Abstract
Brucella abortus and Yersinia enterocolitica serotype O:9 serologically cross-react in the immune response with the host; therefore, our aim was to compare the immune responses to these two pathogens. We selected typical B. abortus and Y. enterocolitica O:9 strains to study the cytokine immune response and the histopathological changes in livers and spleens of BALB/c mice. The data showed the cytokine responses to the two strains of pathogens were different, where the average levels of granulocyte-macrophage colony-stimulating factor (GM-CSF), gamma interferon (IFN-γ), interleukin-12 (IL-12), and tumor necrosis factor alpha (TNF-α) were higher with B. abortus infections than with Y. enterocolitica O:9 infections, especially for IFN-γ, while the IL-10 level was lower and the levels of IL-1β, IL-4, IL-5, and IL-6 were similar. The histopathological effects in the livers and spleens of the BALB/c mice with B. abortus and Y. enterocolitica O:9 infections were similar; however, the pathological changes in the liver were greater with B. abortus infections, while damage in the spleen was greater with Y. enterocolitica O:9 infections. These observations show that different cytokine responses and histopathological changes occur with B. abortus and Y. enterocolitica O:9 infections.
INTRODUCTION
Brucella is a Gram-negative bacterial zoonosis pathogen, causing animal and human infections; the infections in humans can become chronic, and infected people gradually lose their capacity to work (1). Based on DNA-DNA hybridization studies, the genus Brucella comprises a single species, Brucella melitensis, and the names of five other nomenspecies, i.e., Brucella abortus, B. suis, B. ovis, B. neotomae, and B. canis were seen as heterotypic synonyms of Brucella melitensis, resulting in the designation of B. melitensis as a single species comprising 18 biovars. However, the existing vernacular names for the nomenspecies Brucella melitensis, Brucella abortus, etc., can be retained for nontaxonomic purposes to avoid confusion (2). B. abortus is responsible for bovine brucellosis, B. melitensis is the main etiologic agent of ovine and caprine brucellosis, and B. suis is responsible for swine brucellosis. These three species constitute the major pathogenic brucellae causing human clinical infections (1). Brucella infections provoke different kinds of clinical manifestations, e.g., abortion, fever, headache, general malaise, and damage to the liver, spleen, blood, and skeleton. The pathogenic mechanisms for these bacteria are primarily avoidance of killing mechanisms, allowing them to proliferate within macrophages (1).
Yersinia enterocolitica is an important Gram-negative amphixenosis bacterium, causing human and animal infections. The diseases caused by pathogenic Y. enterocolitica are primarily human diarrhea that can lead to systemic diseases, e.g., liver and spleen abscess, reactive arthritis, and erythema nodosum (3, 4). At present, 60 serotypes of Y. enterocolitica have been found, and six biotypes are classified for this bacterium. The highly pathogenic Y. enterocolitica bioserotype 1B/O:8 strain is distributed in the Americas and Japan, while other bioserotypes are less pathogenic or nonpathogenic and are distributed all over the world (5). In China, the bioserotype 2/O:9 is the major pathogenic Y. enterocolitica type and is considered the typical strain (3, 6).
The pathogenic process of Y. enterocolitica is unusual, with the organism having tropism for lymphoid tissue and the ability to invade enteroepithelial cells. In the invasive process, Y. enterocolitica selectively invades the Peyer's patches through M cells, forms microabscesses with polymorphonuclear leukocytes, and finally destroys the cytoarchitecture of the Peyer's patches and then disseminates through the lymphatic vessels (7, 8). This pathogenic ability is similar to that of B. abortus, which penetrates the mucosal epithelium and is transported within or outside phagocytic cells to the regional lymph nodes (1). B. abortus and Y. enterocolitica have the same O side chains of lipopolysaccharide (LPS), as shown by high serological cross-reactivity in several studies (9). Because of the important role of lipopolysaccharide in the cytokine immune responses of bacterial infections, in this study we performed comparisons to further understand whether the lipopolysaccharides of the two bacteria had major effects on cytokine responses. The complication of reactive arthritis caused by Y. enterocolitica infection is similar to that caused by B. abortus infection (10, 11), and the proinflammatory cytokines play a critical role in pathogenesis (12). Therefore, the allergy and immune responses of the two species have similar characteristics. We selected typical B. abortus and Y. enterocolitica serotype O:9 strains isolated from China and compared the cytokine immune responses and histopathology in livers and spleens of infected BALB/c mice.
MATERIALS AND METHODS
Bacterial strains.
B. abortus 2006018 was isolated from a patient in Zhejiang, China. The patient was a cleaning worker for a goat-carrying vehicle. Y. enterocolitica bioserotype 2/O:9 strain Ye8629 was isolated from a patient.
