Abstract
Shigellosis is an important disease in the developing world, where about 90 million people become infected with Shigella spp. each year. We previously demonstrated that the type three secretion apparatus (T3SA) proteins IpaB and IpaD are protective antigens in the mouse lethal pulmonary model. In order to simplify vaccine formulation and process development, we have evaluated a vaccine design that incorporates both of these previously tested Shigella antigens into a single polypeptide chain. To determine if this fusion protein (DB fusion) retains the antigenic and protective capacities of IpaB and IpaD, we immunized mice with the DB fusion and compared the immune response to that elicited by the IpaB/IpaD combination vaccine. Purification of the DB fusion required coexpression with IpgC, the IpaB chaperone, and after purification it maintained the highly α-helical characteristics of IpaB and IpaD. The DB fusion also induced comparable immune responses and retained the ability to protect mice against Shigella flexneri and S. sonnei in the lethal pulmonary challenge. It also offered limited protection against S. dysenteriae challenge. Our results show the feasibility of generating a protective Shigella vaccine comprised of the DB fusion.
INTRODUCTION
Shigellosis is a severe gastrointestinal disease caused by Shigella spp. This disease is characterized by fever, bloody diarrhea, and tenesmus. Recent calculations estimate the annual rate of Shigella infections at ∼90 million, with 100,000 deaths per year (1). An important number of these infections occur in children under 5 years old living in developing countries (2). Other at-risk populations include military personnel deployed abroad (3) and refugees (4). Implications of this disease include severe impairment of child development and nutrition (5), as well as an important mortality index (2).
Four different species of Shigella have been described (6): S. flexneri, S. sonnei, S. boydii, and S. dysenteriae. Modifications in the O antigen give rise to about 50 serotypes (6, 7). The predominance of specific serotypes varies both geographically (8) and during the course of a single outbreak (9), complicating the epidemiology of shigellosis. Immune responses against Shigella during natural infection are predominantly serotype specific, in part due to the high immunodominance of bacterial lipopolysaccharide (LPS) (10, 11). This dominance results in poor cross-reaction between different Shigella serotypes, thus opening the possibility of subsequent reinfections by Shigella organisms bearing different O antigens.
Shigella infection requires the use of a highly conserved type three secretion system (T3SS) encoded on a virulence plasmid present in all Shigella species. After crossing the intestinal barrier via M cells, Shigella is taken up by macrophages; however, it escapes these phagocytes by inducing apoptosis mediated by the T3SS translocator IpaB (12). Shigella then uses the T3SS to inject protein effectors via the basolateral side of epithelial cells to promote bacterial entry. The pathogen lyses the resulting phagosome, replicates, and spreads to adjacent cells. Control of type III secretion occurs at the tip of the secretion apparatus needle by the activity of IpaD along with IpaB (13, 14).
Despite long-standing efforts, a Shigella vaccine is still not available (7, 15). Several groups have explored different approaches in the search of a Shigella vaccine. These include live attenuated strains of Shigella (16), LPS-protein conjugates (17), mixtures of subunit components (18), and recombinant proteins (19). Our hypothesis is that a vaccine comprised of highly conserved protein antigens would provide broad, serotype-independent protection, thus bypassing the need to consider multiple serotypes as are needed for vaccines that target LPS or O antigen. We have previously demonstrated the protective efficacy of S. flexneri 2a-derived IpaB and IpaD, components of the T3SS (20), when included in a vaccine formulation incorporating the novel mucosal adjuvant dmLT and delivered intranasally (20), orogastrically (21), or intramuscularly (22) using monophosphoryl lipid A (MPL) and alum hydroxide as adjuvants. The IpaB/IpaD subunit vaccine elicited a strong systemic immunity, with the presence of antibody-secreting cells in various compartments, as well as eliciting specific cytokine-secreting cells. Protection is achieved against a homologous strain of S. flexneri and a heterologous strain of S. sonnei in the mouse pneumonia model. Given that children in low-resource countries are a primary target of a Shigella vaccine, the ultimate vaccine formulation must be inexpensive. To reduce the cost of an IpaB- and IpaD-based vaccine and simplify manufacture and formulation, we created a genetically fused IpaD-IpaB protein (DB fusion). The approach of using a fusion of protective antigens has been explored successfully with other subunit vaccines, including LcrV, an IpaD homolog (23–25). In this study, the DB fusion elicited immune responses of a magnitude similar to those generated by a combination of separate IpaB and IpaD proteins. Interestingly, higher cytokine levels were detected when cells from mice immunized with the DB fusion were stimulated. In addition, mice were protected in the lethal pulmonary challenge (26, 27) using S. flexneri, S. sonnei, and S. dysenteriae. Therefore, this novel fusion protein represents an efficient alternative for vaccination against shigellosis in humans.
MATERIALS AND METHODS
Materials.
pET plasmids, ligation mix, and competent Escherichia coli were from EMD Millipore (Billerica, MA). Oligonucleotides were from IDT (Coralville, IA). Restriction endonucleases were from New England BioLabs (Ipswich, MA). HisTrap Crude FF immobilized-metal affinity chromatography (IMAC) columns and Q FF anion-exchange columns were from GE Healthcare (Piscataway, NJ). n-Octyl-oligo-oxyethylene (OPOE) was from Enzo Life Sciences (Farmingdale, NY).
Generation of plasmids for expression of the DB fusion in E. coli.
ipaD was amplified from D/pET15b (28) by PCR using a 5′ primer with an NdeI restriction site and a 3′ primer with an XhoI restriction site, while ipaB was amplified from B/pET15b (29) using a 5′ primer with an XhoI site and a 3′ primer with a BamHI site. The PCR products were digested by the appropriate restriction endonucleases and ligated into pET28b. The ligation reaction was used to transform E. coli NovaBlue. The resulting DB/pET28b and ipgC/pACYCDuet-1 were cotransformed into E. coli Tuner(DE3) for coexpression (Fig. 1A).
