Abstract
Both idiopathic and infectious forms of colitis disrupt normal intestinal epithelial cell (IEC) proliferation and differentiation, although the mechanisms involved remain unclear. Recently, we demonstrated that infection by the attaching and effacing murine pathogen Citrobacter rodentium leads to a significant reduction in colonic goblet cell numbers (goblet cell depletion). This pathology depends on T and/or B cells, as Rag1−/− mice do not suffer this depletion during infection, instead suffering high mortality rates. To address the immune mechanisms involved, we reconstituted Rag−/− mice with either CD4+ or CD8+ T cells. Both T cell subsets increased Rag1−/− mouse survival during infection, with mice that received CD8+ T cells developing colonic ulcers but not goblet cell depletion. In contrast, mice that received CD4+ T cells showed goblet cell depletion in concert with exaggerated IEC proliferation. To define the possible involvement of T cell-derived cytokines, we infected gamma interferon receptor gene knockout (IFN-γR−/−) mice and wild-type mice given interleukin 17A (IL-17A) neutralizing antibodies and found that IFN-γ signaling was required for both goblet cell depletion and increased IEC proliferation. Immunostaining revealed that C. rodentium cells preferentially localized to nonhyperplastic crypts containing numerous goblet cells, whereas hyperplastic, goblet cell-depleted crypts appeared protected from infection. To address whether goblet cell depletion benefits the C. rodentium-infected host, we increased goblet cell numbers using the γ-secretase inhibitor dibenzazepine (DBZ), which resulted in greatly increased pathogen burdens and mortality rates. These results demonstrate that goblet cell depletion reflects host immunomodulation of IEC homeostasis and reflects a novel host defense mechanism against mucosal-adherent pathogens.
INTRODUCTION
The intestinal epithelium is comprised of several distinct epithelial cell types, each defined by its individual structure and function and contributing to the overall absorption and secretory capability of the gut. To maintain intestinal health, the gastrointestinal tract requires its epithelial layer to be selectively permeable so as to allow efficient digestion and absorption of nutrients (1). It also needs to provide a stringent defensive and discriminating barrier to ward off potential opportunistic pathogens or even commensal microbes from damaging the underlying mucosa (2, 3). The intestinal epithelium can be delineated into two specialized cell types, absorptive enterocytes, which make up the majority of the epithelium, and the less-abundant secretory cells, which include enteroendocrine cells, Paneth cells, and goblet cells (4). Of these epithelial subtypes, goblet cells are the most numerous in the colon and rectum and play a key role in maintaining intestinal barrier function through the release of structural mucins, such as Muc2, as well as proteins that modulate epithelial repair and function, such as Trefoil factor 3 (TFF3) and RELM-β (5–7).
Another hallmark of the intestinal epithelium is its rapid turnover, with its entire replacement in humans occurring within 3 to 5 days under normal conditions. Such rapid turnover highlights how important the proper regulation of epithelial cell differentiation, migration, and luminal sloughing is to the maintenance of intestinal health and homeostasis (8, 9). Moreover, the typical cellular distribution and arrangement of cell types within the epithelial layer undergo dramatic changes during many forms of gastrointestinal disease. For example, human inflammatory bowel diseases (IBD), as well as many forms of infectious colitis, are associated with the depletion (or loss) of goblet cells and/or a functional diminishment in their secretory profile (10, 11). While goblet cell depletion can also be modeled in murine models of chemically induced (dextran sulfate sodium) colitis, as well as the infectious colitis caused by the attaching/effacing (A/E) bacterial pathogen Citrobacter rodentium, it is unclear at this time why goblet cells are depleted, how the depletion is mediated, and what effect their depletion may have on colitis progression and/or host defense (12–14).
Goblet cells most notably generate the mucus layer that lines the intestinal epithelium through the secretion of the heavily O-glycosylated mucin protein, Muc2. Several groups have shown that the addition of mucus (Muc2) to epithelial cells in culture inhibits the ability of bacterial pathogens to adhere to the epithelial cells and/or invade them (15, 16). Conversely, mice lacking Muc2 show increased susceptibility to colitis and suffer increased contact between their intestinal epithelium and luminal microbes, thereby promoting intestinal inflammation (17). In addition, bacterial pathogens frequently express proteases that are able to cleave Muc2, suggesting that the mucus layer presents a protective barrier that needs to be overcome for successful pathogenesis (18, 19). In addition to the protective mucus layer, goblet cell-derived proteins, such as TFF3 and RELM-β, promote epithelial repair and/or antimicrobial function after their secretion into the neighboring lumen (20–22). Despite these protective actions, it is unclear whether goblet cell function is always protective, since the mucus layer may also provide a nutrient source for pathogenic microbes (23).
We recently showed that C. rodentium infection of mice results in the depletion of colonic goblet cells and their mucins; interestingly, the depletion was not seen in infected mice lacking T and B cells (13). This immune cell requirement led our group to investigate the role of CD4+ versus CD8+ T cells in modulating goblet cell depletion and how this may relate to epithelial turnover and, ultimately, to protection against C. rodentium-induced colitis. In the current study, we demonstrate that CD4+ T cells drive C. rodentium-induced intestinal epithelial cell (IEC) hyperproliferation and goblet cell depletion, likely through a Th1-driven response that depends on gamma interferon (IFN-γ). The epithelial cell hyperproliferation and goblet cell depletion are clearly associated with survival of the host and, ultimately, clearance of the pathogen, whereas use of the notch inhibitor dibenzazepine (DBZ) increased goblet cell numbers, impairing host defense and leading to poor survival of infected hosts.
MATERIALS AND METHODS
Mice.
Six- to 8-week-old C57BL/6 mice were obtained from the UBC Center for Disease Modeling (British Columbia, Canada), and Rag1−/− (on a C57BL/6 background) and gamma interferon receptor gene knockout (IFN-γR−/−) mice with appropriate controls were purchased from Jackson Laboratories (Bar Harbor, ME). Mice were kept in sterilized, filter-topped cages and fed autoclaved food and water while being routinely monitored and tested for common pathogens. The protocols employed were approved by the University of British Columbia's Animal Care Committee and were in direct accordance with guidelines drafted by the Canadian Council on the Use of Laboratory Animals.
Bacterial strains and infection of mice.
Mice were inoculated by oral gavage with 100 μl of wild-type C. rodentium (formerly known as Citrobacter freundii biotype 4280 strain DBS100) culture grown in Luria broth overnight at 37°C and used at a concentration of 2.5 × 108 CFU.
Tissue collection.
Mice were anesthetized with isofluorane and euthanized at 12 to 15 days postinfection or after losing approximately 15% of their initial body weight and showing signs of significant morbidity (piloerection, hunching, and/or shaking). Colons, ceca, spleens, mesenteric lymph nodes, and livers were all excised and stored in either 10% neutral buffered formalin (Fisher) or 4% paraformaldehyde. Formalin-fixed tissues were paraffin embedded and sectioned by the histology laboratory at the Child and Family Research Institute (CFRI). The paraformaldehyde-fixed tissues were washed in phosphate-buffered saline (PBS) and then embedded in Shandon Cryomatrix embedding medium (Thermoelectron Corporation) and subsequently frozen by partial immersion in liquid N2-precooled 2-methylbutane. Additional tissue samples were stored in RNAlater (Qiagen) at −80°C. To enumerate bacterial loads, colon and cecum tissues were collected separately, homogenized in PBS, serially diluted, and plated onto LB agar dishes, and colonies were enumerated.