Animal experiments and cytokine determination.
Healthy female BALB/c mice weighing 18 to 20 g were used. The mice were randomly divided into groups of five mice each. The mice were injected intraperitoneally with 1 ml of bacterial suspension. The experimental groups were divided into three groups (high, medium, and low) based on the injection dose for each strain; every group contained 40 mice. Serum was collected at 3, 6, 9, 12, 15, 24, 48, and 72 h after injection. Each test point was designated according to the infection dose and time; e.g., for B. abortus, the high infection dose at 3 h was BH3, the medium infection dose at 3 h was BM3, and the low infection dose at 3 h was BL3. Likewise, for the Y. enterocolitica O:9 strain, the high infection dose at 3 h was YH3, etc. Physiological saline solution was injected as the control, and serum was collected at the same time for the control groups.
Suspensions were prepared from B. abortus (2006018) cultured for 24 h on brucella agar at 37°C and from Yersinia (Ye8629) cultured for 24 h on brain heart infusion agar at 25°C. The McFarland values of the suspensions were adjusted to 1.0 for 2006018 and 1.2 for Ye8629. The suspensions of 2006018 and Ye8629 were serially diluted to 10−6, and plate counts were performed for each strain. The stock suspension of the bacterium was the high dose, the 10−2 dilution was the medium dose, and the 10−4 dilution was the low dose.
Cytokines were determined using the Fluorokine MAP mouse cytokine custom premix kit using a Luminex 100 Analyzer with the X-Y platform (Luminex, Austin, TX). Cytokine levels were analyzed using Flowmetrix software, Miraibio Masterplex QT2.0 (Luminex, Austin, TX). The cytokines determined in this study were granulocyte-macrophage colony-stimulating factor (GM-CSF), gamma interferon (IFN-γ), interleukin-1β (IL-1β), IL-2, IL-4, IL-5, IL-6, IL-10, IL-12, and tumor necrosis factor alpha (TNF-α).
Histopathology.
The livers and spleens were collected from the infected mice from the cytokine experiment. Two mice in each group were anatomized for the two pathogen strains at different times; the liver and spleen were fixed with 10% neutral formaldehyde solution and then paraffin sectioned and stained with hematoxylin and eosin (H&E). The histopathological changes caused by the two bacterial pathogen strains in the livers and spleens of the mice were compared.
Statistical analysis.
The cytokine levels for each group were expressed as the mean ± standard deviation. When appropriate, the Student t test or analysis of variance (ANOVA) with the Bonferroni posttest was used for statistical comparisons.
Animal experiments.
The animal experiments in this study were approved by the animal ethical review committee of the National Institute of Communicable Disease Control and Prevention, Chinese Center for Disease Control and Prevention, Beijing, People's Republic of China.
RESULTS
Cytokines.
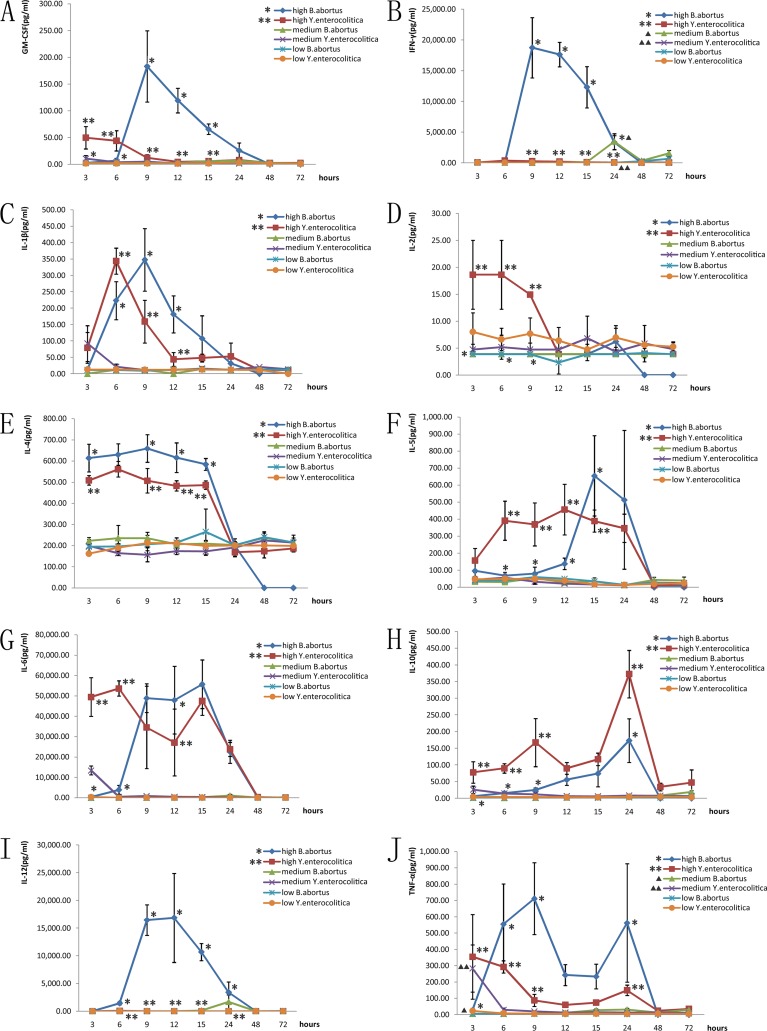
