Abstract
Streptococcus pneumoniae (pneumococcus) frequently colonizes the human nasopharynx and is an important cause of pneumonia, meningitis, sinusitis, and otitis media. The outer cell surface of pneumococcus may assume various degrees of negative charge depending on the polysaccharide capsule, of which more than 90 serotypes have been identified. The negative charge of capsular polysaccharides has been proposed to electrostatically repel pneumococci from phagocytic cells, and avoidance of phagocytosis correlates with higher carriage prevalence. We hypothesized that the surface charge of pneumococcus contributes to its success in nasopharyngeal carriage by modulating resistance to phagocyte-mediated killing. Here, we measured the surface charge (zeta potential) of laboratory-constructed strains that share a genetic background but differ in serotype and of clinical strains that differ in serotype and genetic background. A more negative surface charge correlated with higher resistance to nonopsonic killing by human neutrophils in vitro. In addition, a more negative zeta potential was associated with higher carriage prevalence in human populations before and after the widespread use of the pneumococcal conjugate vaccine PCV7. We also confirmed that capsule is the major determinant of net surface charge in clinical isolates with diverse backgrounds. We noted that exceptions exist to the idea that a higher magnitude of negative charge predicts higher prevalence. The results indicated that zeta potential is strongly influenced by pneumococcal capsule type but is unlikely to be the only important mechanism by which capsule interacts with host.
INTRODUCTION
Streptococcus pneumoniae (pneumococcus) is an important pathogen and is a common cause of pneumonia, meningitis, sinusitis, and otitis media worldwide. Pneumococcus frequently colonizes the nasopharynx, which precedes invasive infections (1, 2), and these colonization events are a prerequisite for disease and serve as a reservoir for bacterial transmission.
Pneumococci typically produce a polysaccharide capsule, of which more than 90 serotypes have been identified. Serotypes differ substantially in their prevalence (3–5), their tendency to cause diseases (6–9), and their degree of antimicrobial resistance (10, 11). Notably, the most common serotypes in both invasive disease and carriage exhibited overall consistency across populations and time (4) until the recent widespread use of pneumococcal conjugate vaccines (PCV7, PCV10, and PCV13) (12, 13). While PCV7 successfully reduced the burden of invasive pneumococcal disease (14), specific serotypes not targeted by the vaccine, such as 19A and serogroup 15, have been reported to increase in both carriage and invasive disease (15–18). Similar trends have appeared with PCV13 (19). These increases in replacement serotypes could partially undermine the public health impact of the vaccine (13). To determine the potential importance of serotype replacement for pneumococcal vaccines, it is critical to understand the factors that determine serotype patterns of carriage.
The outer cell surface of pneumococcus displays various degrees of negative charge, mainly due to the presence of acidic sugars, pyruvate, or phosphate in capsular polysaccharides of different serotypes (20), with additional contributions from cell surface structures (21). Electrostatic interaction between pneumococcal cells and host immune effectors could influence acquisition and clearance rates. Acquisition and clearance rates vary between serotypes (22–24) and are determinants of carriage prevalence. For example, it was proposed that the capsule enhances pneumococcal colonization by electrostatic repulsion against highly negatively charged mucus, which could reduce clearance by mucociliary flow (25). The negative charge of capsule polysaccharides may also electrostatically repel pneumococci from negatively charged phagocytic cells (26), including neutrophils and macrophages that accelerate clearance of pneumococcal carriage in immunized animals (27, 28). Serotypes that are resistant to opsonin-independent neutrophil-mediated killing tend to be more common among healthy carriers (29). Therefore, we hypothesize that the surface charge of pneumococcus contributes to resistance to nonopsonic killing by phagocytic cells and success during nasopharyngeal carriage.
Here, we characterized the effect of serotype and genetic background on the net surface charge of pneumococcus by zeta potential measurement. Zeta potential is the relevant measure of surface charge that mediates the electrostatic interaction between particles, and it can be estimated by measuring particle electrophoretic mobility in an electric field (30). We found that zeta potential varied according to serotype in both isogenic capsule variants and clinical isolates of diverse backgrounds. Lower zeta potential (higher net negative surface charge) was associated with higher resistance to neutrophil-mediated killing as well as higher carriage prevalence in human populations. Two chemically neutral capsules with near-zero zeta potential were exceptions to this general relationship, having moderate resistance to phagocytic killing and intermediate carriage prevalence. Thus, the net surface charge of pneumococcus appears to be strongly influenced by capsule but is unlikely to be the only important mechanism by which capsule interacts with human neutrophils.
MATERIALS AND METHODS
Bacteria and cells.
