Abstract
We confirmed that chlorhexidine decontamination yielded more nontuberculous mycobacteria than did the N-acetyl-l-cysteine-NaOH-oxalic acid procedure from respiratory samples of cystic fibrosis patients on solid cultures. However, this improved recovery is mostly balanced if the latter is combined with liquid culture. Furthermore, none of the 145 cough swabs, used to sample young children, cultured positive, suggesting that swabs are low-quality samples.
TEXT
Since the 1990s, an increasing number of studies have reported the recovery of nontuberculous mycobacteria (NTM) from respiratory samples of cystic fibrosis (CF) patients (1, 2, 3). NTM are ubiquitous environmental organisms that can be isolated from natural water, tap water, and soil (4). Although rapid decline is described in CF patients with NTM infections, the role of NTM as lung pathogens in CF is not well defined and often underestimated because clinical symptoms and radiographic features are nonspecific and overlap considerably with progression of lung disease due to underlying CF (5). In addition, laboratory detection and identification of NTM pose considerable challenges. Albeit NTM are able to grow on many standard culture media, they grow relatively slowly compared to other CF pathogens such as Pseudomonas aeruginosa (5), hampering the recovery of NTM by rapidly overgrowing them. As a consequence, it is essential to use a decontamination procedure prior to culture for acid-fast bacilli to detect NTM in respiratory secretions of CF patients. In the literature, different decontamination procedures are described, with the N-acetyl-l-cysteine-NaOH-oxalic acid (NALC-NaOH-OxA) method being the most widely applied for CF samples. However, some reports suggest that this method affects the viability of mycobacteria (6), with false-negative results for samples with low mycobacterial loads in consequence (7, 8). Ferroni et al. (6) demonstrated that the chlorhexidine (CHX) decontamination method was superior, but to our knowledge—although mentioned in the Clinical and Laboratory Standards Institute (CLSI) guidelines (9)—this method has never been used in a prevalence study.
As part of a multicenter study to evaluate the prevalence of NTM in Belgian CF patients, we compared the CHX and NALC-NaOH-OxA decontamination methods. The protocol of this study was approved by the Ethics Committee of the Universitair Ziekenhuis Brussel (B.U.N. B14320085216).
Respiratory samples from CF patients collected between July 2009 and October 2010 were divided equally into two aliquots. After 0.5% NALC-2% NaOH-5% OxA (7) or CHX decontamination (6), they were processed for auramine staining and NTM culture by inoculating one Löwenstein-Jensen and one Ogawa slant. As CHX decontamination is incompatible with mycobacterial growth indicator tube (MGIT) culture (6), a liquid medium was inoculated only after the NALC-NaOH-OxA procedure. Media were incubated at 37°C and examined weekly for 8 weeks. Species identification was performed by gas-liquid chromatography using the Microbial Identification Systems (MIS, Newark, NJ, USA) database and classical biochemical tests or sequencing. Solid cultures were scored as contaminated if both slants were overgrown.
A total of 795 respiratory samples from 312 CF patients collected in 4 different centers were analyzed: 647 sputum samples, 145 cough swabs, 2 bronchoalveolar lavage fluid samples, and 1 nasopharyngeal aspiration. At the time of sampling, the patients' ages ranged from 16 months to 57 years (median age, 21 years). Three acid-fast bacillus smears were positive after CHX decontamination; two of them were also positive after NALC-NaOH-OxA treatment. In total, 18 sputum cultures from 11 patients were found to be NTM positive. The recovered NTM species were Mycobacterium avium-intracellulare complex (n = 6), Mycobacterium abscessus (n = 6), Mycobacterium chelonae or chelonae-like (n = 2), Mycobacterium gordonae (n = 1), Mycobacterium xenopi (n = 1), Mycobacterium malmoense (n = 1), and Mycobacterium lentiflavum (n = 1). Out of the 18 positive sputum samples, 15 were positive with the CHX method, 5 were positive with the NALC-NaOH-OxA method combined with solid culture, and 12 were positive with the NALC-NaOH-OxA method combined with MGIT culture (chi-square test, P = 0.0819) (Fig. 1). The increased yield of the CHX decontamination method is mostly balanced if NALC-NaOH-OxA decontamination is combined with MGIT culture. With one exception, discrepancies between CHX and NALC-NaOH-OxA/MGIT are unique positive cultures from that CF patient, so the clinical significance of these isolations can be discussed.
Fig 1.