The plate counts of 2006018 and Ye8629 were, respectively, 73 CFU and 64 CFU for the 10−6 dilution; therefore, the infection dose with 2006018 for the BH (high infection dose) group was 7.3 × 108 CFU/ml, that for the BM (medium infection dose) group was 7.3 × 106 CFU/ml, and that for the BL (low infection dose) group was 7.3 × 104 CFU/ml. With Ye8629, the dose for the YH group was 6.4 × 108 CFU/ml, that for the YM group was 6.4 × 106 CFU/ml, and that for the YL group was 6.4 × 104 CFU/ml. In general, the average levels of GM-CSF, IL-12, IFN-γ, and TNF-α with the B. abortus (2006018) infection were significant higher than those with Y. enterocolitica (Ye9629), especially for IFN-γ, while the IL-10 level with B. abortus infection was lower and the levels of IL-1β, IL-4, IL-5, and IL-6 were similar for the two strains.
(i) GM-CSF.
For the 2006018 infection, only the BH9, BH12, and BH15 groups had statistically significantly higher quantities of GM-CSF than the control (P < 0.05), and for Ye8629, the quantities of GM-CSF in the YH3 and YH6 groups were significantly higher than in the control. The high infection dose groups at 3, 6, 9, 12, and 15 h had significant differences in GM-CSF between the two strains. The GM-CSF level with Ye8629 was higher than that with 2006018 before 6 h, and then the situation was reversed after 6 h (Fig. 1A).
Fig 1.
Comparison of cytokines induced by the two pathogens in BALB/c mice. (A) GM-CSF; (B) IFN-γ; (C) IL-1β; (D) IL-2; (E) IL-4; (F) IL-5; (G) IL-6; (H) IL-10; (I) IL-12; (J) TNF-α. *, statistically significant difference between the two strains in the high-infection-dose groups (P < 0.05). ▲, statistically significant difference between the two strains in the medium-infection-dose groups (P < 0.05).
(ii) IFN-γ.
The BH9, BH12, BH15, BH24, and BM24 groups infected with 2006018 showed significant differences for IFN-γ compared to the control, while all the IFN-γ values with Ye8629 were not significantly different from that for the control. The high infection dose groups at 9, 12, 15, and 24 h and the medium infection dose group at 24 h showed a difference in IFN-γ between the two strains. The levels of IFN-γ reached a maximum at 9 h and then decreased gradually, returning to the normal value after 24 h (Fig. 1B).
(iii) IL-1β.
The BH6, BH9, BH12, and BH15 groups infected with 2006108 and the YH6 and YH9 groups of Ye8629 had significantly different IL-1β values than the control. In the high infection dose groups at 6, 9, and 12 h, the IL-1β values were different for the 2006018 and Ye8629 strains. The IL-1β value with Ye8629 was higher than that with 2006018 before 6 h, and after that the values with 2006018 were higher than those with Ye8629 (Fig. 1C).
(iv) IL-2.
All the values for IL-2 with 2006018 were not significantly different from that for the control; however, these values in YH3, YH6, and YH9 groups infected with Ye8629 were significantly different from that for the control. The IL-2 values in the high infection dose groups at 3, 6, and 9 h with Ye8629 were significantly different from those with the 2006018 strain (Fig. 1D).
(v) IL-4.
The BH3, BH6, BH9, BH12, and BH15 groups infected with 2006018 and the YH3, YH6, YH9, YH12, and YH15 groups infected with Ye8629 had IL-4 levels significantly different from that for the control. The high infection dose groups for the two strains were significantly different from each other at 3, 9, 12, and 15 h; all of the IL-4 cytokine values with 2006108 were higher than those with Ye8629 (Fig. 1E).
(vi) IL-5.
