Abstract
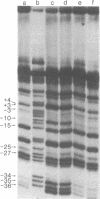
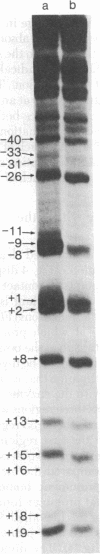
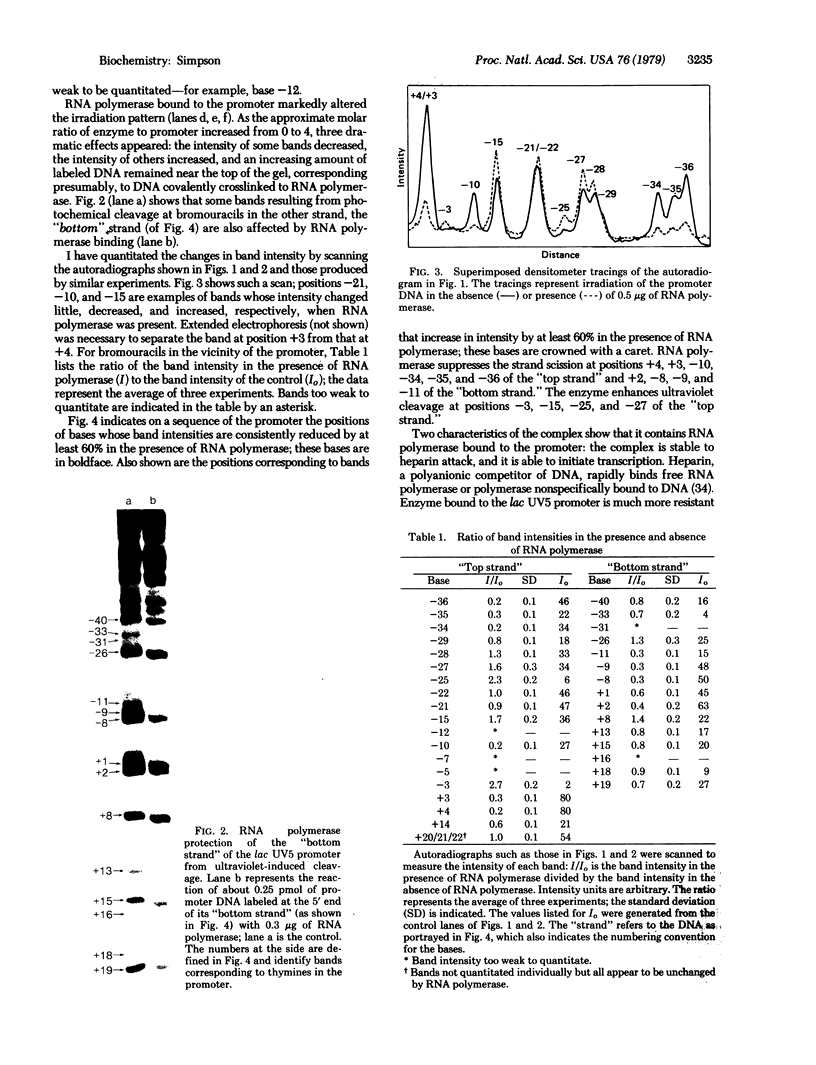
I have identified those 5 positions of thymines in the lac UV5 promoter that lie close to bound Escherichia coli RNA polymerase (nucleosidetriphosphate:RNA nucleotidyltransferase, EC 2.7.7.6). Although ultraviolet irradiation of DNA with 5-bromouracil substituted in place of thymine normally cleaves the DNA at the bromouracils, a protein bound to the DNA can perturb these cleavages at those locations at which the protein lies close to the bromine. In the lac promoter most of these contacts lie in three regions. Four contacts lie in the region where transcription initiates; four lie in the "Pribnow box," which is located about 10 base pairs upstream from the initiation site; and three more lie in the "-35 region," located about 35 base pairs upstream from the initiation site. The "Pribnow box" and the "-35 region" are regions whose sequences are partially conserved between promoters and in which most promoter mutations are located; thus, contacts in these two regions probably represent sites of sequence-specific recognition by RNA polymerase.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allet B., Roberts R. J., Gesteland R. F., Solem R. Class of promotor sites for Escherichia coli DNA-dependent RNA polymerase. Nature. 1974 May 17;249(454):217–221. doi: 10.1038/249217a0. [DOI] [PubMed] [Google Scholar]
- Bick M. D., Devine E. A. Interaction of chromosomal proteins with BrdU substituted DNA as determined by chromatin-DNA competition. Nucleic Acids Res. 1977 Nov;4(11):3687–3700. doi: 10.1093/nar/4.11.3687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown K. D., Bennett G. N., Lee F., Schweingruber M. E., Yanofsky C. RNA polymerase interaction at the promoter--operator region of the tryptophan operon of Escherichia coli and Salmonella typhimurium. J Mol Biol. 1978 May 15;121(2):153–177. doi: 10.1016/s0022-2836(78)80003-5. [DOI] [PubMed] [Google Scholar]
- Burgess R. R., Jendrisak J. J. A procedure for the rapid, large-scall purification of Escherichia coli DNA-dependent RNA polymerase involving Polymin P precipitation and DNA-cellulose chromatography. Biochemistry. 1975 Oct 21;14(21):4634–4638. doi: 10.1021/bi00692a011. [DOI] [PubMed] [Google Scholar]
- Calos M. P. DNA sequence for a low-level promoter of the lac repressor gene and an 'up' promoter mutation. Nature. 1978 Aug 24;274(5673):762–765. doi: 10.1038/274762a0. [DOI] [PubMed] [Google Scholar]
- D'Andrea A. D., Haseltine W. A. Sequence specific cleavage of DNA by the antitumor antibiotics neocarzinostatin and bleomycin. Proc Natl Acad Sci U S A. 1978 Aug;75(8):3608–3612. doi: 10.1073/pnas.75.8.3608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickson R. C., Abelson J., Barnes W. M., Reznikoff W. S. Genetic regulation: the Lac control region. Science. 1975 Jan 10;187(4171):27–35. doi: 10.1126/science.1088926. [DOI] [PubMed] [Google Scholar]
- Hsieh T., Wang J. C. Physicochomecial studies on interactions between DNA and RNA polymerase. Isolation and mapping of a T7 DNA fragment containing the early promoters for Escherichia coli RNA polymerase. Biochemistry. 1976 Dec 28;15(26):5776–5783. doi: 10.1021/bi00671a014. [DOI] [PubMed] [Google Scholar]