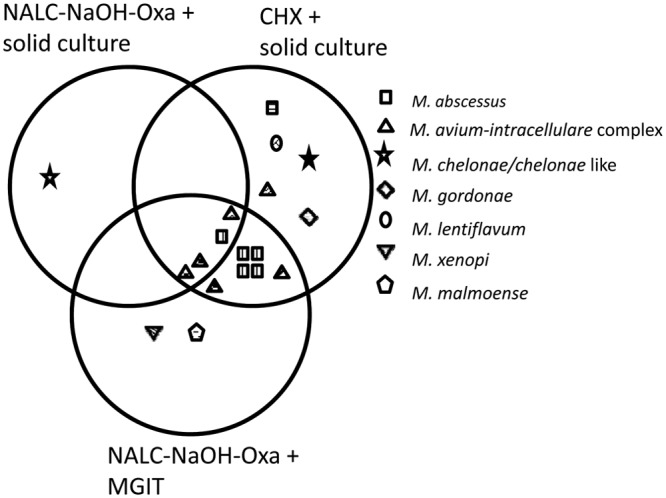
Overview of the recovered nontuberculous mycobacteria species after N-acetyl-l-cysteine-NaOH-oxalic acid (NALC-NaOH-OxA) and chlorhexidine (CHX) decontamination. For each patient with NTM-positive cultures, a different pattern fill was used. MGIT, mycobacterial growth indicator tube.
One hundred twenty-three (15.5%) cultures were overgrown after CHX decontamination, whereas 179 (22.5%) were overgrown after NALC-NaOH-OxA treatment combined with solid culture (chi-square test, P = 0.004). This is in contrast to the work of Ferroni et al. (6), who found a higher contamination rate with the CHX method (20% versus 14.2%). Of MGIT cultures, 23.9% were contaminated.
In this study, we analyzed cough swabs in order to include young children, who are mostly sputum nonproducers. These deep pharyngeal swabs were taken after drainage and cough stimulation by a physiotherapist (10). Albeit swabs are not first-choice samples, they are common practice for routine bacteriology in this age group in CF patients. In the literature, data on their suitability for mycobacterial detection are rare. However, the Clinical Microbiology Procedures Handbook (11) states that swabs should be refused for mycobacterial culture in general, because they absorb too little material and because of their hydrophobic properties, which hinder the release of mycobacteria from the swab. Other authors (12, 13) recommend that swabs for mycobacterial culture should be used only whenever another sample is unavailable, for example, in cases of abscess or tissue, skin, or eye injury. Ahmed et al. (14) state that cough swabs should not be used to isolate nontuberculous mycobacteria in children with CF.
We used elution swabs (ESwabs; Copan, Brescia, Italy), which consist of a flocked nylon swab for sample collection and a liquid transport medium. In general, the use of ESwabs results in better specimen collection and a more efficient release of specimen material compared to woven fiber-tipped swabs (12, 15). However, in the field of CF mycobacteriology, data are scarce. Linscott et al. (16) found a recovery rate of 71% of Mycobacterium fortuitum with the ESwab compared to 45% with other types of swabs. Snyder et al. (17) also reported that the recovery rate of mycobacteria, although lower than those of other organisms, was higher with the ESwab than with other swab types.
In our study, 145/795 (18%) included samples were cough swabs. All were negative. Although it is described elsewhere that in CF patients the prevalence of NTM increases with patient age (18), our data suggest that cough swabs recovered from sputum nonproducers, even if recovery is performed with the ESwab, are low-quality samples.
This work confirms that the CHX decontamination method is more sensitive than the NALC-NaOH-OxA decontamination procedure for the recovery of NTM on solid cultures from respiratory samples of CF patients. However, this improved recovery is mostly nullified if the latter is combined with MGIT culture. In our hands, CHX decontamination yielded a lower contamination rate. As our data suggest that cough swabs are low-quality samples for NTM detection, we will no longer accept them as specimens for NTM culture.
ACKNOWLEDGMENTS
This study was funded by Belgische Vereniging voor Strijd tegen Mucoviscidose (BVSM)/Association Belge de Lutte contre la Mucoviscidose (ABLM).
We thank the patients, CF nurses, and physicians of the participating centers.