RNA extraction and quantitative RT-PCR.
Colon tissues stored in RNAlater (Qiagen) at −86°C were thawed on ice and weighed, and total RNA was extracted using a Qiagen RNeasy kit following the manufacturer's instructions. Total RNA was quantified using a Bio-Rad SmartSpec (Bio-Rad), and 1 to 2 μg of RNA was reverse transcribed using a Qiagen Omniscript reverse transcription (RT) kit (Qiagen) according to the manufacturer's instructions. Agarose gels were stained with SYBR safe DNA gel stain (Molecular Probes) and visualized with a Chemi Doc XRS system (Bio-Rad). For quantitative PCR, Bio-Rad supermix was used at a 1:2 dilution, and real-time PCR was carried out using a Bio-Rad MJ MiniOpticon according to the manufacturer's instructions. Quantitation was carried out using GeneEx Macro OM 3.0 software.
Histological staining.
Briefly, 5-μm paraffin sections were deparaffinized by heating them at 55 to 65°C for 10 min and then cleared with xylene and rehydrated through an ethanol gradient to water. For periodic acid-Schiff (PAS) staining, standard histological techniques were used. Rat antisera against C. rodentium Tir (1:500; a gift from W. Deng), anti-Muc2 (H-300, 1:100), rabbit anti-CD4 (GK 1.5, 1:200), -CD3 (ab5690, 1:100), and -CD8 (53.67, 1:200), and anti-Ki67 (CP249B, 1:100) were used as primary antibodies and were diluted in PBS containing 1% bovine serum albumin. Following 0.2% Triton X-100 (Sigma) permeabilization, immunofluorescent labeling for all stains was carried out with the appropriate secondary antibody using Alexa Fluor 488-conjugated goat anti-rat IgG, Alexa Fluor 568-conjugated goat anti-rabbit IgG, or Alexa Fluor 568-conjugated goat anti-rat IgG (Invitrogen). Tissues were mounted using ProLong gold antifade plus DAPI (4′,6′-diamidino-2-phenylindole) (Invitrogen) for DNA staining. Sections were captured with a Zeiss AxioImager microscope equipped with an AxioCam HRm camera operating through AxioVision software (version 4.4).
Histopathological scoring.
To assess tissue pathology, paraffin-embedded colonic-tissue sections (5 μm) were stained with hematoxylin and eosin (H&E) and then examined by two blinded observers. For C. rodentium infection, tissue sections were assessed for submucosal edema (0 = no change, 1 = mild, 2 = moderate, and 3 = profound), epithelial hyperplasia (scored based on percentage above the height of the control, where 0 = no change, 1 = 1 to 50%, 2 = 51 to 100%, and 3 = >100%), epithelial integrity (0 = no change, 1 = <10 epithelial cells shedding per lesion, 2 = 11 to 20 epithelial cells shedding per lesion, 3 = epithelial ulceration, and 4 = epithelial ulceration with severe crypt destruction), and neutrophil and mononuclear cell infiltration (0 = none, 1 = mild, 2 = moderate, and 3 = severe), as previously described. The maximum score that could be obtained with this system was 13 points.
Reconstitution of Rag1−/− mice with CD4+ and CD8+ T cells.
The adaptive immune system was partially reconstituted in Rag1−/− mice using splenic and mesenteric lymph node (MLN) populations of CD4+ or CD8+ T cells. In brief, wild-type immunocompetent mice were euthanized, and their spleens and MLNs were aseptically removed. Spleens and MLNs were placed in RPMI medium with 10% fetal bovine serum, mashed to a pulp with the rubber end of the plunger from a 1.0-ml syringe, and then forced through a 70-μm-pore-size filter (BD Biosciences), generating a single-cell suspension. Following two washes with RPMI medium, cells were pelleted and then resuspended in PBS. Cells were then bound to biotinylated primary antibodies specific for either CD4 or CD8, which in turn were bound to streptavidin-coated magnetic beads and separated using the Miltenyi MiniMACs apparatus. Cells were then counted, and viability was analyzed by trypan blue exclusion. Recipient Rag1−/− mice were then inoculated via the tail vein with 2 × 108 viable T cells. Mice were left for 8 weeks and then tested for the success of reconstitution by staining colonic tissue sections for the presence of T lymphocytes using the marker CD3.
IL-17A neutralization.
C57BL/6 mice were treated with three injections of monoclonal anti-interleukin 17A (IL-17A) antibody (R&D) at days 5, 7, and 10 postinfection. Each intraperitoneal (i.p.) injection consisted of 100 μg per mouse diluted in 100 μl PBS.
Notch inhibition.
Notch signaling in infected mice was inhibited using the γ-secretase inhibitor DBZ, obtained from Axon MedChem (catalog number Axon 1488). Each mouse was given three i.p. injections of 10 μM/kg of body weight at days 9, 10, and 11 postinfection. DBZ was delivered in a vehicle consisting of 10% dimethyl sulfoxide (DMSO) in PBS.
Statistical analysis.
Statistical significance was calculated by using either a two-tailed Student t test or the Mann-Whitney t test as indicated below, with assistance from GraphPad Prism software (version 4.00; GraphPad Software, San Diego, CA). A P value of less than 0.05 was considered significant. The results were expressed as means and standard errors of the means (SEM) unless indicated otherwise.
RESULTS
CD4+ and CD8+ T cell reconstitution reduces infection-induced mortality.
As outlined previously, C. rodentium infection is known to promote goblet cell depletion in the colon of mice, whereas this pathology is not seen in mice lacking T and B cells. We confirmed this observation, finding that immunocompetent C57BL/6 mice develop an approximately 60% decrease in intestinal goblet cell numbers in the distal colon by day 10 postinfection (13). In contrast, this goblet cell depletion is not seen in infected mice lacking T and B cells (Rag1−/− mice). Moreover, Rag1−/− mice are known to carry significantly heavier C. rodentium burdens than C57BL/6 mice, as well as rapidly succumb to infection (24). To address whether these readouts were attributable to the absence of either CD4+ or CD8+ T cells, we reconstituted Rag1−/− mice with CD4+ and CD8+ T cells isolated from C57BL/6 mice and, after 8 weeks, orally infected these mice with C. rodentium. Throughout the infection, all mice progressively lost weight, but nonreconstituted Rag1−/− mice began to succumb to the infection by day 8, and by day 15, only 53% of the mice survived (Fig. 1A). In contrast, CD4+ and CD8+ T cell reconstitution greatly improved the outcome of infected mice, such that 86% and 93%, respectively, survived (P = 0.004 and P = 0.003), while 100% of C57BL/6 mice survived. Interestingly, this change in survival was not accompanied by changes in pathogen burdens. Regardless of survival, there was no difference in C. rodentium CFU/gram of tissue among nonreconstituted versus CD4+ and CD8+ T cell-reconstituted Rag1−/− mice at day 12 postinfection (Fig. 1B). However, control C57BL/6 mice carried comparatively smaller cecal and colonic C. rodentium burdens. Therefore, while the presence of CD4+ and CD8+ T cells reduced mortality among infected Rag1−/− mice, it was not due to changes in pathogen burdens.
Fig 1.
