Abstract
Streptococcus suis serotype 2 is an important swine bacterial pathogen, and it is also an emerging zoonotic agent. It is unknown how S. suis virulent strains, which are usually found in low quantities in pig tonsils, manage to cross the first host defense lines to initiate systemic disease. Influenza virus produces a contagious infection in pigs which is frequently complicated by bacterial coinfections, leading to significant economic impacts. In this study, the effect of a preceding swine influenza H1N1 virus (swH1N1) infection of swine tracheal epithelial cells (NTPr) on the ability of S. suis serotype 2 to adhere to, invade, and activate these cells was evaluated. Cells preinfected with swH1N1 showed bacterial adhesion and invasion levels that were increased more than 100-fold compared to those of normal cells. Inhibition studies confirmed that the capsular sialic acid moiety is responsible for the binding to virus-infected cell surfaces. Also, preincubation of S. suis with swH1N1 significantly increased bacterial adhesion to/invasion of epithelial cells, suggesting that S. suis also uses swH1N1 as a vehicle to invade epithelial cells when the two infections occur simultaneously. Influenza virus infection may facilitate the transient passage of S. suis at the respiratory tract to reach the bloodstream and cause bacteremia and septicemia. S. suis may also increase the local inflammation at the respiratory tract during influenza infection, as suggested by an exacerbated expression of proinflammatory mediators in coinfected cells. These results give new insight into the complex interactions between influenza virus and S. suis in a coinfection model.
INTRODUCTION
Streptococcus suis is one of the most important postweaning bacterial pathogens in swine, and it is also an emerging zoonotic agent (1). Among the 35 described S. suis serotypes, type 2 is the most virulent one for both pigs and humans (2), although differences in virulence have been described for this serotype (3). Pigs may acquire S. suis very early in life, and some colonized animals may never develop disease (carrier animals); on the other hand, some carrier piglets will eventually develop bacteremia, septicemia, and meningitis following dissemination of S. suis in the bloodstream (1). Human infections with S. suis manifest mainly as meningitis, septicemia, and septic shock (4). It is believed that people can become infected through skin lesions, surface mucosa, and/or the oral route (5).
It is still unknown how low quantities of S. suis virulent serotype 2 strains present in tonsils of pigs manages to cross the first natural line of the host defense to initiate disease. It is believed that the pathogen would breach the mucosal epithelium at the upper respiratory tract (6). Bacterial adhesion and invasion of epithelial cells are usually associated with the first steps of colonization by mucosal pathogens; however, few data are available concerning the interaction between S. suis and swine respiratory epithelial cells. Ferrando and colleagues described for the first time S. suis adhesion to (but not invasion of) porcine tracheal epithelial cells (7).
The S. suis capsular polysaccharide (CPS), which defines the serotype, is essential for the virulence of this pathogen mainly due to its antiphagocytic activity (6). The analysis of the serotype 2 CPS revealed the presence of different sugars, including Neu5Ac and sialic acid. Interestingly, sialic acid was found to be terminal [(2→6)-β-d-galactose], and the CPS can be quantitatively desialylated by mild acid hydrolysis (8). It has been shown previously that expression of CPS interferes with adhesion to and (if any) invasion of epithelial cells by S. suis (9, 10). Classically, the role of this virulence factor has been suggested to be crucial once bacteria reach the bloodstream (6). Among other suggested S. suis virulence factors are secreted proteins, such as the hemolysin (suilysin), surface proteins, and other cell wall components (11).
Secondary bacterial infections associated with influenza virus infection in humans are a leading cause of human morbidity and mortality worldwide (12). Swine influenza virus infections in pigs also cause serious respiratory disease (13). Although this infection is typically self-limited with high morbidity but low mortality, secondary complications substantially increase illness and death (14). In fact, influenza virus is a key contributor to the porcine respiratory disease complex (PRDC), a multifactorial syndrome characterized by severe respiratory disease after infection with two or more agents (15). Pathogens associated with PRDC include (among others) Actinobacillus pleuropneumoniae, Haemophilus parasuis, Mycoplasma hyopneumoniae, S. suis, and porcine reproductive and respiratory syndrome virus (15). Subtypes of swine influenza virus that are most frequently identified in pigs include H1N1 (classical and pandemic), H1N2, and H3N2 (13). Influenza virus strains uniformly recognize cell surface oligosaccharides with a terminal sialic acid, either 2,3 Neu5Ac-galactose or 2,6 Neu5Ac-galactose. However, their receptor specificity varies according to host. Pigs are unique among influenza virus hosts, in that they are susceptible to infection with influenza viruses of human and avian origin as well as to swine influenza virus, because their tracheal epithelium contains these two sialyloligosaccharides (16).
In this study, we demonstrated, for the first time, a novel mechanism used by a bacterial species to facilitate the invasion of respiratory epithelial cells already infected with an influenza virus. More specifically, we showed that the sialic acid moiety present in the CPS of S. suis serotype 2 directly interacts with swine influenza virus, leading to increased bacterial adhesion to, invasion of, and activation of tracheal epithelial cells. This mechanism could explain, at least in part, how secondary bacterial infection with a virulent S. suis strain could be enhanced following influenza virus infection.
MATERIALS AND METHODS
Bacterial strains, epithelial cells, and influenza virus strain.
S. suis strains used in this study are listed in Table 1. The well-characterized S. suis serotype 2 virulent strain 31533 (10, 17) was used throughout this study. Other previously well-characterized isogenic mutants derived from this strain and devoid of either CPS or suilysin production, or modified at either the peptidoglycan (PG) or lipoteichoic acid (LTA) level, were also included (18–21). In addition, serotype 2 field strains with lower (Canadian strain) or higher (epidemic strain isolated from a deadly S. suis human outbreak in China) virulence potential (3), as well as reference strains of serotypes 3 and 14, were also included for comparison purposes (Table 1). A swine H1N1 influenza virus (swH1N1; strain A/swine/St-Hyacinthe/148/1990) isolated from a case of swine flu in Canada was used (22).
Table 1.
List of Streptococcus suis strains used in this study
| Strain | Relevant phenotype and/or description | Reference |
|---|---|---|
| 31533 | Serotype 2 highly pathogenic European strain isolated from a diseased pig | 10 |
| SC84 | Serotype 2 epidemic virulent strain isolated from a human outbreak in China | 3 |
| 89–1591 | Serotype 2 intermediate virulent strain isolated from a disease pig in Canada | 3 |
| CPS− | Nonencapsulated B218 mutant strain derived from strain 31533 | 19 |
| ΔSly | Suilysin-negative SX911 mutant strain derived from strain 31533 | 18 |
| ΔdLTA | D-alanylation of LTA mutant strain derived from strain 31533 | 20 |
| ΔpgdA | N-deacetylation of peptidoglycan mutant strain derived from strain 31533 | 21 |
| 4961 | Reference strain, serotype 3, isolated from a diseased pig | 57 |
| DAN13730 | Reference strain, serotype 14 isolated from a diseased human | 57 |
| S735 | Reference strain, serotype 2, isolated from a diseased pig | 8 |
Bacteria were cultured as previously reported (17). The number of CFU/ml in the final suspension before each experiment was determined by plating samples onto Todd-Hewitt agar (THA) using an Autoplate 4000 automated spiral plater (Spiral Biotech, Norwood, MA). The pig trachea epithelial cell line NPTr was used for virus growth and coinfection studies as described previously (23). For assays, cells were treated with 0.05% trypsin in 0.03% EDTA solution and diluted in culture medium to obtain a final concentration of 105 cells/ml. The cell suspension then was distributed into tissue culture plates and incubated until cell confluence was reached. Twenty-four hours before the assays, culture medium was removed from the wells and replaced by fresh complete medium without antibiotics. Virus was produced by replication in NPTr cells as previously described (23). The titer of the viral production was 107.25 50% tissue culture infectious doses (TCID50)/ml.