The IL-5 levels in the BH15 and BH24 groups infected with 2006018 and in the YH6, YH9, YH12, YH15, and YH24 groups infected with Ye8629 were significantly different from that for the control. The high infection dose groups at 6, 9, 12, and 15 h showed significant differences in IL-5 between the two strains. The IL-5 values with Ye8629 were higher than those with 2006018 before 12 h, and then the IL-5 levels with 2006018 were higher than those with Ye8629 (Fig. 1F).
(vii) IL-6.
All of the IL-6 values for infections with 2006018 and the IL-6 values for the YH3, YH6, YH9, YH12, YH15, and YH24 groups infected with Ye8629 were significantly different from those for the control. In the high infection dose groups at 3, 6 and 12 h, the IL-6 levels were different for the two strains (Fig. 1G).
(viii) IL-10.
The BH12, BH15, and BH24 groups infected with 2006018 and the YH3, YH6, YH9, YH12, YH15, and YH24 groups infected with Ye8629 had significantly different levels of IL-10 than the control group. The levels of IL-10 for the high infection dose groups at 3, 6, 9, and 24 h were significantly different for the two strains; the cytokine levels with Ye8629 were always higher than those with 2006018 (Fig. 1H).
(ix) IL-12.
All of the IL-12 values for infections with 2006018 and Ye8629 were significantly different from the control values; however, only one IL-12 value was shown for the Ye8629 strain. In the high infection dose groups at 6, 9, 12, 15, and 24 h, the medium infection dose groups at 15, 24, 48, and 72 h, and the low infection dose group at 72 h, the IL-12 levels were different for the two strains; the values with 2006018 were higher than those with Ye8629 (Fig. 1I).
(x) TNF-α.
The BH6, BH9, BH12, BH15, and BH24 groups infected with 2006018 and the YH3, YH6, and YM3 groups infected with Ye8629 had TNF-α values significantly different from those for the control. The TNF-α levels in the high infection dose groups at 3, 6, 9, and 24 h and the medium infection dose group at 3 h were different for the 2006018 and Ye8629 strains (Fig. 1J).
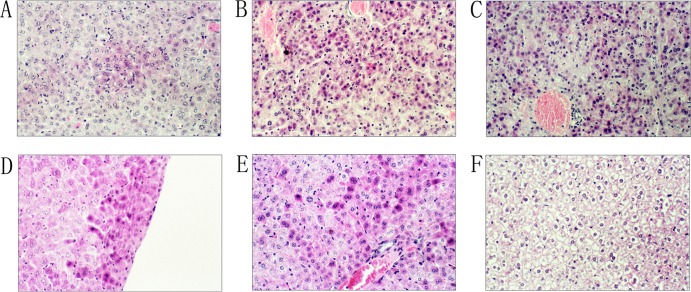
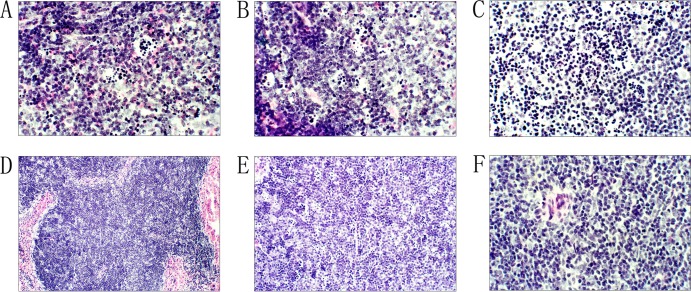
Histopathology. (i) Liver.
The pathological effects on the livers of the BALB/c mice caused by B. abortus infection were observed after 15 h in the high infection dose groups; they included denaturation of the kytoplasm acidophilia, nuclear fusion, condensed nuclei, degradation of the nuclei, liver cell necrosis, and many distributed focal changes (Fig. 2A). At 24 h, the pathological changes in the liver were diffused and distributed (Fig. 2B). At 48 h, a large area of histopathological change was observed, and this was the highest pathological stage (Fig. 2C). The pathological changes in the liver for the medium and low infection doses of B. abortus appeared at 48 h; the degree of change was slight and then disappeared.
Fig 2.
Histopathology of the liver in BALB/c mice after infection with the two pathogens. (A) B. abortus infection at 15 h; (B) B. abortus infection at 24 h; (C) B. abortus infection at 48 h; (D) Y. enterocolitica serotype O:9 infection at 3 h; (E) Y. enterocolitica serotype O:9 infection at 12 h; (F) normal liver tissue. Eyepiece magnification, ×10; objective, ×20.
For the pathogenic Y. enterocolitica serotype O:9, the pathological changes in the liver were observed at 3 h for the high infection dose group. Many focal changes occurred in the liver, including denaturation of the kytoplasm acidophilia, nuclear fusion, and condensed nuclei (Fig. 2D). At 12 h, the infections were diffused, and a large area of pathological change appeared; this was the highest pathological stage for the infection (Fig. 2E). The pathological changes of the liver for medium and low infection dose groups of Y. enterocolitica O:9 appeared at 9 h and then decreased.