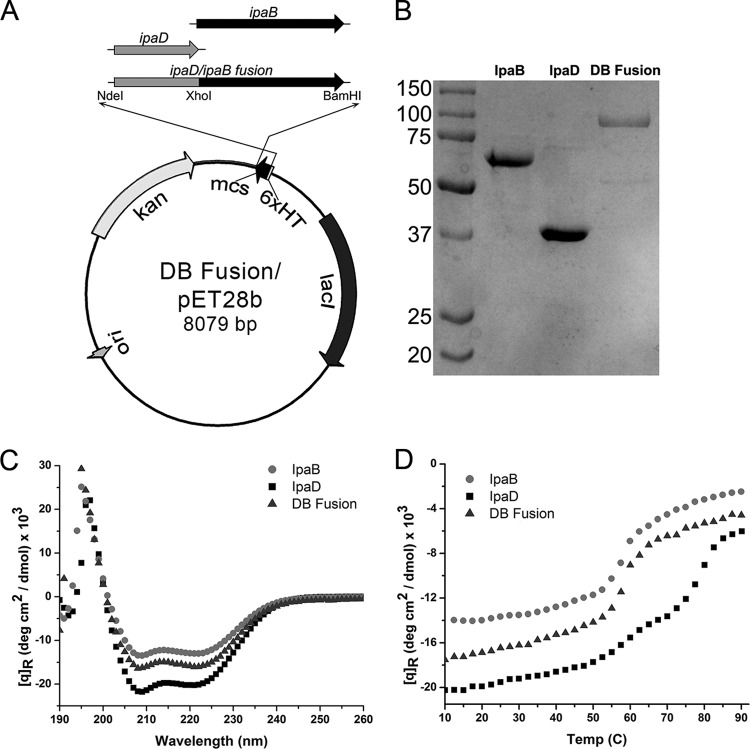
Fig 1.
Biophysical analysis of the DB fusion. (A) Construct harboring the IpaD/IpaB fusion. Restriction sites used for cloning are shown along a map of pET28b. (B) Comparative SDS-PAGE with IpaB, IpaD, and DB fusion proteins is shown with molecular mass markers, in kilodaltons, indicated to the left. (C) The CD spectra for IpaB in 0.5% OPOE, IpaD in PBS, and the DB fusion prepared in 0.5% OPOE all indicate predominantly α-helical content with the mean residue molar ellipticity ([q]R) values (measured in degrees cm2 per decimol). (D) Thermal unfolding of the secondary structures of the DB fusion, IpaB, and IpaD as a function of temperature is shown.
Protein purification and sample preparation.
DB/pET28b+ipgC/pACYCDuet-1//Tuner(DE3) bacteria were grown in autoinduction medium (30) containing kanamycin (50 μg/ml) and chloramphenicol (25 μg/ml) for 16 to 18 h. Purification of the DB fusion was done as previously described for IpaB (20, 31). Briefly, bacteria were collected by centrifugation, resuspended in IMAC binding buffer containing protease inhibitors (Roche, Basel, Switzerland), and lysed; the suspension was clarified by centrifugation and the supernatant containing the DB Fusion/IpgC complex purified by IMAC. After further purification using a Q FF anion-exchange chromatography column, OPOE was added to 0.5% to release the IpgC. The His-tagged DB fusion was separated from IpgC using an IMAC column with OPOE at 0.5% in all buffers and dialyzed into phosphate-buffered saline (PBS) with 0.5% OPOE. IpaB and IpaD were purified (32). Protein concentrations were determined by the A280 (33).
CD.
Far-UV circular-dichroism (CD) spectra were collected (34). Briefly, a Jasco J-815 spectropolarimeter fitted with a Peltier temperature controller (Jasco Inc., Easton, MD) was used to collect spectra from 190 nm to 260 nm through a 0.1-cm-path-length quartz cuvette. Samples were kept at 10°C and scanned at 50 nm/min with a 1-nm spectral resolution and a 2-s data integration time. All spectra are an average of three measurements. Secondary-structure thermal stability was determined by the monitoring the CD signal at 222 nm as the temperature was increased from 10 to 90°C. The temperature ramp rate was 15°C/h, and data were collected every 2.5°C. All protein solutions were made to 0.5 mg/ml in phosphate citrate buffer, pH 7.4, with 0.5% OPOE included for IpaB and the DB fusion. CD signals were converted to mean residue molar ellipticity.
Mice and immunizations.
Six- to eight-week-old female BALB/c mice (Charles River Laboratories, Wilmington, MA) were used. Mice were anesthetized and vaccinated intranasally using 30 μl (20). IpaB (13 μg) combined with IpaD (7 μg) or the DB fusion (20 μg) was admixed with dmLT (2.5 μg). These doses represent equimolar concentrations of antigens. Mice that received an adjuvant or vehicle alone were included as controls. Vaccine was delivered at days 0, 14, and 28.
Specific IgG antibodies.
Antibodies specific for IpaB and IpaD were determined by enzyme-linked immunosorbent assay (ELISA) (20). Briefly, 96-well plates coated with IpaB or IpaD (1 μg/ml in PBS) were blocked overnight with PBS with 10% milk. Each well was incubated with serum for 1 h at 37°C. After the plates were washed with PBS-Tween (0.05%), secondary antibody (KPL, Gaithesburg, MD) was added for 1 h at 37°C. Horseradish peroxidase (HRP) substrate was added, and the reaction was stopped with H3PO4. Endpoint titers were calculated and represented as ELISA units (EU) per ml.
Stool IgA.
Fresh fecal samples (3 to 5 pellets/mouse) were collected. Each sample was resuspended in 10% (wt/vol) PBS with 0.2% NaN3. The supernatant was clarified by centrifugation, and phenylmethylsulfonyl fluoride (PMSF) was added to 1 mM. IgA levels were determined by ELISA with an anti-IgA antibody (Southern Biotech, Birmingham, AL). Endpoint titers were calculated as described above.
ASCs.
Antibody-secreting cells (ASCs) were determined (20). Briefly, cell suspensions were obtained by homogenizing through a nylon mesh (BD Biosciences, San Diego, CA) the organs from five mice per group. Samples were incubated with 5 μg/ml of IpaB or IpaD for 24 h at 37°C. After samples were washed with PBS-Tween, antibodies against IgG or IgA were added. Trueblue (KPL) was used as a substrate in an agarose overlay. Spots were counted under a stereomicroscope by 2 individuals, and a mean of quadruplicate wells was expressed as specific ASCs per 106 cells.
IFN-γ ELISPOT.
Splenocyes were collected from five mice per group at day 56. Cells were incubated for 48 h at 37°C with 5 μg/ml IpaB or IpaD in plates coated with antibodies against IFN-γ. An enzyme-linked immunosorbent spot (ELISPOT) assay was performed (BD Biosciences). Spots were counted as described above and expressed as spot-forming cells (SFC) per 106 cells.
Cytokine determinations.
Splenocytes (obtained at day 56) were incubated with 10 μg/ml of IpaB, IpaD, or PBS for 48 h at 37°C. Secreted interleukin 17 (IL-17) levels were measured using the DuoSet ELISA development kit (22) or using a Th1/Th2 multiplex cytokine plate (Meso Scale Discovery, Gaithersburg, MD).