- Hutchinson F. The lesions produced by ultraviolet light in DNA containing 5-bromouracil. Q Rev Biophys. 1973 May;6(2):201–246. doi: 10.1017/s0033583500001141. [DOI] [PubMed] [Google Scholar]
- Johnsrud L. Contacts between Escherichia coli RNA polymerase and a lac operon promoter. Proc Natl Acad Sci U S A. 1978 Nov;75(11):5314–5318. doi: 10.1073/pnas.75.11.5314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones T. C., Dove W. F. Photosensitization of transcription by bromodeoxyuridine substitution. J Mol Biol. 1972 Mar 14;64(2):409–416. doi: 10.1016/0022-2836(72)90507-4. [DOI] [PubMed] [Google Scholar]
- Kleid D., Humayun Z., Jeffrey A., Ptashne M. Novel properties of a restriction endonuclease isolated from Haemophilus parahaemolyticus. Proc Natl Acad Sci U S A. 1976 Feb;73(2):293–297. doi: 10.1073/pnas.73.2.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin S. Y., Riggs A. D. Lac operator analogues: bromodeoxyuridine substitution in the lac operator affects the rate of dissociation of the lac repressor. Proc Natl Acad Sci U S A. 1972 Sep;69(9):2574–2576. doi: 10.1073/pnas.69.9.2574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin S. Y., Riggs A. D. Photochemical attachment of lac repressor to bromodeoxyuridine-substituted lac operator by ultraviolet radiation. Proc Natl Acad Sci U S A. 1974 Mar;71(3):947–951. doi: 10.1073/pnas.71.3.947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin S. Y., Riggs A. D. The binding of lac repressor and the catabolite gene activator protein to halogen-substituted analogues of poly[d(A-T)]. Biochim Biophys Acta. 1976 May 3;432(2):185–191. doi: 10.1016/0005-2787(76)90160-x. [DOI] [PubMed] [Google Scholar]
- Lin S., Lin D., Riggs A. D. Histones bind more tightly to bromodeoxyuridine-substituted DNA than to normal DNA. Nucleic Acids Res. 1976 Sep;3(9):2183–2191. doi: 10.1093/nar/3.9.2183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe P. A., Hager D. A., Burgess R. R. Purification and properties of the sigma subunit of Escherichia coli DNA-dependent RNA polymerase. Biochemistry. 1979 Apr 3;18(7):1344–1352. doi: 10.1021/bi00574a034. [DOI] [PubMed] [Google Scholar]
- Majors J. Initiation of in vitro mRNA synthesis from the wild-type lac promoter. Proc Natl Acad Sci U S A. 1975 Nov;72(11):4394–4398. doi: 10.1073/pnas.72.11.4394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maniatis T., Ptashne M., Backman K., Kield D., Flashman S., Jeffrey A., Maurer R. Recognition sequences of repressor and polymerase in the operators of bacteriophage lambda. Cell. 1975 Jun;5(2):109–113. doi: 10.1016/0092-8674(75)90018-5. [DOI] [PubMed] [Google Scholar]
- Maquat L. E., Reznikoff W. S. In vitro analysis of the Escherichia coli RNA polymerase interaction with wild-type and mutant lactose promoters. J Mol Biol. 1978 Nov 15;125(4):467–490. doi: 10.1016/0022-2836(78)90311-x. [DOI] [PubMed] [Google Scholar]