Capsule switch variants used in this study were reported previously (29) and were constructed on the TIGR4 genetic background (31). Nasopharyngeal clinical carriage isolates (see Table S1 in the supplemental material) were colony purified prior to use. All strains were grown in Todd Hewitt broth with 0.5% yeast extract (THY) (BD, Franklin Lakes, NJ) at 37°C with 5% CO2. In some experiments, strains were grown in a semidefined minimal medium (32) with 1,000 U/ml catalase (MP Biomedicals, Solon, OH) supplemented with either 10 mM glucose or 10 mM fructose.
Blood was obtained from healthy volunteers according to a protocol approved by the Office of Human Research Administration at the Harvard School of Public Health. Neutrophils were isolated using a Histopaque 10771, 11191 gradient (Sigma-Aldrich, St. Louis, MO) according to the manufacturer's instructions and used immediately.
Zeta potential measurement.
Exponentially growing pneumococcal cultures in THY were frozen in THY plus 10% glycerol at −80°C until use. On the day of measurement, the frozen stocks of bacteria were thawed, washed twice in phosphate-buffered saline (PBS; pH 7.4; Mediatech, Inc., Manassas, VA), and resuspended in PBS to an optical density at 620 nm (OD620) of 0.1 (∼5 × 107 CFU/ml). The zeta potential of the samples was measured at 25°C by an automated Zetasizer apparatus (Zetasizer nano ZS; Malvern Instruments Ltd., Malvern, United Kingdom) with parameters adjusted according to the manufacturer's specifications. The measurements were repeated at least three times, and the mean zeta potential was presented.
Neutrophil surface killing assay.
Neutrophil surface killing assays were performed as described previously, and survival data on the isogenic strains were obtained from a previously published study (29). Briefly, bacteria were grown to mid-log phase and frozen in THY–10% glycerol at −80°C. On the day of the experiment, bacteria were thawed and diluted to 5 × 103 CFU/ml in saline, and 10 μl of this suspension was spotted and allowed to dry at room temperature on Trypticase soy agar with 5% defibrinated sheep blood (TSA II) (BD) with 8 to 10 replicates per plate. Twenty microliters of neutrophils (2 × 106 cells/ml) was then overlaid, allowed to dry, and incubated overnight at 37°C with 5% CO2. Percent survival was calculated by comparing killing of each strain to a duplicate control plate with no neutrophils. For experiments comparing isogenic capsule switch variants, the data were normalized by dividing percent survival for each serotype by percent survival of type 9N to obtain relative survival.
Capsule size determination.
The data on degree of encapsulation were obtained from a previously published study using a fluorescein isothiocyanate (FITC)-dextran exclusion method (29). Briefly, bacteria were grown overnight on TSA II plates and swabbed into PBS, and 20 μl of bacteria was mixed with 2 μl of a 10 mg/ml stock solution of FITC-dextran (2,000 kDa; Sigma) and used to create wet mounts with coverslips. Fluorescence microscopy images were captured and analyzed with UTHSCSA ImageTool for Windows v3.0 (University of Texas Health Science Center in San Antonio). The mean area of FITC exclusion of 100 to 250 cells was determined for each serotype.
Serotype carriage.
Carriage prevalence data were obtained from previous studies (12, 16, 29, 33, 34). We included any serotype whose zeta potential was available in the analysis of correlation between prevalence and surface charge.
Statistics.
Zeta potential between strains was compared using either t tests or analysis of variance (ANOVA), as appropriate. Nonparametric Spearman correlation was used to evaluate the relationship between zeta potential and resistance to neutrophil-mediated killing, carriage prevalence, or degree of encapsulation. Linear regression was used to evaluate the relationship between zeta potential of isogenic capsule switch variants and clinical carriage isolates. Statistical analyses were conducted using the GraphPad Prism V5.0 software (GraphPad Software, San Diego, CA) and the R software package (http://www.r-project.org/).
RESULTS
Negative surface charge correlates with avoidance of nonopsonic killing by human neutrophils.