(ii) Spleen.
The pathological changes in the spleen in the high B. abortus dose groups were observed at 15 h and included lymphocyte degeneration, necrosis, and formation of typical “cat eyes” at the germinal centers (Fig. 3A); the infection persisted at 24 h (Fig. 3B). At 48 h, the degree of change decreased, lymphocyte degeneration stopped, the medulla of the spleen returned, and the “cat eye” phenomenon disappeared (Fig. 3C). The degree of the change for the medium and low infection doses with B. abortus was slight; the pathological effects appeared at 24 h and then disappeared.
Fig 3.
Histopathology of the spleen in BALB/c mice after infection with the two pathogens. (A) B. abortus infection at 15 h; (B) B. abortus infection at 24 h; (C) B. abortus infection at 48 h; (D) Y. enterocolitica serotype O:9 infection at 15 h; (E) Y. enterocolitica serotype O:9 infection at 24 h; (F) normal spleen tissue. Eyepiece magnification, ×10; objective, ×20.
For the Y. enterocolitica serotype O:9 infections, the histopathological changes of the spleen appeared at 12 h for the high infection dose groups, where lesions similar to those for B. abortus were observed in the spleen (Fig. 3D); the infection persisted until 48 h. After that, the pathological changes decreased, the medulla of the spleen proliferated, and the “cat eye” phenomenon disappeared (Fig. 3E). For the medium and low infection dose groups with Y. enterocolitica O:9, slight changes in the spleen appeared at 15 h.
The holopathological effects caused by the two strains were similar for the liver and spleen infections of the BALB/c mice; however, the pathological changes in the liver were greater in the B. abortus infections, while the lesions of the spleen were greater in the Y. enterocolitica serotype O:9 infections.
DISCUSSION
In our previous study, the cross-reactive immune responses to B. abortus and Y. enterocolitica serotype O:9 were determined to be caused by identical 23- to 45-kDa lipopolysaccharides of the O side chains, a linear polymer of 1,2-linked 4,6-dideoxy-4-formamido-α-d-mannopyranosyl units (9). Because lipopolysaccharide influences the pathogenic ability and immune reaction to bacterial infections, we assayed the cytokine immune responses to the two pathogens. The data showed a large difference in cytokine response provoked by the two strains in BALB/c mice; comparing the cytokine values induced in the high infection dose groups by the two strains, the average levels of GM-CSF, IFN-γ, IL-12, and TNF-α induced by B. abortus were significantly higher than those induced by Y. enterocolitica serotype O:9, especially for IFN-γ. The secretion of IFN-γ reached a maximum value (19,000 pg/ml) at 9 h after B. abortus infection and then decreased gradually, while the secretion of IFN-γ after infection with Y. enterocolitica O:9 was not significantly different from that in the control group. B. abortus infections induced IFN-γ levels that were 104 times higher. Several studies have shown the important roles in infection and immunity of IFN-γ for B. abortus; e.g., high levels of IFN-γ were observed in animal and cell infection models, and IFN-γ made a critical contribution in chronic infection by B. abortus, promoting the immunological response of the host, and reducing or eliminating the pathogens in the tissues and cells (13–19). In abortion caused by B. abortus, there is a close relationship with high levels of IFN-γ (20). In our study, IFN-γ was the most important significant cytokine for B. abortus infection, and identical results could be found in other reports. However, those studies were all based on the B. abortus itself; no comparison with other bacteria was done. Therefore, we compared cytokine responses induced by different bacteria in our study. Compared with other cytokines, IL-10 levels induced by Y. enterocolitica O:9 were always higher than those induced by B. abortus; the secretions of IL-1β, IL-4, IL-5, and IL-6 induced by the two strains were not significantly different.
Many studies have reported the major immunogens of B. abortus, where the outer membrane proteins (OMP) were considered the major protective immunity constituents for this bacterium (21–25), with secretion of the cytokines being elicited by outer membrane proteins and not lipopolysaccharides. Our results support this because if the two strains have the same O side chains of lipopolysaccharide and the immune responses were caused by lipopolysaccharide, the same types and levels of cytokine secretion should be observed. On the contrary, a large cytokine difference was observed here between the two strains of pathogens; therefore, the major protective immunogens of B. abortus and Y. enterocolitica O:9 were different.
Brucella abortus is intracellular bacterium, which avoids killing mechanisms and proliferates within macrophages; its ability to survival in macrophages is considered to be responsible for the establishment of chronic infections (1). However, Yersinia enterocolitica is extracellular bacterium, with tropism for lymphoid tissue and resistance to the nonspecific immune response of the host, especially macrophages and polymorphonuclear leukocytes (PMN) (7, 8).
