Abstract
Recent work has identified T cells and the cytokines they produce as important correlates of immune protection during Staphylococcus aureus infections through the ability of these T cells to regulate local neutrophil responses. However, the specific T-cell subsets that are involved in coordinating protection at distinct sites of infection remains to be established. In this study, we identify for the first time an important role for γδT cells in controlling S. aureus surgical site infection (SSI). γδT cells are recruited to the wound site following S. aureus challenge, where they represent the primary source of interleukin 17 (IL-17), with a small contribution from other non-γδT cells. The IL-17 response is entirely dependent upon IL-1 receptor signaling. Using IL-17 receptor-deficient mice, we demonstrate that IL-17 is required to control bacterial clearance during S. aureus SSI. However, we demonstrate a strain-dependent requirement for γδT cells in this process due to the differential abilities of individual strains to activate IL-1β production. IL-1β processing relies upon activation of the Nlrp3 inflammasome complex, and we demonstrate that Nlrp3-deficient and IL-1 receptor-deficient mice have an impaired ability to control S. aureus SSI due to reduced production of IL-17 by γδT cells at the site of infection. Given that IL-17 has been identified as an important correlate of immune protection during S. aureus infection, it is vital that the unique cellular sources of this cytokine and mechanisms inducing its activation are identified at distinct sites of infection. Our study demonstrates that while IL-17 may be critically important for mediating immune protection during S. aureus SSI, the relative contribution of γδT cells to these protective effects may be strain dependent.
INTRODUCTION
Staphylococcus aureus is one of the primary causative organisms of skin and soft-tissue infections, the severity of which can range from minor conditions such as folliculitis, cellulitis, and impetigo to more severe surgical site infections (SSIs) (1). S. aureus is accountable for a significant proportion of all hospital-associated SSIs (2), and dissemination from the initial infecting site can lead to invasive and life-threatening conditions, such as bacteremia and endocarditis (1). Treatment of SSIs has become increasingly complicated by the pervasiveness of antibiotic resistance among strains of S. aureus, particularly in the hospital setting (3). The high prevalence of S. aureus infections in surgical patients and the reduced efficacy of antibiotics in treating these infections has led to vigorous attempts to develop new therapies directed against this pathogen, namely, vaccination strategies and immunomodulatory approaches (4). However, success in this area has been limited in part due to a lack of understanding of what constitutes a protective immune response against S. aureus at specific infection sites.
Interleukin 17A (IL-17A) is the prototype of a family of cytokines produced by Th17 cells. IL-17 is critically important in antimicrobial immunity, particularly during fungal and extracellular bacterial infections, because of this cytokine's ability to activate CXC chemokine production and consequently direct the recruitment of neutrophils to sites of infection (5). Mice deficient in IL-17 are highly susceptible to a number of bacterial and fungal infections, including Klebsiella pneumoniae (6), Escherichia coli (7), and Candida albicans (8). In the context of S. aureus infections, IL-17 is believed to play a significant role, supported by clinical findings that hyper-IgE syndrome patients who have impaired Th17 cell responses suffer from recurrent S. aureus infections (9). In addition, it has been identified that patients with atopic dermatitis have increased susceptibility to colonization by S. aureus (10), and this has in part been ascribed to decreased IL-17 responses (11). These findings have thrust IL-17 to the leading edge of research into S. aureus immunity. The importance of IL-17 in regulating neutrophil responses, which are essential in determining S. aureus infection outcomes (12), identifies IL-17 and the cells that produce it as important potential targets for novel anti-S. aureus vaccine and immunotherapeutic strategies.
However, therapeutic targeting of IL-17 necessitates a more lucid understanding of the cellular sources of this cytokine and, in particular, whether distinct cell types play specific roles depending on the site of infection. Although there is significant interest in targeting Th17 cells for the treatment of opportunistic infections such as S. aureus (4, 13), it must be considered that during host defense against infection, the early innate effects of IL-17 on CXC chemokine production (14) are unlikely to be mediated by the Th17 pathway. In recent years the importance of innate sources of IL-17, such as γδT cells (15, 16), invariant natural killer T cells (iNKTs) (17), and lymphoid tissue inducer (LTi)-like cells (18), have been documented (19). These cells appear to predominate in the skin and at mucosal sites, where they play important sentinel roles and can respond rapidly and nonspecifically to pathogenic insult.
γδT cells have been identified as an important first line of defense in S. aureus cutaneous infection. Initial studies revealed that δTCR−/− mice exhibited more severe skin lesions than wild-type mice, with significantly elevated levels of bacteria recovered from the skin following intradermal administration of S. aureus (20). More recently it was shown that impaired bacterial clearance in δTCR−/− mice was associated with reduced IL-17 production (21), with IL-17 shown to be critical for the recruitment of neutrophils to the site of skin infection. Similarly, during S. aureus-induced pneumonia, local IL-17 production in the lung was significantly reduced in the absence of γδT cells, and this was associated with impaired neutrophil recruitment and elevated bacterial burden in the lungs of δTCR−/− mice compared to wild-type mice (22). Taken together, these studies suggest that γδT cells, as opposed to Th17 cells, are the more important source of IL-17 during S. aureus infection and support further investigation of their role in infection at alternative nonmucosal sites.
To date, few molecules have been identified as direct γδT-cell antigens; it has been hypothesized that the rapid IL-17 response mounted by γδT cells relies upon the activation of pathogen patterns receptors (PPRs) and/or activation by inflammatory cytokines (19). IL-1β and IL-23 are potent activators of IL-17 production by γδT cells (16). IL-1β, together with IL-1α, plays a well-defined role in S. aureus immunity (23–26), although it has been proposed that during more invasive infection IL-1β plays a more predominant role in host defense than IL-1α (25). Mice deficient in MyD88, a signaling adapter for the IL-1 receptor, were found to be highly susceptible to cutaneous S. aureus infection, and this was attributed to impaired neutrophil recruitment (23, 26). These effects were shown to be a consequence of defective IL-1R but not TLR2 signaling (23). During S. aureus cutaneous infection, induction of IL-17 production by γδT cells was shown to be dependent upon IL-1β and IL-23 (21), suggesting that activation of IL-17 production by γδT cells represents a critical mechanism by which IL-1β mediates protective immunity in S. aureus infections.
Production of active IL-1β requires enzymatic processing of pro-IL-1β into its active form, a process which is facilitated by an intracellular protein complex known as the inflammasome (27). Several inflammasome complexes have been identified based on the Nod-like receptors (NLRs) that activate the complex. The Nlrp3 inflammasome complex consists of the proteins Nlrp3, apoptosis-associated speck-like protein (ACS), and procaspase-1 (28). Engagement of this complex leads to activation of procaspase 1, which in turn cleaves pro-IL-1β to its active form, ready for secretion from the cell. During S. aureus infection, activation of the Nlrp3 inflammasome can be induced by alpha toxin and other pore-forming hemolysins (29, 30) and by peptidoglycan following lysosomal degradation (31). Although the Nlrp3 inflammasome has been implicated in host defense against S. aureus, a direct link between Nlrp3 activation and bacterial clearance during S. aureus SSI infection has not been identified.
Using a clinically relevant model of S. aureus SSI, we have previously demonstrated an important role for αβT cells present at the wound site in regulating the local neutrophil responses and, subsequently, infection clearance (32, 33). Given the emerging importance of γδT cells in host defense during S. aureus infection (21), we sought to determine if γδT cells and IL-17 play any role during SSI, where their presence has never previously been described. Here, we demonstrate for the first time that γδT cells are recruited to the surgical wound site upon S. aureus infection and are the primary source of IL-17. Importantly, we demonstrate that activation of these γδT cells is dependent upon Nlrp3 and IL-1 receptor signaling. However, our data demonstrate strain-dependent discrepancies in activation of γδT cells due to the fact that certain strains of S. aureus can differentially activate IL-1β (and potentially IL-1α) production. These studies emphasize the importance of establishing the site-specific role played by individual T-cell subsets in regulating protective immunity to S. aureus and highlights that the choice of infecting strain must be considered when studying cellular immunity to S. aureus infection.
MATERIALS AND METHODS
Bacterial strains.
S. aureus strain PS80 is a capsular polysaccharide 8-expressing strain that has been described previously (32, 34). S. aureus strain SH1000 is an acapsular strain and is a derivative of strain 8325-4 with a restored rsbU gene (35, 36). Bacteria were cultivated from frozen stocks for 24 h at 37°C on agar plates. Bacterial suspensions were then prepared in phosphate-buffered saline (PBS), and the concentrations were estimated by measuring the absorbance of the suspension read at 600 nm. CFU numbers were determined by plating serial dilutions of each inoculum.
Animals.
Wild-type (C57BL/6), IL-1R−/−, δTCR−/−, and Nlrp3−/− mice (6 to 8 weeks old) all were housed under specific-pathogen-free conditions at the TCD Bioresources facility. All mice were maintained according to EU regulations, and experiments were performed under license from the Irish Department of Health and Children and with approval from the Trinity College Dublin Bioresources Ethics Committee.
