequence (mass uncertainty is caused by the presence of two potential start codons) and runs on denaturing protein gels as a 130-kDa protein (19). The protein is required for swimming motility in Synechococcus sp. strain WH8102 (18, 19), has RTX motifs, and has some similarity with the much longer Sll1951 (23% identity and 36% similarity).
Sll1951 is the Synechocystis S-layer protein.
In this paper, we have reported the identification of the sll1951 gene that encodes the Synechocystis S-layer protein. Our interest in Sll1951 was based initially on the analysis of a Synechocystis Δsll1213 mutant (24), which shed significant amounts of Sll1951 into the medium, and our interest was furthered by the characterization of the Δsll1951 deletion strain, which lacks the ability to synthesize the S-layer protein.
As reported by Mohamed et al. (24), Sll1213 is a fucose synthetase that is essential for the biosynthesis of myxoxanthophyll, a native Synechocystis sp. PCC 6803 carotenoid. Deletion of this enzyme resulted not only in an irregular thylakoid organization, but also in an altered cell wall and loss of the S-layer in the Δsll1213 mutant. Fucose has been identified as a building block in the S-layer glycan of Geobacillus tepidamans (40) and may be important for proper anchoring of the S-layer protein to the underlying lipopolysaccharide. Changes in composition of the lipopolysaccharide layer or (if present) the S-layer glycan frequently lead to a loss of the glycocalyx (41–43). The predominant protein in supernatants of Δsll1213 cultures was identified as Sll1951, a hemolysin-like protein that has been characterized by Sakiyama et al. (22, 23). However, these studies did not provide data to demonstrate or suggest that Sll1951 would be the S-layer protein. A predicted extracellular protein (44), Sll1951 is particularly prone to shedding when changes to the structural organization of the cell wall occur (45). It has several characteristics that make it a likely candidate for a surface layer protein. First, its primary structure lacks cysteines and has few methionines; a low abundance of sulfur-containing amino acids is a common feature of S-layer proteins (43). Second, the theoretical pI of Sll1951 is 3.50; this low pI is due mainly to the presence of 193 (11.1%) aspartate and 80 (4.6%) glutamate residues. With few exceptions for archaeal S-layer proteins, pIs in the acidic range are common for the majority of S-layer proteins that have been described to date (5). Third, surface layer proteins are often glycosylated (46). A PROSITE scan of Sll1951 resulted in 19 possible N-glycosylation sites for this protein, and Sll1951 glycosylation indeed was demonstrated experimentally (Fig. 6). Finally, sll1951 has a codon sequence that is obviously highly optimized for rapid translation (26), as well as a transcription initiation region with multiple possible start sites (47). In certain fast-growing strains, S-layer proteins range among the most highly expressed proteins, with synthesis rates of 400 to 500 subunits per second (5, 46). Tiling array data support this observation, as they show high sll1951 transcript levels during logarithmic- and late-linear-phase growth (see Fig. S2 in the supplemental material).
Phenotype of the Δsll1951 mutant.
A mutant strain was created with a chloramphenicol resistance cassette that replaced most of the sll1951 open reading frame. Segregation of this strain to homozygosity at the mutated locus did not require high concentrations of chloramphenicol, and the mutant grew very well under photoautotrophic conditions at rates comparable to or higher than that of the wild type, even though the mutant had a longer lag phase and surprisingly was unable to grow mixotrophically at very low light intensity. At present, we do not have a good explanation for why changes in cell wall structure lead to these phenotypic growth effects. Although the Synechocystis wild-type strain has kept its S-layer over many years of culturing, axenically grown laboratory strains may not have an immediate evolutionary disadvantage from losing their surface layer due to optimized growth conditions and lack of competition. A higher growth rate can most likely be attributed to the reduced metabolic load from the absence of Sll1951. Indeed, several laboratory strains of Synechocystis sp. PCC 6803 carry one of two forms of a truncated sll1951 open reading frame (48, 49), indicating that under laboratory conditions cells with a shorter Sll1951 may have a slight growth advantage.
In contrast to the wild-type strain, densely grown cultures of the Δsll1951 strain exhibited an increased tendency to foam, possibly due to accumulation of cell wall components and excreted metabolites in the growth medium that are usually retained in the wild type. A comparison of the supernatants taken from three strains—the wild type, the Δsll1951 strain, and the Δsll1213 fucose synthetase mutant—demonstrated the absence of significant amounts of pigments, including chlorophyll or fully desaturated carotenoids, in the supernatants of any of the strains. However, absorption under UV was increased in supernatants of the Δsll1951 strain and particularly in supernatants of the Δsll1213 strain (peaks at 312 and 266 nm [Fig. 5]). In the Δsll1951 and Δsll1213 supernatants, the absorption maximum above 300 nm could originate from mycosporin-like amino acids, which are common UV protectants in a wide variety of organisms (50). Such compounds have also been found in Synechocystis (51).