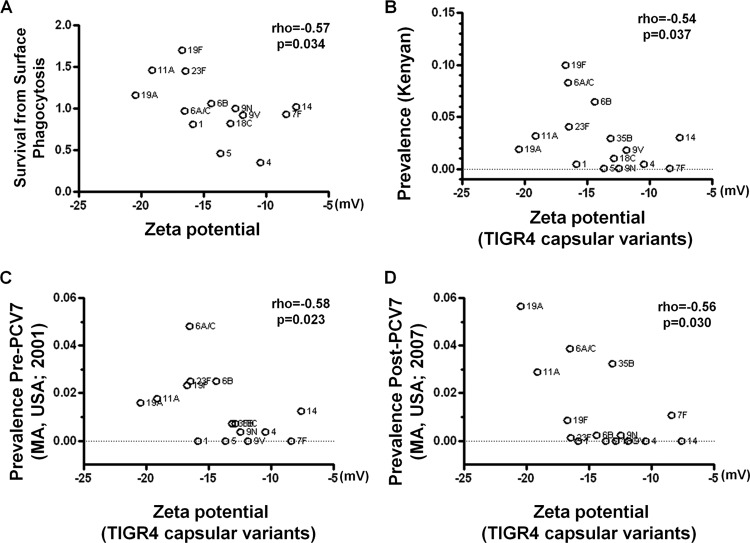
To evaluate whether bacterial surface charge influences resistance to killing by human neutrophils, we tested whether more negatively charged strains are less likely to be killed by neutrophils in a surface killing assay (29). We measured the zeta potential of a group of laboratory-constructed strains that share the TIGR4 genetic background but differ in the capsule polysaccharide and assessed the correlation with the resistance to neutrophil killing that has been reported previously (29). The strains with lower zeta potentials, such as those expressing the serotype 19F or 23F capsule, were more likely to survive in the surface killing assay, while strains with higher zeta potentials, such as those expressing the serotype 4 or 5 capsule, were less resistant to neutrophil killing. (Fig. 1A) (rho = −0.57, n = 14, P = 0.034). Serotypes 7F and 14, which produce neutral polysaccharides, appeared to be exceptions to this relationship, since they conferred the highest zeta potentials (nearly neutral) but only showed intermediate levels of susceptibility to neutrophil-mediated killing. Without these two strains, the relationship becomes much stronger (rho = −0.72, n = 12, P = 0.0082).
Fig 1.
Pneumococcal surface charge predicts avoidance of neutrophil-mediated killing and serotype carriage prevalence. (A) Surface charge of the TIGR4 capsule variants is inversely correlated with avoidance of neutrophil-mediated killing. Resistance to neutrophil-mediated killing is represented by mean survival relative to serotype 9N. Spearman's rank correlation coefficient (rho) and the associated P value (p) are shown. Negative surface charge of the isogenetic TIGR4 capsule variants is correlated with the frequency of each serotype among carriage isolates from a Kenyan cohort (B), a Massachusetts cohort before the widespread use of the PCV7 vaccine (C), and a Massachusetts cohort after the widespread use of the PCV7 vaccine (D).
Lower zeta potential in isogenic capsule switch variants predicts higher carriage prevalence.
It has been reported that serotypes associated with higher resistance to neutrophil killing are likely to show higher carriage prevalence in human populations. Since we observed a link between surface charge and resistance to neutrophil killing, we further tested whether lower zeta potential can predict higher carriage prevalence. In a Kenyan cohort (33), pneumococcal serotype prevalence showed a significant negative correlation with zeta potential measured for capsular variants in the TIGR4 background (Fig. 1B) (rho = −0.54, n = 15, P = 0.037). The more prevalent serotypes, such as 19F and 23F, were the more negatively charged ones in the isogenic strains, while types that are infrequently isolated from carriage, such as types 1 and 4, were less negatively charged. Similarly, strong negative correlation was also evident in a Massachusetts cohort sampled in 2001, prior to the widespread use of the PCV7 vaccine (Fig. 1C) (rho = −0.58, n = 15, P = 0.023), as well as in a Massachusetts cohort sampled in 2007, when the PCV7 vaccine had been widely used for 7 years (Fig. 1D) (rho = −0.56, n = 15, P = 0.030).
Lower zeta potential in clinical isolates predicts higher carriage prevalence.
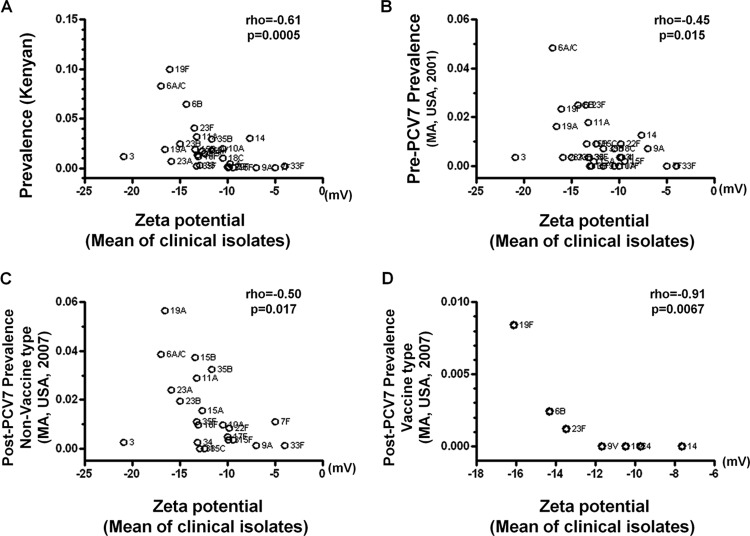
To determine whether the effect of negative surface charge could be generalized to clinical carriage isolates, we measured the zeta potential of 140 clinical strains, including 29 serotypes from diverse bacterial genetic backgrounds. All but 3 isolates were from a Massachusetts cohort (16, 34); the remaining 3 clinical isolates were from Arizona, another location in the United States, and Israel (29) (see Table S1 in the supplemental material). The number of isolates measured for each serotype ranged from 1 to 13, and the average zeta potential of isolates within the same serotype was used as the serotype-specific surface charge. We then examined whether the more prevalent serotypes, such as 23F and 19F, exhibited, on average, lower serotype-specific surface charges. In the Kenyan cohort (33), pneumococcal serotype prevalence showed a highly significant inverse correlation with mean zeta potential measured for clinical isolates of that serotype (Fig. 2A) (rho = −0.51, n = 29, P = 0.0005). In the Massachusetts cohort sampled prior to the widespread use of the PCV7 vaccine (16, 34), a similarly strong negative correlation was also observed (Fig. 2B) (rho = −0.45, n = 29, P = 0.015). In the Massachusetts population exposed to the PCV7 vaccine (16, 34), a significant correlation between zeta potential of clinical isolates and serotype carriage prevalence in 2007 was observed for both serotypes not included in the PCV7 vaccine (Fig. 2C) (rho = −0.50, n = 22, P = 0.017) and for the 7 vaccine serotypes (Fig. 2D) (rho = −0.91, n = 7, P = 0.0067) considered separately.