Mouse model of S. aureus wound infection.
Surgical wound infection was established as previously described (33). Briefly, mice were anesthetized and their right thighs were shaved. The surgical area was disinfected with iodine and 70% ethanol. A 1-cm incision was made in the right thigh muscle and then closed with one 4-0 silk suture. Ten microliters of S. aureus (102 CFU) suspended in sterile PBS was introduced into the incision under the suture. The skin was closed with four Prolene sutures. At specific time points after the induction of infection, the mice were euthanized and wound tissue was excised and analyzed as follows.
Total tissue bacterial burden was established by homogenizing tissue in tryptic soy broth and plating serial dilutions on tryptic soy agar plates. Results were expressed as CFU/g of tissue weight.
Tissue explants were incubated in complete RPMI (cRPMI; supplemented with fetal calf serum [10%], l-glutamine [100 mM], and penicillin/streptomycin [0.1 mg/ml; Gibco]) for 24 h at 37°C. Culture supernatant was then collected, and IL-1β, IL-1α, and IL-18 concentrations were determined by enzyme-linked immunosorbent assay (ELISA) (R&D Systems).
For intracellular cytokine staining, excised wound tissue was collagenase digested (collagenase type IV; 300 U/ml) for 1 h at 37°C with constant rotation. Digested tissue was lysed to remove contaminating erythrocytes. CD45+ leukocytes were purified from digested tissue by magnetically activated cell sorting (MACS; Miltenyi Biotec) and stimulated in vitro for 4 h with phorbol myristate acetate (PMA; 10 ng/ml) and ionomycin (1 μg/ml) in the presence of GolgiStop (BD). Cells were washed with 3% bovine serum albumin (BSA) in PBS and blocked with Fcγ block (1 μg/ml; BD Pharmingen) before extracellular staining with monoclonal antibodies against the T-cell surface markers CD3, CD4, CD8, and γδTCR (BD Pharmingen). Cells were then fixed and permeabilized using the BD Cytofix/Cytoperm plus fixation/permeabilization kit (per the manufacturer's instructions), and intracellular IL-17 was detected using monoclonal antibody (clone eBio 17B7; eBiosciences). Flow-cytometric analysis was performed using a CyANADP flow cytometer (DakoCytomation) and analyzed with FloJo software, with gating set on fluorescence minus one control.
RNA extraction, reverse transcription cDNA synthesis, and quantitative PCR.
Total RNA from homogenized wounds was extracted using TRIzol reagent (Invitrogen) according to the manufacturer's instructions. The RNA yield was measured on a NanoDrop ND-1000. RNA was stored at −80°C until analysis was performed. RNA (1 μg) was reverse transcribed using a high-capacity cDNA reverse transcription kit (Applied Biosystems) with random primers according to the manufacturer's instructions. Quantitative PCR was performed on a CFX96 touch real-time PCR detection system (Bio-Rad) using the SsoFast probes supermix (Bio-Rad) according to the manufacturer's recommendations. Quantitative PCR was performed using a primer/probe set designed to amplify PTX3 (forward, 5′-ACAACGAAATAGACAATGGACTTCAT-3′; reverse, 5′-CTGGCGGCAGTCGCA-3′ probe, 5′-CCACCGAGGACCCCACGCC-3′; Sigma-Genosys) as previously described (37) and an 18S rRNA endogenous control (Life Technologies). Expression of PTX3 mRNA from S. aureus-infected wounds was compared to that of wounds from control mice treated with PBS by the change-in-cycle-threshold (ΔΔCT) method.
Intraperitoneal infection with S. aureus.
Peritoneal infection was established in WT and δTCR−/− mice by intraperitoneal (i.p.) injection of S. aureus strain PS80 (108 CFU) or SH1000 (108 CFU). The peritoneal cavity was lavaged with sterile PBS at 3 h postinfection. Samples were centrifuged at 1,200 rpm for 10 min, and the supernatants were removed and stored at −20°C for ELISA analysis. Isolated leukocyte populations were stained and analyzed by fluorescence-activated cell sorting (FACS) as described above.
Characterization of toxin production by S. aureus strains.
The production of α- and δ-hemolysin by PS80 and SH1000 was determined by cross-streaking perpendicularly to strain RN4220 on sheep blood agar. RN4220 produces β-hemolysin, which enhances red blood cell lysis by δ-hemolysin, but inhibits lysis by α-hemolysin. The production of Panton-Valentine leukocidin and β-hemolysin was determined by Western blotting. Supernatants from overnight cultures of SH1000 and PS80 were separated on 12% (wt/vol) polyacrylamide gels, transferred onto polyvinylidene difluoride (PVDF) membranes (Roche), and blocked in 10% (wt/vol) skim milk proteins. Blots were probed with PVL antiserum (1:200) or β-hemolysin antiserum (1:500). Bound antibodies were detected using horseradish peroxidase-conjugated (HRP) protein A (1:500; Sigma). Reactive bands were visualized using the LumiGLO reagent and peroxide detection system (Cell Signaling Technology). Antiserum was raised in rabbits at Trinity College Dublin against the purified toxins from S. aureus strain BB (β-hemolysin antiserum) or strain V8 (PVL antiserum).
Culture of BMDCs.
Bone marrow-derived dendritic cells (BMDCs) were prepared by culturing bone marrow cells from C57BL/6 mice with granulocyte-macrophage colony-stimulating factor (GM-CSF) as previously described (38). On day 10, loosely adherent cells were collected, washed, and reseeded at a concentration of 2 ×105 cells/well. The following day cells were infected with live S. aureus at multiplicities of infection (MOIs) of 10 and 100 for the indicated time points. Supernatants were collected and IL-1β, IL-1α, tumor necrosis factor alpha (TNF-α), IL-23, and IL-6 concentrations were determined by ELISA (R&D Systems). Cell viability was assessed by propidium iodide staining. Ninety percent viability was maintained up to 6 h postinfection but had decreased by 24 h.
In vitro stimulation of purified γδT cells.
γδT cells were purified from spleen and lymph node cells of WT and IL-1R−/− mice using MACS pan-T-cell isolation, followed by FACS purification of CD3+ γδTCR+ cells, yielding a population with ≥95% purity. Purified γδT cells were resuspended in medium and seeded at a concentration of 1 × 105 cells/well. Cells were stimulated with tissue culture supernatant from dendritic cells, which were previously incubated with live S. aureus for 24 h. Culture supernatant was filter sterilized prior to incubation with γδT cells. After 18 h of culture at 37°C, γδT-cell supernatants were assessed for IL-17 concentration by ELISA.
Statistical analysis was carried out using GraphPad Prism statistical analysis software. Group differences were analyzed by unpaired Student t test or analysis of variance (ANOVA) where applicable. P values of less than 0.05 were considered statistically significant.
RESULTS
γδT cells are the principal producers of IL-17 during S. aureus SSI.
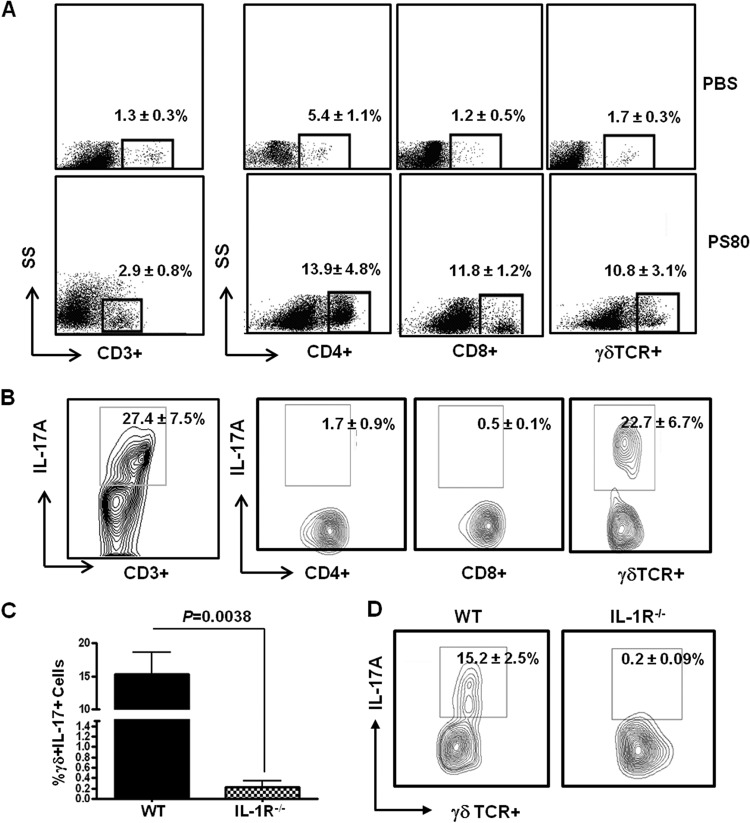
To establish if γδT cells play a significant role in the host immune response to S. aureus SSI, we established surgical wounds in groups of wild-type (WT) mice and infected them with S. aureus strain PS80. On day 3 postinfection, there was a significant increase in the proportions of CD3+, CD3+ CD4+, CD3+ CD8+, and CD3+ γδTCR+ T cells present at the S. aureus wound site compared to PBS-treated wounds (Fig. 1A). Intracellular cytokine staining revealed high levels of IL-17A production by total CD3+ T cells (27.4% ± 7.5%). Further analysis identified γδTCR+ T cells as the primary source of this IL-17A (22.7% ± 6.7%), with CD4+ and CD8+ T cells contributing only 1.7% ± 0.9% and 0.5% ± 0.1%, respectively (Fig. 1B). CD3− cells were not producing any IL-17A (data not shown). No IL-17A production was detected in lymphocytes isolated from PBS-treated wounds (see Fig. S1A in the supplemental material). At this time point during infection, γδT cells were not producing significant levels of gamma interferon (IFN-γ) or IL-17F (see Fig. S1B and C).
