Abstract
Gardnerella vaginalis, the bacterial species most frequently isolated from women with bacterial vaginosis (BV), produces a cholesterol-dependent cytolysin (CDC), vaginolysin (VLY). At sublytic concentrations, CDCs may initiate complex signaling cascades crucial to target cell survival. Using live-cell imaging, we observed the rapid formation of large membrane blebs in human vaginal and cervical epithelial cells (VK2 and HeLa cells) exposed to recombinant VLY toxin and to cell-free supernatants from growing liquid cultures of G. vaginalis. Binding of VLY to its human-specific receptor (hCD59) is required for bleb formation, as antibody inhibition of either toxin or hCD59 abrogates this response, and transfection of nonhuman cells (CHO-K1) with hCD59 renders them susceptible to toxin-induced membrane blebbing. Disruption of the pore formation process (by exposure to pore-deficient toxoids or pretreatment of cells with methyl-β-cyclodextrin) or osmotic protection of target cells inhibits VLY-induced membrane blebbing. These results indicate that the formation of functional pores drives the observed ultrastructural rearrangements. Rapid bleb formation may represent a conserved response of epithelial cells to sublytic quantities of pore-forming toxins, and VLY-induced epithelial cell membrane blebbing in the vaginal mucosa may play a role in the pathogenesis of BV.
INTRODUCTION
The cholesterol-dependent cytolysins (CDCs) are a group of pore-forming toxins (PFTs) targeting eukaryotic cell membranes. CDCs are elaborated by many Gram-positive and, as recently recognized, some Gram-negative bacteria (1). Certain CDCs, including listeriolysin O from Listeria monocytogenes and pneumolysin from Streptococcus pneumoniae, are required for virulence of the producing organism (2, 3), whereas the specific roles of other CDC family members remain less clear. There is substantial diversity among the CDCs, with at least 3 (intermedilysin [ILY], vaginolysin [VLY], and lectinolysin [LLY]) of the more than 20 family members exhibiting species specificity dependent on recognition of human CD59 (hCD59) on target cells (4–6). A cocrystal structure of ILY and hCD59 was recently reported (7).
Gardnerella vaginalis produces VLY and is thought to be an important organism in the pathogenesis of bacterial vaginosis (BV), a mucosal dysbiosis associated with substantial adverse health consequences (8, 9). G. vaginalis induces epithelial cell cytotoxicity (10), but this effect has not yet been linked definitively to any particular virulence factor. The absence of robust genetic systems for manipulation of the G. vaginalis genome has hindered substantial investigations in this area.
If present at high enough concentrations, PFTs (including CDCs) induce target cell lysis and may compromise anatomic barriers or immune function (11). At lower (sublytic) concentrations, PFT exposure induces complex defense pathways that promote cellular survival (12, 13) and may still affect barrier or immune function. Relevant signaling pathways include p38 mitogen-activated protein kinase-dependent cascades, endocytic/vesicle trafficking, and membrane repair and lipid biosynthesis modules (11, 12, 14, 15). Cellular blebbing, a phenomenon generally linked to cell death pathways, was recently described as a protective response to transient disruption of membrane integrity (16). Some of these studies have used purified streptolysin O, a CDC, as a model pore-forming toxin, and recent work has linked pneumolysin (PLY), the CDC from Streptococcus pneumoniae, to host cell actin remodeling and astrocyte bleb formation (17, 18). However, the relevance of such findings to mucosal surfaces and to the setting of bacterial infection or colonization remains unclear in many cases.
Here we show that epithelial cells, which represent the first point of contact between mucosal pathogens and the host, initiate rapid bleb responses to G. vaginalis supernatants (GVS) that are dependent on the presence of VLY. Using antibody inhibition of both toxin and receptor, stimulation with sublytic quantities of recombinant toxin, genetic perturbation of toxin function, osmotic protection, and pharmacologic depletion of target cell membrane cholesterol, we demonstrate that this bleb response requires the formation of functional pores. Other CDCs, including PLY and ILY, which are hCD59-independent and -dependent toxins, respectively, induce similar blebbing, suggesting that rapid bleb formation may represent a conserved response of epithelial cells to sublytic quantities of pore-forming toxins.
MATERIALS AND METHODS
Reagents.
Unless otherwise specified, reagents were purchased from Sigma.
Bacterial strains and epithelial cell lines.
G. vaginalis strains 14018 and 49145 were purchased from ATCC. Strain ARG7 is a clinical isolate of G. vaginalis. Strains were grown in GV medium (GVM; brain heart infusion supplemented with 10% fetal bovine serum [FBS; HyClone], 5% Fildes enrichment [Remel], and 1 μg/ml amphotericin) as described previously (19).
Human cell lines were purchased from ATCC. Human cervical epithelial cells (HeLa cells; ATCC CCL-2) were grown at 37°C and 5% CO2 in minimal essential medium (Invitrogen) supplemented with 10% fetal bovine serum and 10 μg/ml penicillin and streptomycin. Human vaginal epithelial cells (VK2 cells; ATCC CRL-2616) were grown at 37°C and 5% CO2 in serum-free keratinocyte growth medium (Invitrogen) with 0.1 ng/ml epidermal growth factor (EGF), 0.05 mg/ml bovine pituitary extract, and 0.4 mM calcium chloride (20). HeLa cells were weaned from FBS prior to experiments.