CD4+ and CD8+ T cell reconstitution reduces mortality in Rag1−/− mice. (A) Percent survival of Rag1−/− mice during infection is greatly reduced compared to that of CD4+ and CD8+ T cell-reconstituted and C57BL/6 control mice. Each symbol represents the mean of three independent infections. Error bars indicate ±1 SEM. Asterisks show significance at a P value of <0.05. (B) No differences in C. rodentium CFU/gram of tissue were identified among the cecae and distal colons from Rag1−/− and CD4+ and CD8+ T cell-reconstituted mice at day 12 p.i. Cecae and distal colons from control C57BL/6 mice yielded significantly lower bacterial burdens than those of Rag1−/− counterparts. Error bars indicate SEM from at least six mice. Asterisks show significance at a P value of <0.05.
CD4+ and CD8+ T cell reconstitution increases intestinal pathology.
We next addressed what role the specific T cell subsets might play in controlling colonic-tissue pathology during infection. Our initial assessment included mononuclear cell infiltration, epithelial integrity, crypt hyperplasia, and submucosal edema. Interestingly, both CD4+ and CD8+ T cell reconstitution of Rag1−/− mice dramatically worsened histological damage in the distal colon compared to the damage seen in nonreconstituted Rag1−/− mice (Fig. 2A and B). A detailed examination of the resulting pathologies revealed very different outcomes following CD4+ versus CD8+ T cell reconstitution. CD4+ T cell reconstitution led to exaggerated crypt hyperplasia compared to that in CD8+ T cell-reconstituted Rag1−/− mice, as reflected in their histological scores (2.89 ± 0.11 versus 2.2 ± 0.15, respectively; P = 0.002). However, this was offset by severe epithelial damage and mucosal ulceration found in the colons of CD8+ T cell-reconstituted mice but not in those receiving CD4+ T cells (2.89 ± 0.56 versus 0.67 ± 0.17, respectively; P = 0.002). In further analysis, we immunofluorescently stained serial colon sections for CD3 expression to determine the localization of T cells in each group of reconstituted mice. We found that CD4+ T cells tended to accumulate in clusters within the lamina propria and at the base of hyperplastic crypts, whereas CD8+ T cells were found surrounding regions of mucosal ulceration (Fig. 2C and D).
Fig 2.
Increased pathology in Rag1−/− mice reconstituted with CD4+ or CD8+ T cells. (A) Comparative pathological scores of control Rag1−/− and CD4+ and CD8+ T cell-reconstituted mice. CD4+ reconstitution greatly increased hyperplasia, while CD8+ reconstitution resulted in worsened epithelial integrity. Bars represent the average pathology scores of at least 3 experiments, each with 5 to 8 mice. Error bars indicate SEM. Asterisks show significance at a P value of <0.05. (B to D) Representative H&E staining of distal colonic tissues removed on day 12 p.i. from nonreconstituted Rag1−/− (B), CD4+ T cell-reconstituted (C), and CD8+ T cell-reconstituted (D) mice exhibiting different associated pathologies. Yellow-bordered areas are enlarged to the right. Double-headed arrows highlight increased hyperplasia in CD4+ reconstitution, while CD8+ T cell reconstitution exhibits severe focal ulceration. (C and D) Anti-CD3 staining (red) in serial sections showing aggregates of CD3-positive cells (arrows) in the lamina propria and regions of mucosal ulceration. Original magnification was ×200. Scale bars = 50 μm.
Only CD4+ T cell reconstitution restores goblet cell depletion, crypt hyperplasia, and proliferation in Rag1−/− mice.
Our next step was to determine whether CD4+ and CD8+ T cell reconstitution affected goblet cells by examining colon tissue sections processed for H&E staining. Goblet cell numbers, their morphology, and their distribution in the colon, as well as their proliferation in Rag1−/− mice following C. rodentium infection, were markedly different than those seen in infected wild-type C57BL/6 mice. Furthermore, reconstitution of CD4+ and CD8+ T cells yielded dramatically different results (Fig. 3A). We enumerated goblet cells per 100 enterocytes, and as noted previously, Rag1−/− mice maintained baseline-like distribution and numbers of goblet cells in the distal colon by day 12 postinfection (21.8 ± 2.6 goblet cells), whereas C57BL/6 mice showed significant depletion of mucin-filled goblet cells (3.5 ± 1.3 goblet cells; P < 0.001) (Fig. 3B). Strikingly, reconstitution of Rag1−/− mice with CD4+ T cells led to partial but significant restoration of goblet cell depletion by day 12 postinfection (11.0 ± 2.4 goblet cells; P = 0.005). However, the restored goblet cell depletion was patchier than that seen in wild-type mice, since, although most crypt regions showed significant goblet cell depletion, in other crypt regions, numerous mature goblet cells remained. On the other hand, CD8+ T cell reconstitution, regardless of mucosal damage, resulted in no overt change in goblet cell numbers (20.8 ± 2.6 goblet cells). While focal ulcerated regions displayed high levels of goblet cell depletion due to eradication of crypt architecture, overall, CD8+ T cell reconstitution had no effect on goblet cell numbers. Next, we measured colonic crypt lengths in these mice and found that CD4+ T cell reconstitution increased crypt lengths by 54.4% ± 6.2%, which was not seen following CD8+ T cell reconstitution (Fig. 3C). Restoration of crypt lengths was also accompanied by significantly increased numbers of Ki67-positive cells per crypt in mice reconstituted with CD4+ T cells (Fig. 3D). The expression of Ki67 protein is strictly associated with cell proliferation and used by many groups as a putative marker for proliferation (25). Interestingly, this appears to be a partial restoration of the massive proliferative response seen in C57BL/6 mice, again showing a patchy appearance. These results demonstrate that CD4+ T cells but not CD8+ T cells regulate goblet cell depletion, crypt hyperplasia, and IEC proliferation during C. rodentium infection.
Fig 3.
Restored hyperplasia, proliferation, and goblet cell depletion in CD4+ T cell-reconstituted Rag1−/− mice. (A) Representative H&E and anti-Ki67 (red) staining of day-12-p.i. distal colons from nonreconstituted Rag1−/− and CD4+ and CD8+ T cell-reconstituted mice. Carets indicate representative mature goblet cells. Original magnification was ×200. Scale bars = 50 μm. (B) Quantification of goblet cells per 100 intestinal epithelial cells (IEC) in distal colons of mice at day 12 p.i. Bars represent the means of 3 experiments, each with 5 to 8 mice, accounting for 20 sections. (C) Fold increase in crypt lengths in distal colons of mice at day 12 p.i. Bars represent mean fold increase of crypt length relative to crypt length in Rag1−/− mice. (D) Enumeration of Ki67-positive cells per crypt in distal colons of mice at day 12 p.i. Bars represent the mean number of Ki67-positive cells per crypt. Error bars indicate SEM. Asterisks show significance at a P value of <0.05.
CD4+ T cell-reconstituted Rag1−/− mice show decreased Muc2, Tff3, and Relm-β but increased IFN-γ and IL-17A.