Fig 1.
γδT cells are the primary source of IL-17A at the S. aureus surgical wound site, and this IL-17A production is dependent upon IL-1β signaling. S. aureus surgical wounds were established in naive WT mice with S. aureus strain PS80 (102 CFU) or PBS (as a control). Wound tissues were excised on day 3 postinfection, and the T cell infiltrate was evaluated by flow cytometry. (A) The percentage of CD4+, CD8+, or γδTCR+ T cells was determined by gating on total CD3+ cells. Results are expressed as means ± SEM (n = 10 individual mice), with representative FACS plots shown. (B) Infiltrating T cells were also assessed for their capacity to produce IL-17A using intracellular cytokine staining. Results are expressed as means ± SEM (n = 10 individual mice), with a representative FACS plot shown. S. aureus surgical wounds were established in WT and IL-1R−/− mice with S. aureus strain PS80 (102 CFU). Wound tissues were excised on day 3 postinfection, and the percentage of γδTCR+ IL-17A+ cells was determined by flow cytometry. Results are expressed as means ± SEM (n = 4 individual mice) (C), and a representative FACS plot is shown (D).
IL-1β has been described previously as an essential signal for IL-17 production by γδ T cells (16). Therefore, we assessed the production of IL-17A by γδT cells during S. aureus SSI in WT and IL-1R−/− mice. On day 3 postinfection, IL-17A production by γδTCR+ cells present at the wound site was almost completely abolished from IL-1R−/− mice (P = 0.0038) (Fig. 1C and D).
Taken together, our data indicate that γδT cells are the primary source of IL-17A during S. aureus SSI and that IL-17A production by γδT cells is entirely dependent upon IL-1 signaling. Here, we utilize IL-17 to refer to levels of IL-17A, unless otherwise stated.
γδT cells and IL-17 are required to control S. aureus SSI, but this effect may depend on the infecting strain.
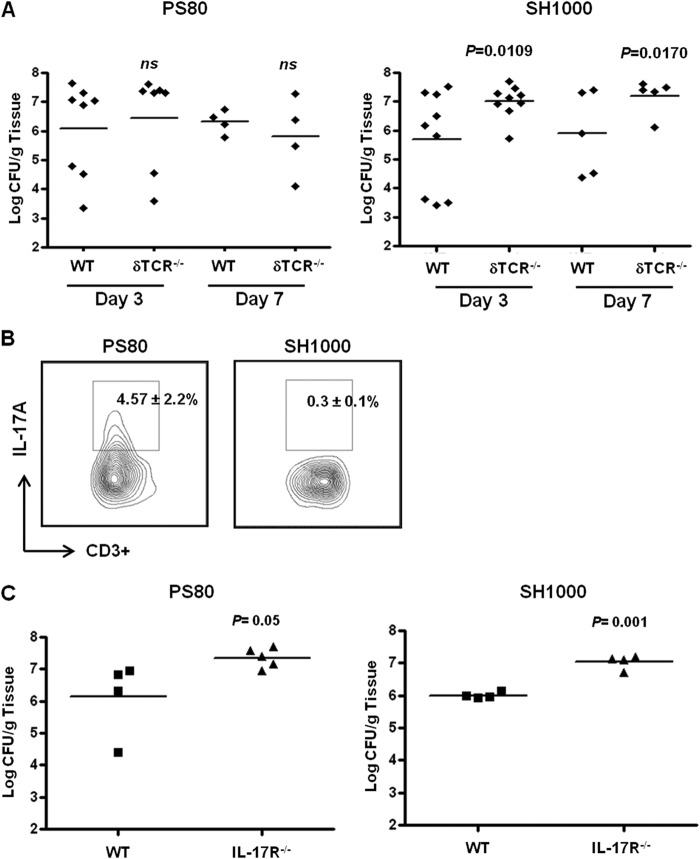
To investigate if γδT cells are important in controlling infection during S. aureus SSI, bacterial burden was assessed in WT and δTCR−/− mice on days 3 and 7 postinfection with two distinct strains of S. aureus. Surprisingly, we observed no significant increase in bacterial burden at the wound site in δTCR−/− mice compared to that of WT mice following infection with S. aureus strain PS80. However, clearance of the infection was significantly impaired in the δTCR−/− mice on days 3 (P = 0.0109) and 7 (P = 0.017) postinfection with S. aureus strain SH1000 (Fig. 2A). To determine the levels of IL-17 being produced in δTCR−/− mice, we analyzed IL-17 production by total CD3+ T cells isolated from the wound site on day 3 postinfection with S. aureus strain PS80 or SH1000. Interestingly, following infection with S. aureus strain PS80, residual IL-17 production by CD3+ T cells could still be detected (4.57% ± 2.2%); however, IL-17 production by CD3+ T cells was undetectable following infection with SH1000 (Fig. 2B). To confirm the absolute requirement for IL-17 in controlling S. aureus wound infection, we compared bacterial clearance in WT and IL-17R−/− mice and observed a significant increase in bacterial burden on day 7 postchallenge following infection with either strain of S. aureus (Fig. 2C). These data imply that IL-17 production by γδT cells is instrumental in controlling S. aureus SSI with strain SH1000; however, different strains of S. aureus (e.g., PS80) may employ different mechanisms to activate IL-17 production by the γδT cells and/or other non-γδT cells present at the wound site.
Fig 2.
Strain-dependent control of infection clearance by γδT cells during S. aureus SSI. Surgical wounds were established in WT and δTCR−/− mice. Wounds were infected with either S. aureus strain PS80 (102 CFU) or S. aureus strain SH1000 (102 CFU). (A) Wound tissues were excised on days 3 and 7 postinfection to determine total tissue bacterial burden. Results are expressed as log CFU per g of tissue, and the median is indicated by a bar (n = 4 to 9 per group). (B) Wound tissues were excised on day 3 postinfection in δTCR−/− mice, and infiltrating CD3+ T cells were assessed for their capacity to produce IL-17. Results are expressed as means ± SEM (n = 3 individual mice), with representative FACS plots shown. Surgical wounds were also established in WT and IL-17R−/− mice and infected with either S. aureus strain PS80 or strain SH1000. (C) Tissue bacterial burden was assessed on day 7 postchallenge. Results are expressed as log CFU per g of tissue, and the median is indicated by a bar (n = 4 to 9 per group).
S. aureus strains SH1000 and PS80 differentially activate γδT cells to produce IL-17 during SSI.
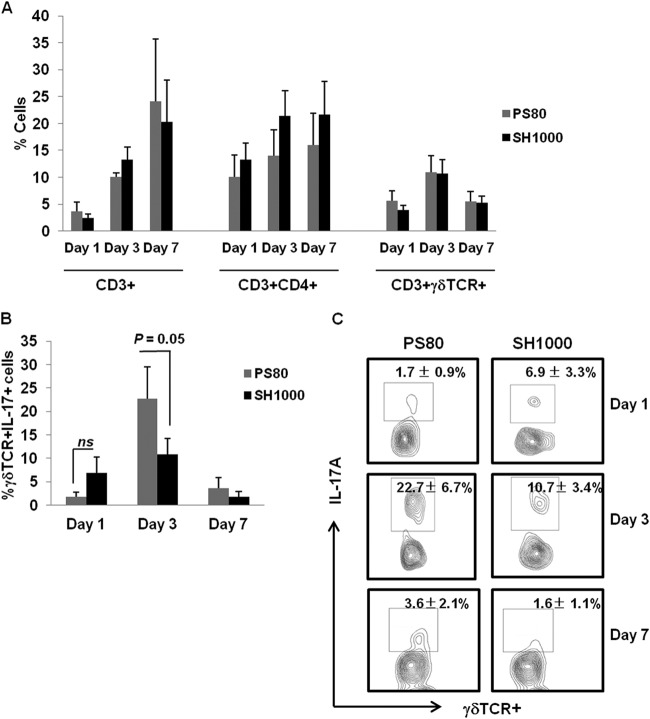
Having observed a strain-dependent requirement for γδT cells during S. aureus SSI, we sought to establish if distinct strains of S. aureus could differentially activate local IL-17 production by γδT cells. S. aureus surgical wound infection was established in WT mice using either S. aureus strain PS80 or SH1000. The overall recruitment of T cells (Fig. 3A) to the surgical wound site was similar following infection with either S. aureus strain. We observed no significant differences in the proportions of CD3+, CD3+ CD4+, or CD3+ γδTCR+ T cells present at the wound site over a 7-day period of infection with either PS80 or SH1000 (Fig. 3A). IL-17 production by γδTCR+ T cells present at the wound site were maximal on day 3 postinfection with either strain of S. aureus, with PS80 inducing significantly more IL-17 production by γδT cells at this time point than SH1000 (22.7% ± 6.7% versus 10.7% ± 3.4%, respectively; P = 0.05) (Fig. 3B and C and Table 1).
