Abstract
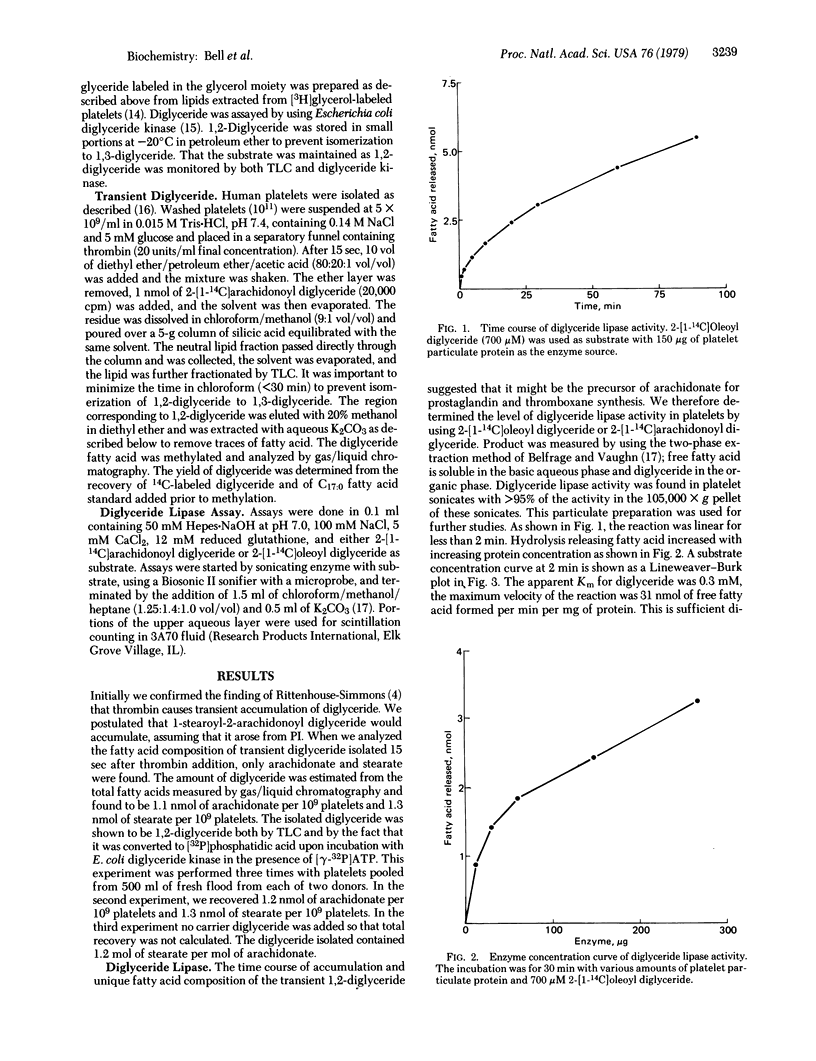
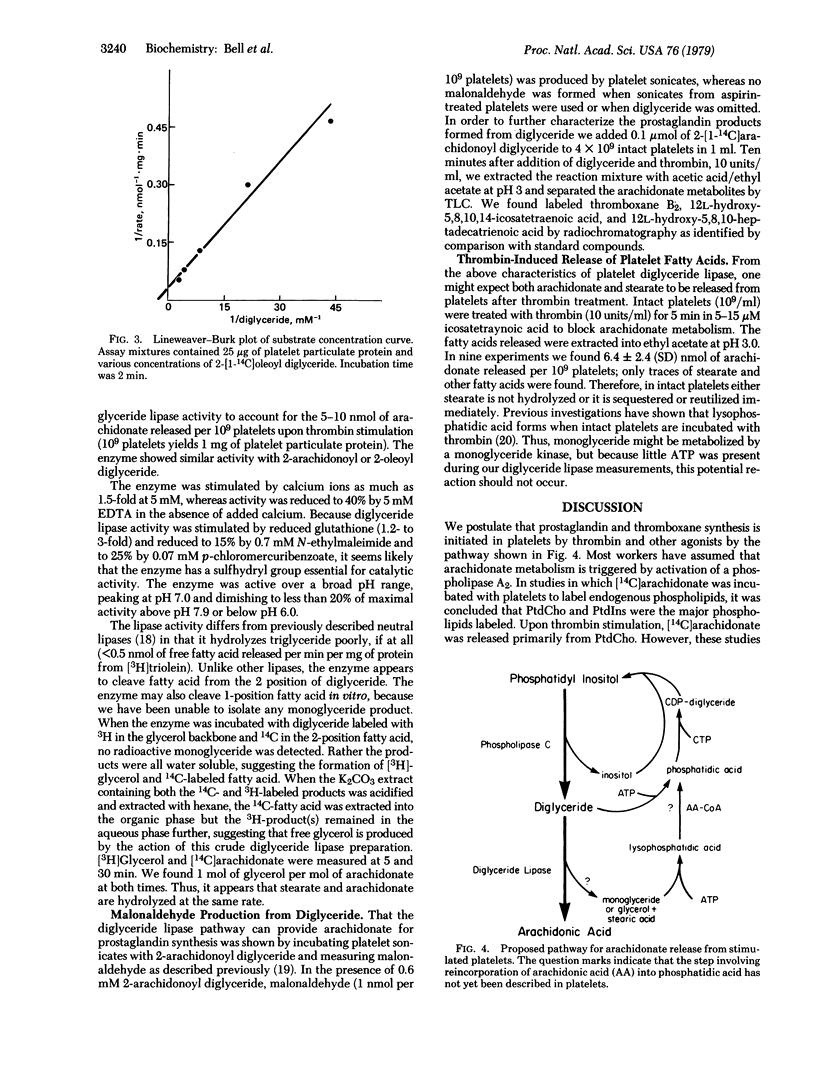
We provide evidence that the mechanism for arachidonate release from stimulated human platelets involves two enzymes: a phosphatidylinositol-specific phospholipase C (EC 3.1.4.10) and a diglyceride lipase. After incubation of platelets with thrombin for 15 seconds, 1.2 nmol of 1-stearoyl-2-arachidonoyl diglyceride per 10(9) platelets, was isolated. Arachidonate was released from this substrate by the action of diglyceride lipase located in the particulate fraction of platelets. The enzyme has a pH optimum of 7.0, is stimulated by calcium ions and reduced glutathione, and liberates 31 nmol of fatty acid per min per mg of platelet particulate protein. The diglyceride lipase has sufficient activity to account for the 5-10 nmol of arachidonate released per 10(9) platelets upon thrombin stimulation. That only arachidonate is released upon thrombin stimulation may be explained by the fact that the diglyceride substrate in platelets contains only arachidonate in the 2 position. The lipase activity found in platelet membranes can also hydrolyze the 1-position fatty acid. Stearate is not released when intact platelets are stimulated with thrombin, and the fate of this fatty acid remains to be elucidated.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baenziger N. L., Majerus P. W. Isolation of human platelets and platelet surface membranes. Methods Enzymol. 1974;31:149–155. doi: 10.1016/0076-6879(74)31015-4. [DOI] [PubMed] [Google Scholar]
- Belfrage P., Vaughan M. Simple liquid-liquid partition system for isolation of labeled oleic acid from mixtures with glycerides. J Lipid Res. 1969 May;10(3):341–344. [PubMed] [Google Scholar]