Fig 2.
Relationship between serotype-specific surface charge in clinical isolates and serotype carriage prevalence. Serotype-specific surface charge, as measured by averaging zeta potentials of clinical isolates with the same serotype, is negatively associated with the frequency of each serotype among carriage isolates from a Kenyan cohort (A) and a Massachusetts cohort before the introduction of the PCV7 vaccine (B). The negative correlation is also evident in a post-PCV7 population for both the non-vaccine type (C) and the vaccine type (D).
Capsule is the major determinant of surface charge in clinical isolates of pneumococcus.
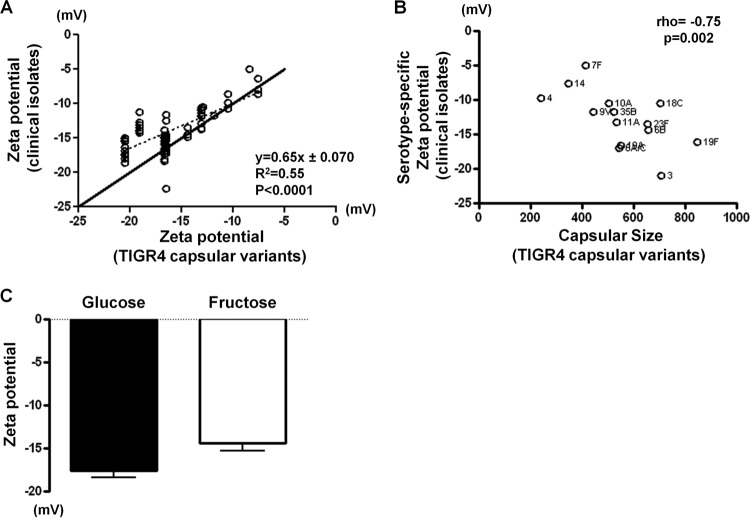
All capsular polysaccharides with known chemical structure carry negative net charges, except for those from serotypes 7F, 7A, 14, 33F, and 37, which are close to neutral (20). In isogenic strains, capsule switch clearly leads to a change of zeta potential (Fig. 1A). To understand how much the capsule type affects pneumococcal surface charge property in diverse backgrounds, we first statistically tested the contribution of the factor serotype to the measured zeta potential in the clinical isolates. An ANOVA on zeta potential demonstrated that the effect of serotype was highly significant [F (28, 111) = 23.9; P < 2 × 10−16], and the majority (85.8%) of the total variance can be explained by serotype (total sum of squares, 1,554; explained sum of squares, 1,332.9).
If capsule is the major determinant of the surface charge, then the zeta potential of the clinical isolates should be similar to that of the isogenic capsular variants of the same serotype. Indeed, we observed a good linear relationship between zeta potential measured for the isogenic capsular variants and zeta potential measured for the clinical isolates (Fig. 3B) (R2 = 0.55; P < 0.0001 by linear regression), with each unit increase of zeta potential in the isogenic strains corresponding to an increase of 0.65 units (95% confidence interval, 0.51, 0.80) in the means for the clinical strains. In addition, the degree of encapsulation measured for the isogenic strains was also highly significantly correlated with the serotype-specific zeta potential of clinical strains (Fig. 3C) (rho = −0.75, n = 14, P = 0.002). Thus, the capsule also appears to be the major determinant of surface charge in the clinical isolates of pneumococcus. We further tested whether experimentally modifying the quantity of capsule produced influences surface charge within a serotype. A TIGR4 variant producing a type 19F (TIGR4:19F) capsule was grown in a semidefined medium with either fructose, which has been reported to reduce capsule production (29, 35), or glucose. The bacteria grown in glucose showed lower zeta potential (mean, −17.6; standard deviation [SD], 0.76) than those grown in fructose (mean, −14.4; SD, 0.92) [t (15) = 8.203; P < 0.0001] (Fig. 3D). Since glucose-grown TIGR4:19F bacteria have been shown to be more resistant to surface killing than the fructose-grown ones (29), the result was consistent with the hypothesis that a more negative surface charge is associated with higher survival from nonopsonic killing by human neutrophils.