Fig 3.
S. aureus strains PS80 and SH1000 have different capacities for inducing IL-17 production by γδT cells at the surgical wound site. S. aureus surgical wounds were established in WT mice with either S. aureus strain PS80 (102 CFU) or SH1000 (102 CFU). (A) Wound tissues were excised on days 1, 3, and 7 postinfection, and the T cell infiltrate was evaluated by flow cytometry. The percentage of CD4+ and γδTCR+ T cells was determined first by gating on total CD3+ cells. Results are expressed as the means ± SEM (n = 10 individual mice). CD3+ γδTCR+ T cells infiltrating wound tissue were also assessed for their capacity to produce IL-17 in response to both S. aureus strains on days 1, 3, and 7 postinfection. Results are expressed as the means ± SEM (n = 10 individual mice) (B), and representative FACS plots are included (C).
Table 1.
Total numbers of γδTCR+ IL-17+ cells present at the S. aureus wound infection site
| Day | No. of CD3+ γδTCR+ IL-17+ cells of strain: |
|
|---|---|---|
| PS80 | SH1000 | |
| 3 | 8.87 × 107 ± 3.3 × 107 | 3.32 × 107 ± 0.93 × 107 |
| 7 | 1.29 × 107 ± 0.66 × 107 | 0.63 × 107 ± 0.32 × 107 |
When we assessed IL-17 production during infection with either strain at an alternative site, we observed a similar effect. Local IL-17 production was significantly (P = 0.013) increased following intraperitoneal challenge with PS80 compared to SH1000 in WT mice (see Fig. S2A in the supplemental material), and this was associated with greater IL-1β production in WT mice in response to challenge with PS80 versus SH1000 (see Fig. S2B in the supplemental material). When intraperitoneal infection was induced in δTCR−/− mice, overall IL-17 production was significantly reduced compared to that of WT mice; however, it was not abolished, similar to what was observed at the surgical wound site (Fig. 2B). Analysis of the cellular source of the residual IL-17 in the δTCR−/− mice revealed that it is produced primarily (74.5%) by a CD3+ CD4− CD8− cell subset with a low level of IL-17 produced by CD4+ T cells at this site (see Fig. S2C).
Differential activation of IL-1β production by APCs following infection with S. aureus strains PS80 and SH1000 influences subsequent IL-17 production by γδT cells.
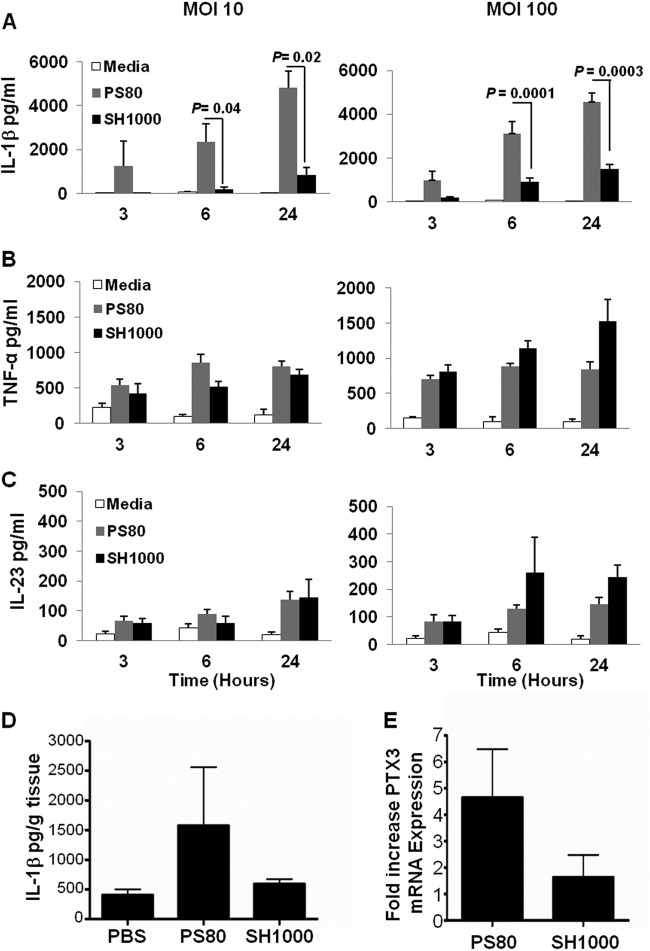
IL-1β and IL-23 derived from antigen-presenting cells (APCs) have been identified as key cytokines that are specifically required for the production of IL-17 by γδTCR+ cells (16). In addition, we demonstrate that IL-17 production by γδT cells at the surgical wound site is entirely dependent upon IL-1R signaling (Fig. 1C). Therefore, we hypothesized that the ability of S. aureus strains to differentially activate IL-17 production by γδT cells is due to their distinct capacity to regulate IL-1β and/or IL-23 production by APCs. To investigate this, BMDCs were cultured in the presence of S. aureus strain PS80 or SH1000 for 3, 6, and 24 h at MOIs of 10 and 100. PS80 induced significantly higher levels of IL-1β from BMDCs at 6 and 24 h than SH1000-treated BMDCs (Fig. 4A). In contrast, IL-23, TNF-α, and IL-6 production by the BMDCs was comparable following infection with either strain (Fig. 4B and C; also see Fig. S3A in the supplemental material), suggesting that both strains were comparable in their abilities to activate the innate signaling pathways (e.g., TLRs) responsible for the induction of these inflammatory cytokines. PS80 also resulted in significantly greater levels of IL-1α production by the BMDCs compared to SH1000 (for PS80 and SH1000 at an MOI of 100 at 6 h, 7,274.8 ± 702.7 and 389.3 ± 202.9 pg/ml, respectively [P = 0.002]; for PS80 and SH1000 at an MOI of 100 at 24 h, 8,614.5 ± 473.4 and 4,580.7 ± 496.2 pg/ml, respectively [P = 0.014]).
Fig 4.
S. aureus strain-dependent effects on IL-1β production. BMDCs from WT mice were infected with S. aureus strain PS80 or SH1000 at MOIs of 10 and 100 for 3, 6, and 24 h. IL-1β (A), TNF-α (B), and IL-23 (C) levels in the supernatant were quantified by ELISA. Results are expressed as means ± SEM (n = 9 individual experiments). S. aureus surgical wounds were established in WT mice with either S. aureus strain PS80 (102 CFU) or SH1000 (102 CFU). Control wounds were established and treated with PBS. (D) Wound tissues were excised at 6 h postinfection, and explants were incubated in vitro for 24 h. IL-1β secretion was quantified by ELISA and expressed as pg/g tissue. Results are expressed as means ± SEM (n = 3 to 4 mice per group). Wound tissue was also excised at 24 h postinfection with S. aureus strain PS80 or SH1000, and mRNA transcription of PTX3 was measured using quantitative PCR. Results are expressed as fold change in gene expression compared to that of PBS-treated wounds (means ± SEM; n = 6 mice per group).
We have shown previously that macrophages are likely to be the most abundant APCs present at the surgical wound infection site (39). When primary peritoneal macrophages were infected with PS80 or SH1000, we observed similar significant differences in their abilities to induce IL-1β production (see Fig. S3B in the supplemental material). These data suggest that different S. aureus strains have differential capacities for specifically activating the innate signaling pathways in APCs that are responsible for IL-1β production. To confirm that these in vitro observations translate to the in vivo situation, we measured IL-1β production by wound explant tissue isolated at 6 h postinfection with PS80 or SH1000. Consistent with the in vitro results, PS80 induced greater IL-1β production in the infected wound tissue than SH1000 (Fig. 4D). To further establish the differential in vivo effects on IL-1β signaling induced by these individual strains, we measured gene expression of pentraxin-related protein (PTX3) at the wound infection site. Expression of PTX3 has been shown to be induced during S. aureus infection (24), and importantly it appears that IL-1β is specifically responsible for its induction (37, 40). Consequently, PTX3 has the potential to be used as a surrogate marker for assessment of IL-1β protein synthesis and bioactivitiy in vivo (37). We observed a greater induction of PTX3 gene expression in the wound tissue at 24 h following infection with PS80 than with SH1000 (Fig. 4E).