Further investigation of goblet cell depletion led us to assess alterations in the mRNA transcript levels of goblet cell mediators. We measured differences in Muc2, Tff3, and Relm-β mRNA levels by quantitative PCR. Nonreconstituted mice displayed high levels of Muc2, Tff3, and Relmβ, while reconstitution with CD4+ significantly decreased Muc2 levels, by 10-fold (Fig. 4). As well, a similar trend of decreased mRNA levels was found for Tff3 and Relmβ in CD4+-reconstituted mice. In addition, we measured mRNA transcript levels for IFN-γ and IL-17A and found an increase in both of these cytokines in mice reconstituted with CD4+ T cells.
Fig 4.
CD4+ T cell reconstitution affects mRNA transcript levels during C. rodentium infection. Bars represent mean mRNA transcript levels of Muc2, Tff3, Relm-β, IFN-γ, and IL-17A relative to transcript levels of control glyceraldehyde-3-phosphate dehydrogenase (GAPDH) determined by quantitative RT-PCR in 3 independent experiments. Significant differences in gene expression of Muc2, IFN-γ, and IL-17A were found with CD4+ reconstitution. Error bars indicate SEM. Asterisks show significance at a P value of <0.05.
IFN-γ receptor signaling regulates goblet cell depletion during C. rodentium infection.
C. rodentium is widely known to induce a Th1 and Th17 immune response, resulting in increased populations of IFN-γ- and IL-17A-producing CD4+ T cells within the infected colonic mucosa (26, 27). To determine whether IFN-γ signaling promotes the goblet cell depletion seen during infection, we analyzed goblet cell numbers in IFN-γR−/− mice under both uninfected and infected conditions. Interestingly, under uninfected conditions, IFN-γR−/− mice had higher numbers of mature goblet cells than uninfected C57BL/6 mice (16.0 ± 0.7 versus 11.2 ± 0.4 goblet cells/100 enterocytes, respectively; P < 0.001) (Fig. 5A). Furthermore, after 12 days of infection, C. rodentium induced neither crypt hyperplasia nor goblet cell depletion in IFN-γR−/− mice compared to the crypt architecture and goblet cell numbers in the wild-type mice (17.4 ± 1.9 versus 2.0 ± 0.3 goblet cells/100 enterocytes; P < 0.001). Interestingly, loss of IFN-γ-dependent signaling abrogated the typical infection-induced crypt hyperplasia and goblet cell depletion (Fig. 5B). In contrast, the induction of other pathologies (mononuclear cell infiltration, loss of epithelial integrity, and submucosal edema) associated with C. rodentium infection was not impaired in IFN-γR−/− mice (data not shown).
Fig 5.
IFN-γ signaling affects goblet cell depletion and IEC proliferation during C. rodentium infection. (A) Quantification of goblet cells per 100 IEC in C57BL/6 and IFN-γR−/− mice with or without C. rodentium infection. Error bars indicate SEM. Asterisks show significance at a P value of <0.05. (B) Distal colons stained with H&E and anti-Ki67 (red), showing high numbers of goblet cells (H&E) and reduced proliferation (Ki67). Carets indicate representative mature goblet cells. Original magnification was ×200. Scale bars = 50 μm. (C) C57BL/6 mice treated with IL-17A neutralizing antibody (anti-IL-17A) carried heavier C. rodentium burdens than control mice at day 12 p.i. Horizontal bars and error bars indicate means and SEM from at least six mice. (D) H&E staining of distal colons of control and IL-17A-neutralized mice at day 12 p.i. No changes in goblet cell number or hyperplasia were observed. Original magnification was ×200. Scale bars = 50 μm.
Mice treated with IL-17A neutralizing antibody still exhibit goblet cell depletion.
In addition to our investigation of IFN-γ, we also examined whether IL-17A modulates intestinal goblet cell numbers and architecture during C. rodentium infection. IL-17A is a critical factor in the development of Th17 responses, as well as providing mucosal protection against C. rodentium (28). By neutralizing IL-17A in infected C57BL/6 mice with a neutralizing antibody against IL-17A, we found significant, 5-fold-increased C. rodentium burdens at 12 days postinfection (1.16 ± 0.51 × 108 versus 5.00 ± 1.32 × 108 CFU/gram; P = 0.03) (Fig. 5C). However, the crypt hyperplasia and other pathology readouts in mice given the neutralizing antibody were markedly similar to those of our vehicle-injected control C57BL/6 mice. Furthermore, there was significant goblet cell depletion seen in these mice, such that the colonic tissues from the IL-17A-depleted mice were visually indistinguishable from those of mice not receiving IL-17A antagonist (4.0 ± 0.9 versus 2.9 ± 0.6 goblet cells/100 enterocytes) (Fig. 5D). Taken together, these data suggest that IL-17A does not play a major role in goblet cell depletion and crypt hyperplasia. Instead, these mechanisms appear to be governed by IFN-γ-dependent signaling.
Goblet cell depletion is not associated with changes in notch pathways.
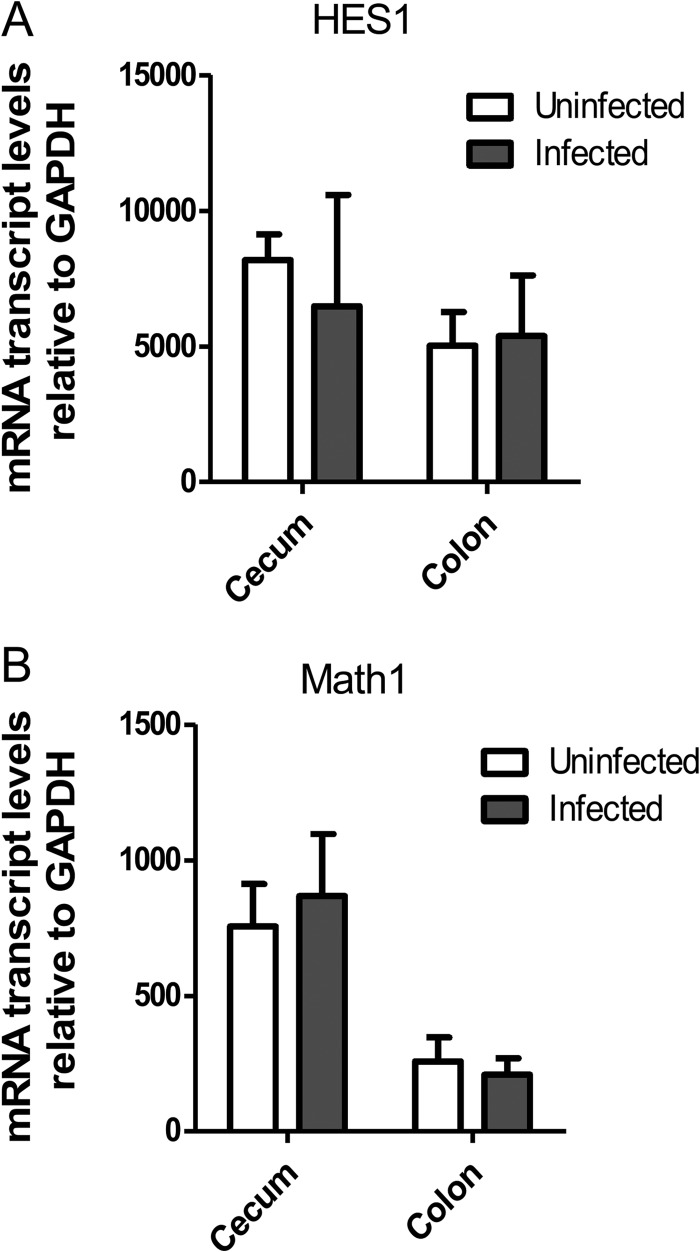
Our results show that infection-induced goblet cell depletion is driven by CD4+ T cells and IFN-γ-dependent signaling. However, it remains unclear how the host immune system alters the intestinal epithelium to induce the loss of mature goblet cells. A potential candidate is the notch pathway, as it governs the differentiation of intestinal stem cells by promoting progenitor epithelial cells toward a goblet cell lineage (29). Since notch signaling regulates HES1 expression, which suppresses the downstream factor Math1 (30), we examined the gene expression of these notch signaling components using quantitative PCR (qPCR). Notably, we found no significant differences in the HES1 and Math1 mRNA levels in uninfected and infected C57BL/6 mice (Fig. 6). In addition, we performed immunofluorescent staining for HES1 in the distal colon and found that C. rodentium infection caused no differences in the localization or abundance of the protein (data not shown). These results indicate that the changes in goblet cell numbers seen during C. rodentium infection are not governed at the level of notch-dependent differentiation.