Fig 3.
Capsule is the major determinant of the surface charge in clinical isolates. (A) Zeta potential of clinical isolates is linearly correlated with zeta potential of the isogenic TIGR4 capsular variants. The dashed line represents the regression line. The solid line indicates where y = x. (B) Zeta potential of clinical isolates is correlated with degree of encapsulation that was measured by the area of the FITC-dextran exclusion zone in pixels. Spearman's rank correlation coefficient (rho) and the associated P value (p) are shown. (C) Growth in fructose leads to decreased surface charge in a TIGR4 variant producing a type 19F capsule. Error bars represent SD. P < 0.001 by t test (n = 9 for each group).
DISCUSSION
In this study, we focused on the contribution of surface charge to the prevalence of different pneumococcal serotypes in carriage. We found that serotype-specific zeta potential was a good predictor of carriage prevalence in each of the three cohorts examined. The Kenyan and the Massachusetts 2001 cohorts were sampled prior to widespread use of PCV7. The correlation between lower zeta potential and higher prevalence suggested that surface charge is linked to a factor(s) maintaining the naturally occurring serotype pattern. The Massachusetts 2007 cohort was sampled after widespread use of PCV7, and evidence suggested that serotype replacement had been completed by then (12). The serotype pattern in this cohort was clearly different from that in other cohorts, because PCV7 effectively decreased the prevalence of vaccine types while some non-vaccine types increased in frequency. Nonetheless, in both vaccine types and non-vaccine types, a negative correlation between zeta potential and prevalence was observed. The results suggested that surface charge is a useful predictor for the outcome of serotype rearrangement following intervention, given that serotypes affected by such intervention were known. We noted that outliers exist in this relationship, such as serotype 19A and 11A in Fig. 1B and C. A possible reason, besides uncertainty in measurement, is that factors other than surface charge that are associated with serotype also contribute to host-pneumococcus interaction and influence prevalence. For example, serotype 19A may compete with serotype 19F for a shared colonization niche (24) and could be outcompeted by 19F in unvaccinated populations. In addition, surface protein antigens may vary among serotypes and mediate variable recognition by host immunity. It will be interesting to test these possibilities in future investigations.
Negative surface charge is thought to facilitate pneumococcal colonization by reducing association with, and subsequent clearance by, host immune effectors that are also negatively charged, including mucus (25) and neutrophils (36). Avoidance of neutrophil-mediated surface killing in particular was shown to correlate with increased carriage prevalence (29). Therefore, we evaluated the relationship between zeta potential and resistance to neutrophil-mediated killing as a possible mechanism of the observed link between surface charge and carriage prevalence. We found that lower zeta potential of a serotype indeed correlated strongly with higher levels of survival from neutrophil-mediated killing. Serotype 3, which has the least costly repeat unit structure and is known for its mucoid colony phenotype, which is attributed to extensive capsule production, was an exception in this relationship, with relatively low carriage prevalence.
Capsule is unlikely to be the only important determinant of surface charge, as indicated by the within-serotype variation in zeta potential (Fig. 3A). The source of such variation may include either phenotypic differences within serotype, e.g., the well-characterized opaque/transparent switches (37, 38), or genetic differences within serotype, e.g., the expression of variable pneumococcal surface proteins that carry charge (18), or both. In the human nasopharyngeal environment, host factors that bind to pneumococcal cell surface, such as secretory IgA, lactoferrin, and factor H (39), also may influence zeta potential and modulate the interaction with neutrophils in vivo. Additionally, the pH of the nasopharynx ranges from approximately 6 to 8 (40), which could have an effect on the level of surface charge, as higher pH generally led to lower zeta potential. We observed, however, that the relative rank order of zeta potential among strains, which may contribute to the relative strength of interaction between the bacterium and nasopharyngeal cells, remained largely unchanged at different pHs (data not shown). Further characterization of noncapsule determinants of surface charge and their impacts on resistance to neutrophil killing would be an interesting focus for future investigation.