IL-18 has also been implicated in activation of IL-17 production by γδT cells (41); however, when we assessed IL-18 production by the explanted wound tissue, we found that it was undetectable (data not shown), suggesting that the levels of IL-18 present at the S. aureus surgical wound infection site are too low to contribute to γδT-cell activation. IL-1α production by the explanted wound tissue was detectable, and we observed increased levels of IL-1α production by PS80-infected wound tissue compared to SH1000-infected tissue (710.4 ± 439 and 99.81 ± 25 pg/g tissue for PS80- and SH1000-infected wound explant tissue, respectively).
Having established that S. aureus strains SH1000 and PS80 had differential capacities to activate IL-1β and IL-1α, we wanted to confirm this as the mechanism responsible for the differential IL-17 production by γδT cells induced by these strains during S. aureus infection. Purified γδT cells from naive WT or IL-1R−/− mice were cultured in vitro in the presence of cell culture supernatants from BMDCs that had been infected with either S. aureus strain PS80 or SH1000 at an MOI of 10. γδT cells stimulated with supernatants from PS80-infected DCs produced significantly higher levels of IL-17, as detected by ELISA, compared to γδT cells stimulated with supernatants from SH1000-infected BMDCs (Fig. 5). Importantly, in the absence of IL-1R, γδT cells were unable to respond to the S. aureus conditioned culture media, confirming the absolute requirement for IL-1β and/or IL-1α in regulating IL-17 production by γδT cells following S. aureus infection. BMDCs were incapable of producing IL-17 following infection with S. aureus (levels were below the limits of detection by ELISA; data not shown). Taken together, these data point to differential induction of IL-1β and/or IL-1α synthesis as a pathological difference between infecting strains of S. aureus, which can subsequently influence the activation of IL-17 production by γδT cells during the course of infection.
Fig 5.
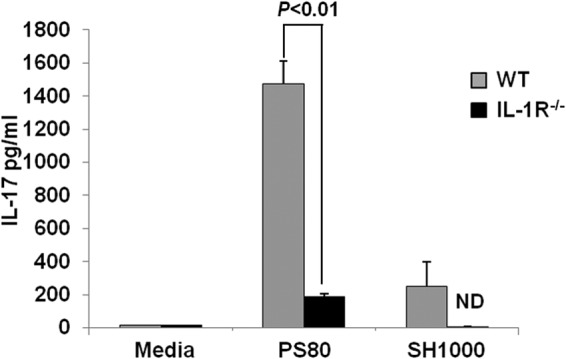
Strain-dependent effects of IL-1β production by BMDCs regulate downstream IL-17 production by γδT cells. Purified γδT cells were isolated from WT and IL-1R−/− mice and stimulated with supernatant from BMDCs infected with S. aureus strain PS80 or SH1000 (MOI, 10). After 18 h, IL-17 levels in the supernatants were determined by ELISA. Results are expressed as means ± SEM (n = 3 individual experiments).
Activation of IL-17 production by γδT cells during S. aureus SSI requires activation of the Nlrp3 inflammasome.
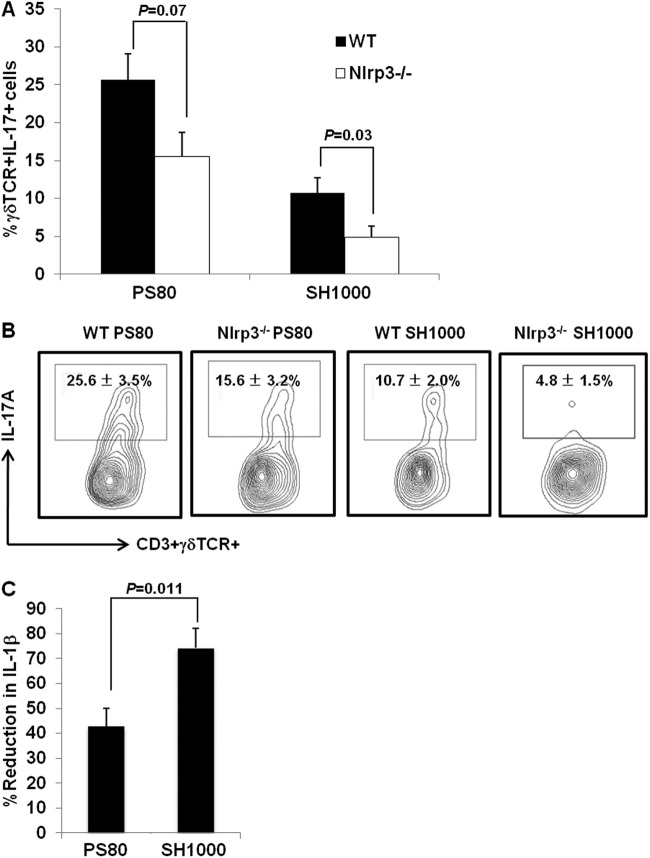
Overall, the proportions of CD3+ γδTCR+ cells present at the wound infection site were comparable in WT and Nlrp3−/− mice on day 3 postinfection with either strain (see Fig. S4 in the supplemental material), suggesting that activation of the Nlrp3 inflammasome does not regulate γδT-cell recruitment to the site of S. aureus infection. However, IL-17 production by these γδT cells does appear to be dependent upon Nlrp3 signaling. Following infection with SH1000, there was a significant (P = 0.03) reduction in the proportions of CD3+ γδTCR+ IL-17+ T cells present at the wound site in Nlrp3−/− mice compared to WT mice, with only background levels of IL-17 staining detected (Fig. 6A and B). Although the proportions of CD3+ γδTCR+ IL-17+ cells were also reduced in the wounds of Nlrp3−/− mice infected with PS80 compared to WT mice, this reduction was not statistically significant (P = 0.07) (Fig. 6A and B). These data suggest that PS80 is capable of activating alternative non-Nlrp3-dependent pathways, which can contribute to IL-1-driven activation of IL-17 production by γδT cells.
Fig 6.
S. aureus-induced IL-1β production is partially Nlrp3 dependent. S. aureus surgical wounds were established in WT and Nlrp3−/− mice with either S. aureus strain PS80 (102 CFU) or SH1000 (102 CFU). (A) Wound tissues were excised on day 3 postinfection. CD3+ γδTCR+ T cells infiltrating the wound tissue were assessed for their capacity to produce IL-17A. (B) Results are expressed as the means ± SEM from 7 individual mice with representative FACS plots shown. BMDCs were isolated from WT and Nlrp3−/− mice and infected with S. aureus strain PS80 or SH1000 at an MOI of 100 for 6 h. IL-1β levels in the supernatant were quantified by ELISA. Results are expressed as percent reduction in IL-1β production in the absence of Nlrp3 (means ± SEM; n = 8 individual experiments).
To investigate Nlrp3-driven IL-1β production in response to individual S. aureus strains, BMDCs were isolated from WT and Nlrp3−/− mice and infected with S. aureus strain SH1000 or PS80 for 6 h. IL-1β production was reduced in Nlrp3−/− BMDCs following infection with both strains of S. aureus; however, the percentage of reduction in IL-1β production in the absence of Nlrp3 was significantly greater following infection with SH1000 than with PS80 (P = 0.011) (Fig. 6C). These data suggest that SH1000 has a significant dependency on Nlrp3 for IL-1β production (IL-1β levels were reduced by 75% in the absence of Nlrp3). PS80, on the other hand, only partially relies on Nlrp3 for IL-1β production. TNF-α and IL-23 production by WT and Nlrp3−/− BMDCs were comparable following infection with either strain (data not shown).
Activation of Nlrp3 and IL-R signaling pathways are required to control S. aureus SSI.
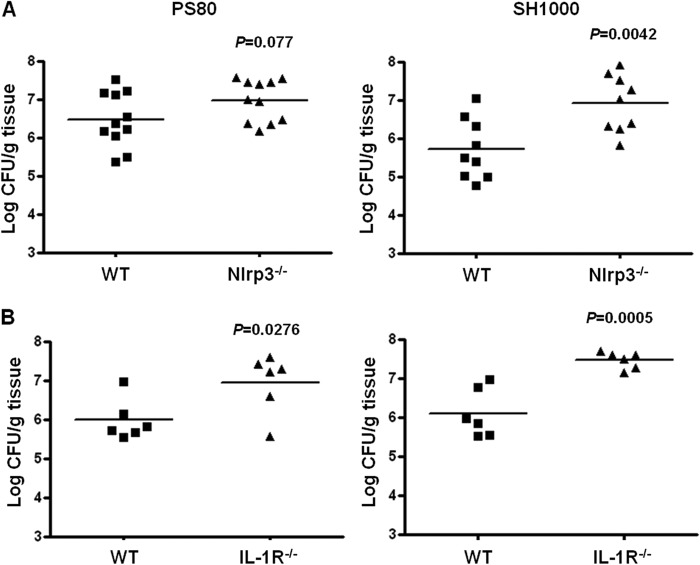
To determine if the reduction in IL-17 production by γδT cells in the absence of Nlrp3 had any effect on infection outcomes, we evaluated bacterial burden at the wound site in WT and Nlrp3−/− mice on day 3 postinfection with SH1000 or PS80. Wound tissue bacterial burden was significantly (P = 0.0042) elevated in Nlrp3−/− mice compared to WT mice infected with SH1000 (Fig. 7A). Although there was an ∼0.7 log increase in bacterial burden in the Nlrp3−/− mice compared to WT mice following infection with PS80, this difference did not reach statistical significance (Fig. 7A). This was likely because appreciable levels of IL-17 were still being produced by γδT cells in these animals (Fig. 6).