Fig 6.
mRNA transcript levels of HES1 (A) and Math1 (B) during C. rodentium infection. Bars represent mean expression of HES1 and Math1 relative to expression of control GAPDH by quantitative RT-PCR from 3 independent experiments. No significant differences were found between tissues compared (colon versus colon, cecum versus cecum). Error bars indicate SEM.
Goblet cell depletion and crypt hyperplasia protect against C. rodentium infection.
The dramatic crypt hyperplasia seen only in CD4+ T cell-reconstituted mice led us to next examine whether increased proliferation of IEC (and their more rapid turnover) might be responsible for the goblet cell depletion. Through detailed analysis using serial sections of both H&E- and Ki67-stained tissue, we determined that 89% of crypts exhibiting goblet cell depletion also exhibit increased proliferation and hyperplasia (Fig. 7A, yellow-bordered area). On the other hand, crypts exhibiting few Ki67-positive cells and decreased hyperplasia contain mature goblet cells in high numbers (Fig. 7A, green-bordered area). We next examined whether C. rodentium preferentially localized to either goblet cell-depleted or goblet cell-filled crypts. Through costaining for the C. rodentium translocated effector Tir and the proliferative marker Ki67, we determined that C. rodentium penetrated deeply into crypts with little hyperplasia and high numbers of goblet cells (such as those in nonreconstituted Rag1−/− mice). Interestingly, crypts displaying high levels of goblet cell depletion, such as those found in Rag1−/− mice reconstituted with CD4+ T cells, only showed C. rodentium colonization on the most-apical epithelial cells at the luminal surface (Fig. 7B).
Fig 7.
Impaired goblet cell depletion is associated with deep penetration of crypts by C. rodentium cells. (A) Serial sections stained with H&E and Ki67 (red) exhibited patchy goblet cell depletion in the distal colons of CD4+ T cell-reconstituted mice at day 12 p.i. Crypts displaying goblet cell depletion also display high numbers of Ki67-positive cells (yellow-bordered area), while crypts with mature goblet cells (carets) exhibit low numbers of Ki67-positive cells (green-bordered area). Original magnification, ×200. Scale bars = 50 μm. (B and C) Immunofluorescence staining for C. rodentium translocated effector Tir (green), Ki67 (red in panel B), Muc2 (red in panel C), and DNA (blue) in distal colonic tissues at day 12 p.i. Nonreconstituted Rag1−/− mice have deeply filled crypts containing C. rodentium cells (arrows) and few Ki67-positive cells (B, left) but increased Muc2 expression (C, left). CD4+ T cell reconstitution prevents C. rodentium cells from associating deep within crypts while dramatically increasing the numbers of Ki67-positive IEC (B, right) but decreasing Muc2 expression (C, right). Original magnification was ×200. Scale bars = 50 μm.
As previously noted, colonic goblet cells produce large amounts of the mucin Muc2. To address the functional impact of goblet cell depletion, we fluorescently stained Muc2 in infected tissues and found not only decreased numbers of Muc2-expressing goblet cells in the crypts of CD4+ T cell-reconstituted mice but also decreased Muc2 staining within the colonic lumen compared to that in nonreconstituted mice (Fig. 7C). Interestingly, C. rodentium cells, visualized by staining tissues for Tir, appeared to be highly colocalized to those crypts filled with Muc2 and lined with large numbers of Muc2-positive goblet cells. Ninety-five percent of crypts deeply colonized with Tir also contained more than 10 mature goblet cells. These results suggest that either C. rodentium preferentially colonizes these Muc2-containing crypts or the pathogen is at least partially cleared from crypts that undergo increased epithelial cell proliferation and goblet cell depletion.
Overdifferentiation of goblet cells increases pathogen burdens and mortality rates.
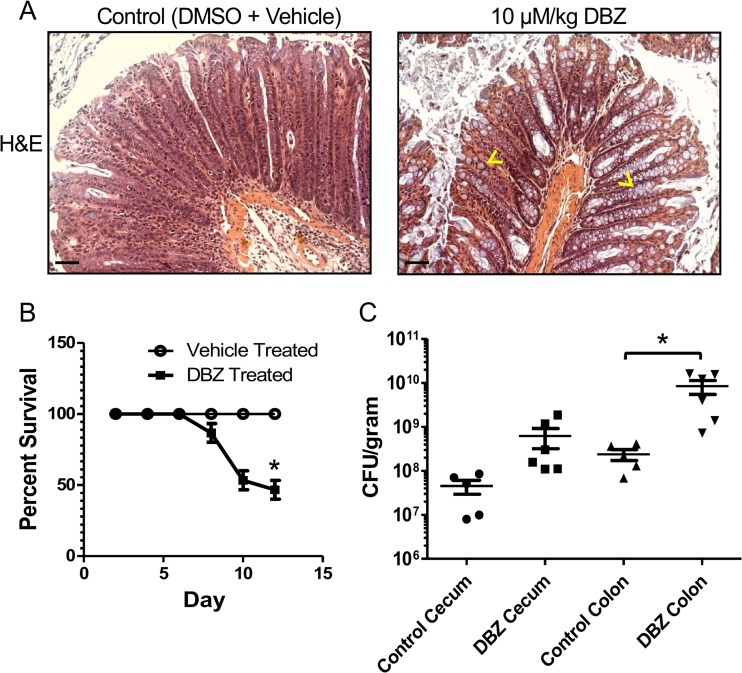
While our studies indicate that goblet cell depletion and increased epithelial cell proliferation are driven by CD4+ T cells and associated with protection against C. rodentium, the other actions of CD4+ T cells complicate our assessment of the role of these processes in providing host defense against C. rodentium. Though we already determined that notch signaling is not overtly altered in this model, notch inhibition has been shown to dramatically increase goblet cell numbers and mucus production in the intestine (31). We therefore tested the impact of notch inhibition and increasing intestinal goblet cell numbers on the course of C. rodentium infection. Notch was inhibited in mice by using serial injections of the γ-secretase inhibitor dibenzazepine (DBZ; Axon Medchem) at days 9, 10, and 11 postinfection. DBZ-treated mice exhibited a dramatic 6-fold increase in mature goblet cells, a 65% reduction in crypt length, and a thickened mucus layer (Fig. 8A). Moreover, only 46.7% ± 6.7% of mice treated with DBZ survived to day 12 postinfection, compared to 100% of vehicle-treated mice (Fig. 8B). Treatment with DBZ also led to significantly greater pathogen burdens in the colon but not the cecum (Fig. 8C), again indicating that infection-induced goblet cell depletion and increase in epithelial proliferation play an important role in protecting the host during C. rodentium infection.