NPTr coinfection by swH1N1 and S. suis.
swH1N1 (multiplicity of infection [MOI], 1) was inoculated onto NPTr cell monolayers in 24-well culture plates and incubated with 2% fetal bovine serum (FBS) (as standardized in preliminary experiments) and antibiotic-free minimal essential medium (MEM; Invitrogen, Burlington, Ontario, Canada) for 1 h at 37°C in 5% CO2. The virus-infected cells were then washed twice with phosphate-buffered saline (PBS), and fresh media containing 10% FBS without antibiotic was added. The increased serum concentration did not affect virus replication and kept cells healthy for the whole experiment. Following a 12-h incubation at 37°C in 5% CO2, cells were infected with S. suis (106 CFU/well; MOI, 10). Plates were centrifuged at 800 × g for 10 min in order to bring bacteria into close contact with the cells (24). Bacterium-infected cells were then incubated at 37°C in 5% CO2 for different incubation times (see below). The infectious viral load profile was determined in cell cultures for virus-infected cells and for virus-bacterium-coinfected cells by virus titration evaluation as described above. Cell cytotoxicity levels were determined using a Cytotox 96 kit (Promega, Madison, WI) from culture supernatants according to the manufacturer's instructions. In selected experiments, swH1N1 and S. suis cells were preincubated for 1 h at 4°C (S. suis, 106 CFU; swH1N1, 106 TCID50; final bacterium/virus ratio of 1). Afterwards, the virus-S. suis mixture was washed twice with PBS and resuspended with complete medium, inoculated into cells, and incubated at 37°C in 5% CO2 for bacterial adhesion and invasion assays as described below. Mock-treated bacteria were used as a control.
The invasion assay was performed as previously described (17), with some modifications. After 2 or 4 h of incubation with S. suis, the NPTr cell monolayers were washed twice with PBS, and 1 ml of cell culture medium containing 100 μg of gentamicin and 5 μg of penicillin G (Invitrogen) was added to each well. The plates were then further incubated for 1 h at 37°C with 5% CO2 to kill extracellular and surface-adherent bacteria. After washing, cells were disrupted with sterile ice-cold deionized water followed by cell scraping from the bottom of the well in order to liberate intracellular bacteria. Bacterial CFU numbers were determined by plating serial dilutions as described above. Levels of invasion were expressed as the total number of CFU recovered per well. A so-called adhesion assay, which in fact quantifies total cell-associated bacteria (intracellular bacteria and surface-adherent bacteria), was performed similarly to the invasion assay. However, the cells were vigorously washed five times to eliminate nonspecific bacterial attachment, and no antibiotic treatment to kill the extracellular bacteria was used. At different incubation times (see Results), the levels of adhesion (total associated bacteria) were expressed as the total number of CFU recovered per well.
For the inhibition studies, after removing the cell supernatant and twice washing the wells with fresh PBS, 100 μg of purified native CPS or desialylated CPS (prepared as described below) resuspended in cell culture medium was added to swH1N1-infected cells. Control cells were treated similarly but without addition of CPS. After 1 h of incubation, cells were washed twice with PBS and infected with S. suis as previously described. Bacterial adhesion and invasion studies were performed as described above and compared to nontreated cells. Results were expressed in percentages compared to bacterial adhesion and invasion of untreated swH1N1-preinfected cells (considered 100%). Swine polyclonal antibody serum against the whole swH1N1 virus strain (serum from a convalescent animal) was used as a positive inhibition control. Supernatants of swH1N1-infected cells were removed, and the serum (diluted 1/40 in cell culture medium) was added to the wells.
HI assay.
A hemagglutination inhibition (HI) test was carried out as previously described (25), with the modification that swine sera were replaced by different concentrations of S. suis. Serial dilutions of S. suis strains (wild-type strain 31533 or nonencapsulated B218 mutant strain) were used for the HI assay. Briefly, 50-μl bacterial suspensions (grown as described above) were dispensed at different concentrations in triplicate in a 96-well round-bottom plate. Fifty μl of swH1N1 (2 × 106.25 TCID50/ml) was then added to each well and incubated for 1 h at room temperature. Different wells represented a 2-fold dilution of S. suis/swH1N1 virus ratios, beginning at a ratio of 200 for the wild-type encapsulated strain and 10,000 for the nonencapsulated B218 mutant. Afterward, 50 μl of a 0.5% suspension of rooster whole red blood cells (RBC) in PBS was added to each well and gently mixed. The HI test was evaluated after incubating the plate at room temperature for 1 h. For this experiment, PBS was used as a negative RBC control, and serial dilutions of reference heat-inactivated anti-swH1N1 serum were used as a positive HI control. Under the conditions tested, capsulated and nonencapsulated S. suis strains did not induce any hemagglutination (results not shown).
S. suis CPS purification and CPS desialylation.
The CPS of S. suis serotype 2 reference strain S735 was prepared and purified as previously described (8). For quality controls, CPS was analyzed by nuclear magnetic resonance. Lack of protein and RNA/DNA contamination was verified by the Lowry method and by spectrophotometry, respectively. CPS was also desialylated by mild acid hydrolysis. CPS (8 mg) was heated in 1 ml of HCl (70 mM) at 60°C for 4 h, neutralized with NH4OH (2 M), and purified on a Sephadex G10 column (1.5 by 10 cm). The presence (native CPS) or absence (desialylated CPS) of sialic acid was verified by gas chromatography after methanolysis and acetylation and by nuclear magnetic resonance, as well as by a reaction with an enzyme-linked lectin assay as previously described (26).
Confocal and electron microscopy.
For confocal microscopy analysis, cells were placed on coverslips and infected (or not) with swH1N1 and either S. suis strain 31533 or its nonencapsulated mutant strain (B218) as described above and further incubated for 2 h at 37°C in 5% CO2. Coverslips were washed with PBS to remove nonassociated bacteria, and cells were fixed with 4% paraformaldehyde solution for 10 min. Cells were then washed and permeabilized with PBS containing 0.2% Triton X-100 (Thermo HyClone, Burlington, Ontario, Canada) for 2 min. The coverslips were blocked for 10 min with PBS containing 2% bovine serum albumin and 0.2% gelatin (Sigma-Aldrich, Oakville, Ontario, Canada). Coverslips were then incubated for 1 h with a mouse monoclonal antibody against an epitope within influenza virus A nucleoprotein H1N1 (1/500 dilution; US Biological, Swampscott, MA) and a rabbit anti-S. suis serum against either wild-type strain 31533 (1/5,000) or its nonencapsulated B218 mutant strain (1/1,000) (27). After washing with PBS, coverslips were incubated with the secondary antibodies Alexa Fluor 568 goat anti-mouse IgG (for swH1N1) and Alex-Fluor 488 goat anti-rabbit IgG (for S. suis) (both from Invitrogen) for 30 min. Coverslips were then washed and mounted on glass slides with Mowiol containing Dabco.
For transmission electron microscopy (TEM) and scanning electron microscopy (SEM), samples were fixed for 1 h at room temperature with 2% (vol/vol) glutaraldehyde in 0.1 M cacodylate buffer (pH 7.3) and then were postfixed for 45 min at room temperature with 2% osmium tetroxide. Specimens for TEM were dehydrated in a graded series of ethanol solutions and embedded with LR white resin. Thin sections were cut with a diamond knife and poststained with uranyl acetate and lead citrate. Samples were observed with an electron microscope (model JEM-1230; JEOL, Tokyo, Japan). Samples for SEM were dehydrated in a graded series of ethanol solutions and covered with gold after critical point drying and were examined with a Hitachi S-3000N microscope.
qRT-PCR for cytokine and chemokine expression.
Quantitative RT-PCR (qRT-PCR) assays were performed as previously described (28). Primers (IDT DNA, Coralville, IA) used for detection of genes all were verified to have PCR amplification efficiency ranked between 90 and 110% using a CFX96 rapid thermal cycler system (Bio-Rad, Hercules, CA) (Table 2). The GeNorm applet, v.3.5 (http://medgen.ugent.be/∼jvdesomp/genorm/), was used to initially determine the two most stable reference genes from a set of six reference genes using random samples from the cDNA panel generated for the quantitative PCR (qPCR) analysis of cytokine/chemokine gene expression. Therefore, normalization of data was done using the reference genes hypoxanthine phosphoribosyltransferase 1 (Hprt1) and peptidylprolyl isomerase A (Ppia). Fold change of gene expression was calculated using the normalized gene expression (ΔΔCq) calculation method of the CFX software manager (v.2.1; Bio-Rad). Mock-infected samples were used as the calibrator, and consequently the relative fold differences were calculated for the rest of the samples compared to the means from the calibrator samples.
Table 2.