Footnotes
Published ahead of print 18 September 2013
REFERENCES
- 1.Aitken ML, Burke W, McDonald G, Wallis C, Ramsey B, Nolan C. 1993. Nontuberculous mycobacterial disease in adult cystic fibrosis patients. Chest 4:1096–1099 [DOI] [PubMed] [Google Scholar]
- 2.Hjelt K, Højlyng N, Howitz P, Illum N, Munk E, Valerius NH, Fursted K, Hansen KN, Heltberg I, Koch C. 1994. The role of mycobacteria other than tuberculosis (MOTT) in patients with cystic fibrosis. Scand. J. Infect. Dis. 26:569–576 [DOI] [PubMed] [Google Scholar]
- 3.Kilby JM, Gilligan PH, Yankaskas JR, Highsmith WE, Edwards LJ, Knowles MR. 1992. Nontuberculous mycobacteria in adult patients with cystic fibrosis. Chest 1:70–75 [DOI] [PubMed] [Google Scholar]
- 4.Kazda JK. 1983. The principles of ecology of mycobacteria, p 26–29 In Stanford JL, Ratledge C. (ed), Biology of mycobacteria, vol 2 Academic Press, London, United Kingdom [Google Scholar]
- 5.Griffith DE, Aksamit T, Brown-Elliott BA, Catanzaro A, Daley C, Gordin F, Holland SM, Horsburgh R, Huitt G, Iademarco MF, Iseman M, Olivier K, Ruoss C, von Reyn F, Richard JR, Jr, Winthrop K. 2007. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am. J. Respir. Crit. Care Med. 175:367–416 [DOI] [PubMed] [Google Scholar]
- 6.Ferroni A, Vu-Thien H, Lanotte P, Le Bourgeois M, Sermet-Gaudelus I, Fauroux B, Marchand S, Varaigne F, Berche P, Gaillard JL, Offredo C. 2006. Value of the chlorhexidine decontamination method for recovery of nontuberculous mycobacteria from sputum samples of patients with cystic fibrosis. J. Clin. Microbiol. 44:2237–2399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Whittier S, Hopfer RL, Knowles MR, Gilligan PH. 1993. Improved recovery of mycobacteria from respiratory secretions of patients with cystic fibrosis. J. Clin. Microbiol. 31:861–864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bange FC, Kirschner P, Bottger EC. 1999. Recovery of mycobacteria from patients with cystic fibrosis. J. Clin. Microbiol. 37:3761–3763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clinical and Laboratory Standards Institute 2008. Laboratory detection and identification of mycobacteria; approved guideline. CLSI document M48-A. Clinical and Laboratory Standards Institute, Wayne, PA [Google Scholar]
- 10.Gilligan P. 2010. Respiratory cultures from cystic fibrosis, p 3.11.3.1–3.11.3.9 In Garcia LS. (ed), Clinical microbiology procedures handbook, 3rd ed. ASM Press, Washington, DC [Google Scholar]
- 11.Della-Latta P. 2010. Mycobacteriology and antimycobacterial susceptibility testing, p 7.0.1–7.8.8.2 In Garcia LS. (ed), Clinical microbiology procedures handbook, 3rd ed. ASM Press, Washington, DC [Google Scholar]
- 12.Daley P, Castriciano S, Chernesky M, Smieja M. 2006. Comparison of flocked and rayon swabs for collection of respiratory epithelial cells from uninfected volunteers and symptomatic patients. J. Clin. Microbiol. 44:2265–2267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pfyffer GE, Palicova F. 2011. Mycobacterium: general characteristics, laboratory detection, and staining procedures, p 472–502 In Versalovic J, Carroll KC, Jorgensen JH, Funke G, Landry ML, Warnock WD. (ed), Manual of clinical microbiology, 10th ed, vol 2 ASM Press, Washington, DC [Google Scholar]
- 14.Ahmed B, Balfour-Lynn IM, Alshafi K. 2012. Cough swabs should not be used to isolate non-tuberculous mycobacteria in children with cystic fibrosis. Arch. Dis. Child. 97:854–855 [DOI] [PubMed] [Google Scholar]
- 15.Van Horn KG, Audette CD, Sebeck D, Tucker KA. 2008. Comparison of 3 swab transport systems for direct release and recovery of aerobic and anaerobic bacteria. J. Diagn. Microbiol. Infect. Dis. 62:471–473 [DOI] [PubMed] [Google Scholar]
- 16.Linscott A, Pollen M, Matthews-Greer J. 2008. Comparison of three transport systems for the viability of clinically relevant fastidious organisms, abstr D-4014, p 270 Abstr. 48th Intersci. Conf. Antimicrob. Agents Chemother. (ICAAC)-Infect. Dis. Soc. Am. (IDSA) 46th Annu. Meet. American Society for Microbiology and Infectious Diseases Society of America, Washington, DC [Google Scholar]
- 17.Snyder GK, Munier C, Schiavi M, Johnson CL. 2010. Evaluation of the Copan liquid Amies elution swab (ESwab) for maintaining the viability of selected fungi and Mycobacterium spp., abstr F-2141 Abstr. 110th Gen. Meet. Am. Soc. Microbiol. American Society for Microbiology, Washington, DC [Google Scholar]
- 18.Olivier KN, Weber DJ, Wallace RJ, Faiz AR, Lee JH, Zhang Y, Brown-Elliot BA, Handler A, Wilson RW, Schechter MS, Edwards LJ, Chakraborti S, Knowles MR. 2002. Nontuberculous mycobacteria. I. Multicenter prevalence study in cystic fibrosis. Am. J. Respir. Crit. Care Med. 167:828–834 [DOI] [PubMed] [Google Scholar]