Chinese hamster ovary K1 (CHO-K1) cells transfected with plasmid pIRES2-EGFP/hCD59 or pIRES2-EGFP (vector control) were grown as described previously (4).
Preparation of G. vaginalis supernatants.
G. vaginalis strains were grown in GVM at 37°C in the presence of 5% CO2 overnight. Supernatants were collected by centrifugation and sterilized using a 0.20-μm syringe filter. Sterility of supernatants was confirmed by culture of 100-μl aliquots on human blood-bilayer Tween (HBT) agar (BD Biosciences) and in GVM. Supernatants had 1 to 5 ng/ml VLY as measured by enzyme-linked immunosorbent assay (ELISA) (data not shown). Control preparations included GVM without bacteria and were incubated, centrifuged, and filtered under identical conditions.
Preparation of recombinant toxins.
Purification of recombinant VLY, VLY-P480W, ILY, and PLY was performed as described previously (4, 21). PLY lacks a secretion signal, and VLY was purified as the native protein (without a signal sequence) from a codon-optimized construct (21, 22).
We constructed a new toxoid form of VLY by introducing mutations into the codon-optimized VLY construct via overlap-extension PCR. We first deleted the domain 1 cysteine (C44), which did not affect toxin activity (data not shown). We then mutated the threonine-leucine pair in domain 4 that corresponds to the CDC cholesterol-binding site (T474G and L475G mutations) (23) and subsequently introduced cysteines at positions predicted to lock the protein in a monomeric state (T333C and I348C mutations), based on data from LaChapelle et al. (24). All constructs were confirmed by sequencing. This monomer-locked toxoid (VLY-ML) was expressed as a hexahistidine fusion from Escherichia coli T7-ExpressIq (New England BioLabs) and purified as described for VLY and PLY (21). Stability of the protein pre- and postdialysis was checked by SDS-PAGE with Coomassie blue staining.
We also constructed a recombinant fusion protein consisting of a transcriptional fusion between green fluorescent protein and VLY domain 4 (GFP-D4), produced in E. coli and purified using an N-terminal hexahistidine tag as described for VLY and PLY (21).
Measurement of cell viability.
Twenty-four-well plates were seeded with VK2 or HeLa cells and grown to 90% confluence prior to treatment. Trypan blue exclusion was assessed by visual inspection of at least 30 cells counted in duplicate wells 4 and 24 h after exposure to GVS. For cells treated with recombinant toxins, plates were incubated for 45 min, medium was removed, and the concentration of lactate dehydrogenase (LDH) was determined using a commercial kit (Roche) according to the manufacturer's instructions.
Live-cell fluorescence microscopy.
Glass-bottom plates (MatTek) were seeded with VK2 or HeLa cells in appropriate medium and grown to ∼60% confluence. Cells were treated with sublytic concentrations of purified recombinant VLY (18 ng/ml), PLY (50 ng/ml), VLY-ML (18 ng/ml), or vehicle control diluted in medium. For experiments using bacterial supernatants, the volume of supernatant or GVM control (100 μl) did not exceed 5% of the total volume in the well (2 ml).
To evaluate ultrastructural changes, cells were stained using an Image-iT Live plasma membrane and nuclear labeling kit (Invitrogen) containing Hoechst 33342 and Alexa Fluor 594-wheat germ agglutinin. Real-time microscopy (Zeiss AxioObserver; 37°C stage) was utilized to observe discrete high-power (63× oil-immersion objective) fields of cells following 10 min of exposure to toxins. Composite digital images were taken of 5 different high-power fields per glass-bottom plate. Bleb formation was scored by counting the number and percentage of cells with membrane blebs of >10 μm in diameter for each field. For each field, the lower limit of detection was counted as 1/N, expressed as a percentage, where N represents the total number of cells in the field. For fields in which no blebbing was observed, the percentage was set as the lower limit of detection. Images were processed in AxioVision (Zeiss), with histogram adjustment using Photoshop CS3 (Adobe). Images from the same experiment were adjusted identically in all cases. Composite figures were assembled in Illustrator CS3 (Adobe). Experiments were performed in triplicate and were repeated at least three times.
Pharmacologic inhibition of bleb formation.
Where indicated, cells were pretreated with methyl-β-cyclodextrin (MβCD) (5 mM final concentration) or anti-CD59 antibody (clone MEM-43) (10 μg/ml final concentration; Santa Cruz Biotechnology) for 1 h at 37°C and 5% CO2 prior to toxin exposure. In the indicated experiments, VLY was preincubated with rabbit polyclonal anti-VLY (1:50) or control rabbit serum for 30 min prior to use (25). For osmotic protection experiments, the cells were exposed to 12% dextran T500 or vehicle control immediately prior to toxin exposure.
Statistical analysis.
For microscopy experiments, the mean percentage of blebbed cells per high-power field was calculated for each condition. All data were expressed as means ± standard errors of the means (SEM) and compared using Student's t test or one-way analysis of variance (ANOVA) with the Tukey posttest for comparison of individual groups when indicated (Prism; GraphPad Software).