Challenge.
Shigella flexneri 2457T, Shigella sonnei 53G, and Shigella dysenteriae serotype Sd1617 were grown overnight at 37°C in tryptic soy agar with 0.05% Congo red. Ten colonies were picked and grown in tryptic soy broth (EMD Millipore) at 37°C in agitation until reaching an A600 of ∼1. Bacteria were centrifuged and resuspended in PBS. On day 56, mice were challenged by delivery of Shigella intranasally (26, 27). The doses, administered in 30 μl, were 6 ×106 CFU for S. flexneri, 2.1 ×106 CFU for S. sonnei, and 7.5 ×106 CFU for S. dysenteriae. Changes in health and weight loss were closely monitored for 14 days. Mice that became too sick or remained below 80% of their starting weight for more than 48 h were humanely euthanized. Animals were housed and handled in agreement with Oklahoma State University Institutional Animal Care and Use Committee practices (protocol AS-10-6).
Statistical analysis.
GraphPad Prism 5.04 was used to generate graphics and statistical comparisons. Differences were analyzed using t test. Survival plots were analyzed using log rank tests. A P value of less than 0.05 was considered significant for all comparisons. Vaccine efficacy was calculated by using the formula efficacy = (ARU − ARV)/ARU × 100, where ARU is attack rate in the unvaccinated group and ARV is attack rate in the vaccinated group (35).
RESULTS
DB fusion protein is expressed and folded.
Following coexpression with the Shigella chaperone protein IpgC, the DB fusion was isolated using the mild nonionic detergent OPOE, resulting in a dominant 101.2-kDa product comprised of both IpaD and IpaB (Fig. 1B). Like IpaB, the isolated DB fusion remains soluble in buffer containing 0.5% OPOE. Far-UV circular-dichroism (CD) measurements of IpaD, IpaB, and the DB fusion all showed spectra exhibiting dominant minima at 208 and 222 nm, characteristic of proteins with highly α-helical secondary structures (Fig. 1C). This suggests that the fusion maintained a proper and organized secondary structure following purification and separation from IpgC. The secondary-structure thermal stabilities for all three proteins were determined using CD spectroscopy by monitoring mean residue molar ellipticity at 222 nm as a function of temperature. The resulting plots indicated a transition at ∼58°C for IpaB and two transitions at 60°C and 80°C for IpaD (Fig. 1D), findings in agreement with previously published data (13, 34). Interestingly, the thermal unfolding curve for the DB fusion protein exhibits characteristics intermediate to both IpaD and IpaB, with a major transition at 60°C and a minor one around 78°C. Furthermore, the DB fusion mean residue molar ellipticity values for both the far-UV scans and the thermal unfolding curves lie between those for IpaD and IpaB alone, further suggesting that the IpaD and IpaB domains of the fusion protein both maintain a substantial portion of their original structural characteristics.
DB fusion protein generates antibody titers similar to those generated by the combination of IpaB and IpaD.
Mice were vaccinated intranasally three times, at days 0, 14, and 28, and serum titers of IgG against IpaB and IpaD were determined by ELISA (Fig. 2A and B). The titers of antibody against IpaB and IpaD elicited by the DB fusion in the presence of dmLT were comparable to those generated by vaccination with IpaB and IpaD with dmLT. The peak antibody levels and the kinetics follow very similar patterns, with no significant differences observed over time. In both cases, IpaD responses were delayed until day 28 (after two immunizations). Although the DB fusion protein administered without dmLT is able to generate detectable antibodies against IpaB and IpaD, adjuvant is required for generation of consistent titers higher than 103 to 104 EU/ml. No specific IgG was detected in mice immunized with PBS.
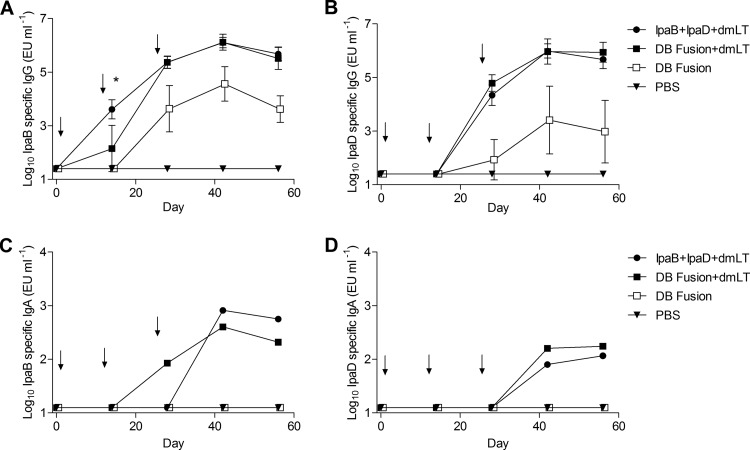
Fig 2.
Serum IgG titer kinetics. Mice were vaccinated three times, at the time points indicated by the arrows. Blood samples were collected and serum was separated. IgG antibodies specific for IpaB (A) or IpaD (B) were measured by ELISA. The individual titers are represented as EU ml−1, and each point represents mean ± SD of 10 mice per group. *, P < 0.05 comparing groups that received IpaB plus IpaD plus dmLT and the DB fusion plus dmLT using t test. IgA antibodies specific for IpaB (C) or IpaD (D) were measured by ELISA. The pool titers are represented as EU ml−1, and each point represents pooled samples of 10 mice per group.
To assess the intestinal mucosal antibody responses, fecal IgA antibody titers were determined by ELISA (Fig. 2C and D). Both the DB fusion and the combination of IpaB and IpaD administered with dmLT elicited specific IgA titers in stool. IgA antibodies against IpaB were detected in the group immunized with the DB fusion with adjuvant at day 28, one time point ahead of the group that received IpaB and IpaD. The stool IgA titer was 10-fold higher for IpaB than for IpaD in the DB fusion group, and the IpaD antibodies were not detected until day 42 rather than at day 28, as was the case for the IpaB antibodies. No stool IgA specific for these proteins was detected in the group immunized with the DB fusion without dmLT or in the group immunized with PBS.
The DB fusion protein generates ASCs.