A previous study (29) has shown that less metabolic cost of capsule production, measured by the number of high-energy bonds (ATP equivalents) that are required to generate one polysaccharide repeat unit, is associated with higher colonization prevalence. In fact, there is also a correlation between metabolic cost and surface charge (see Fig. S2 in the supplemental material), with high metabolic cost leading to lower negative surface charge. It is possible that higher metabolic cost may result in the production of lower numbers of polysaccharide repeat units on each pneumococcal cell due to constraints on the amount of energy that is available for capsule synthesis. For most serotypes, each polysaccharide repeat unit carries one anionic group and no cationic group (20). Thus, capsules composed of fewer polysaccharide repeat units due to high metabolic cost would contain smaller amounts of negatively charged groups per cell and display less negative zeta potential.
In summary, serotype appears to be an important, but not the sole, determinant of surface charge (41). In turn, surface charge is an important, but not the sole, determinant of survival from nonopsonic killing by neutrophils. Finally, resistance to the action of phagocytes is a correlate of carriage prevalence by serotype, although this relationship may reflect other effects of negative charge, such as the ability to escape from other immune effectors. Given the importance of serotype replacement in the pneumococcal conjugate vaccine era, understanding the mechanism of the link between pneumococcal surface charge and serotype prevalence is an important direction for future research.
Supplementary Material
ACKNOWLEDGMENTS
Strains used in this study are made available through the Conjugate Vaccine Impact on Pneumococcal Carriage, Disease, and Population Genetics project, which is supported by NIH grant R01AI066304 (principal investigator: Jonathan Finkelstein, Boston Children's Hospital and Harvard Medical School). We thank Richard Malley at Boston Children's Hospital for helpful discussions and assistance with the Zetasizer nano instrument. We are grateful to Bernice Sim for technical assistance. We also thank Anna Moore and Peter Jarzyna at Massachusetts General Hospital for their assistance with the Zetasizer nano instrument.
The study was supported in part by NIH grant R01 AI048935 to M.L.
Footnotes
Published ahead of print 30 September 2013
Supplemental material for this article may be found at http://dx.doi.org/10.1128/IAI.00724-13.
REFERENCES
- 1.Simell B, Auranen K, Kayhty H, Goldblatt D, Dagan R, O'Brien KL, Pneumococcal Carriage Group 2012. The fundamental link between pneumococcal carriage and disease. Exp. Rev. Vaccines 11:841–855 [DOI] [PubMed] [Google Scholar]
- 2.Bogaert D, De Groot R, Hermans PW. 2004. Streptococcus pneumoniae colonisation: the key to pneumococcal disease. Lancet Infect. Dis. 4:144–154 [DOI] [PubMed] [Google Scholar]
- 3.Hausdorff WP, Feikin DR, Klugman KP. 2005. Epidemiological differences among pneumococcal serotypes. Lancet Infect. Dis. 5:83–93 [DOI] [PubMed] [Google Scholar]
- 4.Babl FE, Pelton SI, Theodore S, Klein JO. 2001. Constancy of distribution of serogroups of invasive pneumococcal isolates among children: experience during 4 decades. Clin. Infect. Dis. 32:1155–1161 [DOI] [PubMed] [Google Scholar]
- 5.Hausdorff WP, Bryant J, Paradiso PR, Siber GR. 2000. Which pneumococcal serogroups cause the most invasive disease: implications for conjugate vaccine formulation and use, part I. Clin. Infect. Dis. 30:100–121 [DOI] [PubMed] [Google Scholar]
- 6.Brueggemann AB, Griffiths DT, Meats E, Peto T, Crook DW, Spratt BG. 2003. Clonal relationships between invasive and carriage Streptococcus pneumoniae and serotype- and clone-specific differences in invasive disease potential. J. Infect. Dis. 187:1424–1432 [DOI] [PubMed] [Google Scholar]
- 7.Brueggemann AB, Peto TE, Crook DW, Butler JC, Kristinsson KG, Spratt BG. 2004. Temporal and geographic stability of the serogroup-specific invasive disease potential of Streptococcus pneumoniae in children. J. Infect. Dis. 190:1203–1211 [DOI] [PubMed] [Google Scholar]
- 8.Hanage WP, Kaijalainen TH, Syrjanen RK, Auranen K, Leinonen M, Makela PH, Spratt BG. 2005. Invasiveness of serotypes and clones of Streptococcus pneumoniae among children in Finland. Infect. Immun. 73:431–435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Austrian R. 1981. Some observations on the pneumococcus and on the current status of pneumococcal disease and its prevention. Rev. Infect. Dis. 3(Suppl):S1–S17 [DOI] [PubMed] [Google Scholar]