RESULTS
G. vaginalis supernatants induce membrane blebbing in epithelial cells via vaginolysin.
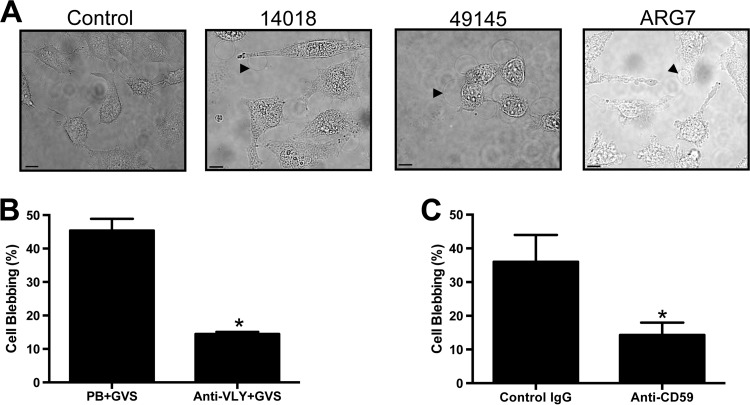
In order to determine the effect of G. vaginalis products on relevant cell types, we exposed human vaginal (VK2) and cervical (HeLa) epithelial cells to cell-free G. vaginalis supernatant from strain 14018, 49145, or ARG7. Such stimulation led to the formation of membrane blebs in more than 75% of cells within 10 min (Fig. 1A). Because VLY is hypothesized to be an important factor in Gardnerella-host interactions, we explored the effect of VLY inhibition on this response. Preincubation of G. vaginalis 49145 cell-free supernatants with anti-VLY polyclonal rabbit antiserum, but not control serum, significantly decreased the observed blebbing (Fig. 1B). Because VLY requires the availability of cell surface hCD59 for activity (4, 25), we assessed whether pretreatment with a blocking antibody prior to toxin exposure would also inhibit these ultrastructural responses by using a protocol to quantify bleb formation in epithelial cells exposed to bacterial toxins. Anti-hCD59 markedly inhibited VLY-induced membrane blebbing, while an isotype control antibody had no protective effect (Fig. 1C). At a 10-fold lower dose (1 μg/ml), the anti-CD59 antibody still inhibited formation of large blebs, but membrane ruffling and formation of small blebs were noted (data not shown). These results suggested that the VLY-hCD59 interaction is required for induction of epithelial cell bleb responses to G. vaginalis supernatants. These blebs persisted over the course of hours but were not associated with appreciable cellular death or detachment. When washed with phosphate-buffered saline (PBS) and allowed to recover in fresh medium, cells were viable and appeared to have recovered at 4 and 24 h post-VLY exposure. Trypan blue was excluded from >90% of cells at both time points (data not shown).
Fig 1.
G. vaginalis conditioned medium induces epithelial bleb formation without lysis in a VLY-dependent manner. (A) HeLa cells were exposed to G. vaginalis cell-free supernatants (GVS) from the indicated strains and photographed under bright-field exposure after 10 min. Black arrowheads indicate representative single blebs. Bars, 10 μm. (B) GVS from strain 49145 was pretreated with rabbit anti-VLY polyclonal serum (1:50 dilution, 1 h) or control serum (prebleed [PB]) prior to exposure of VK2 vaginal epithelial cells. Bleb formation was quantified with live-cell imaging. Anti-VLY inhibited bleb formation (P = 0.001; t test). (C) Pretreatment of VK2 cells with monoclonal antibody to the hCD59 receptor inhibited GVS-induced bleb formation (P = 0.013), while an isotype control antibody had no effect.
Sublytic quantities of recombinant VLY drive membrane blebbing.
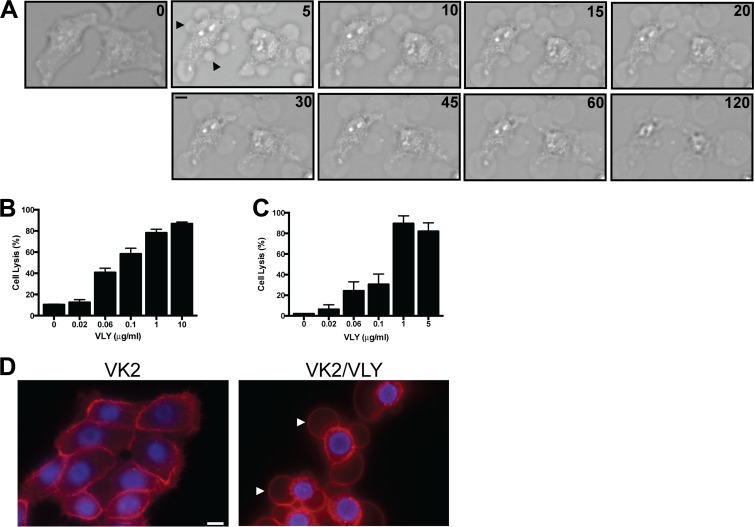
Having determined that VLY activity was necessary for induction of epithelial cell blebbing, we investigated whether it was also sufficient to initiate such responses. We treated human genital tract epithelial cells (HeLa and VK2) with sublytic concentrations of purified VLY and noted the rapid (<5 min) induction of membrane blebs in essentially 100% of cells observed (Fig. 2A). Small (<10 μm) blebs tended to resolve over 15 to 30 min and on serial images could be seen to shrink, presumably with reabsorption of contents into the cytoplasm (Fig. 2A). Larger blebs persisted over the course of hours (Fig. 2A). Occasionally, these were observed to “pinch off,” but they generally remained associated with a single location on a target cell and were resistant to gentile agitation of the medium. The concentrations of VLY used for these experiments did not cause appreciable cell lysis as measured by a sensitive lactate dehydrogenase assay (Fig. 2B and C). Similar bleb morphology was noted in VLY-exposed VK2 cells (Fig. 2D).