Sequences of porcine-specific real-time PCR primers
| Gene | GenBank accession no. | Amplicon size (bp) | Sequence |
% efficiency (qPCR) | |
|---|---|---|---|---|---|
| Forward | Reverse | ||||
| Hprt1 | NM_001032376 | 142 | GCAGCCCCAGCGTCGTGATT | CGAGCAAGCCGTTCAGTCCTGT | 99 |
| Ppia | NM_214353 | 133 | TGCAGACAAAGTTCCAAAGACAG | GCCACCAGTGCCATTATGG | 97 |
| Ccl2 | NM_214214 | 169 | CAGGTCCTTGCCCAGCCAGATG | CACAGATCTCCTTGCCCGCGA | 90 |
| Ccl4 | NM_213779 | 125 | TCCCACCTCCTGCTGCTTCACAT | GCCTGCCCTTTTTGGTCTGGAA | 100 |
| Il6 | NM_214399 | 105 | ACTCCCTCTCCACAAGCGCCTT | TGGCATCTTCTTCCAGGCGTCCC | 97 |
| Il8 | NM_003300390 | 80 | TGTGAGGCTGCAGTTCTGGCAAG | GGGTGGAAAGGTGTGGAATGCGT | 95 |
| Ifnβ | NM_001003923.1 | 150 | TGCAACCACCACAATTCCAGAAGG | TCTGCCCATCAAGTTCCACAAGGA | 96 |
| Tnf | NM_214022 | 112 | GCCACCACGCTCTTCTGCCTA | ACGATGATCTGAGTCCTTGGGCCA | 91 |
Statistical analysis.
All data are expressed as means ± standard errors of the means. Prism statistical software (v.5; GraphPad, San Diego, CA) was employed for data analysis. Data from the adhesion and invasion assays were analyzed for significance using Student's unpaired t test. Data from qPCR assays were subjected to one-way analysis of variance (ANOVA), followed by Tukey's post hoc test. A P value of <0.01 was used as the threshold for statistical significance. Results reflect mean values from at least three independent experiments.
RESULTS
S. suis serotype 2 adhesion and invasion are significantly increased when cells are previously infected by swH1N1 independently of the virulence of the S. suis strain.
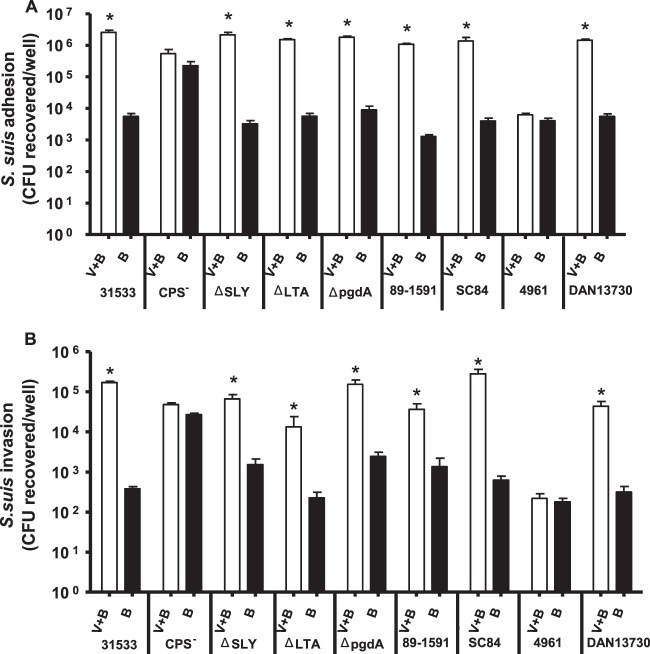
The kinetics of adhesion of the highly virulent S. suis serotype 2 strain 31533 to NPTr cells was studied. As shown in Fig. 1A, in the absence of virus infection, adhesion was time dependent, increasing from 30 min to 4 h of incubation. After 4 h of incubation, a plateau was reached (data not shown). Results of the kinetics and levels of adhesion are similar to those previously obtained with porcine endothelial and other epithelial cells (10, 17). However, when cells were preinfected with swH1N1 for 12 h, the adhesion levels increased more than 100-fold compared to those observed in the absence of virus (Fig. 1A). In addition, adhesion levels immediately reached a plateau (Fig. 1A), even after 5 min of incubation (data not shown). When strains of serotype 2 with lower or higher virulence potential than that of strain 31533 were tested (intermediate virulence Canadian strain 1591 or epidemic strain SC84 from a Chinese human outbreak) (3), bacterial adhesion levels were statistically similar to those obtained with the virulent strain 31533, either in the absence or presence of swH1N1 infection (Fig. 2A).
Fig 1.
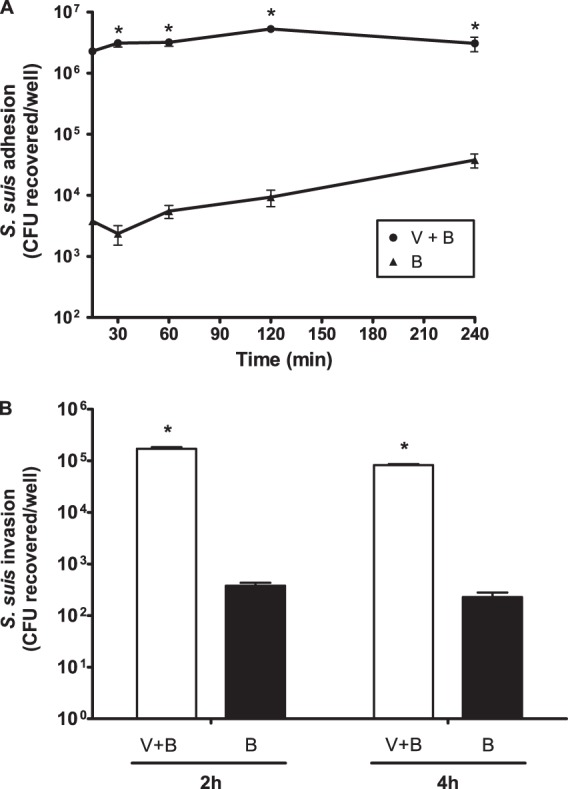
Adhesion to and invasion of virus-free or swH1N1-infected NPTr cells by S. suis serotype 2 strain 31533. NPTr cells were either preinfected with swH1N1 for 12 h (MOI, 1) or left untreated, and cells were subsequently infected with S. suis serotype 2 strain 31533 (MOI, 10). (A) Kinetics of adhesion of S. suis to virus-infected (V+B) or control (B) NPTr cells. After S. suis infection, cells were extensively washed to remove nonadherent bacteria and then lysed to determine S. suis viable counts. (B) S. suis invasion of swH1N1-infected (V+B) or control (B) NPTr cells at bacterial incubation times of 2 and 4 h. Results were determined as described above, except that after washing, cells were exposed to antibiotics to kill extracellular bacteria. Data are expressed as means ± standard errors of the means from at least four independent experiments, each done in triplicate. An asterisk indicates significant differences between samples infected with bacteria alone and those coinfected with virus and bacteria (P < 0.01).
Fig 2.
Adhesion to and invasion of virus-free or swH1N1-infected NPTr cells by different strains of S. suis. NPTr cells were either preinfected with swH1N1 for 12 h (MOI, 1) or left untreated, and subsequently the cells were infected with S. suis strains (MOI, 10). (A) Adhesion (incubation time of 2 h) of different S. suis strains to NPTr cells. Results were determined after exposure of swH1N1-infected (V+B) or control (B) NPTr cells to S. suis, followed by extensive washing of nonadherent bacteria and cell lysis to obtain S. suis viable counts. (B) Invasion (incubation time of 1 h) of swH1N1-infected (V+B) or control (B) NPTr cells by different S. suis strains. Results were determined as described above, except that after washing, cells were exposed to antibiotics to kill extracellular bacteria. Table 1 describes the strains. Data are expressed as means ± standard errors of the means from at least four independent experiments, each done in triplicate. An asterisk indicates significant difference between samples infected with bacteria alone and those coinfected with virus and bacteria (P < 0.01).
Surprisingly, and different from what has been previously reported for other epithelial cells of swine origin (10), encapsulated S. suis serotype 2 clearly was able to invade NPTr cells (Fig. 1B). However, when cells were preinfected with the swH1N1 strain, invasion rates also increased more than 100-fold at both 2 and 4 h of incubation (P < 0.01) (Fig. 1B). Similar to the adhesion results, invasion rates of the two additional S. suis serotype 2 strains were statistically similar to those obtained with strain 31533 in the presence or absence of swH1N1 preinfection (Fig. 2B). For all adhesion and invasion experiments, cells presented cytotoxicity levels of less than 20% (data not shown). Interestingly, virus replication levels in NPTr cells were similar in the presence or absence of bacterial infection (see Fig. S1 in the supplemental material).
Critical role of the CPS in the increased S. suis adhesion to/invasion of swH1N1-preinfected NPTr cells.