Fig 8.
Treatment with γ-secretase inhibitor DBZ increases mortality and C. rodentium burden. (A) Representative H&E-stained images from infected distal colons showing increased goblet cell numbers in DBZ-treated C57BL/6 mice. Carets indicate representative mature goblet cells. Original magnification was ×200. Scale bars = 50 μm. (B) Percent survival of DBZ-treated mice is reduced beginning at day 9 p.i. Each symbol indicates the mean of three independent infections. Error bars indicate SEM. Asterisks show significance at a P value of <0.05. (C) DBZ-treated mice carry increased C. rodentium CFU/gram of tissue in distal colons at day 12 p.i. Error bars indicate SEM from at least six mice. Asterisks show significance at a P value of <0.05.
DISCUSSION
Our previous work determined that C. rodentium-induced goblet cell depletion depends on the presence of T and B cells, although the specific cell types and mechanisms driving this pathology were not determined. Here, we examined the key components and mechanisms that govern goblet cell depletion by reconstituting immunodeficient Rag1−/− mice with either CD4+ or CD8+ T cells. Our findings show that it is CD4+ T cells that drive goblet cell depletion, potentially through the actions of IFN-γ, since loss of IFN-γ signaling (but not IL-17A) abrogated the infection-induced depletion. Our results also indicate that it is increased IEC proliferation rather than alterations in notch signaling that causes goblet cell depletion. In addition, by staining tissue sections for C. rodentium, we determined that this pathogen preferentially colocalizes to crypts containing large numbers of goblet cells rather than to hyperplastic, goblet cell-depleted crypts. These data suggest that goblet cell depletion and increased IEC proliferation protect against C. rodentium infection, and correspondingly, when infected mice were given a notch inhibitor that dramatically increased goblet cell numbers and reduced IEC proliferation, the treated mice showed increased susceptibility to infection, carrying significantly heavier pathogen burdens and suffering high mortality rates.
In our reconstitution studies, we confirmed that Rag1−/− mice frequently succumb to C. rodentium infection, whereas immunocompetent C57BL/6 mice do not. Previous studies infecting mice lacking T and B cells with C. rodentium typically found such infections to be fatal, which was attributed to heavier pathogen burdens and to an inability to clear the infections (24, 32). Moreover, we previously showed that reconstituting Rag1−/− mice with both T and B cells reduced C. rodentium burdens, as well as mortality rates (13). Interestingly, we found that reconstituting Rag1−/− mice with either CD4+ or CD8+ T cells reduced their infection-induced mortality rates but did not alter pathogen burdens. This suggests that it is B cells that likely control pathogen burdens, whereas T cell reconstitution reduces mortality in this model through other mechanisms. With no change in pathogen load, we focused our attention toward the effects of reconstitution on colon architecture. Overall, we demonstrated that CD4+ and CD8+ T cell reconstitution led to distinct colonic pathologies not found in nonreconstituted Rag1−/− mice. Reconstitution with either T cell subtype led to grossly enhanced histological damage, with CD8+ T cell-reconstituted mice suffering patchy ulceration in their mid- and distal colon regions. Associated with these focal ulcers were aggregates of CD8+ T cells. While it is unclear whether these ulcers were directly the result of the actions of the CD8+ T cells, there was no evidence of goblet cell depletion in this group of mice.
In contrast, reconstitution with CD4+ T cells led to increased IEC proliferation, as well as dramatic goblet cell depletion and crypt hyperplasia, similar to, albeit patchier than, the colonic pathologies seen in infected C57BL/6 mice. Aside from a reduction in goblet cell numbers, we also noted a reduction in Muc2 mRNA levels, as well as reduced expression of immunoreactive Muc2 protein within goblet cells and within the colonic lumen. Since the goblet cell depletion was patchy, we wondered whether it might reflect regions where intestinal epithelial cell proliferation and turnover were greatest. Indeed, we did find a correlation between goblet cell depletion and regions showing significant crypt hyperplasia and increased IEC proliferation, as revealed by Ki67 staining. Notably, the localization of C. rodentium cells was altered by the CD4+ T cell reconstitution since, although C. rodentium cells were noted to penetrate deeply into the colonic crypts of Rag1−/− mice, they were predominantly limited to the luminal surface of those hyperplastic crypts showing goblet cell depletion in the CD4+ T cell-reconstituted mice. These findings suggest that increased IEC proliferation and/or goblet cell depletion may play a host-protective role during these infections.
C. rodentium has been widely shown to induce a strong Th1 and Th17 immune response in infected mice. While many inflammatory cytokines are upregulated in this model, we elected to study IFN-γ and IL-17A (28, 33), as these cytokines play critical host-protective roles against C. rodentium (34). While our results confirm that IL-17A limits C. rodentium burdens, neutralizing this cytokine had little impact on the colonic pathology suffered during infection. In contrast, much of the infection-induced IEC proliferation, crypt hyperplasia, and goblet cell depletion were dependent on IFN-γ signaling, since these responses were largely abrogated in infected IFN-γR−/− mice. IFN-γ is typically produced in large amounts by CD4+ T cells, and indeed, we observed that CD4+ T cell-reconstituted mice expressed much higher mRNA levels of IFN-γ than nonreconstituted Rag1−/− mice. Interestingly, goblet cell function has also been shown to be altered in Salmonella enterica serovar Typhimurium infection, albeit much more rapidly than the alteration seen in the C. rodentium model (35). Studies by Songhet et al. (35) established a role for IFN-γ receptor signaling in regulating the loss of goblet cell mucin production. While the exact mechanisms involved remain unclear, they showed that it was expression of the IFN-γ receptor by nonhematopoietic cell types (such as goblet cells) that mediated the goblet cell depletion.
The concept that loss of goblet cells and their function would protect the host from an intestinal pathogen is of course surprising, considering that many studies, including those from our laboratory, have shown that intestinal mucus plays a protective role in host defense (17, 36). For example, mice deficient in Muc2 and therefore lacking an intestinal mucus layer are highly susceptible to C. rodentium colonization, frequently succumbing to infection. Moreover, many bacterial pathogens are equipped with flagella or mucin-cleaving proteolytic enzymes that aid microbes in passing through the intestinal mucus barrier (18, 37). In fact, the success of pathogens in bypassing the intestinal mucus layer and, perhaps, even using it as a nutrient source could explain why goblet cell depletion may prove beneficial. As we noted, the goblet cell depletion is overt by days 10 to 12 postinfection, long after the pathogen has successfully colonized its host. Although the mucus layer may have delayed and limited the extent of initial pathogen colonization, by this later stage of infection, the mucus layer has failed in its role of preventing pathogen colonization. We hypothesize that in the face of heavy pathogen colonization of the intestinal mucosal surface, the exaggerated microbial stimulation of the mucosal immune system drives increased IEC proliferation as a generalized host defense mechanism. The rapid turnover of IEC, as well as the sloughing of infected IEC into the intestinal lumen, may aid in clearing mucosal-adherent pathogens like C. rodentium. Moreover, the loss of mature goblet cells and the reduced production of mucus may reduce nutrient availability for C. rodentium, again promoting its clearance.