- 10.McCormick AW, Whitney CG, Farley MM, Lynfield R, Harrison LH, Bennett NM, Schaffner W, Reingold A, Hadler J, Cieslak P, Samore MH, Lipsitch M. 2003. Geographic diversity and temporal trends of antimicrobial resistance in Streptococcus pneumoniae in the United States. Nat. Med. 9:424–430 [DOI] [PubMed] [Google Scholar]
- 11.Hyams C, Trzcinski K, Camberlein E, Weinberger DM, Chimalapati S, Noursadeghi M, Lipsitch M, Brown JS. 2013. Streptococcus pneumoniae capsular serotype invasiveness correlates with the degree of factor H binding and opsonization with C3b/iC3b. Infect. Immun. 81:354–363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hanage WP, Finkelstein JA, Huang SS, Pelton SI, Stevenson AE, Kleinman K, Hinrichsen VL, Fraser C. 2010. Evidence that pneumococcal serotype replacement in Massachusetts following conjugate vaccination is now complete. Epidemics 2:80–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Weinberger DM, Malley R, Lipsitch M. 2011. Serotype replacement in disease after pneumococcal vaccination. Lancet 378:1962–1973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Whitney CG, Farley MM, Hadler J, Harrison LH, Bennett NM, Lynfield R, Reingold A, Cieslak PR, Pilishvili T, Jackson D, Facklam RR, Jorgensen JH, Schuchat A, Active Bacterial Core Surveillance of the Emerging Infections Program Network 2003. Decline in invasive pneumococcal disease after the introduction of protein-polysaccharide conjugate vaccine. N. Engl. J. Med. 348:1737–1746 [DOI] [PubMed] [Google Scholar]
- 15.Hicks LA, Harrison LH, Flannery B, Hadler JL, Schaffner W, Craig AS, Jackson D, Thomas A, Beall B, Lynfield R, Reingold A, Farley MM, Whitney CG. 2007. Incidence of pneumococcal disease due to non-pneumococcal conjugate vaccine (PCV7) serotypes in the United States during the era of widespread PCV7 vaccination, 1998–2004. J. Infect. Dis. 196:1346–1354 [DOI] [PubMed] [Google Scholar]
- 16.Huang SS, Platt R, Rifas-Shiman SL, Pelton SI, Goldmann D, Finkelstein JA. 2005. Post-PCV7 changes in colonizing pneumococcal serotypes in 16 Massachusetts communities, 2001 and 2004. Pediatrics 116:e408–e413 [DOI] [PubMed] [Google Scholar]
- 17.Frazao N, Brito-Avo A, Simas C, Saldanha J, Mato R, Nunes S, Sousa NG, Carrico JA, Almeida JS, Santos-Sanches I, de Lencastre H. 2005. Effect of the seven-valent conjugate pneumococcal vaccine on carriage and drug resistance of Streptococcus pneumoniae in healthy children attending day-care centers in Lisbon. Pediatr. Infect. Dis. J. 24:243–252 [DOI] [PubMed] [Google Scholar]
- 18.Singleton RJ, Hennessy TW, Bulkow LR, Hammitt LL, Zulz T, Hurlburt DA, Butler JC, Rudolph K, Parkinson A. 2007. Invasive pneumococcal disease caused by nonvaccine serotypes among Alaska native children with high levels of 7-valent pneumococcal conjugate vaccine coverage. JAMA 297:1784–1792 [DOI] [PubMed] [Google Scholar]
- 19.Gladstone RA, Jefferies JM, Faust SN, Clarke SC. 2012. Pneumococcal 13-valent conjugate vaccine for the prevention of invasive pneumococcal disease in children and adults. Exp. Rev. Vaccines 11:889–902 [DOI] [PubMed] [Google Scholar]
- 20.Kamerling J. 2000. Pneumococcal polysaccharides: a chemical view, p 85–93 In Tomasz A. (ed), Streptococcus pneumoniae: molecular biology & mechanisms of disease. Mary Ann Liebert, New York, NY [Google Scholar]
- 21.Swiatlo E, Champlin FR, Holman SC, Wilson WW, Watt JM. 2002. Contribution of choline-binding proteins to cell surface properties of Streptococcus pneumoniae. Infect. Immun. 70:412–415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hogberg L, Geli P, Ringberg H, Melander E, Lipsitch M, Ekdahl K. 2007. Age- and serogroup-related differences in observed durations of nasopharyngeal carriage of penicillin-resistant pneumococci. J. Clin. Microbiol. 45:948–952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sleeman KL, Griffiths D, Shackley F, Diggle L, Gupta S, Maiden MC, Moxon ER, Crook DW, Peto TE. 2006. Capsular serotype-specific attack rates and duration of carriage of Streptococcus pneumoniae in a population of children. J. Infect. Dis. 194:682–688 [DOI] [PubMed] [Google Scholar]