In general (26), intracellular bacterial pathogens activate NK cells and tend to induce a cell-mediated immune response. In this response, cytokines secreted by CD4+ T cells are important, notably IFN-γ, which activates macrophages to kill pathogens. In contrast, infection by extracellular bacteria induces production of humoral antibodies, and the humoral immune response is the main protective response against extracellular bacteria. Typically, the TH1 profile of cytokines is higher in response to intracellular pathogens, and the TH2 profile is higher for allergic and extracellular pathogens. The TH1 subset induces mainly IL-2, IFN-γ, TNF-α, and GM-CSF secretion, while the TH2 subset induces mainly IL-4, IL-5, IL-10, and IL-13. In particular, IL-4 is essential for the development of the TH2 response, and IFN-γ, IL-12, and IL-18 are all important in the physiology of the development of TH1 cells. The source of IL-12, one of the key mediators of TH1 differentiation, is typically macrophages or dendritic cells activated by an encounter with intracellular bacteria, with bacterial products such as LPS. TH1 development is also critically dependent on IFN-γ, which induces a number of changes, including the upregulation of IL-12 production by macrophages and dendritic cells. Just as TH1 cells require IL-12 and IFN-γ, the generation of TH2 cells depends critically on IL-4.
Several studies had shown the important roles of the cytokine response for B. abortus and Y. enterocolitica infections (15, 27–30), Cytokines regarded as key players in brucellosis are IL-12, IFN-γ, and TNF-α. IL-12 is a key cytokine produced by B cells and macrophages and leads the TH1 immune response in the host that ultimately induces the secretion of IFN-γ from T cells. For Yersinia enterocolitica, TNF-α acts on various cell types involved in the host defense mechanisms. It stimulates both macrophage and PMN microbicidal activity and acts on natural killer cells together with IL-12 to provoke the release of IFN-γ (31). Infection of monolayers of human colon epithelial cells with invasive bacteria, including Y. enterocolitica, results in the coordinate expression and upregulation of a specific array of four proinflammatory cytokines, namely, IL-8, monocyte chemotactic protein-1, GM-CSF, and TNF-α (32). The same cytokine, IL-6, is also expressed by freshly isolated human colon epithelial cells and upregulated upon infection with invasive bacteria, including Y. enterocolitica (32). It seemed that both B. abortus and Y. enterocolitica had similar cytokine response patterns with the hosts; however, little was known about the comparative degree of response for the two species.
Our results showed that the cytokine responses provoked by these two bacteria were in accord with immunological principles. The secretion of GM-CSF, IFN-γ, IL-12, and TNF-α with B. abortus was statistically higher than that with Y. enterocolitica, especially for IFN-γ; therefore, the different cytokine response reflected the different growth styles and pathogenic mechanisms of intracellular and extracellular bacteria. The similar structure of lipopolysaccharide for O-antigen or other pathogenic characteristics of the two species may contribute little to the cytokine responses to the infections.
In our previous research (33), the histopathological changes in the liver and spleen caused by different pathogenic Y. enterocolitica strains were similar: necrosis of the liver cells, condensed nuclei, and degradation were observed in liver, and lymphocyte degeneration, necrosis, and formation of typical “cat eyes” were observed in the spleen. The highly pathogenic Y. enterocolitica strain had a “cytokine storm” phenomenon, whereas the low-pathogenicity strains were unable to elicit the storm, showing that this contributed to the pathogenic abilities of the bacterium. However, in this study, the same histopathological changes in the liver and spleen were seen for B. abortus and Y. enterocolitica O:9 infections, and the typical changes were focused in the high infection dose groups; the medium and low infection dose groups did not show obvious changes. The histopathological changes in the liver were greater in B. abortus infection; the lesions of the liver appeared later but persisted for a long time and showed significant changes compared to those with Y. enterocolitica O:9. Greater pathological changes of the spleen were observed for Y. enterocolitica O:9 infection; the lesions in the spleen appeared synchronous with those for B. abortus but continued for a long time and were significant. Therefore, we consider that the histopathological changes caused by the two species possibly show a preference for tissue selection.
ACKNOWLEDGMENTS
This work was supported by the National Sci-Tech Key Project (2012ZX10004-201, 2012ZX10004-212, and 2013ZX10004203-002).
We thank Liuying Tang and Jim Nelson for critical reading of and helpful comments on the manuscript.