Our findings suggest that C. rodentium-induced goblet cell depletion does not rely on notch signaling-mediated changes in goblet cell differentiation. This was somewhat unexpected, since the notch pathway and bHLH transcription factors, such as Math1, are known to play a key role in controlling the fate of intestinal epithelial progenitor cells, determining whether they differentiate into secretory lineages, such as goblet cells (30). Moreover, studies have shown that notch inhibition can prevent goblet cell depletion during dextran sulfate sodium-induced colitis (38). Instead, our findings indicate that the goblet cell depletion we observe reflects increased IEC proliferation. Our analysis of crypts displaying goblet cell depletion identified the presence of small and weakly PAS-stained cells, which we hypothesize are immature goblet cells. This observation leads us to believe that IEC proliferation may reduce the opportunity for goblet cells to mature, hence the observed goblet cell depletion in highly proliferating crypts. Proliferation, as opposed to differentiation, controls rapid expansion of the cellular pool, giving rise to increased cell numbers and accelerated IEC turnover kinetics. IEC proliferation is governed by the canonical Wnt pathway and functions both independently and cooperatively with the notch pathway (39). Other groups have provided insight into the role played by changes in IEC turnover kinetics in goblet cell maturation. For example, deletion of Kruppel-like factor 4 can result in the upregulation of genes in the Wnt pathway, increasing IEC proliferation and thereby leading to quicker migration of goblet cells along the crypt axis, ultimately leading to their shedding into the lumen (40). While more work is required to determine the interplay and potential interdependence between IEC proliferation and goblet cell depletion, our study shows a clear association and protective role for both processes.
In conclusion, we demonstrate that the goblet cell depletion seen during C. rodentium infection reflects the immunomodulation of the intestinal epithelium by CD4+ T cells. The IFN-γ-signaling-dependent goblet cell depletion appears to reflect increased IEC proliferation and turnover, thereby limiting the ability of goblet cells to reach maturity. Ultimately, the goblet cell depletion and increased IEC proliferation are associated with protection against C. rodentium and may reflect a generalized host response against mucosal pathogens. Defining the exact mechanisms by which these responses help clear C. rodentium infection from the mucosal surface may offer insights into the development of new approaches to combat mucosal-adherent bacterial pathogens.
ACKNOWLEDGMENTS
This work was supported by operating grants to B.A.V. from the Canadian Institutes for Health Research (CIHR) and the Crohn's and Colitis Foundation of Canada (CCFC). J.M.C. and G.B. were both funded by CIHR Masters awards. H.P.S. was funded by a CFRI studentship, while N.R. was funded by a CIHR Vanier award. B.A.V. is the Children with Intestinal and Liver Disorders (CHILD) Foundation Chair in Pediatric Gastroenterology Research and the Canada Research Chair in Pediatric Gastroenterology.
Footnotes
Published ahead of print 7 October 2013
REFERENCES
- 1.Ashida H, Ogawa M, Kim M, Mimuro H, Sasakawa C. 2012. Bacteria and host interactions in the gut epithelial barrier. Nat. Chem. Biol. 8:36–45 [DOI] [PubMed] [Google Scholar]
- 2.Moens E, Veldhoen M. 2012. Epithelial barrier biology: good fences make good neighbours. Immunology 135:1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goto Y, Kiyono H. 2012. Epithelial barrier: an interface for the cross-communication between gut flora and immune system. Immunol. Rev. 245:147–163 [DOI] [PubMed] [Google Scholar]
- 4.Karam SM. 1999. Lineage commitment and maturation of epithelial cells in the gut. Front. Biosci. 4:D286–D298 [DOI] [PubMed] [Google Scholar]
- 5.Allen A, Hutton DA, Pearson JP. 1998. The MUC2 gene product: a human intestinal mucin. Int. J. Biochem. Cell Biol. 30:797–801 [DOI] [PubMed] [Google Scholar]
- 6.Taupin D, Podolsky DK. 2003. Trefoil factors: initiators of mucosal healing. Nat. Rev. Mol. Cell Biol. 4:721–732 [DOI] [PubMed] [Google Scholar]
- 7.Artis D, Wang ML, Keilbaugh SA, He W, Brenes M, Swain GP, Knight PA, Donaldson DD, Lazar MA, Miller HR, Schad GA, Scott P, Wu GD. 2004. RELMbeta/FIZZ2 is a goblet cell-specific immune-effector molecule in the gastrointestinal tract. Proc. Natl. Acad. Sci. U. S. A. 101:13596–13600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van der Flier LG, Clevers H. 2009. Stem cells, self-renewal, and differentiation in the intestinal epithelium. Annu. Rev. Physiol. 71:241–260 [DOI] [PubMed] [Google Scholar]
- 9.Cliffe LJ, Humphreys NE, Lane TE, Potten CS, Booth C, Grencis RK. 2005. Accelerated intestinal epithelial cell turnover: a new mechanism of parasite expulsion. Science 308:1463–1465 [DOI] [PubMed] [Google Scholar]
- 10.Smithson JE, Campbell A, Andrews JM, Milton JD, Pigott R, Jewell DP. 1997. Altered expression of mucins throughout the colon in ulcerative colitis. Gut 40:234–240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Morita H, Kettlewell MG, Jewell DP, Kent PW. 1993. Glycosylation and sulphation of colonic mucus glycoproteins in patients with ulcerative colitis and in healthy subjects. Gut 34:926–932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Johansson ME, Gustafsson JK, Sjoberg KE, Petersson J, Holm L, Sjovall H, Hansson GC. 2010. Bacteria penetrate the inner mucus layer before inflammation in the dextran sulfate colitis model. PLoS One 5:e12238. 10.1371/journal.pone.0012238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bergstrom KS, Guttman JA, Rumi M, Ma C, Bouzari S, Khan MA, Gibson DL, Vogl AW, Vallance BA. 2008. Modulation of intestinal goblet cell function during infection by an attaching and effacing bacterial pathogen. Infect. Immun. 76:796–811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Luperchio SA, Schauer DB. 2001. Molecular pathogenesis of Citrobacter rodentium and transmissible murine colonic hyperplasia. Microbes Infect. 3:333–340 [DOI] [PubMed] [Google Scholar]
- 15.Deplancke B, Gaskins HR. 2001. Microbial modulation of innate defense: goblet cells and the intestinal mucus layer. Am. J. Clin. Nutr. 73:1131S–1141S [DOI] [PubMed] [Google Scholar]
- 16.Meyer-Hoffert U, Hornef MW, Henriques-Normark B, Axelsson LG, Midtvedt T, Putsep K, Andersson M. 2008. Secreted enteric antimicrobial activity localises to the mucus surface layer. Gut 57:764–771 [DOI] [PubMed] [Google Scholar]