- 24.Lipsitch M, Abdullahi O, D'Amour A, Xie W, Weinberger DM, Tchetgen E, Scott JA. 2012. Estimating rates of carriage acquisition and clearance and competitive ability for pneumococcal serotypes in Kenya with a Markov transition model. Epidemiology 23:510–519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nelson AL, Roche AM, Gould JM, Chim K, Ratner AJ, Weiser JN. 2007. Capsule enhances pneumococcal colonization by limiting mucus-mediated clearance. Infect. Immun. 75:83–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.van Oss CJ. 1978. Phagocytosis as a surface phenomenon. Annu. Rev. Microbiol. 32:19–39 [DOI] [PubMed] [Google Scholar]
- 27.Lu YJ, Gross J, Bogaert D, Finn A, Bagrade L, Zhang Q, Kolls JK, Srivastava A, Lundgren A, Forte S, Thompson CM, Harney KF, Anderson PW, Lipsitch M, Malley R. 2008. Interleukin-17A mediates acquired immunity to pneumococcal colonization. PLoS Pathog. 4:e1000159. 10.1371/journal.ppat.1000159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang Z, Clarke TB, Weiser JN. 2009. Cellular effectors mediating Th17-dependent clearance of pneumococcal colonization in mice. J. Clin. Investig. 119:1899–1909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Weinberger DM, Trzcinski K, Lu YJ, Bogaert D, Brandes A, Galagan J, Anderson PW, Malley R, Lipsitch M. 2009. Pneumococcal capsular polysaccharide structure predicts serotype prevalence. PLoS Pathog. 5:e1000476. 10.1371/journal.ppat.1000476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wilson WW, Wade MM, Holman SC, Champlin FR. 2001. Status of methods for assessing bacterial cell surface charge properties based on zeta potential measurements. J. Microbiol. Methods 43:153–164 [DOI] [PubMed] [Google Scholar]
- 31.Trzcinski K, Thompson CM, Lipsitch M. 2003. Construction of otherwise isogenic serotype 6B, 7F, 14, and 19F capsular variants of Streptococcus pneumoniae strain TIGR4. Appl. Environ. Microbiol. 69:7364–7370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Adams MH, Roe AS. 1945. A partially defined medium for cultivation of pneumococcus. J. Bacteriol. 49:401–409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Abdullahi O, Karani A, Tigoi CC, Mugo D, Kungu S, Wanjiru E, Jomo J, Musyimi R, Lipsitch M, Scott JA. 2012. The prevalence and risk factors for pneumococcal colonization of the nasopharynx among children in Kilifi District, Kenya. PLoS One 7:e30787. 10.1371/journal.pone.0030787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hanage WP, Huang SS, Lipsitch M, Bishop CJ, Godoy D, Pelton SI, Goldstein R, Huot H, Finkelstein JA. 2007. Diversity and antibiotic resistance among nonvaccine serotypes of Streptococcus pneumoniae carriage isolates in the post-heptavalent conjugate vaccine era. J. Infect. Dis. 195:347–352 [DOI] [PubMed] [Google Scholar]
- 35.Bernheimer AW. 1953. Synthesis of type III pneumococcal polysaccharide by suspensions of resting cells. J. Exp. Med. 97:591–600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Klinke A, Nussbaum C, Kubala L, Friedrichs K, Rudolph TK, Rudolph V, Paust HJ, Schroder C, Benten D, Lau D, Szocs K, Furtmuller PG, Heeringa P, Sydow K, Duchstein HJ, Ehmke H, Schumacher U, Meinertz T, Sperandio M, Baldus S. 2011. Myeloperoxidase attracts neutrophils by physical forces. Blood 117:1350–1358 [DOI] [PubMed] [Google Scholar]
- 37.Weiser JN, Austrian R, Sreenivasan PK, Masure HR. 1994. Phase variation in pneumococcal opacity: relationship between colonial morphology and nasopharyngeal colonization. Infect. Immun. 62:2582–2589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Arai J, Hotomi M, Hollingshead SK, Ueno Y, Briles DE, Yamanaka N. 2011. Streptococcus pneumoniae isolates from middle ear fluid and nasopharynx of children with acute otitis media exhibit phase variation. J. Clin. Microbiol. 49:1646–1649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mook-Kanamori BB, Geldhoff M, van der Poll T, van de Beek D. 2011. Pathogenesis and pathophysiology of pneumococcal meningitis. Clin. Microbiol. Rev. 24:557–591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Brunworth JD, Garg R, Mahboubi H, Johnson B, Djalilian HR. 2012. Detecting nasopharyngeal reflux: a novel pH probe technique. Ann. Otol. Rhinol. Laryngol. 121:427–430 [DOI] [PubMed] [Google Scholar]
- 41.Kozel TR, Reiss E, Cherniak R. 1980. Concomitant but not causal association between surface charge and inhibition of phagocytosis by cryptococcal polysaccharide. Infect. Immun. 29:295–300 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.