Fig 2.
Recombinant VLY recapitulates the effect of G. vaginalis supernatants on epithelial cells. (A) HeLa cells were treated with VLY (18 ng/ml), and live-cell imaging was performed. A single field of cells is depicted, with numbers indicating the amount of time (min) following addition of VLY. Widespread membrane blebbing was noted within 5 min, with some smaller blebs (arrowheads) resolving during the 120-min exposure. (B and C) HeLa (B) and VK2 (C) cells were treated with a range of concentrations of VLY (45-min exposure), and lactate dehydrogenate release was quantified. (D) VK2 cells were labeled with fluorescent dyes (red, membrane; blue, nucleus) and exposed to VLY (18 ng/ml; 10 min). Bars, 10 μm.
hCD59 binding is required for VLY-mediated responses.
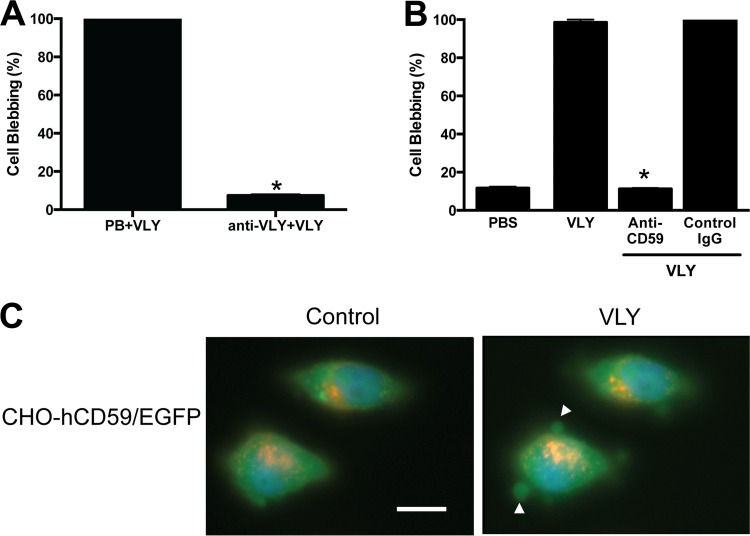
Using live-cell microscopy, we noted that the number of blebs per high-power field was greatest within 10 min of exposure to recombinant VLY. Thus, we chose this time point and a threshold size (10 μm) that was easily observable with the membrane dye used and was distinct from the small (<5 μm) blebs occasionally observed in vehicle control-treated cells. Treatment of cells with sublytic concentrations of VLY led to bleb formation in 100% of cells, but preincubation of VLY with a polyclonal antiserum significantly inhibited this response (Fig. 3A).
Fig 3.
VLY-mediated epithelial bleb formation requires host cell hCD59. (A) VLY-specific antiserum significantly inhibited HeLa cell blebbing compared to control antiserum treatment (P < 0.0001; ANOVA). (B) Monoclonal anti-hCD59 inhibited blebbing compared to an isotype control antibody (P < 0.0001; ANOVA). (C) CHO-K1 cells transfected with pIRES-eGFP/hCD59 (enhanced GFP [EGFP] signal localizes to cytoplasm; membrane hCD59 not labeled) formed blebs in response to VLY, with cytoplasmic GFP observed within the bleb structures (white arrowheads). Bar, 10 μm.
Consistent with our results with bacterial supernatants described above, anti-hCD59 markedly inhibited VLY-induced membrane blebbing, while normal mouse IgG had no protective effect, suggesting that hCD59 is required for this response (Fig. 3B). In order confirm the requirement of the VLY-hCD59 interaction, we used CHO-K1 cells transfected with an expression vector encoding hCD59 cDNA and a cytoplasmic GFP marker (pIRES-eGFP/hCD59). We observed bleb formation in GFP-positive cells that were exposed to VLY, though the blebs were generally smaller than those observed in either HeLa or VK2 cells. Notably, the GFP filled the blebs and appeared contiguous with the cytoplasm, suggesting that these structures likely contained cytoplasmic contents (Fig. 3C). CHO-K1 cells transfected with control vector (pIRES-eGFP) did not form blebs in response to VLY (data not shown).
hCD59 ligation is not sufficient to induce epithelial blebbing.