Fig 7.
Nlrp3 and IL-1R signaling are required to control bacterial burden during S. aureus SSI. Surgical wounds were established in WT and Nlrp3−/− (A) or IL-1R−/− (B) mice. Wounds were infected with either S. aureus strain PS80 (102 CFU) or S. aureus strain SH1000 (102 CFU). Wound tissues were excised on day 3 postinfection to determine total tissue bacterial burden. Results are expressed as log CFU per g of tissue, and medians are indicated by bars (n = 5 to 11 mice per group).
We then investigated infection outcomes in the absence of IL-1R signaling. S. aureus surgical wound infection was compared in WT and IL-1R−/− mice following infection with S. aureus strain SH1000 or PS80. Wound tissue bacterial burden was significantly increased in IL-1R−/− mice on day 3 following infection with both strains of S. aureus (Fig. 7B). Taken together, these results suggest that IL-1R signaling is critical for containing S. aureus SSI regardless of the infecting strain. In the case of SH1000, Nlrp3-driven IL-1β production is crucial for controlling infection. However, in the case of PS80 infection, the Nlrp3 signaling pathway is only partially required.
DISCUSSION
In this study, we identify γδT cells as the primary source of IL-17 during S. aureus SSI. At the surgical wound site, γδT cells are activated indirectly in response to IL-1β production by APCs. Our data also demonstrate for the first time the importance of Nlrp3 signaling in controlling S. aureus SSI outcomes through a mechanism that depends upon Nlrp3-driven activation of IL-17 production by γδT cells. Given that IL-17 has been identified as an important correlate of immune protection during S. aureus infection (13), it is vital that the unique cellular sources of this cytokine and mechanisms inducing its activation are identified at distinct sites of infection. Our data support recent murine model studies that suggest γδT cells are a more important source of IL-17 during S. aureus infection than Th17 cells (21, 22). Furthermore, our study demonstrates that while IL-17 may be critically important for mediating immune protection during S. aureus infection through this cytokine's well-documented ability to modulate downstream phagocytic cell functions (42), the relative contribution of γδT cells to these protective effects may be critically dependent upon the capacities of individual strains to activate IL-1β production.
We previously identified a novel role for αβTCR+ T cells in regulating neutrophil trafficking and subsequent infection clearance during S. aureus SSI (33). To expand upon these studies, we wanted to explore a role for γδT cells in this model, because to date, the role played by γδT cells in protection against S. aureus infections on nonmucosal surfaces has not been described. γδT cells are rapidly recruited to the S. aureus-infected wound site and are the predominant source of IL-17 at this site. These γδT cells were not producing IFN-γ or IL-17F at any of the time points tested. Previously we demonstrated that IFN-γ is produced very early during S. aureus surgical wound infection. Whole-tissue cytokine levels were measured by ELISA, and IFN-γ was detected as early as 6 h postinfection (32); in contrast, when we measured whole-tissue cytokine levels of IL-17, we detected peak IL-17 levels at 48 h postinfection (271 ± 47, 309 ± 57, and 614 ± 307 IL-17 pg/g tissue at 6, 24, and 48 h, respectively). Consistent with this, our intracellular cytokine staining demonstrated that IL-17 production by γδT cells at the wound infection site is maximal at 3 days postinfection. Further studies are required to ascertain the relative contribution of IFN-γ and IL-17 to immune protection during S. aureus SSI and to establish if these cytokines can regulate one another by either paracrine or autocrine mechanisms.
Having identified γδT cells as the source of IL-17 at the S. aureus surgical wound infection site and given the previously documented role for IL-17 in controlling S. aureus infection (21), we predicted that δTCR−/− mice would have an impaired ability to control wound infection compared to that of WT mice. We observed a significant increase in tissue bacterial burden at the wound site in the δTCR−/− mice compared to WT mice following infection with S. aureus strain SH1000, demonstrating for the first time that γδT cells are required to control S. aureus infection during invasive (nonmucosal) infections. To our surprise, however, bacterial burden at the wound site was similar in WT and δTCR−/− mice when infection was established with S. aureus strain PS80. Importantly, IL-17R−/− mice had an impaired ability to control wound infection with either strain of S. aureus, demonstrating the absolute requirement for IL-17 in host protection against S. aureus infection but suggesting that distinct strains of S. aureus have differential abilities to activate γδT cells. Consistent with this, we demonstrated that although both strains showed similar virulence in the wound infection model, IL-17 production by γδT cells was significantly enhanced following infection with PS80 compared to SH1000. These data suggest that the two strains provide distinct or disproportionate signals to γδT cells.
Differential activation of γδT cells by S. aureus strains PS80 and SH1000 can be explained by the distinct capacities of these strains to activate IL-1β and also, potentially, IL-1α production by APCs. While PS80 and SH1000 induced comparable levels of IL-23, TNF-α, and IL-6 production by APCs, PS80 resulted in significantly higher levels of IL-1β and IL-1α production by these cells than SH1000. Furthermore, IL-1β production and activity (as assessed by downstream activation of PTX3) at the surgical wound site was significantly greater following infection with PS80 than with SH1000. When we stimulated purified γδT cells with cell culture supernatants collected from S. aureus-infected BMDCs, culture supernatants from PS80-treated cells induced significantly more IL-17 production by γδT cells than culture supernatants from SH1000-infected BMDCs. Direct stimulation of γδT cells with either bacterial strain did not result in any IL-17 production (data not shown). Importantly, IL-17 production by γδT cells was completely abolished in the absence of IL-1R, confirming the absolute requirement for IL-1 in controlling IL-17 production by γδT cells during S. aureus infection. Taken together, these results suggest that differences in the abilities of these strains to activate innate signaling pathways involved in driving IL-1β and potentially IL-1α, but not other proinflammatory cytokines, exclusively predicts their abilities to activate IL-17 production by γδT cells. Given the invasive nature of the S. aureus wound infection model, it is likely that IL-1β plays a more predominant role than IL-1α, because previous studies have demonstrated that while IL-1α and IL-1β are both important for controlling infection outcomes during superficial skin infections, IL-1β plays a more dominant role during deeper, more invasive infections (25).
Activation of IL-1β processing during S. aureus infection is primarily mediated through the Nlrp3 inflammasome complex (26, 29, 31). Therefore, we sought to establish if Nlrp3-mediated signaling could control activation of IL-17 production by γδT cells during S. aureus infection. In the absence of Nlrp3, IL-17 production by γδT cells was significantly reduced following infection with SH1000, with only background levels of IL-17 staining detected. Similarly, when we challenged Nlrp3−/− mice with PS80, IL-17 production by γδT cells was diminished compared to that of WT mice; however, this reduction was not statistically significant. These data establish for the first time that γδT-cell activation during S. aureus infection is critically dependent on Nlrp3. In addition, it suggests that certain strains of S. aureus are capable of activating IL-1β production (and, subsequently, IL-17 production by γδT cells) through an alternative non-Nlrp3-dependent inflammasome or through non-inflammasome-mediated pathways, which in turn are sufficient to induce some IL-17 production from γδT cells, even in the absence of Nlrp3. Consistent with this, we observed an absolute requirement for Nlrp3 in controlling IL-1β production by APCs following infection with SH1000, but IL-1β production induced in response to PS80 was only partially Nlrp3 dependent.
To establish the consequences of these effects on infection outcomes, surgical wounds were established in groups of Nlrp3−/− and IL-1R−/− mice. We demonstrate a critical requirement for Nlrp3 and IL-1R signaling to control S. aureus SSI. Bacterial burden at the wound site was significantly elevated in IL-1R−/− mice following challenge with either strain of S. aureus. Consistent with this, challenge with SH1000 resulted in a significant increase in tissue bacterial burden in the absence of Nlrp3. Although challenge with PS80 also resulted in elevated bacterial burden, this increase did not reach statistical significance. These data correlate with the fact that Nlrp3−/− mice still had appreciable levels of IL-17-producing γδT cells present at the wound site following infection with PS80.