The Δsll1213 strain exported large amounts of Sll1951 into the medium. Mass spectrometry confirmed the presence of the S-layer protein in supernatants of the wild-type strain as well, yet to a much lower degree. Sll1951 obviously was absent in the S-layer mutant. Based on the electron microscopy data as well as the observed loss of compounds into the growth medium, the Δsll1951 strain may be capable of assembling a mature, fully functional lipopolysaccharide/exopolysaccharide (EPS) as its outermost cell layer, whereas a structurally crippled lipopolysaccharide with limited functionality is likely to be present in the Δsll1213 strain.
The Synechocystis S-layer polycrystalline structure was examined by negative staining of the surface of whole cells. Negative staining of the Synechocystis wild type displayed Sll1951 as a regularly ordered protein layer that was predictably absent in the Δsll1951 strain (Fig. 2). Thin sections of high-pressure-frozen cells demonstrated altered cell surface features in the mutants, which appeared fuzzy in the Δsll1951 mutant but smooth in the Δsll1213 mutant (Fig. 1). The weakly electron-dense surface of the Δsll1951 mutant likely represents an intact lipopolysaccharide/EPS layer and hence the point of contact with the S-layer glycan. Even though the Δsll1213 mutant expressed sll1951, it appeared to be unable to anchor the exported protein to its lipopolysaccharide layer (43, 52). Electron microscopy images also showed that the peptidoglycan layer of the Δsll1213 strain was less electron dense than in the Δsll1951 strain or the Synechocystis wild type (Fig. 1). Of the three strains examined in this study, the cell periphery of the fucose-deficient strain was structurally most altered, and this may be the reason for its loss of cellular components into the growth medium.
Increased sensitivity of the Δsll1951 strain to osmotic stress.
Assessment of the viability of the Δsll1951 strain upon exposure to environmental stresses suggested a protective but noncritical role of Sll1951. While treatment of cyanobacterial cells with lysozyme usually has little effect due to their robust peptidoglycan layers (37), the protective presence of the S-layer may aid in reducing the effectiveness of lysozyme as well. Wild-type and Δsll1951 cells did not show any lysis prior to lysozyme treatment, but hyperosmotic and particularly hypo-osmotic shock of lysozyme-treated Δsll1951 cells resulted in a high degree of cell lysis, while wild-type cells appeared largely unaffected (Fig. 3). However, access by smaller molecules is not hindered by the S-layer: growth inhibition of wild-type cells and of the S-layer mutant by the antibiotics carbenicillin (an inhibitor of peptidoglycan synthesis), polymyxin B (a cationic ionophor that binds to lipid A), and colistin (binds to cell membrane lipids and disrupts the cell wall integrity) was identical for both strains (see Fig. S1 in the supplemental material [data not shown for carbenicillin]). Hence, the S-layer may not pose a barrier to low-molecular-weight antibiotics, such as the ones tested, regardless of their charge; however, lysozyme is more than 10 times larger than polymyxin B (molecular weight, 1,386), and its diffusion to the peptidoglycan layer may be limited in the wild-type strain.
Taken together, these results show that Sll1951 is the S-layer protein in Synechocystis sp. PCC 6803. Sll1951 is not required for growth or maintenance of the cyanobacterium under laboratory conditions, but the continued presence of this large protein indicates that it does serve an important function in growth and survival, at least under some physiological conditions.
Supplementary Material
ACKNOWLEDGMENTS
We thank Vicki Moore for performing the glycosylation assay and for critical evaluation of the manuscript, David Lowry for his help with preparing samples for electron microscopy, Daniel Brune for his help with mass spectroscopy, and Hongliang Wang and Yih-Kuang Lu for their contribution of the tiling microarray data.
This work was supported by a grant from Science Foundation Arizona (competitive advantage award CAA 0117-07).
Footnotes
Published ahead of print 27 September 2013
Supplemental material for this article may be found at http://dx.doi.org/10.1128/JB.00615-13.