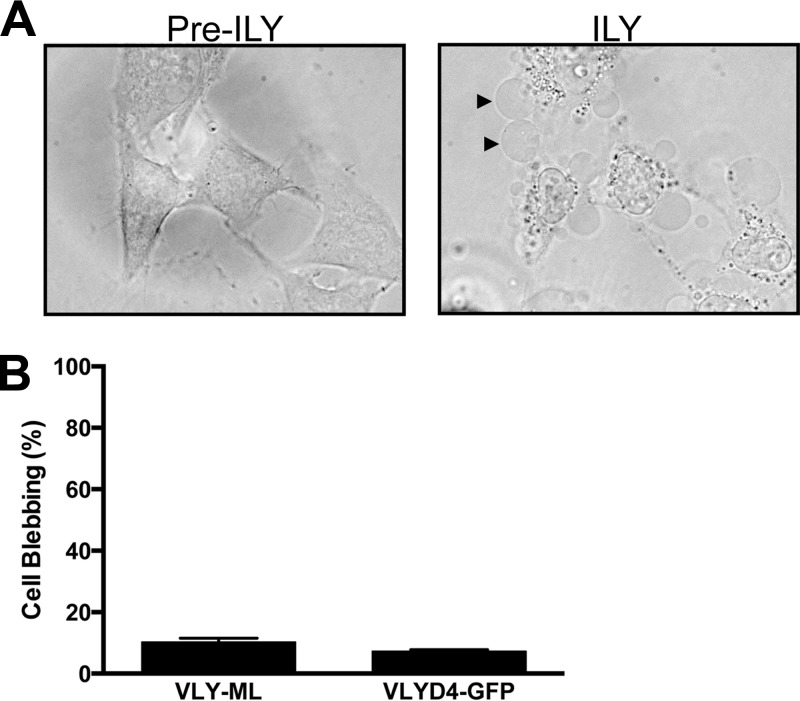
In order to determine the specificity of this response for VLY, we treated HeLa cells with similar concentrations of recombinant ILY (another hCD59-dependent CDC) and noted an essentially identical cellular response (Fig. 4A), indicating that the bleb response is not specific to VLY. Because hCD59 was required for VLY-mediated epithelial blebbing, we questioned whether binding of this cell surface receptor alone was sufficient to induce such responses. Treatment of cells with anti-hCD59 did not induce bleb formation (Fig. 3B). In addition, neither the monomer-locked recombinant VLY toxoid (VLY-ML) nor the GFP-D4 fusion protein, both of which bind hCD59, resulted in appreciable membrane blebbing (Fig. 4B), indicating that hCD59 ligation itself was insufficient to initiate bleb formation. The previously described VLY-P480W toxoid (4) also did not cause blebbing in HeLa cells (data not shown).
Fig 4.
Intermedilysin (ILY) induces membrane blebbing, but hCD59 ligation alone is not sufficient. (A) HeLa cells were treated with recombinant ILY (18 ng/ml) and imaged after 10 min (right). Black arrowheads indicate representative blebs. (B) HeLa cells were treated with VLY-ML toxoid (18 ng/ml) or with GFP-D4 (18 ng/ml), and bleb formation was quantified following 10 min of exposure.
Epithelial bleb responses to VLY require formation of functional pores.
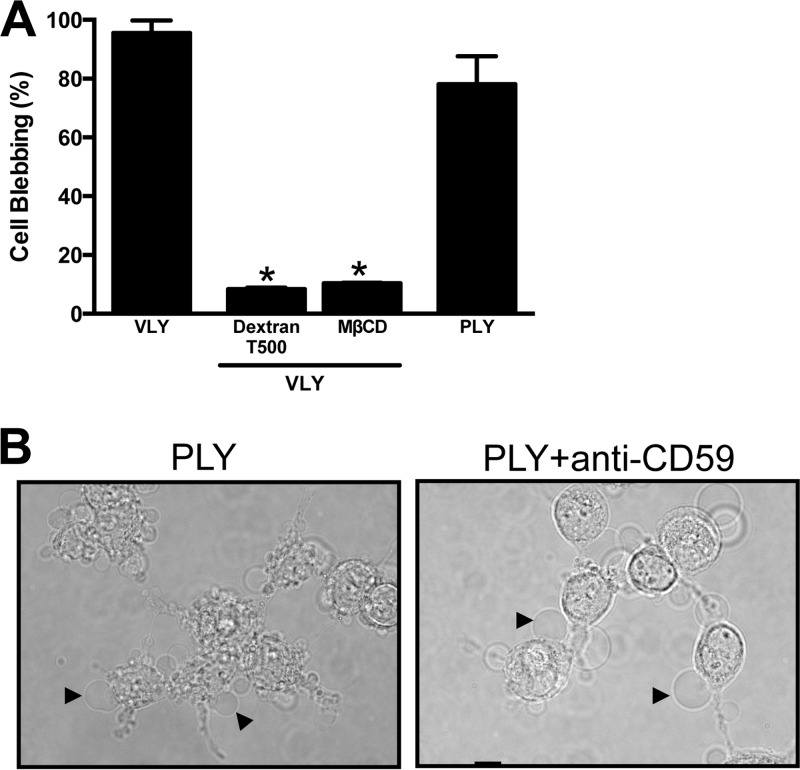
We tested whether PLY, a non-species-specific member of the CDC family, led to similar membrane changes. Treatment of epithelial cells with sublytic quantities of recombinant PLY efficiently induced bleb formation (Fig. 5A), suggesting that pore formation might be the crucial factor in this response. The formation of functional pores in epithelial cells induces osmotic stress, which can be relieved in the presence of large dextran molecules. Osmotic protection with an ∼28-nm-diameter dextran (dextran T500) potently inhibited VLY-induced membrane blebbing (Fig. 5A), consistent with a role for osmotic stress sensing or ion flux as a potential mechanism linking membrane damage to bleb formation (14, 26–28). Similar inhibition was observed following pretreatment of target cells with methyl-β-cyclodextrin (MβCD) in order to remove cholesterol, which is an essential cofactor in CDC-mediated pore formation (Fig. 5A) (29, 30). Consistent with prior data demonstrating hCD59 independence for PLY function (4), no inhibition of PLY-induced blebbing was noted in the presence of an hCD59-blocking antibody (Fig. 5B).