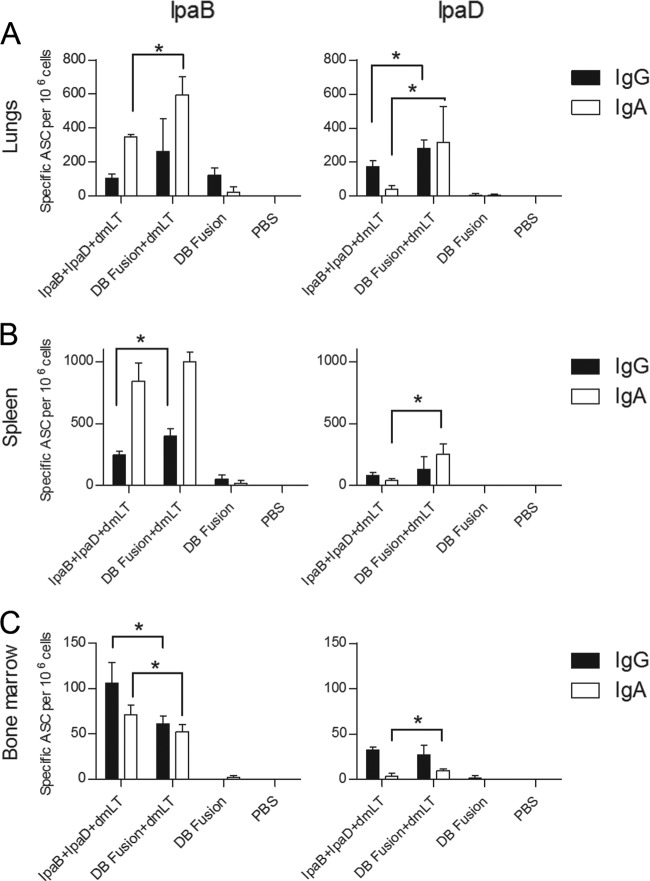
At day 56, the frequency of IgG- and IgA-secreting cells specific for each antigen was determined by ELISPOT assay. In the lungs (Fig. 3A), the frequency of ASCs specific for IpaB was higher for the DB Fusion plus dmLT, especially for IgA-secreting cells. This tendency was also observed for ASCs specific for IpaD. Only IgG-secreting cells specific for IpaB were detected in lungs from mice immunized with the DB fusion without adjuvant. In spleens (Fig. 3B), we found a higher frequency of IgA-secreting cells specific for IpaB than IgG-secreting cells, and when the groups that received IpaB plus IpaD and the DB fusion are compared, no major differences are observed. In general, the responses against IpaB were higher than responses against IpaD in spleens. The DB fusion without dmLT failed to elicit ASCs in the spleens. Finally, the frequencies of ASCs in the bone marrow (Fig. 3C) specific for IpaB were higher in the group that received IpaB plus IpaD plus dmLT, while for IpaD a higher IgA response was observed in the group that received the DB fusion plus dmLT. For this organ, a more balanced IgG/IgA response was observed.
Fig 3.
Antibody-secreting cells. Immunized mice (n = 5 per group) were euthanized at day 56 and organs were collected. Single-cell suspensions obtained from lungs (A), spleen (B), and bone marrow (C) were incubated in plates with IpaB or IpaD. IgG- and IgA-secreting cells were detected by ELISPOT assay and plotted as mean specific ASCs per 106 cells ± SD. *, P < 0.05 comparing groups that received IpaB plus IpaD plus dmLT and DB Fusion plus dmLT using t test.
The DB fusion protein generates higher frequencies of specific IFN-γ-secreting cells.
The frequency of gamma interferon (IFN-γ)-secreting cells was analyzed by ELISPOT assay using cells extracted from spleens of immunized mice at day 56 (Fig. 4). The DB fusion plus dmLT elicited higher numbers of specific IFN-γ-secreting cells than did IpaB plus IpaD plus dmLT. This was more evident for IpaD-specific IFN-γ-secreting cells, for which a 3-fold-higher frequency was seen in the group that received the DB fusion plus dmLT (∼20 SFC/106 cells for IpaB plus IpaD plus dmLT, compared to ∼70 SFC/106 cells for the DB fusion plus dmLT). The DB fusion without dmLT failed to generate IpaB-specific IFN-γ-secreting cells but managed to elicit a moderate number of IpaD-specific IFN-γ-secreting cells. No specific IFN-γ-secreting cells were detected in mice treated with PBS.
Fig 4.
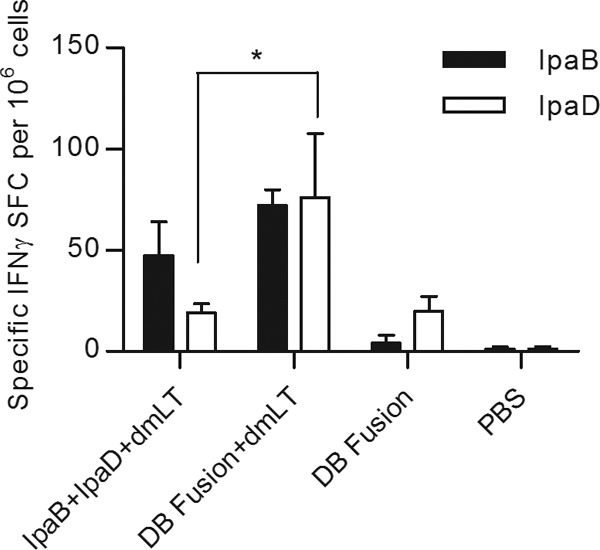
IFN-γ-secreting cells. Splenocytes obtained at day 56 from immunized animals were incubated with 10 μg/ml of IpaB and IpaD. IFN-γ-secreting cells were determined by ELISPOT, and SFC per 106 cells were calculated and plotted as means ± SD of quadruplicate wells. *, P < 0.05 comparing groups that received IpaB plus IpaD plus dmLT and the DB fusion plus dmLT using t test.
DB fusion protein generates a distinct profile of cytokine secretion.
Spleen cells were stimulated with IpaB or IpaD and the resulting supernatants analyzed for cytokine secretion. IL-2 levels varied depending on the antigen used for stimulation (Fig. 5A). For IpaB, the group that received the DB fusion plus dmLT showed higher cytokine secretion levels than the group that received IpaB plus IpaD plus dmLT. The opposite was observed for IpaD, for which cells obtained from animals that received IpaB plus IpaD plus dmLT secreted higher levels of IL-2 than cells obtained from animals that received the DB fusion plus dmLT. For IL-4, cells from mice that were vaccinated with IpaB plus IpaD plus dmLT showed higher cytokine secretion when stimulated with either IpaB or IpaD (Fig. 5B). In contrast, no significant differences were detected in levels of IL-5 secretion between the groups immunized with IpaB plus IpaD plus dmLT or the DB fusion plus dmLT (Fig. 5C). Secretion of the KC chemokine in response to IpaB was higher in cells from mice that received the DB fusion plus dmLT, with no differences being observed between these two treatments when IpaD was used to stimulate these cells (Fig. 5D). In the case of tumor necrosis factor alpha (TNF-α), significant differences were detected when IpaD was used to stimulate these cells, with the mice immunized with the DB fusion plus dmLT showing a greater response (Fig. 5E). In contrast, no differences in TNF-α secretion were observed with IpaB stimulation. Levels of IL-17 secretion in response to IpaB and IpaD stimulation of spleen cells were also measured (Fig. 6). Cells from mice that received the DB fusion plus dmLT secreted larger amounts of IL-17 in response to IpaB than cells from mice that received IpaB plus IpaD plus dmLT.