Footnotes
Published ahead of print 16 September 2013
REFERENCES
- 1.Ko J, Splitter GA. 2003. Molecular host-pathogen interaction in brucellosis: current understanding and future approaches to vaccine development for mice and humans. Clin. Microbiol. Rev. 16:65–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gargani G, Lopez-Merino A. 2006. International Committee on Systematic Bacteriology, Subcommittee on the Taxonomy of Brucella correspondence report (interim report 1991-1993). Int. J. Syst. Evol. Microbiol. 56:1167–1168 [DOI] [PubMed] [Google Scholar]
- 3.Gu W, Wang X, Qiu H, Luo X, Xiao D, Xiao Y, Tang L, Kan B, Jing H. 2012. Comparative antigenic proteins and proteomics of pathogenic Yersinia enterocolitica bio-serotypes 1B/O:8 and 2/O:9 cultured at 25 degrees C and 37 degrees C. Microbiol. Immunol. 56:583–594 [DOI] [PubMed] [Google Scholar]
- 4.Wang X, Gu W, Cui Z, Luo L, Cheng P, Xiao Y, Tang L, Kan B, Jing H. 2012. Multiple-locus variable-number tandem-repeat analysis of pathogenic Yersinia enterocolitica in China. PLoS One 7:e37309. 10.1371/journal.pone.0037309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang X, Li Y, Jing H, Ren Y, Zhou Z, Wang S, Kan B, Xu J, Wang L. 2011. Complete genome sequence of a Yersinia enterocolitica “Old World” (3/O:9) strain and comparison with the “New World” (1B/O:8) strain. J. Clin. Microbiol. 49:1251–1259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang X, Qiu H, Jin D, Cui Z, Kan B, Xiao Y, Xu Y, Xia S, Wang H, Yang J, Hu W, Xu J, Jing H. 2008. O:8 serotype Yersinia enterocolitica strains in China. Int. J. Food Microbiol. 125:259–266 [DOI] [PubMed] [Google Scholar]
- 7.Autenrieth IB, Kempf V, Sprinz T, Preger S, Schnell A. 1996. Defense mechanisms in Peyer's patches and mesenteric lymph nodes against Yersinia enterocolitica involve integrins and cytokines. Infect. Immun. 64:1357–1368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cornelis GR, Boland A, Boyd AP, Geuijen C, Iriarte M, Neyt C, Sory MP, Stainier I. 1998. The virulence plasmid of Yersinia, an antihost genome. Microbiol. Mol. Biol. Rev. 62:1315–1352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gu WP, Wang X, Qiu HY, Luo X, Xiao YC, Tang LY, Kan B, Xu JG, Jing HQ. 2012. Comparison of lipopolysaccharide and protein immunogens from pathogenic Yersinia enterocolitica bio-serotype 1B/O:8 and 2/O:9 using SDS-PAGE. Biomed. Environ. Sci. 25:282–290 [DOI] [PubMed] [Google Scholar]
- 10.Lahesmaa-Rantala R, Granfors K, Isomaki H, Toivanen A. 1987. Yersinia specific immune complexes in the synovial fluid of patients with yersinia triggered reactive arthritis. Ann. Rheum. Dis. 46:510–514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Toivanen A, Lahesmaa-Rantala R, Stahlberg TH, Merilahti-Palo R, Granfors K. 1987. Do bacterial antigens persist in reactive arthritis? Clin. Exp. Rheumatol. 5(Suppl 1):S25–S27 [PubMed] [Google Scholar]
- 12.Scian R, Barrionuevo P, Giambartolomei GH, De Simone EA, Vanzulli SI, Fossati CA, Baldi PC, Delpino MV. 2011. Potential role of fibroblast-like synoviocytes in joint damage induced by Brucella abortus infection through production and induction of matrix metalloproteinases. Infect. Immun. 79:3619–3632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brandao AP, Oliveira FS, Carvalho NB, Vieira LQ, Azevedo V, Macedo GC, Oliveira SC. 2012. Host susceptibility to Brucella abortus infection is more pronounced in IFN-gamma knockout than IL-12/beta2-microglobulin double-deficient mice. Clin. Dev. Immunol. 2012:589494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jiang X, Baldwin CL. 1993. Effects of cytokines on intracellular growth of Brucella abortus. Infect. Immun. 61:124–134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ko J, Gendron-Fitzpatrick A, Splitter GA. 2002. Susceptibility of IFN regulatory factor-1 and IFN consensus sequence binding protein-deficient mice to brucellosis. J. Immunol. 168:2433–2440 [DOI] [PubMed] [Google Scholar]