Taken together, our results demonstrate the absolute requirement for Nlrp3-driven IL-1β in activating IL-17 production by γδT cells, which in turn regulates infection outcomes during S. aureus SSI. It appears, however, that certain strains of S. aureus are capable of activating IL-1β production through alternative Nlrp3-independent pathways, which can activate a level of IL-17 production by γδT cells in the absence of Nlrp3 that may be sufficient to control the infection. In line with this, recent studies have demonstrated the existence of alternative Nlrp3-independent mechanisms for IL-1β processing in microglia following exposure to live S. aureus (43). S. aureus strains PS80 and SH1000 do not differ significantly in terms of their abilities to produce the secreted toxins (Table 2) that have previously been associated with inflammasome activation (29, 30). Significant studies beyond the scope of this paper are required to ascertain the nature of this Nlrp3-independent induction of IL-1β by S. aureus strain PS80. However, preliminary data demonstrate that PS80 activation of IL-1β is caspase-1 dependent, because inhibition of caspase-1 signaling in BMDCs reduced IL-1β production by these cells in response to PS80 infection (data not shown). A recent study speculates on alternative mechanisms of inflammasome activation and suggests that cathepsin B release occurring as a consequence of lysosomal damage can lead to IL-1β processing via caspase-1-dependent pathways (43). This would depend upon strains of S. aureus being capable of phagosomal escape (44, 45). Given that we have previously demonstrated the propensity of PS80 to survive intracellularly within neutrophils at the wound infection site (32), it is tempting to speculate that this results in PS80 escaping the phagolysosome and activating alternative mechanisms of IL-1β processing. A systematic dissection of the bacterial factors involved in Nrlp3-independent induction of IL-1β is warranted.
Table 2.
Cytotoxin production by S. aureus strains
| Cytotoxin | Production in strain: |
|
|---|---|---|
| PS80 | SH1000 | |
| α-Hemolysin | + | + |
| δ-Hemolysin | + | + |
| β-Hemolysin | − | + |
| Panton-Valentine leukocidin | − | − |
Due to their distinct abilities to activate IL-1β processing, S. aureus strains PS80 and SH1000 induced differing degrees of γδT-cell activation, which likely explains the disparate results obtained following infection of δTCR−/− mice with these two strains. The δTCR−/− mice are impaired in their development of T cells bearing TCRδ chains (46); however, it is possible that they still contain subsets of γδT cells that can respond to cytokine stimulation in the absence of TCR engagement or other innate-like lymphocytes that are capable of responding to inflammatory cytokine stimulation. In support of this, we identified a population of CD3+ IL-17+ cells present at the wound site in the δTCR−/− mice during PS80 infection. Furthermore, following i.p. injection of S. aureus PS80, IL-17 release, although significantly reduced compared to that of the WT, was still detectable in δTCR−/− mice. The cellular source of this IL-17 was identified within the peritoneal cavity to be a population of CD3+ CD4− CD8− cells, suggesting that a subset of innate-like lymphocytes is still present in these animals that can respond to IL-1β and produce IL-17. Limitations in recovering sufficient numbers of rare T-cell subsets prevented similar analysis of innate-like lymphocytes at the surgical wound site. Importantly, in the absence of IL-1β signaling, IL-17 production was completely abolished both in the peritonitis model (data not shown) and in the SSI model. These data confirm that a certain level of IL-17 production can still occur in the absence of cells expressing a functional δTCR but that there is an absolute requirement for IL-1β signaling, and they suggest that the δTCR−/− mice still possess “γδT-cell-like” cells that are capable of responding to IL-1β with the production of IL-17.
γδT-cell-based immunotherapies, either through adoptive transfer of ex vivo-activated autologous γδT cells or administration of γδT-cell antigens, which induce in vivo activation of γδT cells, are already showing promise in clinical trials for the treatment of various advanced cancers that are refractory to conventional treatments (47). In the context of an infectious disease, it has been shown that administration of the α-glucans, which are known γδT-cell antigens, attenuated viremia and mortality following West Nile virus infection in mice through enhancement of γδT-cell responses (48). Consistent with other recent findings (21), our studies support the notion that γδT cells are an important target for novel anti-S. aureus immunomodulatory therapies. However, it is important that we interpret all preclinical mouse model data with caution, as our studies demonstrate that distinct strains of S. aureus have differential capacities to activate γδT cells. Taken together, our results highlight the importance of strain selection for all studies investigating cellular immune responses to S. aureus infection and suggest that a disparity of virulence factor expression by distinct strains will influence the mechanisms by which these strains interact with and modulate T-cell activation and thereby affect infection outcomes.
Supplementary Material
ACKNOWLEDGMENTS
This project was funded by a Wellcome Trust RCDF (WT086515MA) to R.M.L.
We thank Colm Cunningham and Carol Murray for their assistance with the PTX3 gene analysis.
Footnotes
Published ahead of print 30 September 2013
Supplemental material for this article may be found at http://dx.doi.org/10.1128/IAI.01026-13.
REFERENCES
- 1.Lowy FD. 1998. Staphylococcus aureus infections. N. Engl. J. Med. 339:520–532 [DOI] [PubMed] [Google Scholar]
- 2.Hidron AI, Edwards JR, Patel J, Horan TC, Sievert DM, Pollock DA, Fridkin SK. 2008. NHSN annual update: antimicrobial-resistant pathogens associated with healthcare-associated infections: annual summary of data reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2006–2007. Infect. Control Hosp. Epidemiol. 29:996–1011 [DOI] [PubMed] [Google Scholar]
- 3.Shukla S, Nixon M, Acharya M, Korim MT, Pandey R. 2009. Incidence of MRSA surgical-site infection in MRSA carriers in an orthopaedic trauma unit. J. Bone Joint Surg. Br. 91:225–228 [DOI] [PubMed] [Google Scholar]
- 4.Spellberg B, Daum R. 2012. Development of a vaccine against Staphylococcus aureus. Semin. Immunopathol. 34:335–348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Korn T, Bettelli E, Oukka M, Kuchroo VK. 2009. IL-17 and Th17 Cells. Annu. Rev. Immunol. 27:485–517 [DOI] [PubMed] [Google Scholar]
- 6.Ye P, Garvey PB, Zhang P, Nelson S, Bagby G, Summer WR, Schwarzenberger P, Shellito JE, Kolls JK. 2001. Interleukin-17 and lung host defense against Klebsiella pneumoniae infection. Am. J. Respir. Cell Mol. Biol. 25:335–340 [DOI] [PubMed] [Google Scholar]
- 7.Shibata K, Yamada H, Hara H, Kishihara K, Yoshikai Y. 2007. Resident Vdelta1+ gammadelta T cells control early infiltration of neutrophils after Escherichia coli infection via IL-17 production. J. Immunol. 178:4466–4472 [DOI] [PubMed] [Google Scholar]
- 8.Kagami S, Rizzo HL, Kurtz SE, Miller LS, Blauvelt A. 2010. IL-23 and IL-17A, but not IL-12 and IL-22, are required for optimal skin host defense against Candida albicans. J. Immunol. 185:5453–5462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Milner JD, Brenchley JM, Laurence A, Freeman AF, Hill BJ, Elias KM, Kanno Y, Spalding C, Elloumi HZ, Paulson ML, Davis J, Hsu A, Asher AI, O'Shea J, Holland SM, Paul WE, Douek DC. 2008. Impaired T(H)17 cell differentiation in subjects with autosomal dominant hyper-IgE syndrome. Nature 452:773–776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ong PY, Ohtake T, Brandt C, Strickland I, Boguniewicz M, Ganz T, Gallo RL, Leung DY. 2002. Endogenous antimicrobial peptides and skin infections in atopic dermatitis. N. Engl. J. Med. 347:1151–1160 [DOI] [PubMed] [Google Scholar]
- 11.Guttman-Yassky E, Lowes MA, Fuentes-Duculan J, Zaba LC, Cardinale I, Nograles KE, Khatcherian A, Novitskaya I, Carucci JA, Bergman R, Krueger JG. 2008. Low expression of the IL-23/Th17 pathway in atopic dermatitis compared to psoriasis. J. Immunol. 181:7420–7427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rigby KM, DeLeo FR. 2012. Neutrophils in innate host defense against Staphylococcus aureus infections. Semin. Immunopathol. 34:237–259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Miller LS, Cho JS. 2011. Immunity against Staphylococcus aureus cutaneous infections. Nat. Rev. Immunol. 11:505–518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Onishi RM, Gaffen SL. 2010. Interleukin-17 and its target genes: mechanisms of interleukin-17 function in disease. Immunology 129:311–321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Korn T, Petermann F. 2012. Development and function of interleukin 17-producing gammadelta T cells. Ann. N. Y. Acad. Sci. 1247:34–45 [DOI] [PubMed] [Google Scholar]