Fig 5.
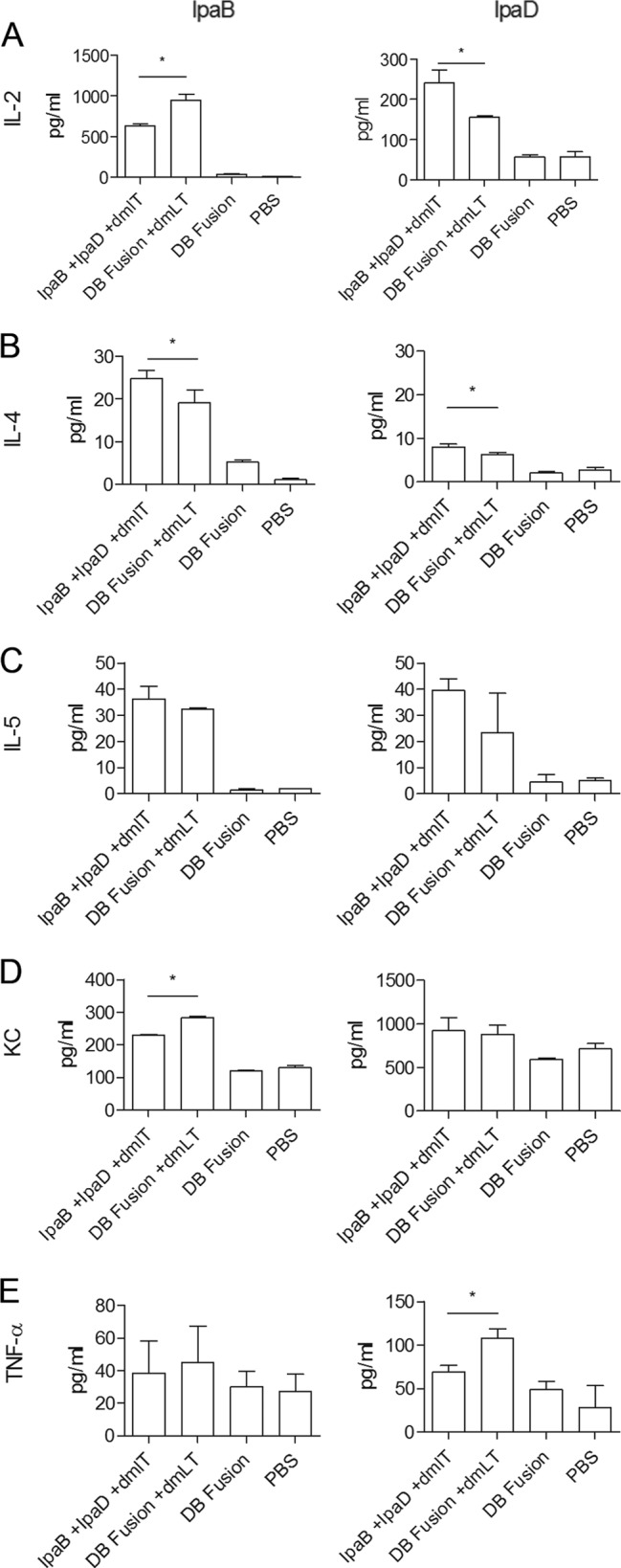
Cytokines. Splenocytes obtained at day 56 from immunized animals were incubated with 10 μg/ml of IpaB and IpaD. After 48 h, supernatants were collected and levels of cytokine secretion in response to IpaB (left) and IpaD (right) were then measured (in pg/ml) using a cytokine detection plate. Each bar represents the mean of quadruplicate wells ± SD. *, P < 0.05 comparing groups that received IpaB plus IpaD plus dmLT and the DB fusion plus dmLT using t test.
Fig 6.
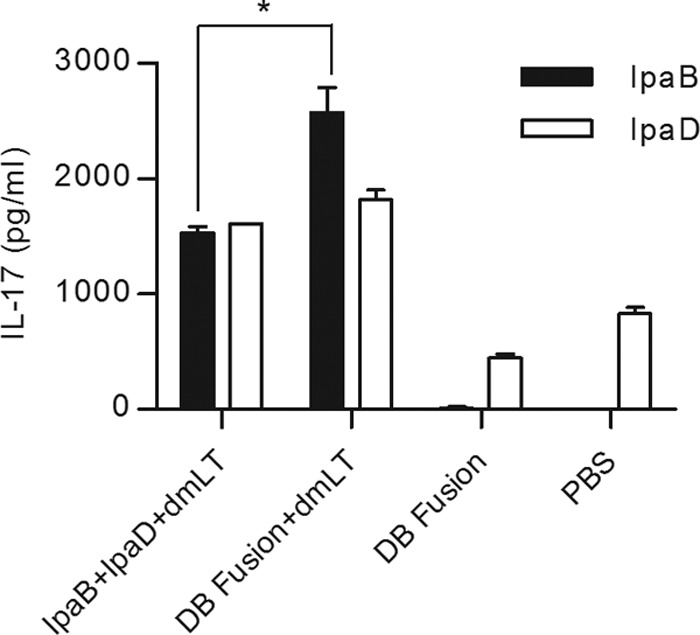
IL-17 secretion. Splenocytes obtained at day 56 from immunized animals were incubated with 10 μg/ml of IpaB and IpaD. After 48 h, supernatants were collected and levels of IL-17 secretion were measured using an ELISA kit. Each bar represents the mean of quadruplicate wells ± SD. *, P < 0.05 comparing groups that received IpaB plus IpaD plus dmLT and the DB fusion plus dmLT using t test.
DB fusion protein protects against Shigella homologous and heterologous challenges.