- Bills T. K., Smith J. B., Silver M. J. Selective release of archidonic acid from the phospholipids of human platelets in response to thrombin. J Clin Invest. 1977 Jul;60(1):1–6. doi: 10.1172/JCI108745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackwell G. J., Duncombe W. G., Flower R. J., Parsons M. F., Vane J. R. The distribution and metabolism of arachidonic acid in rabbit platelets during aggregation and its modification by drugs. Br J Pharmacol. 1977 Feb;59(2):353–366. doi: 10.1111/j.1476-5381.1977.tb07500.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derksen A., Cohen P. Patterns of fatty acid release from endogenous substrates by human platelet homogenates and membranes. J Biol Chem. 1975 Dec 25;250(24):9342–9347. [PubMed] [Google Scholar]
- HOKIN M. R., HOKIN L. E. Enzyme secretion and the incorporation of P32 into phospholipides of pancreas slices. J Biol Chem. 1953 Aug;203(2):967–977. [PubMed] [Google Scholar]
- Kennerly D. A., Sullivan T. J., Parker C. W. Activation of phospholipid metabolism during mediator release from stimulated rat mast cells. J Immunol. 1979 Jan;122(1):152–159. [PubMed] [Google Scholar]
- Lapetina E. G., Schmitges C. J., Chandrabose K., Cuatrecases P. Cyclic adenosine 3',5'-monophosphate and prostacyclin inhibit membrane phospholipase activity in platelets. Biochem Biophys Res Commun. 1977 Jun 6;76(3):828–835. doi: 10.1016/0006-291x(77)91575-3. [DOI] [PubMed] [Google Scholar]
- Lewis N., Majerus P. W. Lipid metabolism in human platelets. II. De novo phospholipid synthesis and the effect of thrombin on the pattern of synthesis. J Clin Invest. 1969 Nov;48(11):2114–2123. doi: 10.1172/JCI106178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little C., Aurebekk B., Otnaess A. B. Purification by affinity chromatography of phospholipase C from Bacillus cereus. FEBS Lett. 1975 Apr 1;52(2):175–179. doi: 10.1016/0014-5793(75)80800-3. [DOI] [PubMed] [Google Scholar]
- Lloyd J. V., Nishizawa E. E., Mustard J. F. Effect of ADP-induced shape change on incorporation of 32P into platelet phosphatidic acid and mono-, di- and triphosphatidyl inositol. Br J Haematol. 1973 Jul;25(1):77–99. doi: 10.1111/j.1365-2141.1973.tb01718.x. [DOI] [PubMed] [Google Scholar]