Isogenic mutants defective in suilysin production, D-alanylation of LTA, or N-deacetylation of PG behaved statistically similarly to the wild-type strain 31533 either in the presence or absence of swH1N1 preinfection. Only the nonencapsulated (CPS−) mutant presented a different pattern. In the absence of virus infection, the adhesion and invasion levels of the mutant strain were significantly higher (P < 0.01) than those of the wild-type strain (Fig. 2A and B), confirming previously published results which indicated that the CPS interferes with S. suis-host cell interactions (9, 10). However, these adhesion and invasion levels were unmodified after a swH1N1 preinfection. These data suggest that the CPS plays a role in the observed increased levels of wild-type S. suis adhesion to/invasion of virus-infected cells (Fig. 2A and B). Since the antigenic characteristics of the CPS define the serotype (1), two additional S. suis serotypes (3 and 14) were tested. Although both strains are well encapsulated (29), the adhesion and invasion of the S. suis serotype 14 reference strain (DAN13730), but not those of serotype 3 (strain 4961), were significantly affected by preinfection with swH1N1 (Fig. 2A and B). This indicates that the CPS structure and/or composition directly influences the interactions between S. suis and swH1N1-preinfected cells.
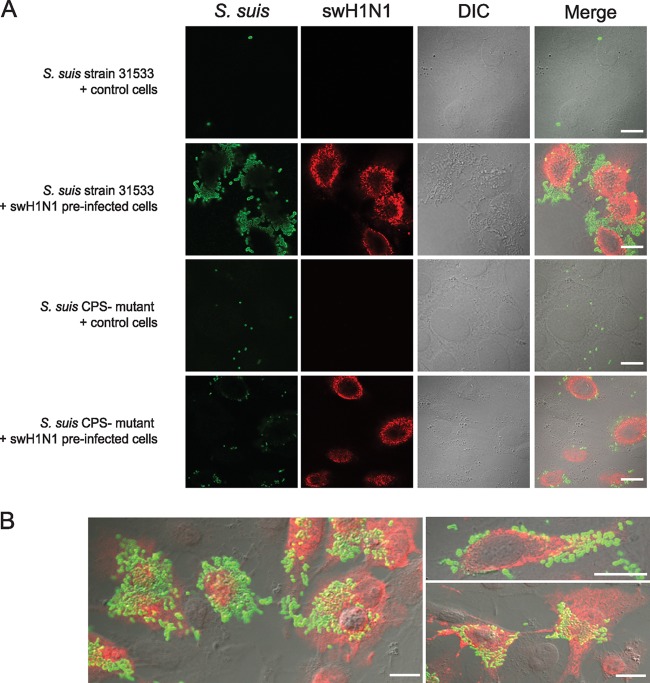
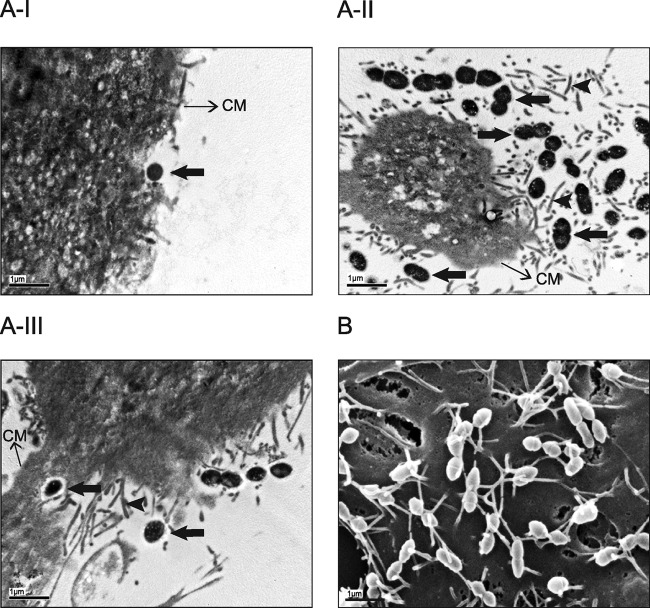
Influence of epithelial cell swH1N1 preinfection on adhesion/invasion abilities of S. suis serotype 2 was confirmed by microscopy. First, confocal microscopy revealed that very few encapsulated wild-type bacteria could be observed interacting with epithelial cells in the absence of virus preinfection (Fig. 3). However, after 12 h of swH1N1 preinfection, levels of wild-type encapsulated S. suis adhesion were clearly higher and grouped around the cells (in grape-like shape), especially where the red staining with anti-H1N1 monoclonal antibody was present, indicating a colocalization of virus and bacteria. In the absence of virus infection, the nonencapsulated mutant showed a higher level of adhesion than the wild-type strain, although bacteria were randomly distributed on the cell surface (diffuse adhesion). A similar adhesion pattern for the mutant strain was observed when cells were preinfected with swH1N1. Electron microscopy (TEM and SEM) confirmed the influence of a preinfection with influenza virus on S. suis-cell interactions (Fig. 4). In the absence of virus infection, very few cocci (if any) could be observed interacting with cells (Fig. 4A-I). In the presence of a virus preinfection, cells were highly activated (clearly showing cilia at their surface), and large numbers of cocci were at the cell surface (closely interacting with cilia) (Fig. 4A-II and 4B) and, sometimes, inside the cells (Fig. 4A-III).
Fig 3.
Confocal microscopy showing association of wild-type encapsulated S. suis serotype 2 strain 31533 and its nonencapsulated mutant (CPS−) with virus-free cells (control) or swH1N1-preinfected NPTr cells. Cells were noninfected (control) or virus infected for 12 h with swH1N1 (MOI, 1) and then infected with either the S. suis wild-type strain or the CPS− mutant strain (MOI, 10) for 2 h. Samples were labeled using polyclonal antibodies conjugated with Alexa Fluor 488 against S. suis (green) and a mouse monoclonal antibody against influenza virus A nucleoprotein H1N1 conjugated with Alexa Fluor 568 against swH1N1 (red). (A) Wild-type S. suis strain 31533 shows a high level of interactions with cells only when they are preinfected with swH1N1. Nonencapsulated S. suis-cell interaction is not altered by preinfection with influenza virus. DIC, differential interference contrast. (B) High level of adhesion to/invasion of swH1N1-preinfected cells by S. suis strain 31533. Scale bar, 10 μm. Original magnification, ×100.
Fig 4.
TEM and SEM showing interactions between S. suis serotype 2 and NTPr cells. (A-I) TEM of S. suis serotype 2 strain 31533 infection of virus-free (control) NPTr cells showing very few cocci at the cell surface. (A-II and A-III) TEM of S. suis strain 31533 infection of swH1N1-preinfected NPTr cells showing high numbers of cocci interacting with epithelial cells (A-II) and intracellular bacteria (A-III). Scale bar, 1 μm. Original magnification, ×5,000. (B) SEM of S. suis serotype 2 strain 31533 infection of swH1N1-preinfected NPTr cells showing high numbers of cocci intimately interacting with cell cilia. Scale bar, 1 μm. Original magnification, ×10,000. No bacteria could be found in the observed SEM fields of control NPTr cells infected with S. suis strain 31533 only (data not shown). Black arrows show bacterial cells, and arrowheads show cilia. CM, cell membrane.
Bacterial capsular sialic acid is responsible for bacterium-virus interactions in infected cells.
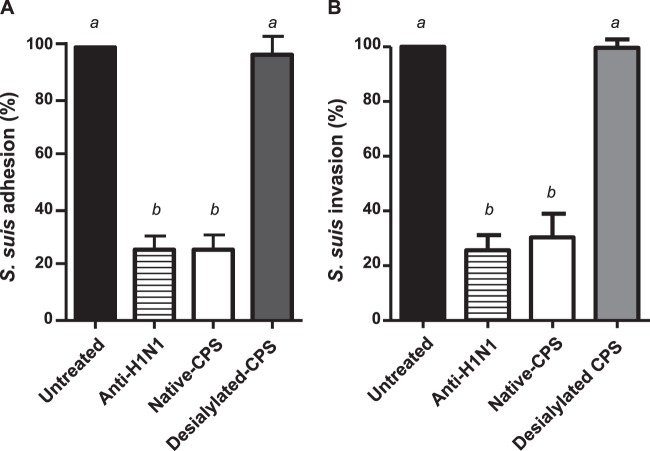
Since the CPS of S. suis serotype 2 was shown to be implicated in the increased bacterium-cell interactions when cells were preinfected with swH1N1, it was hypothesized that the sialic acid moiety present in the CPS of this serotype is involved through interactions with viral hemagglutinin. In fact, the reference strain of serotype 14 CPS (which also interacted with swH1N1-preinfected cells) possesses an identical sialic acid-containing side chain (also with a 2,6 link to the adjacent galactose) as serotype 2 CPS (30), whereas the reference strain of serotype 3 lacks this sugar (31–33). To confirm such a hypothesis, inhibition studies were performed. Interestingly, when wells were simply washed before adding the bacterial suspension and used as a control, no differences could be obtained with previous results for nonwashed wells, indicating that free viruses either were not present at significant numbers or that they did not significantly interfere with bacterial adhesion to/invasion of epithelial cells. A pretreatment of swH1N1-NPTr-preinfected cells with purified native CPS inhibits >75% of adhesion and invasion by S. suis serotype 2. This inhibition was similar to that obtained with a pretreatment with an anti-swH1N1-specific antibody (Fig. 5). When the same amount of desialylated CPS was used, no inhibition of bacterial adhesion/invasion could be observed, confirming the involvement of the CPS sialic acid in the interactions of S. suis with swH1N1-preinfected cells (Fig. 5).