- Maurer R., Maniatis T., Ptashne M. Promoters are in the operators in phage lambda. Nature. 1974 May 17;249(454):221–223. doi: 10.1038/249221a0. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. A new method for sequencing DNA. Proc Natl Acad Sci U S A. 1977 Feb;74(2):560–564. doi: 10.1073/pnas.74.2.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer B. J., Kleid D. G., Ptashne M. Lambda repressor turns off transcription of its own gene. Proc Natl Acad Sci U S A. 1975 Dec;72(12):4785–4789. doi: 10.1073/pnas.72.12.4785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miozzari G., Yanofsky C. Naturally occurring promoter down mutation: nucleotide sequence of the trp promoter/operator/leader region of Shigella dysenteriae 16. Proc Natl Acad Sci U S A. 1978 Nov;75(11):5580–5584. doi: 10.1073/pnas.75.11.5580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musso R. E., Di Lauro R., Adhya S., de Crombrugghe B. Dual control for transcription of the galactose operon by cyclic AMP and its receptor protein at two interspersed promoters. Cell. 1977 Nov;12(3):847–854. doi: 10.1016/0092-8674(77)90283-5. [DOI] [PubMed] [Google Scholar]
- Ogata R., Gilbert W. Contacts between the lac repressor and the thymines in the lac operator. Proc Natl Acad Sci U S A. 1977 Nov;74(11):4973–4976. doi: 10.1073/pnas.74.11.4973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Post L. E., Arfsten A. E., Nomura M., Jaskunas S. R. Isolation and characterization of a promoter mutant in the str ribosomal protein operon in E. coli. Cell. 1978 Sep;15(1):231–236. doi: 10.1016/0092-8674(78)90097-1. [DOI] [PubMed] [Google Scholar]
- Pribnow D. Bacteriophage T7 early promoters: nucleotide sequences of two RNA polymerase binding sites. J Mol Biol. 1975 Dec 15;99(3):419–443. doi: 10.1016/s0022-2836(75)80136-7. [DOI] [PubMed] [Google Scholar]
- Pribnow D. Nucleotide sequence of an RNA polymerase binding site at an early T7 promoter. Proc Natl Acad Sci U S A. 1975 Mar;72(3):784–788. doi: 10.1073/pnas.72.3.784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ptashne M., Backman K., Humayun M. Z., Jeffrey A., Maurer R., Meyer B., Sauer R. T. Autoregulation and function of a repressor in bacteriophage lambda. Science. 1976 Oct 8;194(4261):156–161. doi: 10.1126/science.959843. [DOI] [PubMed] [Google Scholar]
- Schaller H., Gray C., Herrmann K. Nucleotide sequence of an RNA polymerase binding site from the DNA of bacteriophage fd. Proc Natl Acad Sci U S A. 1975 Feb;72(2):737–741. doi: 10.1073/pnas.72.2.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siebenlist U. Nucleotide sequence of the three major early promoters of bacteriophage T7. Nucleic Acids Res. 1979;6(5):1895–1907. doi: 10.1093/nar/6.5.1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverstone A. E., Arditti R. R., Magasanik B. Catabolite-insensitive revertants of lac promoter mutants. Proc Natl Acad Sci U S A. 1970 Jul;66(3):773–779. doi: 10.1073/pnas.66.3.773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka T., Weisblum B. Construction of a colicin E1-R factor composite plasmid in vitro: means for amplification of deoxyribonucleic acid. J Bacteriol. 1975 Jan;121(1):354–362. doi: 10.1128/jb.121.1.354-362.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walz A., Pirrotta V. Sequence of the PR promoter of phage lambda. Nature. 1975 Mar 13;254(5496):118–121. doi: 10.1038/254118a0. [DOI] [PubMed] [Google Scholar]
- Weintraub H. The assembly of newly replicated DNA into chromatin. Cold Spring Harb Symp Quant Biol. 1974;38:247–256. doi: 10.1101/sqb.1974.038.01.028. [DOI] [PubMed] [Google Scholar]