At day 56, vaccinated animals (n = 10 per bacterial strain) were challenged with S. flexneri 2a, S. sonnei, or S. dysenteriae, and protection was monitored for 14 days after infection. For the homologous challenge using S. flexneri, mice that received IpaB plus IpaD plus dmLT showed a protection level of 90%, while the mice that received the DB fusion plus dmLT showed a protection level of 70%. Mice that received the DB fusion without dmLT showed 20% protection after 14 days. No protection was observed for mice immunized with PBS (Fig. 7A). When S. sonnei was used to challenge vaccinated animals, we observed 100% protection in animals vaccinated with IpaB plus IpaD plus dmLT or the DB fusion plus dmLT. Mice that received the DB fusion alone showed 80% protection, while mice treated with PBS showed 20% protection. These numbers result in a calculated vaccine efficacy of 80% for both groups that received vaccine formulated with dmLT, and 55% for the group that received the DB fusion alone Fig. 7B). For S. dysenteriae, the group that received IpaB plus IpaD plus dmLT showed only 10% protection, while the group that received the DB fusion plus dmLT showed a protection level of 40%. No protection was observed in mice vaccinated with the DB fusion without dmLT or in mice that received PBS (Fig. 7C).
Fig 7.
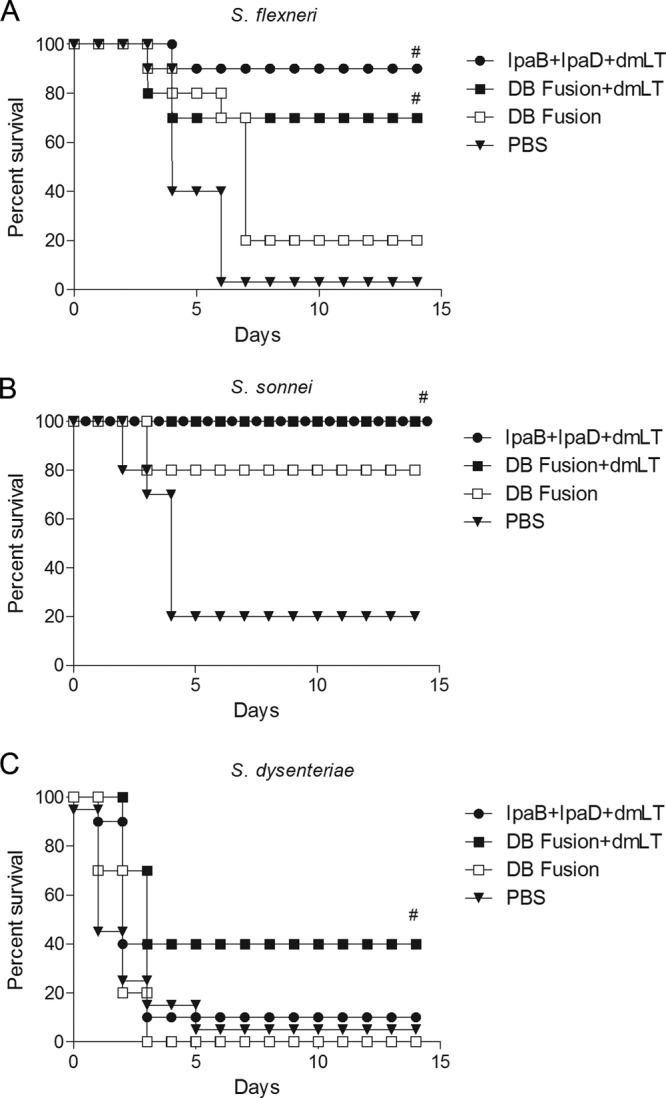
Challenge. Mice were vaccinated at days 0, 14, and 28 with the indicated treatments. After 56 days, 6 × 106 CFU of S. flexneri 2457T (A), 2.1 × 106 CFU of S. sonnei 53G (B), or 7.5 × 106 CFU of S. dysenteriae 1617 (C) were administered intranasally. Survival was monitored for 14 days. #, P < 0.05 compared to survival of mice vaccinated with PBS using log rank test.
DISCUSSION
Despite progress using different approaches, a Shigella vaccine is still not available. When the main target group for a Shigella vaccine is children living in developing countries, variables that impact the cost of production should be evaluated to diminish the vaccine cost. Taking this into consideration, we generated a fusion protein consisting of IpaD and IpaB. The DB fusion shared characteristics of IpaB. DB fusion expression was achieved only in the presence of IpaB's chaperone, IpgC, which is removed using 0.5% OPOE during chromatography purification. Although this indicates that the IpaD portion of the DB fusion is not sufficient to generate an independently soluble polypeptide, the subsequent purification step allows for a highly pure protein. Therefore, this purification step may be advantageous. Additionally, the DB fusion maintains a highly α-helical secondary structure in solution, with a stability similar to that of IpaB. While IpaD undergoes two thermal transitions, these transitions are not seen in the DB fusion. After three immunizations with equimolar concentrations, the DB fusion plus dmLT was able to elicit titers of serum IgG and stool IgA against both IpaB and IpaD at a magnitude similar to that elicited by administering the combination of IpaB and IpaD with dmLT. Therefore, recognition and generation of antibody responses against the components of the DB fusion remain at comparable levels. The presence of antibody-secreting cells in the same organ compartments supports this statement. Both IgG- and IgA-secreting cells were observed in the lungs, spleens, and bone marrow, specific for both IpaB and IpaD. The differences observed in the frequencies of ASCs, however, suggest that there are some small differences in how the proteins are able to activate B cells, which could impact the fate and distribution of plasmatic and memory cells. While mice immunized with the DB fusion plus dmLT showed higher frequencies of ASCs in the lungs and spleens, a lower frequency was observed in the bone marrow, suggesting differences in effector versus long-term memory ratios. Although the DB fusion without dmLT was able to elicit serum IgG responses against IpaB and IpaD, these responses were of a lower magnitude and highly variable between the individuals. Furthermore, it failed to induce IgA secretion in stool and generation of specific cytokine-secreting cells. This highlights the requirement of the dmLT adjuvant for these responses.