REFERENCES
- 1.Houwink AL. 1953. A macromolecular monolayer in the cell wall of Spirillum spec. Biochim. Biophys. Acta 10:360–366 [DOI] [PubMed] [Google Scholar]
- 2.Linhartova I, Bumba L, Masin J, Basler M, Osicka R, Kamanova J, Prochazkova K, Adkins I, Hejnova-Holubova J, Sadilkova L, Morova J, Sebo P. 2010. RTX proteins: a highly diverse family secreted by a common mechanism. FEMS Microbiol. Rev. 34:1076–1112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Awram P, Smit J. 1998. The Caulobacter crescentus paracrystalline S-layer protein is secreted by an ABC transporter (type I) secretion apparatus. J. Bacteriol. 180:3062–3069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thompson SA, Shedd OL, Ray KC, Beins MH, Jorgensen JP, Blaser MJ. 1998. Campylobacter fetus surface layer proteins are transported by a type I secretion system. J. Bacteriol. 180:6450–6458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sleytr UB, Beveridge TJ. 1999. Bacterial S-layers. Trends Microbiol. 6:253–260 [DOI] [PubMed] [Google Scholar]
- 6.Engelhardt H. 2007. Are S-layers exoskeletons? The basic function of protein surface layers revisited. J. Struct. Biol. 160:115–124 [DOI] [PubMed] [Google Scholar]
- 7.Mescher MF, Strominger JL. 1976. Structural (shape-maintaining) role of the cell surface glycoprotein of Halobacterium salinarium. Proc. Natl. Acad. Sci. U. S. A. 73:2687–2691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Peters J, Nitsch M, Kuhlmorgen B, Golbik R, Lupas A, Kellermann J, Engelhardt H, Pfander JP, Muller S, Goldie K, Engel A, Stetter KO, Baumeister W. 1995. Tetrabrachion: a filamentous archaebacterial surface protein assembly of unusual structure and extreme stability. J. Mol. Biol. 245:385–401 [DOI] [PubMed] [Google Scholar]
- 9.Buck BL, Alterman E, Svinegerod T, Klaenhammer TR. 2005. Functional analysis of putative adhesion factors in Lactobacillus acidophilus NCFM. Appl. Environ. Microbiol. 71:8344–8351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Calabi E, Calabi F, Phillips AD, Fairweather NF. 2002. Binding of Clostridium difficile surface layer proteins to gastrointestinal tissues. Infect. Immun. 70:5770–5778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kern J, Schneewind O. 2010. BslA, the S-layer adhesin of B. anthracis, is a virulence factor for anthrax pathogenesis. Mol. Microbiol. 75:324–332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jansson C, Northen T. 2010. Calcifying cyanobacteria: the potential of biomineralization for carbon capture and storage. Curr. Opin. Biotechnol. 21:365–371 [DOI] [PubMed] [Google Scholar]
- 13.Schultze-Lam S, Beveridge TJ. 1994. Nucleation of celestite and strontianite on a bacterial S-layer. Appl. Environ. Microbiol. 60:447–453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sleytr UB, Huber C, Ilk N, Pum D, Schuster B, Egelseer EM. 2007. S-layers as a tool kit for nanobiotechnological applications. FEMS Microbiol. Lett. 267:131–144 [DOI] [PubMed] [Google Scholar]
- 15.Jensen TE, Sicko LM. 1973. Fine structure of cell wall of Gloeocapsa alpicola, a blue-green alga. Cytobiologie 6:439–446 [Google Scholar]
- 16.Karlsson B, Vaara T, Lounatmaa K, Gyllenberg H. 1983. Three-dimensional structure of the regularly constructed surface layer from Synechocystis sp. strain CLII. J. Bacteriol. 156:1338–1343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Smarda J. 2002. S-layers on cell walls of cyanobacteria. Micron 33:257–277 [DOI] [PubMed] [Google Scholar]
- 18.McCarren J, Heuser J, Roth R, Yamada N, Martone M, Brahamsha B. 2005. Inactivation of swmA results in the loss of an outer cell layer in a swimming Synechococcus strain. J. Bacteriol. 187:224–230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brahamsha B. 1996. An abundant cell-surface polypeptide is required for swimming by the nonflagellated marine cyanobacterium Synechococcus. Proc. Natl. Acad. Sci. U. S. A. 93:6504–6509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McCarren J, Brahamsha B. 2007. SwmB, a 1.12-megadalton protein that is required for nonflagellar swimming motility in Synechococcus. J. Bacteriol. 189:1158–1162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Strom SL, Brahamsha B, Fredrickson KA, Apple JK, Gutierrez Rodriguez A. 2012. A giant cell surface protein in Synechococcus WH8102 inhibits feeding by a dinoflagellate predator. Environ. Microbiol. 14:807–816 [DOI] [PubMed] [Google Scholar]