- Marcus A. J., Ullman H. L., Safier L. B. Lipid composition of subcellular particles of human blood platelets. J Lipid Res. 1969 Jan;10(1):108–114. [PubMed] [Google Scholar]
- Mauco G., Chap H., Simon M. F., Douste-Blazy L. Phosphatidic and lysophosphatidic acid production in phospholipase C-and thrombin-treated platelets. Possible involvement of a platelet lipase. Biochimie. 1978 Sep 29;60(6-7):653–661. doi: 10.1016/s0300-9084(78)80784-6. [DOI] [PubMed] [Google Scholar]
- Michell R. H. Inositol phospholipids and cell surface receptor function. Biochim Biophys Acta. 1975 Mar 25;415(1):81–47. doi: 10.1016/0304-4157(75)90017-9. [DOI] [PubMed] [Google Scholar]
- Minkes M., Stanford N., Chi M. M., Roth G. J., Raz A., Needleman P., Majerus P. W. Cyclic adenosine 3',5'-monophosphate inhibits the availability of arachidonate to prostaglandin synthetase in human platelet suspensions. J Clin Invest. 1977 Mar;59(3):449–454. doi: 10.1172/JCI108659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nugteren D. H., Hazelhof E. Isolation and properties of intermediates in prostaglandin biosynthesis. Biochim Biophys Acta. 1973 Dec 20;326(3):448–461. doi: 10.1016/0005-2760(73)90145-8. [DOI] [PubMed] [Google Scholar]
- ROBERTSON A. F., LANDS W. E. Positional specificites in phospholipid hydrolyses. Biochemistry. 1962 Sep;1:804–810. doi: 10.1021/bi00911a012. [DOI] [PubMed] [Google Scholar]
- Rittenhouse-Simmons S. Production of diglyceride from phosphatidylinositol in activated human platelets. J Clin Invest. 1979 Apr;63(4):580–587. doi: 10.1172/JCI109339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth G. J., Stanford N., Majerus P. W. Acetylation of prostaglandin synthase by aspirin. Proc Natl Acad Sci U S A. 1975 Aug;72(8):3073–3076. doi: 10.1073/pnas.72.8.3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell F. A., Deykin D. The effect of thrombin on the uptake and transformation of arachidonic acid by human platelets. Am J Hematol. 1976;1(1):59–70. doi: 10.1002/ajh.2830010107. [DOI] [PubMed] [Google Scholar]