Fig 5.
Pore formation drives epithelial bleb responses to CDCs. (A) Osmotic protection of HeLa cells with dextran or depletion of membrane cholesterol with MβCD inhibits blebbing in response to VLY (P < 0.01; ANOVA). (B) PLY (50 ng/ml, 10 min) induces blebbing in HeLa cells with or without hCD59 antibody pretreatment (10 μg/ml, 1 h).
DISCUSSION
Disruption of membrane integrity constitutes a cellular emergency. Microbes produce a vast array of protein toxins that can puncture host cell membranes, with the CDCs representing one of the largest and best-characterized families (31–33). The interaction of PFT with host cells has a number of potential outcomes (11, 34). High PFT concentrations can lead to rapid and complete cellular lysis due to osmotic imbalance. It has been hypothesized that PFT-mediated lysis of host cells might provide access to nutrients for infecting bacteria, though available in vivo evidence is limited (11). At lower bacterial densities and toxin concentrations, such as might be encountered during colonization or early stages of infection, host cells can detect PFT activity at the membrane, activate signaling pathways that promote survival, and initiate membrane repair. Lipid metabolism, vesicle trafficking, calcium or potassium signaling, and mitogen-activated protein kinase pathways have all been implicated in such responses (14, 15, 35–38). It was recently recognized that cellular blebbing may represent an additional early defense mechanism for eukaryotic cells (14, 16, 37, 39). Such membrane blebs may promote survival by physically sequestering PFT and/or receptors and may also activate distinct cellular signaling pathways that promote healing of membrane wounds (14, 27, 35).
G. vaginalis is an enigmatic bacterial species with a strong link to reproductive health in humans (9, 40–42). Mechanistic studies of specific gene products in G. vaginalis pathogenesis have been limited by the lack of strategies for genetic manipulation of this organism. In the absence of such tools, we employed a variety of techniques to explore the interaction between VLY, the G. vaginalis hCD59-dependent CDC, and host cells. Using live-cell imaging, we discovered the rapid formation of membrane blebs following exposure of genital tract epithelial cells to cell-free supernatants. Antibody inhibition of toxin and receptor demonstrated that this response requires VLY-hCD59 interaction. Receptor binding is necessary but not sufficient to initiate membrane blebbing, and it is the assembly of functional pores, as demonstrated with a pore-deficient toxoid and with biochemical inhibition, as well as sensation of osmotic imbalance or ion fluxes, as demonstrated by osmotic protection, that drives these ultrastructural rearrangements.
G. vaginalis is present at the vaginal mucosal surface at high concentrations during BV (43). VLY has been hypothesized to be a contributor to BV pathogenesis, and its detection by reproductive tract epithelial cells may represent an important initial step in this host-pathogen interaction. Because this epithelial response occurs in response to both hCD59-dependent and -independent CDCs, we hypothesize that bleb formation represents a conserved response to this group and to other PFT families and that it may be relevant to the outcome of host-pathogen interactions at mucosal surfaces.
ACKNOWLEDGMENTS
This work was supported by the NIH (grants R01-AI092743 and R21-AI098654 to A.J.R. and K23-HD065844 to T.M.R.).