The analysis of the cytokine secretion profiles elicited by each group showed some differences between the immunized groups. Some cytokine responses were higher when the DB fusion was used for immunization. In particular, the frequencies of IFN-γ-secreting cells and IL-17 secretion levels were higher in cells obtained from mice immunized with the DB fusion. Even if dmLT has the capacity of eliciting IL-17 responses by itself (36), the presence of the adjuvant in both formulations indicates the possibility that the fusion could be recognized by the immune system in a different manner than the individual proteins. Most importantly, this demonstrates that the DB fusion has a unique advantage in the generation of cell-mediated immunity, which can be important for control of Shigella. Indeed, both IFN-γ and IL-17 have been described as important cytokines during Shigella infection (37, 38). The challenge experiments show that both proteins are able to provide heterologous protection. In the case of S. dysenteriae, only the DB fusion with dmLT was able to provide significant protection. This particular challenge is more stringent, as we used a strain that expresses Shiga toxin. The ability of the DB fusion to protect in contrast to the combination of IpaB and IpaD could be related to the cytokine profile elicited by this protein. Even with this tendency of higher protection with higher cytokine secretion, the role of antibodies cannot be ruled out. The protective efficacy of the DB fusion without adjuvant in the S. sonnei challenge could, then, relate to antibodies generated by this protein. Even if humoral responses could be less involved in protection, we still detected a 55% protective efficacy. This was probably observed only for S. sonnei given that the challenge dose that was used is lower than for S. flexneri and S. dysenteriae. In conclusion, we provide evidence that a fusion protein comprised of IpaB and IpaD is able to generate immune responses against the two subcomponents, retaining heterologous protection capabilities and generating higher IFN-γ and IL-17 responses, which could be important for protection against shigellosis in humans.
ACKNOWLEDGMENTS
We thank Atticus Mullon and Micah Scobey for technical support and Olivia Arizmendi for helping with figure preparation.
This work was funded by a grant from PATH-EVI.
Footnotes
Published ahead of print 23 September 2013
REFERENCES
- 1.WHO 2009. Initiative for vaccine research: diarrheal diseases (updated February 2009). WHO, Geneva, Switzerland: www.who.int/vaccine_research/diseases/diarrhoeal/en/index6.html [Google Scholar]
- 2.Kotloff KL, Winickoff JP, Ivanoff B, Clemens JD, Swerdlow DL, Sansonetti PJ, Adak GK, Levine MM. 1999. Global burden of Shigella infections: implications for vaccine development and implementation of control strategies. Bull. World Health Organ. 77:651–666 [PMC free article] [PubMed] [Google Scholar]
- 3.Kasper MR, Lescano AG, Lucas C, Gilles D, Biese BJ, Stolovitz G, Reaves EJ. 2012. Diarrhea outbreak during U.S. military training in El Salvador. PLoS One 7:e40404. 10.1371/journal.pone.0040404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kernéis S, Guerin PJ, von Seidlein L, Legros D, Grais RF. 2009. A look back at an ongoing problem: Shigella dysenteriae type 1 epidemics in refugee settings in Central Africa (1993–1995). PLoS One 4:e4494. 10.1371/journal.pone.0004494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alam AN, Sarker SA, Wahed MA, Khatun M, Rahaman MM. 1994. Enteric protein loss and intestinal permeability changes in children during acute shigellosis and after recovery: effect of zinc supplementation. Gut 35:1707–1711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Niyogi SK. 2005. Shigellosis. J. Microbiol. 43:133–143 [PubMed] [Google Scholar]
- 7.Barry EM, Pasetti MF, Sztein MB, Fasano A, Kotloff KL, Levine MM. 2013. Progress and pitfalls in Shigella vaccine research. Nat. Rev. Gastroenterol. Hepatol. 10:245–255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gu B, Cao Y, Pan S, Zhuang L, Yu R, Peng Z, Qian H, Wei Y, Zhao L, Liu G, Tong M. 2012. Comparison of the prevalence and changing resistance to nalidixic acid and ciprofloxacin of Shigella between Europe-America and Asia-Africa from 1998 to 2009. Int. J. Antimicrob. Agents 40:9–17 [DOI] [PubMed] [Google Scholar]
- 9.Ye C, Lan R, Xia S, Zhang J, Sun Q, Zhang S, Jing H, Wang L, Li Z, Zhou Z, Zhao A, Cui Z, Cao J, Jin D, Huang L, Wang Y, Luo X, Bai X, Wang P, Xu Q, Xu J. 2010. Emergence of a new multidrug-resistant serotype X variant in an epidemic clone of Shigella flexneri. J. Clin. Microbiol. 48:419–426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Formal SB, Oaks EV, Olsen RE, Wingfield-Eggleston M, Snoy PJ, Cogan JP. 1991. Effect of prior infection with virulent Shigella flexneri 2a on the resistance of monkeys to subsequent infection with Shigella sonnei. J. Infect. Dis. 164:533–537 [DOI] [PubMed] [Google Scholar]
- 11.Rasolofo-Razanamparany V, Cassel-Beraud AM, Roux J, Sansonetti PJ, Phalipon A. 2001. Predominance of serotype-specific mucosal antibody response in Shigella flexneri-infected humans living in an area of endemicity. Infect. Immun. 69:5230–5234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zychlinsky A, Kenny B, Menard R, Prevost MC, Holland IB, Sansonetti PJ. 1994. IpaB mediates macrophage apoptosis induced by Shigella flexneri. Mol. Microbiol. 11:619–627 [DOI] [PubMed] [Google Scholar]
- 13.Espina M, Olive AJ, Kenjale R, Moore DS, Ausar SF, Kaminski RW, Oaks EV, Middaugh CR, Picking WD, Picking WL. 2006. IpaD localizes to the tip of the type III secretion system needle of Shigella flexneri. Infect. Immun. 74:4391–4400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stensrud KF, Adam PR, La Mar CD, Olive AJ, Lushington GH, Sudharsan R, Shelton NL, Givens RS, Picking WL, Picking WD. 2008. Deoxycholate interacts with IpaD of Shigella flexneri in inducing the recruitment of IpaB to the type III secretion apparatus needle tip. J. Biol. Chem. 283:18646–18654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Camacho AI, Irache JM, Gamazo C. 2013. Recent progress towards development of a Shigella vaccine. Expert Rev. Vaccines 12:43–55 [DOI] [PubMed] [Google Scholar]