- 22.Sakiyama T, Ueno H, Homma H, Numata O, Kuwabara T. 2006. Purification and characterization of a hemolysin-like protein, Sll1951, a nontoxic member of the RTX protein family from the cyanobacterium Synechocystis sp. strain PCC 6803. J. Bacteriol. 188:3535–3542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sakiyama T, Araie H, Suzuki I, Shiraiwa Y. 2011. Functions of a hemolysin-like protein in the cyanobacterium Synechocystis sp. PCC 6803. Arch. Microbiol. 193:565–571 [DOI] [PubMed] [Google Scholar]
- 24.Mohamed HE, van de Meene AML, Roberson RW, Vermaas WFJ. 2005. Myxoxanthophyll is required for normal cell wall structure and thylakoid organization in the cyanobacterium Synechocystis sp. strain PCC 6803. J. Bacteriol. 187:6883–6892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hihara Y, Kamei A, Kanehisa M, Kaplan A, Ikeuchi M. 2001. DNA microarray analysis of cyanobacterial gene expression during acclimation to high light. Plant Cell 13:793–806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mrázek J, Bhaya D, Grossman AR, Karlin S. 2001. Highly expressed and alien genes of the Synechocystis genome. Nucleic Acids Res. 29:1590–1601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hynönen U, Åvall-Jääskeläinen S, Palva A. 2010. Characterization and separate activities of the two promoters of the Lactobacillus brevis S-layer protein gene. Appl. Microbiol. Biotechnol. 87:657–668 [DOI] [PubMed] [Google Scholar]
- 28.Novotny N, Berger H, Schinko T, Messner P, Schäffer C, Strauss J. 2008. A temperature-sensitive expression system based on the Geobacillus stearothermophilus NRS 2004/3a sgsE surface-layer gene promoter. Biotechnol. Appl. Biochem. 49:35–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rippka R, Deruelles J, Waterbury JB, Herdman M, Stanier RY. 1979. Generic assignments, strain histories and properties of pure cultures of cyanobacteria. J. Gen. Microbiol. 111:1–61 [Google Scholar]
- 30.Kaneko T, Sato S, Kotani H, Tanaka A, Asamizu E, Nakamura Y, Miyajima N, Hirosawa M, Sugiura M, Sasamoto S, Kimura T, Hosouchi T, Matsuno A, Muraki A, Nakazaki N, Naruo K, Okumura S, Shimpo S, Takeuchi C, Wada T, Watanabe A, Yamada M, Yasuda M, Tabata S. 1996. Sequence analysis of the genome of the unicellular cyanobacterium Synechocystis sp. strain PCC 6803. II. Sequence determination of the entire genome and assignment of potential protein-coding regions. DNA Res. 3:109–136 [DOI] [PubMed] [Google Scholar]
- 31.Vermaas WFJ, Williams JGK, Arntzen CJ. 1987. Sequencing and modification of psbB, the gene encoding the CP-47 protein of photosystem II, in the cyanobacterium Synechocystis sp. PCC 6803. Plant Mol. Biol. 8:317–326 [DOI] [PubMed] [Google Scholar]
- 32.Spurr AR. 1969. A low-viscosity epoxy resin embedding medium for electron microscopy. J. Ultrastruct. Res. 26:31–43 [DOI] [PubMed] [Google Scholar]
- 33.Sato T. 1967. A modified method for lead staining of thin sections. J. Electron Microsc. (Tokyo) 16:133. [PubMed] [Google Scholar]
- 34.Hanaichi T, Sato T, Iwamoto T, Malavasi-Yamashiro J, Hoshino M, Mizuno N. 1986. A stable lead by modification of Sato's method. J. Electron Microsc. (Tokyo) 35:304–306 [PubMed] [Google Scholar]
- 35.Ikeuchi M, Inoue Y. 1988. A new 4.8-kDa polypeptide intrinsic to the PS II reaction center, as revealed by modified SDS-PAGE with improved resolution of low-molecular-weight proteins. Plant Cell Physiol. 29:1233–1239 [Google Scholar]