- 17.Bergstrom KS, Kissoon-Singh V, Gibson DL, Ma C, Montero M, Sham HP, Ryz N, Huang T, Velcich A, Finlay BB, Chadee K, Vallance BA. 2010. Muc2 protects against lethal infectious colitis by disassociating pathogenic and commensal bacteria from the colonic mucosa. PLoS Pathog. 6:e1000902. 10.1371/journal.ppat.1000902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lidell ME, Moncada DM, Chadee K, Hansson GC. 2006. Entamoeba histolytica cysteine proteases cleave the MUC2 mucin in its C-terminal domain and dissolve the protective colonic mucus gel. Proc. Natl. Acad. Sci. U. S. A. 103:9298–9303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moncada D, Keller K, Ankri S, Mirelman D, Chadee K. 2006. Antisense inhibition of Entamoeba histolytica cysteine proteases inhibits colonic mucus degradation. Gastroenterology 130:721–730 [DOI] [PubMed] [Google Scholar]
- 20.Podolsky DK, Gerken G, Eyking A, Cario E. 2009. Colitis-associated variant of TLR2 causes impaired mucosal repair because of TFF3 deficiency. Gastroenterology 137:209–220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.He W, Wang ML, Jiang HQ, Steppan CM, Shin ME, Thurnheer MC, Cebra JJ, Lazar MA, Wu GD. 2003. Bacterial colonization leads to the colonic secretion of RELMbeta/FIZZ2, a novel goblet cell-specific protein. Gastroenterology 125:1388–1397 [DOI] [PubMed] [Google Scholar]
- 22.Hogan SP, Seidu L, Blanchard C, Groschwitz K, Mishra A, Karow ML, Ahrens R, Artis D, Murphy AJ, Valenzuela DM, Yancopoulos GD, Rothenberg ME. 2006. Resistin-like molecule beta regulates innate colonic function: barrier integrity and inflammation susceptibility. J. Allergy Clin. Immunol. 118:257–268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.O'Hara AM, Shanahan F. 2006. The gut flora as a forgotten organ. EMBO Rep. 7:688–693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vallance BA, Deng W, Knodler LA, Finlay BB. 2002. Mice lacking T and B lymphocytes develop transient colitis and crypt hyperplasia yet suffer impaired bacterial clearance during Citrobacter rodentium infection. Infect. Immun. 70:2070–2081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Scholzen T, Gerdes J. 2000. The Ki-67 protein: from the known and the unknown. J. Cell. Physiol. 182:311–322 [DOI] [PubMed] [Google Scholar]
- 26.Mangan PR, Harrington LE, O'Quinn DB, Helms WS, Bullard DC, Elson CO, Hatton RD, Wahl SM, Schoeb TR, Weaver CT. 2006. Transforming growth factor-beta induces development of the T(H)17 lineage. Nature 441:231–234 [DOI] [PubMed] [Google Scholar]
- 27.Ishigame H, Kakuta S, Nagai T, Kadoki M, Nambu A, Komiyama Y, Fujikado N, Tanahashi Y, Akitsu A, Kotaki H, Sudo K, Nakae S, Sasakawa C, Iwakura Y. 2009. Differential roles of interleukin-17A and -17F in host defense against mucoepithelial bacterial infection and allergic responses. Immunity 30:108–119 [DOI] [PubMed] [Google Scholar]
- 28.Schwartz S, Beaulieu JF, Ruemmele FM. 2005. Interleukin-17 is a potent immuno-modulator and regulator of normal human intestinal epithelial cell growth. Biochem. Biophys. Res. Commun. 337:505–509 [DOI] [PubMed] [Google Scholar]
- 29.Milano J, McKay J, Dagenais C, Foster-Brown L, Pognan F, Gadient R, Jacobs RT, Zacco A, Greenberg B, Ciaccio PJ. 2004. Modulation of notch processing by gamma-secretase inhibitors causes intestinal goblet cell metaplasia and induction of genes known to specify gut secretory lineage differentiation. Toxicol. Sci. 82:341–358 [DOI] [PubMed] [Google Scholar]
- 30.Yang Q, Bermingham NA, Finegold MJ, Zoghbi HY. 2001. Requirement of Math1 for secretory cell lineage commitment in the mouse intestine. Science 294:2155–2158 [DOI] [PubMed] [Google Scholar]
- 31.van Es JH, van Gijn ME, Riccio O, van den Born M, Vooijs M, Begthel H, Cozijnsen M, Robine S, Winton DJ, Radtke F, Clevers H. 2005. Notch/gamma-secretase inhibition turns proliferative cells in intestinal crypts and adenomas into goblet cells. Nature 435:959–963 [DOI] [PubMed] [Google Scholar]
- 32.Simmons CP, Clare S, Ghaem-Maghami M, Uren TK, Rankin J, Huett A, Goldin R, Lewis DJ, MacDonald TT, Strugnell RA, Frankel G, Dougan G. 2003. Central role for B lymphocytes and CD4+ T cells in immunity to infection by the attaching and effacing pathogen Citrobacter rodentium. Infect. Immun. 71:5077–5086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Simmons CP, Goncalves NS, Ghaem-Maghami M, Bajaj-Elliott M, Clare S, Neves B, Frankel G, Dougan G, MacDonald TT. 2002. Impaired resistance and enhanced pathology during infection with a noninvasive, attaching-effacing enteric bacterial pathogen, Citrobacter rodentium, in mice lacking IL-12 or IFN-gamma. J. Immunol. 168:1804–1812 [DOI] [PubMed] [Google Scholar]
- 34.Shiomi H, Masuda A, Nishiumi S, Nishida M, Takagawa T, Shiomi Y, Kutsumi H, Blumberg RS, Azuma T, Yoshida M. 2010. Gamma interferon produced by antigen-specific CD4+ T cells regulates the mucosal immune responses to Citrobacter rodentium infection. Infect. Immun. 78:2653–2666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Songhet P, Barthel M, Stecher B, Muller AJ, Kremer M, Hansson GC, Hardt WD. 2011. Stromal IFN-gammaR-signaling modulates goblet cell function during Salmonella Typhimurium infection. PLoS One 6:e22459. 10.1371/journal.pone.0022459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Johansson ME, Phillipson M, Petersson J, Velcich A, Holm L, Hansson GC. 2008. The inner of the two Muc2 mucin-dependent mucus layers in colon is devoid of bacteria. Proc. Natl. Acad. Sci. U. S. A. 105:15064–15069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Young KT, Davis LM, Dirita VJ. 2007. Campylobacter jejuni: molecular biology and pathogenesis. Nat. Rev. Microbiol. 5:665–679 [DOI] [PubMed] [Google Scholar]
- 38.Shinoda M, Shin-Ya M, Naito Y, Kishida T, Ito R, Suzuki N, Yasuda H, Sakagami J, Imanishi J, Kataoka K, Mazda O, Yoshikawa T. 2010. Early-stage blocking of Notch signaling inhibits the depletion of goblet cells in dextran sodium sulfate-induced colitis in mice. J. Gastroenterol. 45:608–617 [DOI] [PubMed] [Google Scholar]
- 39.Nakamura T, Tsuchiya K, Watanabe M. 2007. Crosstalk between Wnt and Notch signaling in intestinal epithelial cell fate decision. J. Gastroenterol. 42:705–710 [DOI] [PubMed] [Google Scholar]
- 40.Ghaleb AM, McConnell BB, Kaestner KH, Yang VW. 2011. Altered intestinal epithelial homeostasis in mice with intestine-specific deletion of the Kruppel-like factor 4 gene. Dev. Biol. 349:310–320 [DOI] [PMC free article] [PubMed] [Google Scholar]