Fig 5.
S. suis native, but not desialylated, capsular polysaccharide (CPS) inhibits S. suis adhesion to (A) and invasion of (B) NPTr cells preinfected with swH1N1. NPTr cells were infected with swH1N1 (MOI, 1) for 12 h and then incubated with the native CPS (100 μg/well), desialylated CPS (100 μg/well), or a polyclonal antibody serum against SIV H1N1 (1/40 dilution; positive control) for 1 h at 37°C. S. suis strain 31533 (MOI, 10) was then added to pretreated NPTr cells. Two hours postinfection, adhesion and invasion of S. suis were assessed as described in Materials and Methods. Results are expressed (in percentages) compared to bacterial adhesion and invasion of untreated swH1N1-preinfected cells (considered 100%). Data are expressed as means ± standard errors of the means from at least three independent experiments. Groups that are significantly different from each other are indicated by different letters (a and b), as determined by one-way ANOVA with P ≤ 0.01.
In vitro binding of swH1N1 to S. suis enhances bacterial adhesion to and invasion of epithelial cells.
To investigate if well-encapsulated S. suis directly interacts with the swH1N1 strain, a test of hemagglutination inhibition was performed. Results showed that a 1-h preincubation of S. suis serotype 2 strain 31533 and swH1N1 virus (in a bacterium/virus ratio of >50) resulted in the complete inhibition of RBC hemagglutination (see Fig. S2 in the supplemental material). Lower concentrations of bacteria did not present any visual inhibition. Interestingly, no inhibition of RBC hemagglutination was observed when the nonencapsulated mutant was used, even at a bacterium/virus ratio of 10,000 (see Fig. S2). Finally, a preincubation of S. suis serotype 2 strain 31533 with the swH1N1 strain significantly increases the interaction between S. suis and NPTr cells, since bacterial adhesion to and invasion of epithelial cells presented up to 10-fold-increased values compared to those of cells infected with S. suis without a preincubation with swH1N1 (Fig. 6). These results suggest that S. suis also uses swH1N1 virus as a vehicle to adhere to and invade epithelial cells. The possibility that some bacteria aggregate with virus (forming microclumps), somehow enhancing the total number of bacteria adhering to cells, cannot be ruled out. No increase in bacterium-cell interactions was observed when the nonencapsulated mutant was used (data not shown).
Fig 6.
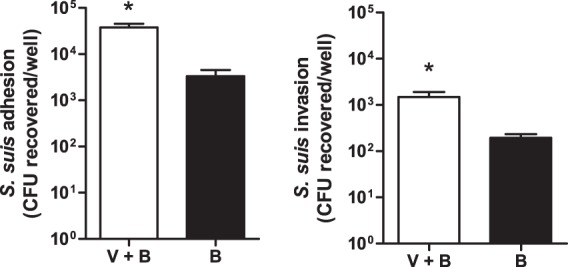
Preincubation of S. suis and swH1N1 significantly increases bacterial adhesion to and invasion of NPTr cells. swH1N1 and S. suis serotype 2 strain 31533 cells (1:1 ratio; TCID50/CFU) were preincubated for 1 h at 4°C. This mixture (V+B) was then added to NPTr cells for an incubation time of 1 or 2 h for adhesion or invasion assays, respectively, as described in Materials and Methods. Mock-treated bacteria were used as the control (B). Data are expressed as means ± standard errors of the means from at least three independent experiments. An asterisk indicates a significant difference (P < 0.01).
Coinfected NPTr cells express higher levels of proinflammatory genes than singly infected cells.
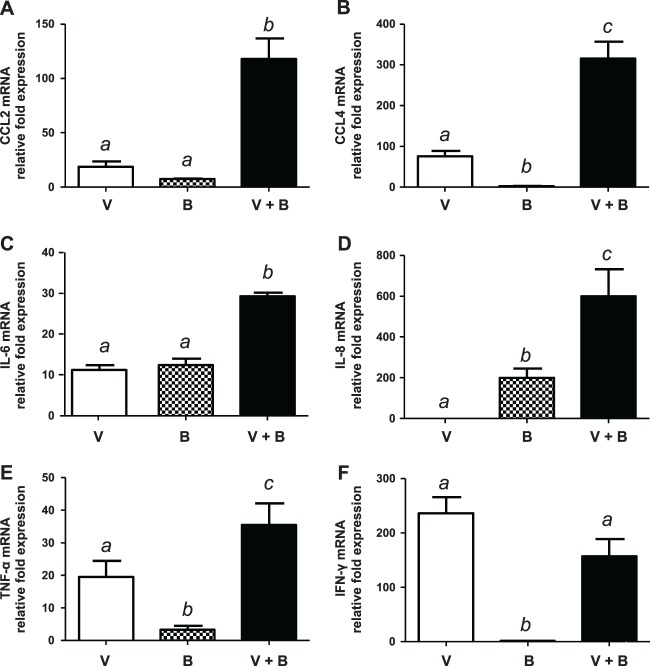
Although the complete kinetics were studied (results not shown), results showed that 24 h after bacterial infection (36 h after virus infection) reflected optimal differences among groups. NPTr cells infected with bacteria alone showed the absence or low expression levels of CCL2 (MCP-1), CCL4 (MIP-1β), beta interferon (IFN-β), and tumor necrosis factor alpha (TNF-α), intermediate expression levels of interleukin-6 (IL-6), and high levels of IL-8 expression (Fig. 7). Virus-mediated NPTr cell activation at that incubation time showed the absence of IL-8 expression. On the other hand, the swH1N1 strain activated gene expression of other mediators at similar levels (CCL2 and IL-6) or at significantly higher levels (CCL4, TNF-α, and IFN-β) than those obtained after activation with S. suis alone (Fig. 7). Interestingly, swH1N1-S. suis coinfection significantly increased the expression of CCL2, CCL4, IL-6, IL-8, and TNF-α mRNA. In some cases, an additive effect seemed responsible for such differences (IL-6 and TNF-α). However, the increase of mRNA expression of CCL2 and CCL4, as well as IL-8 mRNA expression, was clearly ahead of a simple additive effect. Expression of IFN-β mRNA was probably attributed solely to the effect of swH1N1 (Fig. 7).
Fig 7.
Gene expression of proinflammatory mediators by NPTr cells. NPTr cells were either preinfected with swH1N1 for 12 h (MOI, 1) or left untreated, and subsequently the cells were infected with S. suis serotype 2 strain 31533 (MOI, 10) for 24 h. Total RNA was extracted from S. suis- and virus-coinfected cells (V+B), virus-infected cells (V), or bacterium-infected cells (B), and quantitative PCR analysis of selected genes was performed. Normalization of the data was done using the reference genes Hprt1 and Ppia. Mock noninfected samples were used as the calibrator, and relative fold differences were calculated for the rest of the samples and compared to the means from the calibrator samples. Data represent mean values ± standard errors of the means from relative fold expression. Groups that are significantly different from each other are indicated by different letters (a, b, and c), as determined by one-way ANOVA with P ≤ 0.01.
DISCUSSION
The pathogenesis of the infection caused by S. suis is far from being completely understood (6). In swine, S. suis is mainly transmitted by aerosols, and airborne transmission among pigs has been clearly demonstrated (34). S. suis cells play a certain role in mixed respiratory infections, although it is not considered a primary cause of swine pneumonia (1), indicating that it also uses the respiratory tract as a transient passage before reaching the bloodstream and causing bacteremia, which is essential for the pathogen to cause meningitis (35). The actual early mechanisms used by this pathogen to interact with epithelial cells and to further invade the bloodstream are poorly understood.