Footnotes
Published ahead of print 30 September 2013
REFERENCES
- 1.Hotze EM, Le HM, Sieber JR, Bruxvoort C, McInerney MJ, Tweten RK. 2013. Identification and characterization of the first cholesterol-dependent cytolysins from Gram-negative bacteria. Infect. Immun. 81:216–225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gaillard JL, Berche P, Sansonetti P. 1986. Transposon mutagenesis as a tool to study the role of hemolysin in the virulence of Listeria monocytogenes. Infect. Immun. 52:50–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Berry AM, Yother J, Briles DE, Hansman D, Paton JC. 1989. Reduced virulence of a defined pneumolysin-negative mutant of Streptococcus pneumoniae. Infect. Immun. 57:2037–2042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gelber SE, Aguilar JL, Lewis KLT, Ratner AJ. 2008. Functional and phylogenetic characterization of vaginolysin, the human-specific cytolysin from Gardnerella vaginalis. J. Bacteriol. 190:3896–3903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Giddings KS, Zhao J, Sims PJ, Tweten RK. 2004. Human CD59 is a receptor for the cholesterol-dependent cytolysin intermedilysin. Nat. Struct. Mol. Biol. 11:1173–1178 [DOI] [PubMed] [Google Scholar]
- 6.Wickham SE, Hotze EM, Farrand AJ, Polekhina G, Nero TL, Tomlinson S, Parker MW, Tweten RK. 2011. Mapping the intermedilysin-human CD59 receptor interface reveals a deep correspondence with the binding site on CD59 for complement binding proteins C8alpha and C9. J. Biol. Chem. 286:20952–20962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Johnson S, Brooks NJ, Smith RAG, Lea SM, Bubeck D. 2013. Structural basis for recognition of the pore-forming toxin intermedilysin by human complement receptor CD59. Cell Rep. 3:1369–1377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eschenbach DA. 1993. History and review of bacterial vaginosis. Am. J. Obstet. Gynecol. 169:441–445 [DOI] [PubMed] [Google Scholar]
- 9.Marrazzo JM, Martin DH, Watts DH, Schulte J, Sobel JD, Hillier SL, Deal C, Fredricks DN. 2010. Bacterial vaginosis: identifying research gaps. Proceedings of a workshop sponsored by DHHS/NIH/NIAID. Sex. Transm. Dis. 37:732–744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Patterson JL, Stull-Lane A, Girerd PH, Jefferson KK. 2010. Analysis of adherence, biofilm formation and cytotoxicity suggests a greater virulence potential of Gardnerella vaginalis relative to other bacterial-vaginosis-associated anaerobes. Microbiology (Reading, Engl.) 156:392–399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Los FCO, Randis TM, Aroian RV, Ratner AJ. 2013. Role of pore-forming toxins in bacterial infectious diseases. Microbiol. Mol. Biol. Rev. 77:173–207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gurcel L, Abrami L, Girardin S, Tschopp J, van der Goot FG. 2006. Caspase-1 activation of lipid metabolic pathways in response to bacterial pore-forming toxins promotes cell survival. Cell 126:1135–1145 [DOI] [PubMed] [Google Scholar]
- 13.Cassidy SKB, O'Riordan MXD. 2013. More than a pore: the cellular response to cholesterol-dependent cytolysins. Toxins (Basel) 5:618–636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Idone V, Tam C, Goss JW, Toomre D, Pypaert M, Andrews NW. 2008. Repair of injured plasma membrane by rapid Ca2+-dependent endocytosis. J. Cell Biol. 180:905–914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huffman DL, Abrami L, Sasik R, Corbeil J, van der Goot FG, Aroian RV. 2004. Mitogen-activated protein kinase pathways defend against bacterial pore-forming toxins. Proc. Natl. Acad. Sci. U. S. A. 101:10995–11000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Keyel PA, Loultcheva L, Roth R, Salter RD, Watkins SC, Yokoyama WM, Heuser JE. 2011. Streptolysin O clearance through sequestration into blebs that bud passively from the plasma membrane. J. Cell Sci. 124:2414–2423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Iliev AI, Djannatian JR, Nau R, Mitchell TJ, Wouters FS. 2007. Cholesterol-dependent actin remodeling via RhoA and Rac1 activation by the Streptococcus pneumoniae toxin pneumolysin. Proc. Natl. Acad. Sci. U. S. A. 104:2897–2902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Förtsch C, Hupp S, Ma J, Mitchell TJ, Maier E, Benz R, Iliev AI. 2011. Changes in astrocyte shape induced by sublytic concentrations of the cholesterol-dependent cytolysin pneumolysin still require pore-forming capacity. Toxins (Basel) 3:43–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hymes SR, Randis TM, Sun TY, Ratner AJ. 2013. DNase inhibits Gardnerella vaginalis biofilms in vitro and in vivo. J. Infect. Dis. 207:1491–1497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fichorova RN, Rheinwald JG, Anderson DJ. 1997. Generation of papillomavirus-immortalized cell lines from normal human ectocervical, endocervical, and vaginal epithelium that maintain expression of tissue-specific differentiation proteins. Biol. Reprod. 57:847–855 [DOI] [PubMed] [Google Scholar]
