Abstract
Canine pneumovirus (CnPnV) was recently identified during a retrospective survey of kenneled dogs in the United States. In this study, archived samples from pet and kenneled dogs in the United Kingdom were screened for CnPnV to explore the relationship between exposure to CnPnV and the development of canine infectious respiratory disease (CIRD). Within the pet dog population, CnPnV-seropositive dogs were detected throughout the United Kingdom and Republic of Ireland, with an overall estimated seroprevalence of 50% (n = 314/625 dogs). In the kennel population, there was a significant increase in seroprevalence, from 26% (n = 56/215 dogs) on the day of entry to 93.5% (n = 201/215 dogs) after 21 days (P <0001). Dogs that were seronegative on entry but seroconverted while in the kennel were 4 times more likely to develop severe respiratory disease than those that did not seroconvert (P < 0.001), and dogs with preexisting antibodies to CnPnV on the day of entry were significantly less likely to develop respiratory disease than immunologically naive dogs (P < 0.001). CnPnV was detected in the tracheal tissues of 29/205 kenneled dogs. Detection was most frequent in dogs with mild to moderate respiratory signs and histopathological changes and in dogs housed for 8 to 14 days, which coincided with a significant increase in the risk of developing respiratory disease compared to the risk of those housed 1 to 7 days (P < 0.001). These findings demonstrate that CnPnV is present in the United Kingdom dog population; there is a strong association between exposure to CnPnV and CIRD in the kennel studied and a potential benefit in vaccinating against CnPnV as part of a wider disease prevention strategy.
INTRODUCTION
Canine infectious respiratory disease (CIRD) is a highly prevalent multiagent disease that presents a considerable disease control challenge in kenneled dogs, despite the availability of multivalent vaccines which target several of the viral and bacterial pathogens implicated (1, 2). The spectrum of infectious agents involved, the rapid spread of infection, and the dynamic population of many kennel facilities make preventing and treating CIRD difficult and costly.
Clinical signs of CIRD range from nasal discharge and a dry cough to bronchopneumonia and, in severe cases, death (3). As one of the major health and welfare issues affecting domestic dogs, CIRD has attracted considerable interest in recent years. As a result, a number of novel viral agents have been identified (4–7), many of which have since been shown to be important in the development of CIRD (2, 4, 7). Identifying and understanding the pathogenesis of all the infectious agents involved in CIRD is important for improving the management and treatment of this complex disease.
Canine pneumovirus (CnPnV) is one such novel virus, recently identified in a retrospective study of respiratory disease in dogs from two animal shelters in the United States (8). Since the initial report, CnPnV has also been detected in dogs with respiratory disease in eight other U.S. states (9).
Genome analysis places CnPnV in the family Paramyxoviridae, subfamily Pneumovirinae, genus Pneumovirus (9), most closely related to murine pneumovirus (MPV) (9). The type species and best-characterized member of the Pneumovirus genus is human respiratory syncytial virus (hRSV) (10). hRSV is associated with significant human morbidity and mortality and is considered the most important agent of lower respiratory tract illness in infants (11, 12) and immunocompromised patients (13–15). Other members of the genus include bovine (16), ovine (17), and caprine (18) RSV species. Bovine RSV is of particular importance as a primary agent in the multifactorial bovine respiratory disease complex (BRDC), a disease analogous to CIRD which presents a major economic and welfare issue for the cattle and dairy industry (reviewed in reference 19).
MPV is a natural pathogen of rodents, common in research and commercial rodent colonies (20). Serological evidence indicates that many rodent species can be infected by MPV (21, 22). However, little is known about its natural host range or its prevalence and association with disease among wild rodent populations. A close genetic and antigenic relationship between CnPnV and MPV has been reported (9, 23). Following an experimental challenge of mice, CnPnV was shown to replicate effectively in the lungs, with severe respiratory sequelae similar to those observed with MPV. Convalescent-phase serum from CnPnV-inoculated mice cross-reacted with MPV antigens by enzyme-linked immunosorbent assay (ELISA), and CnPnV-exposed mice were protected from subsequent lethal infection with MPV (23).
Despite frequent detection of CnPnV in dogs with CIRD, its role as a causative agent of canine disease is yet to be proven. However, its relationship with a number of important pneumoviruses and its ability to cause severe respiratory disease in mice suggests there is a strong pathogenic potential in dogs.