- 16.Ranallo RT, Fonseka S, Boren TL, Bedford LA, Kaminski RW, Thakkar S, Venkatesan MM. 2012. Two live attenuated Shigella flexneri 2a strains WRSf2G12 and WRSf2G15: a new combination of gene deletions for 2nd generation live attenuated vaccine candidates. Vaccine 30:5159–5171 [DOI] [PubMed] [Google Scholar]
- 17.Shim DH, Chang SY, Park SM, Jang H, Carbis R, Czerkinsky C, Uematsu S, Akira S, Kweon MN. 2007. Immunogenicity and protective efficacy offered by a ribosomal-based vaccine from Shigella flexneri 2a. Vaccine 25:4828–4836 [DOI] [PubMed] [Google Scholar]
- 18.Riddle MS, Kaminski RW, Williams C, Porter C, Baqar S, Kordis A, Gilliland T, Lapa J, Coughlin M, Soltis C, Jones E, Saunders J, Keiser PB, Ranallo RT, Gormley R, Nelson M, Turbyfill KR, Tribble D, Oaks EV. 2011. Safety and immunogenicity of an intranasal Shigella flexneri 2a Invaplex 50 vaccine. Vaccine 29:7009–7019 [DOI] [PubMed] [Google Scholar]
- 19.Pore D, Mahata N, Pal A, Chakrabarti MK. 2011. Outer membrane protein A (OmpA) of Shigella flexneri 2a, induces protective immune response in a mouse model. PLoS One 6:e22663. 10.1371/journal.pone.0022663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Martinez-Becerra FJ, Kissmann JM, Diaz-McNair J, Choudhari SP, Quick AM, Mellado-Sanchez G, Clements JD, Pasetti MF, Picking WL. 2012. Broadly protective Shigella vaccine based on type III secretion apparatus proteins. Infect. Immun. 80:1222–1231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Heine SJ, Diaz-McNair J, Martinez-Becerra FJ, Choudhari SP, Clements JD, Picking WL, Pasetti MF. 2013. Evaluation of immunogenicity and protective efficacy of orally delivered Shigella type III secretion system proteins IpaB and IpaD. Vaccine 31:2919–2929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Martinez-Becerra FJ, Scobey M, Harrison K, Choudhari SP, Quick AM, Joshi SB, Middaugh CR, Picking WL. 2013. Parenteral immunization with IpaB/IpaD protects mice against lethal pulmonary infection by Shigella. Vaccine 31:2667–2672 [DOI] [PubMed] [Google Scholar]
- 23.Liu KY, Shi Y, Luo P, Yu S, Chen L, Zhao Z, Mao XH, Guo G, Wu C, Zou QM. 2011. Therapeutic efficacy of oral immunization with attenuated Salmonella typhimurium expressing Helicobacter pylori CagA, VacA and UreB fusion proteins in mice model. Vaccine 29:6679–6685 [DOI] [PubMed] [Google Scholar]
- 24.de Sousa EM, da Costa AC, Trentini MM, de Araujo Filho JA, Kipnis A, Junqueira-Kipnis AP. 2012. Immunogenicity of a fusion protein containing immunodominant epitopes of Ag85C, MPT51, and HspX from Mycobacterium tuberculosis in mice and active TB infection. PLoS One 7:e47781. 10.1371/journal.pone.0047781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Heath DG, Anderson GW, Jr, Mauro JM, Welkos SL, Andrews GP, Adamovicz J, Friedlander AM. 1998. Protection against experimental bubonic and pneumonic plague by a recombinant capsular F1-V antigen fusion protein vaccine. Vaccine 16:1131–1137 [DOI] [PubMed] [Google Scholar]
- 26.Mallett CP, VanDeVerg L, Collins HH, Hale TL. 1993. Evaluation of Shigella vaccine safety and efficacy in an intranasally challenged mouse model. Vaccine 11:190–196 [DOI] [PubMed] [Google Scholar]
- 27.van de Verg LL, Mallett CP, Collins HH, Larsen T, Hammack C, Hale TL. 1995. Antibody and cytokine responses in a mouse pulmonary model of Shigella flexneri serotype 2a infection. Infect. Immun. 63:1947–1954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Marquart ME, Picking WL, Picking WD. 1995. Structural analysis of invasion plasmid antigen D (IpaD) from Shigella flexneri. Biochem. Biophys. Res. Commun. 214:963–970 [DOI] [PubMed] [Google Scholar]
- 29.Picking WL, Mertz JA, Marquart ME, Picking WD. 1996. Cloning, expression, and affinity purification of recombinant Shigella flexneri invasion plasmid antigens IpaB and IpaC. Protein Expr. Purif. 8:401–408 [DOI] [PubMed] [Google Scholar]
- 30.Studier FW. 2005. Protein production by auto-induction in high density shaking cultures. Protein Expr. Purif. 41:207–234 [DOI] [PubMed] [Google Scholar]
- 31.Choudhari SP, Kramer R, Barta ML, Greenwood JC, II, Geisbrecht BV, Joshi SB, Picking WD, Middaugh CR, Picking WL. 2013. Studies of the conformational stability of invasion plasmid antigen B from Shigella. Protein Sci. 22:666–670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Birket SE, Harrington AT, Espina M, Smith ND, Terry CM, Darboe N, Markham AP, Middaugh CR, Picking WL, Picking WD. 2007. Preparation and characterization of translocator/chaperone complexes and their component proteins from Shigella flexneri. Biochemistry 46:8128–8137 [DOI] [PubMed] [Google Scholar]
- 33.Mach H, Volkin DB, Burke CJ, Middaugh CR. 1995. Ultraviolet absorption spectroscopy. Methods Mol. Biol. 40:91–114 [DOI] [PubMed] [Google Scholar]
- 34.Dickenson NE, Choudhari SP, Adam PR, Kramer RM, Joshi SB, Middaugh CR, Picking WL, Picking WD. 2013. Oligomeric states of the Shigella translocator protein IpaB provide structural insights into formation of the type III secretion translocon. Protein Sci. 22:614–627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Orenstein WA, Bernier RH, Dondero TJ, Hinman AR, Marks JS, Bart KJ, Sirotkin B. 1985. Field evaluation of vaccine efficacy. Bull. World Health Organ. 63:1055–1068 [PMC free article] [PubMed] [Google Scholar]
- 36.Norton EB, Lawson LB, Mahdi Z, Freytag LC, Clements JD. 2012. The A subunit of Escherichia coli heat-labile enterotoxin functions as a mucosal adjuvant and promotes IgG2a, IgA, and Th17 responses to vaccine antigens. Infect. Immun. 80:2426–2435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sellge G, Magalhaes JG, Konradt C, Fritz JH, Salgado-Pabon W, Eberl G, Bandeira A, Di Santo JP, Sansonetti PJ, Phalipon A. 2010. Th17 cells are the dominant T cell subtype primed by Shigella flexneri mediating protective immunity. J. Immunol. 184:2076–2085 [DOI] [PubMed] [Google Scholar]
- 38.Le-Barillec K, Magalhaes JG, Corcuff E, Thuizat A, Sansonetti PJ, Phalipon A, Di Santo JP. 2005. Roles for T and NK cells in the innate immune response to Shigella flexneri. J. Immunol. 175:1735–1740 [DOI] [PubMed] [Google Scholar]