- 21.Rampersaud R, Planet PJ, Randis TM, Kulkarni R, Aguilar JL, Lehrer RI, Ratner AJ. 2011. Inerolysin, a cholesterol-dependent cytolysin produced by Lactobacillus iners. J. Bacteriol. 193:1034–1041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jost BH, Lucas EA, Billington SJ, Ratner AJ, McGee DJ. 2011. Arcanolysin is a cholesterol-dependent cytolysin of the human pathogen Arcanobacterium haemolyticum. BMC Microbiol. 11:239. 10.1186/1471-2180-11-239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Farrand AJ, LaChapelle S, Hotze EM, Johnson AE, Tweten RK. 2010. Only two amino acids are essential for cytolytic toxin recognition of cholesterol at the membrane surface. Proc. Natl. Acad. Sci. U. S. A. 107:4341–4346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.LaChapelle S, Tweten RK, Hotze EM. 2009. Intermedilysin-receptor interactions during assembly of the pore complex: assembly intermediates increase host cell susceptibility to complement-mediated lysis. J. Biol. Chem. 284:12719–12726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Randis TM, Kulkarni R, Aguilar JL, Ratner AJ. 2009. Antibody-based detection and inhibition of vaginolysin, the Gardnerella vaginalis cytolysin. PLoS One 4:e5207. 10.1371/journal.pone.0005207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ratner AJ, Hippe KR, Aguilar JL, Bender MH, Nelson AL, Weiser JN. 2006. Epithelial cells are sensitive detectors of bacterial pore-forming toxins. J. Biol. Chem. 281:12994–12998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Charras GT, Hu C-K, Coughlin M, Mitchison TJ. 2006. Reassembly of contractile actin cortex in cell blebs. J. Cell Biol. 175:477–490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tam C, Idone V, Devlin C, Fernandes MC, Flannery A, He X, Schuchman E, Tabas I, Andrews NW. 2010. Exocytosis of acid sphingomyelinase by wounded cells promotes endocytosis and plasma membrane repair. J. Cell Biol. 189:1027–1038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Giddings KS, Johnson AE, Tweten RK. 2003. Redefining cholesterol's role in the mechanism of the cholesterol-dependent cytolysins. Proc. Natl. Acad. Sci. U. S. A. 100:11315–11320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hotze EM, Tweten RK. 2012. Membrane assembly of the cholesterol-dependent cytolysin pore complex. Biochim. Biophys. Acta 1818:1028–1038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tweten RK. 2005. Cholesterol-dependent cytolysins, a family of versatile pore-forming toxins. Infect. Immun. 73:6199–6209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Heuck AP, Moe PC, Johnson BB. 2010. The cholesterol-dependent cytolysin family of gram-positive bacterial toxins. Subcell. Biochem. 51:551–577 [DOI] [PubMed] [Google Scholar]
- 33.Rosado CJ, Kondos S, Bull Kuiper TEMJ, Law RHP, Buckle AM, Voskoboinik I, Bird PI, Trapani JA, Whisstock JC, Dunstone MA. 2008. The MACPF/CDC family of pore-forming toxins. Cell. Microbiol. 10:1765–1774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bischofberger M, Iacovache I, van der Goot FG. 2012. Pathogenic pore-forming proteins: function and host response. Cell Host Microbe 12:266–275 [DOI] [PubMed] [Google Scholar]
- 35.Corrotte M, Fernandes MC, Tam C, Andrews NW. 2012. Toxin pores endocytosed during plasma membrane repair traffic into the lumen of MVBs for degradation. Traffic 13:483–494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Husmann M, Beckmann E, Boller K, Kloft N, Tenzer S, Bobkiewicz W, Neukirch C, Bayley H, Bhakdi S. 2009. Elimination of a bacterial pore-forming toxin by sequential endocytosis and exocytosis. FEBS Lett. 583:337–344 [DOI] [PubMed] [Google Scholar]
- 37.Los FCO, Kao C-Y, Smitham J, McDonald KL, Ha C, Peixoto CA, Aroian RV. 2011. RAB-5- and RAB-11-dependent vesicle-trafficking pathways are required for plasma membrane repair after attack by bacterial pore-forming toxin. Cell Host Microbe 9:147–157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kao C-Y, Los FCO, Huffman DL, Wachi S, Kloft N, Husmann M, Karabrahimi V, Schwartz J-L, Bellier A, Ha C, Sagong Y, Fan H, Ghosh P, Hsieh M, Hsu C-S, Chen L, Aroian RV. 2011. Global functional analyses of cellular responses to pore-forming toxins. PLoS Pathog. 7:e1001314. 10.1371/journal.ppat.1001314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Babiychuk EB, Monastyrskaya K, Potez S, Draeger A. 2011. Blebbing confers resistance against cell lysis. Cell Death Differ. 18:80–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Catlin BW. 1992. Gardnerella vaginalis: characteristics, clinical considerations, and controversies. Clin. Microbiol. Rev. 5:213–237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Harwich MD, Alves JM, Buck GA, Strauss JF, Patterson JL, Oki AT, Girerd PH, Jefferson KK. 2010. Drawing the line between commensal and pathogenic Gardnerella vaginalis through genome analysis and virulence studies. BMC Genomics 11:375. 10.1186/1471-2164-11-375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yeoman CJ, Yildirim S, Thomas SM, Durkin AS, Torralba M, Sutton G, Buhay CJ, Ding Y, Dugan-Rocha SP, Muzny DM, Qin X, Gibbs RA, Leigh SR, Stumpf R, White BA, Highlander SK, Nelson KE, Wilson BA. 2010. Comparative genomics of Gardnerella vaginalis strains reveals substantial differences in metabolic and virulence potential. PLoS One 5:e12411. 10.1371/journal.pone.0012411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fredricks DN, Fiedler TL, Marrazzo JM. 2005. Molecular identification of bacteria associated with bacterial vaginosis. N. Engl. J. Med. 353:1899–1911 [DOI] [PubMed] [Google Scholar]