The clinical association of S. suis with virus infections have been reported already (36, 37). More recently, several outbreaks in swine due to swine influenza virus with a significant level of systemic coinfection due to S. suis have been reported in England (38). In humans, it is well known that influenza cases are heavily complicated by bacterial infections (12). In fact, it was previously reported that influenza virus as well as other respiratory viruses increase the adhesion/invasion capacities of bacterial pathogens (including streptococci) to epithelial cells, although the mechanisms have not been fully elucidated (39). The goal of the present work was to study interactions between S. suis and tracheal epithelial cells either preinfected with swH1N1 or left untreated.
Results showed that S. suis is able to not only adhere to but also invade swine tracheal epithelial cells. In the absence of virus infections, adhesins involved in such interactions seem to be located in the bacterial cell wall, since they are hindered by the presence of the CPS, as previously suggested (6, 9, 10). Indeed, significantly higher levels of adhesion and, most important, invasion rates were observed with a nonencapsulated S. suis mutant. Interestingly, results obtained with isogenic mutants showed that alteration at the LTA and PG, as well as the lack of suilysin production, did not influence the adhesion/invasion capacities of S. suis. Different S. suis surface-exposed proteins have been described as bacterial adhesins to extracellular matrix proteins present in host cells (6, 11). In fact, ApuA, a surface protein with bifunctional amylopullulanase activity, was described to play an important role in such adhesion to tracheal epithelial cells (7). No differences could be observed between strains of serotype 2 of different virulence potentials or strains belonging to other serotypes, showing that those adhesins are probably common to most strains of S. suis independent of their virulence/serotype.
In the presence of a prior swH1N1 infection, more than 100-fold increases in S. suis adhesion and invasion could be observed. This increased interaction was confirmed by confocal microscopy, TEM, and SEM. Increased cell susceptibility to S. suis adhesion and invasion following a virus infection may have different explanations. One of the most well-known interactions is that between influenza virus and Streptococcus pneumoniae (40). In vivo-increased susceptibility has been attributed to an alteration of antibacterial phagocyte functions through diminished bactericidal activity and/or damage to the respiratory epithelium, resulting in defective mucociliary clearance mechanisms, which in turn leads to increased numbers of bacteria that remain in the respiratory tract (41). In vitro studies suggested damage to the respiratory epithelium by exposing surface molecules and cell receptors to which pneumococci more readily adhere and use to invade cells. This effect would be done mainly by the viral neuraminidase (42), although a certain synergistic role of neuraminidase produced by S. pneumoniae cannot be ruled out (43).
Results from the present study indicate that interactions between influenza virus and S. suis are clearly different from those between influenza virus and S. pneumoniae. In fact, no neuraminidase activities have been demonstrated so far for S. suis. On the other hand, a clear role of the surface-exposed CPS in the S. suis interactions with swH1N1-infected cells could be established. Similar results were previously obtained with group A Streptococcus (GAS) and A549 epithelial cells (44). Although a certain direct binding between GAS and influenza virus could be observed, molecules involved in such interactions have not been elucidated so far (45). Interestingly, GAS lacks sialic acid on its surface. In the present study, the main serotypes of S. suis containing sialic acid (serotypes 2 and 14) clearly interact with swH1N1-infected cells, whereas interactions of a serotype lacking this sugar (serotype 3) were not affected by a virus preinfection. In addition, serotype 2 strains of different virulence potentials behaved similarly due to the fact that capsular composition of the three strains most probably is identical. In an inhibition assay using highly purified native and desialylated CPS purified from the reference strain of serotype 2, it was clearly shown that the bacterial sialic acid moiety was responsible for the virus-bacterium interactions. It was then hypothesized that the S. suis sialic acid binds to the hemagglutinin of the swH1N1 virus. This was further demonstrated by the fact that well-encapsulated S. suis (but not its nonencapsulated mutant) incubated with the swH1N1 strain was able to inhibit the RBC hemagglutination activity of the virus. The binding of S. suis CPS to influenza virus hemagglutinin was not exclusive to the H1N1 strain used. Another swine influenza virus field strain (H3N2) used in parallel studies offered results identical to those obtained with the H1N1 strain (unpublished observations). Interestingly, direct binding of group B Streptococcus (GBS) to influenza virus has also been described previously (46). It was hypothesized that the sialyl-galactose linkage in GBS was responsible for binding to the virus (46). We suggest that GBS behaves similarly to S. suis, since the structures of the CPS of both pathogens are similar (8). Interestingly, not all bacterial pathogens possessing capsular sialic acid use a similar mechanism. For example, it has been proposed that a direct interaction between the neuraminidase of influenza virus and the CPS of Neisseria meningitidis enhances bacterial adhesion to cultured epithelial cells, most likely through cleavage of capsular sialic acid-containing bacterial polysaccharides (47).
Although a typical preinfection with influenza virus is believed to be followed by a bacterial complication, a simultaneous infection with both pathogens cannot be disregarded. In pigs, for example, both pathogens may infect animals at the same age range (1). In this study, binding between free S. suis serotype 2 and free swH1N1 promotes enhanced bacterial adhesion to and invasion of swine epithelial cells, similar to what has been shown for GAS (45). Similarly, previous in vitro binding of nonidentified surface-exposed proteins of Staphylococcus aureus to the viral hemagglutinin enhances bacterial invasion to virus-uninfected cells (48). Hence, influenza virus infection may promote adhesion and internalization of S. suis not only by binding of bacteria to the membrane-associated hemagglutinin but also by binding of bacteria to free virions, followed by internalization of virus-coated bacteria into noninfected epithelial cells. Therefore, a possible synergy between the two pathogens cannot be ruled out. However, further studies on the exact mechanisms involved should be performed.
Influenza virus is able to stimulate epithelial cells and induce the overproduction of different inflammatory mediators. In addition, it may directly or indirectly interfere with the balance of cytokine/chemokine production (49). In coinfection studies, activation of epithelial cells by influenza virus enhances the induction of cytokine and chemokine gene transcripts by S. pneumoniae (50). Inflammation has been reported to be highly important in S. suis infections (51). So far, the inflammatory response of respiratory epithelial cells generated by S. suis has not been addressed. In the present study, S. suis was shown to strongly upregulate gene expression of mainly IL-6 and IL-8, similar to that observed with epithelial cells of the choroid plexus (52). In contrast to what was described with these cells, relatively low levels of TNF-α expression were observed with S. suis-activated NPTr cells, even at shorter incubation times (data not shown), indicating some differences between the two cell types. When NPTr cells were preinfected with swH1N1, the significant increase of IL-8 expression that was observed may be explained by a higher number of bacteria interacting with influenza virus-infected cells. It has been shown that IL-8 expression by S. suis-activated endothelial cells is bacterium concentration dependent (53). In the case of IL-6 and TNF-α, the increased expression observed under the coinfection conditions also may be explained by an additive effect of swH1N1 and S. suis. On the other hand, S. suis alone did not produce significant levels of CCL2 and CCL4. However, when cells were preinfected with swH1N1, between 100- and 300-fold increases in mRNA expression of these mediators were detected. Influenza virus replicates in the respiratory epithelium and induces an inflammatory infiltrate comprised of mononuclear cells and neutrophils (54) to which S. suis possesses antiphagocytic capacities (6). Since S. suis is not a primary pulmonary pathogen, an exacerbated production of proinflammatory mediators during a coinfection with influenza virus may be important in the pathogenesis of the influenza infection.
In conclusion, a new role of S. suis CPS, other than that of an antiphagocytic factor (55), has been demonstrated in the present study. Although it was previously reported that the presence of sialic acid in S. suis could not be directly related to virulence (56), we demonstrated that its presence plays a major role in the interactions with respiratory epithelial cells previously infected by swine influenza virus, acting as a bacterial receptor for the virus. Simultaneous coinfections with both pathogens may also be mutually beneficial due to direct bacterium-virus interaction. Binding of bacteria to influenza virus-infected cells or directly to influenza virus could play an important role in allowing bacteria to move toward the lower airways, initiating the systemic invasion that characterizes the pathogenesis of the infection caused by S. suis. The increased production of local proinflammatory mediators in the presence of both pathogens may also play an important role in the pathogenesis of the pneumonia caused by swine influenza.
Supplementary Material
ACKNOWLEDGMENTS
We thank Sonia Lacouture for technical assistance.
Y.W. was a recipient of China Scholarship Council funding. This work was supported by Natural Sciences and Engineering Research Council of Canada (NSERC) grant 154280 to M.G. C.S. was a recipient of a Canadian Swine Health Board postdoctoral fellowship.
Footnotes
Published ahead of print 30 September 2013
Supplemental material for this article may be found at http://dx.doi.org/10.1128/IAI.00818-13.