- 16.Murphy EA, Sathiyaseelan J, Parent MA, Zou B, Baldwin CL. 2001. Interferon-gamma is crucial for surviving a Brucella abortus infection in both resistant C57BL/6 and susceptible BALB/c mice. Immunology 103:511–518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ritchie JA, Rupper A, Cardelli JA, Bellaire BH. 2012. Host interferon-gamma inducible protein contributes to Brucella survival. Front. Cell Infect. Microbiol. 2:55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stevens MG, Pugh GW, Jr, Tabatabai LB. 1992. Effects of gamma interferon and indomethacin in preventing Brucella abortus infections in mice. Infect. Immun. 60:4407–4409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhan Y, Cheers C. 1993. Endogenous gamma interferon mediates resistance to Brucella abortus infection. Infect. Immun. 61:4899–4901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim S, Lee DS, Watanabe K, Furuoka H, Suzuki H, Watarai M. 2005. Interferon-gamma promotes abortion due to Brucella infection in pregnant mice. BMC Microbiol. 5:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Baldi PC, Giambartolomei GH, Goldbaum FA, Abdon LF, Velikovsky CA, Kittelberger R, Fossati CA. 1996. Humoral immune response against lipopolysaccharide and cytoplasmic proteins of Brucella abortus in cattle vaccinated with B. abortus S19 or experimentally infected with Yersinia enterocolitica serotype 0:9. Clin. Diagn. Lab. Immunol. 3:472–476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cha SB, Rayamajhi N, Kang ML, Lee WJ, Shin MK, Yoo HS. 2010. Comparative study of gamma interferon production in mice immunized with outer membrane proteins and whole bacteria of Brucella abortus. Jpn. J. Infect. Dis. 63:49–51 [PubMed] [Google Scholar]
- 23.Pasquevich KA, Ibanez AE, Coria LM, Garcia Samartino C, Estein SM, Zwerdling A, Barrionuevo P, Oliveira FS, Seither C, Warzecha H, Oliveira SC, Giambartolomei GH, Cassataro J. 2011. An oral vaccine based on U-Omp19 induces protection against B. abortus mucosal challenge by inducing an adaptive IL-17 immune response in mice. PLoS One 6:e16203. 10.1371/journal.pone.0016203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pollak CN, Delpino MV, Fossati CA, Baldi PC. 2012. Outer membrane vesicles from Brucella abortus promote bacterial internalization by human monocytes and modulate their innate immune response. PLoS One 7:e50214. 10.1371/journal.pone.0050214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vemulapalli R, Cravero S, Calvert CL, Toth TE, Sriranganathan N, Boyle SM, Rossetti OL, Schurig GG. 2000. Characterization of specific immune responses of mice inoculated with recombinant vaccinia virus expressing an 18-kilodalton outer membrane protein of Brucella abortus. Clin. Diagn. Lab. Immunol. 7:114–118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Goldsby RA, Kindt TJ, Osborne BA, Kuby J. 2003. Immunology, 5th ed. W. H. Freeman & Co, New York, NY [Google Scholar]
- 27.Doyle AG, Halliday WJ, Barnett CJ, Dunn TL, Hume DA. 1992. Effect of recombinant human macrophage colony-stimulating factor 1 on immunopathology of experimental brucellosis in mice. Infect. Immun. 60:1465–1472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stevens MG, Olsen SC, Palmer MV, Pugh GW., Jr 1996. Immune responses and resistance to brucellosis in mice vaccinated orally with Brucella abortus RB51. Infect. Immun. 64:4534–4541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhan Y, Cheers C. 1995. Endogenous interleukin-12 is involved in resistance to Brucella abortus infection. Infect. Immun. 63:1387–1390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhan Y, Kelso A, Cheers C. 1993. Cytokine production in the murine response to brucella infection or immunization with antigenic extracts. Immunology 80:458–464 [PMC free article] [PubMed] [Google Scholar]
- 31.Autenrieth IB, Heesemann J. 1992. In vivo neutralization of tumor necrosis factor-alpha and interferon-gamma abrogates resistance to Yersinia enterocolitica infection in mice. Med. Microbiol. Immunol. 181:333–338 [DOI] [PubMed] [Google Scholar]
- 32.Jung HC, Eckmann L, Yang SK, Panja A, Fierer J, Morzycka-Wroblewska E, Kagnoff MF. 1995. A distinct array of proinflammatory cytokines is expressed in human colon epithelial cells in response to bacterial invasion. J. Clin. Invest. 95:55–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang X, Gu W, Qiu H, Xia S, Zheng H, Xiao Y, Liang J, Jing H. 2013. Comparison of the cytokine immune response to pathogenic Yersinia enterocolitica bioserotype 1B/O:8 and 2/O:9 in susceptible BALB/C and resistant C57BL/6 mice. Mol. Immunol. 55:365–371 [DOI] [PubMed] [Google Scholar]