- 36.Graham LL, Beveridge TJ. 1990. Effect of chemical fixatives on accurate preservation of Escherichia coli and Bacillus subtilis structure in cells prepared by freeze-substitution. J. Bacteriol. 172:2150–2159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hoiczyk E, Hansel A. 2000. Cyanobacterial cell walls: news from an unusual prokaryotic envelope. J. Bacteriol. 182:1191–1199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vermaas WFJ, Timlin JA, Jones HD, Sinclair MB, Nieman LT, Hamad SW, Melgaard DK, Haaland DM. 2008. In vivo hyperspectral confocal fluorescence imaging to determine pigment localization and distribution in cyanobacterial cells. Proc. Natl. Acad. Sci. U. S. A. 105:4050–4055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lupas A, Engelhardt H, Peters J, Santarius U, Volker S, Baumeister W. 1994. Domain structure of Acetogenium kivui surface layer revealed by electron crystallography and sequence analysis. J. Bacteriol. 176:1224–1233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kahlig H, Kolarich D, Zayni S, Scheberl A, Kosma P, Schäffer C, Messner P. 2005. N-acetylmuramic acid as capping element of α-D-fucose-containing S-layer glycoprotein glycans from Geobacillus tepidamans GS59-7T. J. Biol. Chem. 280:20292–20299 [DOI] [PubMed] [Google Scholar]
- 41.Belland RJ, Trust TJ. 1985. Synthesis, export and assembly of Aeromonas salmonicida A-layer analyzed by transposon mutagenesis. J. Bacteriol. 163:877–881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Garduno RA, Phipps BM, Kay WW. 1995. Physical and functional S-layer reconstitution in Aeromonas salmonicida. J. Bacteriol. 177:2684–2694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Walker SG, Karunaratne DN, Ravenscroft N, Smit J. 1994. Characterization of mutants of Caulobacter crescentus defective in surface attachment of the paracrystalline surface layer. J. Bacteriol. 176:6312–6323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yu NY, Wagner JR, Laird MR, Melli G, Rey S, Lo R, Dao P, Sahinalp SC, Ester M, Foster LJ, Brinkman FSL. 2010. PSORTb 3.0: improved protein subcellular localization prediction with refined localization subcategories and predictive capabilities for all prokaryotes. Bioinformatics 26:1608–1615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bhaya D, Watanabe N, Ogawa T, Grossman AR. 1999. The role of an alternative sigma factor in motility and pilus formation in the cyanobacterium Synechocystis sp. strain PCC 6803. Proc. Natl. Acad. Sci. U. S. A. 96:3188–3193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sleytr UB, Messner P. 1983. Crystalline surface layers on bacteria. Annu. Rev. Microbiol. 37:311–339 [DOI] [PubMed] [Google Scholar]
- 47.Adachi T, Yamagata H, Tsukagoshi N, Udaka S. 1989. Multiple and tandemly arranged promoters of the cell wall protein operon in Bacillus brevis 47. J. Bacteriol. 171:1010–1016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kanesaki Y, Shiwa Y, Tajima N, Suzuki M, Watanabe S, Sato N, Ikeuchi M, Yoshikawa H. 2012. Identification of sub-strain specific mutations by massively parallel whole-genome resequencing of Synechocystis sp. PCC 6803. DNA Res. 19:67–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Trautmann D, Voss B, Wilde A, Al-Babili S, Hess W. 2012. Microevolution in cyanobacteria: re-sequencing a motile substrain of Synechocystis sp. PCC 6803. DNA Res. 19:435–448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cockell CS, Knowland J. 1999. Ultraviolet radiation screening compounds. Biol. Rev. Camb. Philos. Soc. 3:311–345 [DOI] [PubMed] [Google Scholar]
- 51.Zhang L, Li L, Wu Q. 2007. Protective effects of mycosporine-like amino acids of Synechocystis sp. PCC 6803 and their partial characterization. J. Photochem. Photobiol. B 86:240–245 [DOI] [PubMed] [Google Scholar]
- 52.Awram P, Smit J. 2001. Identification of lipopolysaccharide O antigen synthesis genes required for attachment of the S-layer of Caulobacter crescentus. Microbiology 147:1451–1460 [DOI] [PubMed] [Google Scholar]
- 53.Claus H, Akca E, Debaerdemaeker T, Evrard C, Declercq J, Harris J, Schlott B, Konig H. 2005. Molecular organization of selected prokaryotic S-layer proteins. Can. J. Microbiol. 51:731–743 [DOI] [PubMed] [Google Scholar]
- 54.Zhang LF, Yang HM, Cui SX, Hu J, Wang J, Kuang TY, Norling B, Huang F. 2009. Proteomic analysis of plasma membranes of cyanobacterium Synechocystis sp. strain PCC 6803 in response to high pH stress. J. Proteome Res. 6:2892–2902 [DOI] [PubMed] [Google Scholar]
- 55.Huang F, Hedman E, Funk C, Kieselbach T, Schröder WP, Norling B. 2004. Isolation of outer membrane of Synechocystis sp. PCC 6803 and its proteomic characterization. Mol. Cell Proteomics 6:586–595 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.