REFERENCES
- 1.Gottschalk M. 2012. Streptococcocis, p 841–855 In Straw BE, Zimmerman JJ, D'Allaire S, Taylor DJ. (ed), Diseases of swine, 10th ed. Blackwell Publishing, Ames, IA [Google Scholar]
- 2.Gottschalk M, Xu J, Calzas C, Segura M. 2010. Streptococcus suis: a new emerging or an old neglected zoonotic pathogen? Future Microbiol. 5:371–391 [DOI] [PubMed] [Google Scholar]
- 3.Ye C, Zheng H, Zhang J, Jing H, Wang L, Xiong Y, Wang W, Zhou Z, Sun Q, Luo X, Du H, Gottschalk M, Xu J. 2009. Clinical, experimental, and genomic differences between intermediately pathogenic, highly pathogenic, and epidemic Streptococcus suis. J. Infect. Dis. 199:97–107 [DOI] [PubMed] [Google Scholar]
- 4.Lun ZR, Wang QP, Chen XG, Li AX, Zhu XQ. 2007. Streptococcus suis: an emerging zoonotic pathogen. Lancet Infect. Dis. 7:201–209 [DOI] [PubMed] [Google Scholar]
- 5.Wertheim HF, Nghia HD, Taylor W, Schultsz C. 2009. Streptococcus suis: an emerging human pathogen. Clin. Infect. Dis. 48:617–625 [DOI] [PubMed] [Google Scholar]
- 6.Fittipaldi N, Segura M, Grenier D, Gottschalk M. 2012. Virulence factors involved in the pathogenesis of the infection caused by the swine pathogen and zoonotic agent Streptococcus suis. Future Microbiol. 7:259–279 [DOI] [PubMed] [Google Scholar]
- 7.Ferrando ML, Fuentes S, de Greeff A, Smith H, Wells JM. 2010. ApuA, a multifunctional alpha-glucan-degrading enzyme of Streptococcus suis, mediates adhesion to porcine epithelium and mucus. Microbiology 156:2818–2828 [DOI] [PubMed] [Google Scholar]
- 8.Van Calsteren MR, Gagnon F, Lacouture S, Fittipaldi N, Gottschalk M. 2010. Structure determination of Streptococcus suis serotype 2 capsular polysaccharide. Biochem. Cell Biol. 88:513–525 [DOI] [PubMed] [Google Scholar]
- 9.Benga L, Goethe R, Rohde M, Valentin-Weigand P. 2004. Non-encapsulated strains reveal novel insights in invasion and survival of Streptococcus suis in epithelial cells. Cell Microbiol. 6:867–881 [DOI] [PubMed] [Google Scholar]
- 10.Lalonde M, Segura M, Lacouture S, Gottschalk M. 2000. Interactions between Streptococcus suis serotype 2 and different epithelial cell lines. Microbiology 146:1913–1921 [DOI] [PubMed] [Google Scholar]
- 11.Baums CG, Valentin-Weigand P. 2009. Surface-associated and secreted factors of Streptococcus suis in epidemiology, pathogenesis and vaccine development. Anim. Health Res. Rev. 10:65–83 [DOI] [PubMed] [Google Scholar]
- 12.Smith AM, Adler FR, Ribeiro RM, Gutenkunst RN, McAuley JL, McCullers JA, Perelson AS. 2013. Kinetics of coinfection with influenza A virus and Streptococcus pneumoniae. PLoS Pathog. 9:e1003238. 10.1371/journal.ppat.1003238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jung K, Ha Y, Chae C. 2005. Pathogenesis of swine influenza virus subtype H1N2 infection in pigs. J. Comp. Pathol. 132:179–184 [DOI] [PubMed] [Google Scholar]
- 14.Loving CL, Brockmeier SL, Vincent AL, Palmer MV, Sacco RE, Nicholson TL. 2010. Influenza virus coinfection with Bordetella bronchiseptica enhances bacterial colonization and host responses exacerbating pulmonary lesions. Microb. Pathog. 49:237–245 [DOI] [PubMed] [Google Scholar]
- 15.Opriessnig T, Gimenez-Lirola LG, Halbur PG. 2011. Polymicrobial respiratory disease in pigs. Anim. Health Res. Rev. 12:133–148 [DOI] [PubMed] [Google Scholar]
- 16.Ito T, Couceiro JN, Kelm S, Baum LG, Krauss S, Castrucci MR, Donatelli I, Kida H, Paulson JC, Webster RG, Kawaoka Y. 1998. Molecular basis for the generation in pigs of influenza A viruses with pandemic potential. J. Virol. 72:7367–7373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vanier G, Segura M, Friedl P, Lacouture S, Gottschalk M. 2004. Invasion of porcine brain microvascular endothelial cells by Streptococcus suis serotype 2. Infect. Immun. 72:1441–1449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lun S, Perez-Casal J, Connor W, Willson PJ. 2003. Role of suilysin in pathogenesis of Streptococcus suis capsular serotype 2. Microb. Pathog. 34:27–37 [DOI] [PubMed] [Google Scholar]
- 19.Fittipaldi N, Harel J, D'Amours B, Lacouture S, Kobisch M, Gottschalk M. 2007. Potential use of an unencapsulated and aromatic amino acid-auxotrophic Streptococcus suis mutant as a live attenuated vaccine in swine. Vaccine 25:3524–3535 [DOI] [PubMed] [Google Scholar]
- 20.Fittipaldi N, Sekizaki T, Takamatsu D, Harel J, Dominguez-Punaro Mde L, Von Aulock S, Draing C, Marois C, Kobisch M, Gottschalk M. 2008. D-alanylation of lipoteichoic acid contributes to the virulence of Streptococcus suis. Infect. Immun. 76:3587–3594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fittipaldi N, Sekizaki T, Takamatsu D, Dominguez-Punaro Mde L, Harel J, Bui NK, Vollmer W, Gottschalk M. 2008. Significant contribution of the pgdA gene to the virulence of Streptococcus suis. Mol. Microbiol. 70:1120–1135 [DOI] [PubMed] [Google Scholar]
- 22.Bikour MH, Frost EH, Deslandes S, Talbot B, Elazhary Y. 1995. Persistence of a 1930 swine influenza A (H1N1) virus in Quebec. J. Gen. Virol. 76:2539–2547 [DOI] [PubMed] [Google Scholar]
- 23.Ferrari M, Scalvini A, Losio MN, Corradi A, Soncini M, Bignotti E, Milanesi E, Ajmone-Marsan P, Barlati S, Bellotti D, Tonelli M. 2003. Establishment and characterization of two new pig cell lines for use in virological diagnostic laboratories. J. Virol. Methods 107:205–212 [DOI] [PubMed] [Google Scholar]
- 24.Bouchet B, Vanier G, Jacques M, Auger E, Gottschalk M. 2009. Studies on the interactions of Haemophilus parasuis with porcine epithelial tracheal cells: limited role of LOS in apoptosis and pro-inflammatory cytokine release. Microb. Pathog. 46:108–113 [DOI] [PubMed] [Google Scholar]
- 25.Tremblay D, Allard V, Doyon JF, Bellehumeur C, Spearman JG, Harel J, Gagnon CA. 2011. Emergence of a new swine H3N2 and pandemic (H1N1) 2009 influenza A virus reassortant in two Canadian animal populations, mink and swine. J. Clin. Microbiol. 49:4386–4390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lecours MP, Fittipaldi N, Takamatsu D, Okura M, Segura M, Goyette-Desjardins G, Van Calsteren MR, Gottschalk M. 2012. Sialylation of Streptococcus suis serotype 2 is essential for capsule expression but is not responsible for the main capsular epitope. Microbes Infect. 14:941–950 [DOI] [PubMed] [Google Scholar]
- 27.Higgins R, Gottschalk M. 1990. An update on Streptococcus suis identification. J. Vet. Diagn. Investig. 2:249–252 [DOI] [PubMed] [Google Scholar]
- 28.Lecours MP, Segura M, Lachance C, Mussa T, Surprenant C, Montoya M, Gottschalk M. 2011. Characterization of porcine dendritic cell response to Streptococcus suis. Vet. Res. 42:72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jacques M, Gottschalk M, Foiry B, Higgins R. 1990. Ultrastructural study of surface components of Streptococcus suis. J. Bacteriol. 172:2833–2838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Van Calsteren MR, Gagnon F, Calzas C, Goyette-Desjardins G, Okura M, Takamatsu D, Gottschalk M, Segura M. 2013. Structure determination of Streptococcus suis serotype 14 capsular polysaccharide. Biochem. Cell Biol. 91:49–58 [DOI] [PubMed] [Google Scholar]
- 31.Charland N, Kellens JT, Caya F, Gottschalk M. 1995. Agglutination of Streptococcus suis by sialic acid-binding lectins. J. Clin. Microbiol. 33:2220–2221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Smith HE, de Vries R, van't Slot R, Smits MA. 2000. The cps locus of Streptococcus suis serotype 2: genetic determinant for the synthesis of sialic acid. Microb. Pathog. 29:127–134 [DOI] [PubMed] [Google Scholar]