- 16.Sutton CE, Lalor SJ, Sweeney CM, Brereton CF, Lavelle EC, Mills KH. 2009. Interleukin-1 and IL-23 induce innate IL-17 production from gammadelta T cells, amplifying Th17 responses and autoimmunity. Immunity 31:331–341 [DOI] [PubMed] [Google Scholar]
- 17.Michel ML, Keller AC, Paget C, Fujio M, Trottein F, Savage PB, Wong CH, Schneider E, Dy M, Leite-de-Moraes MC. 2007. Identification of an IL-17-producing NK1.1(neg) iNKT cell population involved in airway neutrophilia. J. Exp. Med. 204:995–1001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Takatori H, Kanno Y, Watford WT, Tato CM, Weiss G, Ivanov II, Littman DR, O'Shea JJ. 2009. Lymphoid tissue inducer-like cells are an innate source of IL-17 and IL-22. J. Exp. Med. 206:35–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cua DJ, Tato CM. 2010. Innate IL-17-producing cells: the sentinels of the immune system. Nat. Rev. Immunol. 10:479–489 [DOI] [PubMed] [Google Scholar]
- 20.Molne L, Corthay A, Holmdahl R, Tarkowski A. 2003. Role of gamma/delta T cell receptor-expressing lymphocytes in cutaneous infection caused by Staphylococcus aureus. Clin. Exp. Immunol. 132:209–215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cho JS, Pietras EM, Garcia NC, Ramos RI, Farzam DM, Monroe HR, Magorien JE, Blauvelt A, Kolls JK, Cheung AL, Cheng G, Modlin RL, Miller LS. 2010. IL-17 is essential for host defense against cutaneous Staphylococcus aureus infection in mice. J. Clin. Investig. 120:1762–1773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cheng P, Liu T, Zhou WY, Zhuang Y, Peng LS, Zhang JY, Mao XH, Guo G, Shi Y, Zou QM. 2012. Role of gamma-delta T cells in host response against Staphylococcus aureus-induced pneumonia. BMC Immunol. 13:38. 10.1186/1471-2172-13-38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miller LS, O'Connell RM, Gutierrez MA, Pietras EM, Shahangian A, Gross CE, Thirumala A, Cheung AL, Cheng G, Modlin RL. 2006. MyD88 mediates neutrophil recruitment initiated by IL-1R but not TLR2 activation in immunity against Staphylococcus aureus. Immunity 24:79–91 [DOI] [PubMed] [Google Scholar]
- 24.Cho JS, Guo Y, Ramos RI, Hebroni F, Plaisier SB, Xuan C, Granick JL, Matsushima H, Takashima A, Iwakura Y, Cheung AL, Cheng G, Lee DJ, Simon SI, Miller LS. 2012. Neutrophil-derived IL-1beta is sufficient for abscess formation in immunity against Staphylococcus aureus in mice. PLoS Pathog. 8:e1003047. 10.1371/journal.ppat.1003047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cho JS, Zussman J, Donegan NP, Ramos RI, Garcia NC, Uslan DZ, Iwakura Y, Simon SI, Cheung AL, Modlin RL, Kim J, Miller LS. 2011. Noninvasive in vivo imaging to evaluate immune responses and antimicrobial therapy against Staphylococcus aureus and USA300 MRSA skin infections. J. Investig. Dermatol. 131:907–915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Miller LS, Pietras EM, Uricchio LH, Hirano K, Rao S, Lin H, O'Connell RM, Iwakura Y, Cheung AL, Cheng G, Modlin RL. 2007. Inflammasome-mediated production of IL-1beta is required for neutrophil recruitment against Staphylococcus aureus in vivo. J. Immunol. 179:6933–6942 [DOI] [PubMed] [Google Scholar]
- 27.Franchi L, Eigenbrod T, Munoz-Planillo R, Nunez G. 2009. The inflammasome: a caspase-1-activation platform that regulates immune responses and disease pathogenesis. Nat. Immunol. 10:241–247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bryant C, Fitzgerald KA. 2009. Molecular mechanisms involved in inflammasome activation. Trends Cell Biol. 19:455–464 [DOI] [PubMed] [Google Scholar]
- 29.Craven RR, Gao X, Allen IC, Gris D, Bubeck Wardenburg J, McElvania-Tekippe E, Ting JP, Duncan JA. 2009. Staphylococcus aureus alpha-hemolysin activates the NLRP3-inflammasome in human and mouse monocytic cells. PLoS One 4:e7446. 10.1371/journal.pone.0007446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Munoz-Planillo R, Franchi L, Miller LS, Nunez G. 2009. A critical role for hemolysins and bacterial lipoproteins in Staphylococcus aureus-induced activation of the Nlrp3 inflammasome. J. Immunol. 183:3942–3948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shimada T, Park BG, Wolf AJ, Brikos C, Goodridge HS, Becker CA, Reyes CN, Miao EA, Aderem A, Gotz F, Liu GY, Underhill DM. 2010. Staphylococcus aureus evades lysozyme-based peptidoglycan digestion that links phagocytosis, inflammasome activation, and IL-1beta secretion. Cell Host Microbe 7:38–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McLoughlin RM, Lee JC, Kasper DL, Tzianabos AO. 2008. IFN-gamma regulated chemokine production determines the outcome of Staphylococcus aureus infection. J. Immunol. 181:1323–1332 [DOI] [PubMed] [Google Scholar]
- 33.McLoughlin RM, Solinga Rich J, Zaleski KJ, Cocchiaro JL, Risley A, Tzianabos AO, Lee JC. 2006. CD4+ T cells and CXC chemokines modulate the pathogenesis of Staphylococcus aureus wound infections. Proc. Natl. Acad. Sci. U. S. A. 103:10408–10413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tzianabos AO, Wang JY, Lee JC. 2001. Structural rationale for the modulation of abscess formation by Staphylococcus aureus capsular polysaccharides. Proc. Natl. Acad. Sci. U. S. A. 98:9365–9370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wann ER, Dassy B, Fournier JM, Foster TJ. 1999. Genetic analysis of the cap5 locus of Staphylococcus aureus. FEMS Microbiol. Lett. 170:97–103 [DOI] [PubMed] [Google Scholar]
- 36.Horsburgh MJ, Aish JL, White IJ, Shaw L, Lithgow JK, Foster SJ. 2002. SigmaB modulates virulence determinant expression and stress resistance: characterization of a functional rsbU strain derived from Staphylococcus aureus 8325-4. J. Bacteriol. 184:5457–5467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Skelly DT, Hennessy E, Dansereau MA, Cunningham C. 2013. A systematic analysis of the peripheral and CNS effects of systemic LPS, IL-1Beta, TNF-alpha and IL-6 challenges in C57BL/6 mice. PLoS One 8:e69123. 10.1371/journal.pone.0069123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Higgins SC, Lavelle EC, McCann C, Keogh B, McNeela E, Byrne P, O'Gorman B, Jarnicki A, McGuirk P, Mills KH. 2003. Toll-like receptor 4-mediated innate IL-10 activates antigen-specific regulatory T cells and confers resistance to Bordetella pertussis by inhibiting inflammatory pathology. J. Immunol. 171:3119–3127 [DOI] [PubMed] [Google Scholar]
- 39.Lee JC, Greenwich JL, Zhanel GG, Han X, Cumming A, Saward L, McLoughlin RM. 2010. Modulation of the local neutrophil response by a novel hyaluronic acid-binding peptide reduces bacterial burden during staphylococcal wound infection. Infect. Immun. 78:4176–4186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Field R, Campion S, Warren C, Murray C, Cunningham C. 2010. Systemic challenge with the TLR3 agonist poly I:C induces amplified IFNalpha/beta and IL-1beta responses in the diseased brain and exacerbates chronic neurodegeneration. Brain Behav. Immun. 24:996–1007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lalor SJ, Dungan LS, Sutton CE, Basdeo SA, Fletcher JM, Mills KH. 2011. Caspase-1-processed cytokines IL-1beta and IL-18 promote IL-17 production by gammadelta and CD4 T cells that mediate autoimmunity. J. Immunol. 186:5738–5748 [DOI] [PubMed] [Google Scholar]
- 42.Xu S, Cao X. 2010. Interleukin-17 and its expanding biological functions. Cell Mol. Immunol. 7:164–174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hanamsagar R, Torres V, Kielian T. 2011. Inflammasome activation and IL-1beta/IL-18 processing are influenced by distinct pathways in microglia. J. Neurochem. 119:736–748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gresham HD, Lowrance JH, Caver TE, Wilson BS, Cheung AL, Lindberg FP. 2000. Survival of Staphylococcus aureus inside neutrophils contributes to infection. J. Immunol. 164:3713–3722 [DOI] [PubMed] [Google Scholar]
- 45.Kubica M, Guzik K, Koziel J, Zarebski M, Richter W, Gajkowska B, Golda A, Maciag-Gudowska A, Brix K, Shaw L, Foster T, Potempa J. 2008. A potential new pathway for Staphylococcus aureus dissemination: the silent survival of S. aureus phagocytosed by human monocyte-derived macrophages. PLoS One 3:e1409. 10.1371/journal.pone.0001409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Itohara S, Mombaerts P, Lafaille J, Iacomini J, Nelson A, Clarke AR, Hooper ML, Farr A, Tonegawa S. 1993. T cell receptor delta gene mutant mice: independent generation of alpha beta T cells and programmed rearrangements of gamma delta TCR genes. Cell 72:337–348 [DOI] [PubMed] [Google Scholar]
- 47.Gomes AQ, Martins DS, Silva-Santos B. 2010. Targeting gammadelta T lymphocytes for cancer immunotherapy: from novel mechanistic insight to clinical application. Cancer Res. 70:10024–10027 [DOI] [PubMed] [Google Scholar]
- 48.Wang S, Welte T, Fang H, Chang GJ, Born WK, O'Brien RL, Sun B, Fujii H, Kosuna K, Wang T. 2009. Oral administration of active hexose correlated compound enhances host resistance to West Nile encephalitis in mice. J. Nutr. 139:598–602 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.