- 33.Okura M, Takamatsu D, Maruyama F, Nozawa T, Nakagawa I, Osaki M, Sekizaki T, Gottschalk M, Kumagai Y, Hamada S. 2013. Genetic analysis of capsular polysaccharide synthesis gene clusters from all serotypes of Streptococcus suis: potential mechanisms for the generation of capsular variation. Appl. Environ. Microbiol. 79:2796–2806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Berthelot-Herault F, Gottschalk M, Labbe A, Cariolet R, Kobisch M. 2001. Experimental airborne transmission of Streptococcus suis capsular type 2 in pigs. Vet. Microbiol. 82:69–80 [DOI] [PubMed] [Google Scholar]
- 35.Dominguez-Punaro MC, Koedel U, Hoegen T, Demel C, Klein M, Gottschalk M. 2012. Severe cochlear inflammation and vestibular syndrome in an experimental model of Streptococcus suis infection in mice. Eur. J. Clin. Microbiol. Infect. Dis. 31:2391–2400 [DOI] [PubMed] [Google Scholar]
- 36.Pallares FJ, Halbur PG, Opriessnig T, Sorden SD, Villar D, Janke BH, Yaeger MJ, Larson DJ, Schwartz KJ, Yoon KJ, Hoffman LJ. 2002. Porcine circovirus type 2 (PCV-2) coinfections in US field cases of postweaning multisystemic wasting syndrome (PMWS). J. Vet. Diagn. Investig. 14:515–519 [DOI] [PubMed] [Google Scholar]
- 37.Schmitt CS, Halbur PG, Roth JA, Kinyon JM, Kasorndorkbua C, Thacker B. 2001. Influence of ampicillin, ceftiofur, attenuated live PRRSV vaccine, and reduced dose Streptococcus suis exposure on disease associated with PRRSV and S. suis coinfection. Vet. Microbiol. 78:29–37 [DOI] [PubMed] [Google Scholar]
- 38.Williamson SM, Tucker AW, McCrone IS, Bidewell CA, Brons N, Habernoll H, Essen SC, Brown IH, Cosi Wood JL. 2012. Descriptive clinical and epidemiological characteristics of influenza A H1N1 2009 virus infections in pigs in England. Vet. Rec. 171:271. [DOI] [PubMed] [Google Scholar]
- 39.Avadhanula V, Rodriguez CA, Devincenzo JP, Wang Y, Webby RJ, Ulett GC, Adderson EE. 2006. Respiratory viruses augment the adhesion of bacterial pathogens to respiratory epithelium in a viral species- and cell type-dependent manner. J. Virol. 80:1629–1636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bosch AA, Biesbroek G, Trzcinski K, Sanders EA, Bogaert D. 2013. Viral and bacterial interactions in the upper respiratory tract. PLoS Pathog. 9:e1003057. 10.1371/journal.ppat.1003057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pittet LA, Hall-Stoodley L, Rutkowski MR, Harmsen AG. 2010. Influenza virus infection decreases tracheal mucociliary velocity and clearance of Streptococcus pneumoniae. Am. J. Resp. Cell Mol. Biol. 42:450–460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McCullers JA, Bartmess KC. 2003. Role of neuraminidase in lethal synergism between influenza virus and Streptococcus pneumoniae. J. Infect. Dis. 187:1000–1009 [DOI] [PubMed] [Google Scholar]
- 43.Nishikawa T, Shimizu K, Tanaka T, Kuroda K, Takayama T, Yamamoto T, Hanada N, Hamada Y. 2012. Bacterial neuraminidase rescues influenza virus replication from inhibition by a neuraminidase inhibitor. PLoS One 7:e45371. 10.1371/journal.pone.0045371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Okamoto S, Kawabata S, Terao Y, Fujitaka H, Okuno Y, Hamada S. 2004. The Streptococcus pyogenes capsule is required for adhesion of bacteria to virus-infected alveolar epithelial cells and lethal bacterial-viral superinfection. Infect. Immun. 72:6068–6075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Swildens B, Stockhofe-Zurwieden N, van der Meulen J, Wisselink HJ, Nielen M, Niewold TA. 2004. Intestinal translocation of Streptococcus suis type 2 EF+ in pigs. Vet. Microbiol. 103:29–33 [DOI] [PubMed] [Google Scholar]
- 46.Hosaka Y, Kuroda K, Ikeura A, Iwamoto T, Suzuki Y. 1998. Binding of influenza and paramyxoviruses to group B Streptococcus with the terminal sialyl-galactose linkage. J. Electron Microsc. 47:169–174 [DOI] [PubMed] [Google Scholar]
- 47.Rameix-Welti MA, Zarantonelli ML, Giorgini D, Ruckly C, Marasescu M, van der Werf S, Alonso JM, Naffakh N, Taha MK. 2009. Influenza A virus neuraminidase enhances meningococcal adhesion to epithelial cells through interaction with sialic acid-containing meningococcal capsules. Infect. Immun. 77:3588–3595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Passariello C, Nencioni L, Sgarbanti R, Ranieri D, Torrisi MR, Ripa S, Garaci E, Palamara AT. 2011. Viral hemagglutinin is involved in promoting the internalisation of Staphylococcus aureus into human pneumocytes during influenza A H1N1 virus infection. Int. J. Med. Microbiol. 301:97–104 [DOI] [PubMed] [Google Scholar]
- 49.Lam WY, Yeung AC, Chu IM, Chan PK. 2010. Profiles of cytokine and chemokine gene expression in human pulmonary epithelial cells induced by human and avian influenza viruses. Virol. J. 7:344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tong HH, Long JP, Shannon PA, DeMaria TF. 2003. Expression of cytokine and chemokine genes by human middle ear epithelial cells induced by influenza A virus and Streptococcus pneumoniae opacity variants. Infect. Immun. 71:4289–4296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dominguez-Punaro MC, Segura M, Plante MM, Lacouture S, Rivest S, Gottschalk M. 2007. Streptococcus suis serotype 2, an important swine and human pathogen, induces strong systemic and cerebral inflammatory responses in a mouse model of infection. J. Immunol. 179:1842–1854 [DOI] [PubMed] [Google Scholar]
- 52.Schwerk C, Adam R, Borkowski J, Schneider H, Klenk M, Zink S, Quednau N, Schmidt N, Stump C, Sagar A, Spellerberg B, Tenenbaum T, Koczan D, Klein-Hitpass L, Schroten H. 2011. In vitro transcriptome analysis of porcine choroid plexus epithelial cells in response to Streptococcus suis: release of pro-inflammatory cytokines and chemokines. Microb. Infect. 13:953–962 [DOI] [PubMed] [Google Scholar]
- 53.Vadeboncoeur N, Segura M, Al-Numani D, Vanier G, Gottschalk M. 2003. Pro-inflammatory cytokine and chemokine release by human brain microvascular endothelial cells stimulated by Streptococcus suis serotype 2. FEMS Immun. Med. Microbiol. 35:49–58 [DOI] [PubMed] [Google Scholar]
- 54.Wareing MD, Lyon AB, Lu B, Gerard C, Sarawar SR. 2004. Chemokine expression during the development and resolution of a pulmonary leukocyte response to influenza A virus infection in mice. J. Leukoc. Biol. 76:886–895 [DOI] [PubMed] [Google Scholar]
- 55.Smith HE, Damman M, van der Velde J, Wagenaar F, Wisselink HJ, Stockhofe-Zurwieden N, Smits MA. 1999. Identification and characterization of the cps locus of Streptococcus suis serotype 2: the capsule protects against phagocytosis and is an important virulence factor. Infect. Immun. 67:1750–1756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Charland N, Kobisch M, Martineau-Doize B, Jacques M, Gottschalk M. 1996. Role of capsular sialic acid in virulence and resistance to phagocytosis of Streptococcus suis capsular type 2. FEMS Immun. Med. Microbiol. 14:195–203 [DOI] [PubMed] [Google Scholar]
- 57.Gottschalk M, Higgins R, Jacques M, Mittal KR, Henrichsen J. 1989. Description of 14 new capsular types of Streptococcus suis. J. Clin. Microbiol. 27:2633–2636 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.