Here, we present the first study to demonstrate a strong relationship between CnPnV and respiratory disease in dogs. Furthermore, this is also the first study to report CnPnV outside the United States. We present evidence that CnPnV is highly prevalent within a large United Kingdom rehoming center where it has a strong association with disease, as well as evidence that it is widely circulating within the dog population of the United Kingdom and Republic of Ireland.
MATERIALS AND METHODS
Two different dog populations were investigated in this retrospective study.
Pet dog population.
A total of 625 archived serum samples obtained from Axiom Veterinary Laboratories, Devon, United Kingdom, were obtained in 2005 and stored at −20°C. The samples had been received by Axiom Laboratories from veterinary practices across the United Kingdom and Republic of Ireland for various biochemical or hematological screens (approximately 5% for rabies testing, 25% for allergy testing, and 70% from other clinical investigations, including those of the liver and kidney, anemia, and infectious disease). The age (years) and location (country, county, town) of the dogs had been recorded.
Kennel population.
Archived tissue and serum samples collected from dogs housed at a well-established rehoming kennel with a history of endemic CIRD were analyzed. As reported previously (4), samples were collected over a 3-year study period from April 1999 to August 2001. All dogs were vaccinated upon entry into the kennel with KAVAK DA2 PiP69 (Fort Dodge), a live attenuated vaccine for distemper virus, canine adenovirus type 2, canine parainfluenza virus, and canine parvovirus, and a killed leptospirosis vaccine (Fort Dodge). The estimated age (years), preentry housing status (gifts or strays), date of entry, and date of sample collection had been recorded for each dog.
Kennel population—serological survey.
Paired serum samples collected from 215 dogs on the day of entry into the kennel and on day 21 after entry were stored at −20°C until analyzed by ELISA for the presence of CnPnV antibodies, providing estimates of day 1 seroprevalence and 21-day-period seroprevalence (total seroprevalence on day 21).
The occurrence of seroconversion was determined from day 1 and day 21 serological results and categorized as follows: (i) presence or absence of seroconversion, seroconversion (dogs that were negative on the day of entry and positive on day 21), and no seroconversion (dogs that were positive on day 1 and remained positive on day 21 and dogs that were negative on day 1 and remained negative on day 21); and (ii) seroconversion substatuses of seropositive-positive (PP) (dogs that were positive on day 1 and remained positive on day 21), seronegative-negative (NN) (dogs that were negative on day 1 and remained negative on day 21), and seronegative-positive (NP) (dogs that were negative on day 1 and positive on day 21).
The health of each dog had been assessed twice daily by a veterinary clinician, and respiratory signs were scored as follows: 1, no respiratory signs; 2, mild cough; 3, cough and nasal discharge; 4, cough, nasal discharge, and inappetence; and 5, evidence of bronchopneumonia (4). From this, the maximum respiratory score recorded for each dog during the 21-day study period was recategorized to indicate the occurrence of any clinical disease as no respiratory disease (maximum respiratory score = 1) and respiratory disease (maximum respiratory score = 2 to 5) and the occurrence of severe respiratory disease as no severe disease (maximum respiratory score = 1 to 2) and severe respiratory disease (maximum respiratory score = 3 to 5).
To assess seasonal trends, the month of entry was categorized into seasons, as follows: winter, December to February; spring, March to May; summer, June to August; autumn, September to November.
Kennel population—virological survey.
Tracheal tissues from an additional 205 dogs were analyzed for the presence of CnPnV by real-time reverse transcription (RT)-PCR. The dogs had been euthanized by a veterinary clinician for welfare and ethical reasons unrelated to this study, ranging from behavioral problems to signs of severe disease. The health status of each dog had been assessed by a veterinary clinician on the day of euthanasia and any respiratory signs were scored and recategorized as described for the serological survey. The length of time that each dog was housed in the kennel at the time of euthanasia was recorded in days and collapsed into categories of 0 to 7, 8 to 14, 15 to 21, 22 to 28, and >28 days.
Immediately following euthanasia, a full postmortem examination was performed. Tissue samples were collected with informed consent and stored at −70°C for subsequent analysis by RT-PCR or fixed in 10% buffered formalin.
Histology.
Formalin-fixed tissues were processed, and sections were stained using hematoxylin and eosin. The histological sections were examined by a veterinary pathologist, blinded to the respiratory score and molecular analyses. Each tissue was graded for several features (neutrophils, lymphocytes, plasma cells, macrophage, mucosa-associated lymphoid tissue [MALT] reactivity, edema, fibrin, and repair) from 0 (none or normal level) to 3 (severely affected). An overall grade (0 to 3) for the respiratory tract as a whole was determined based on an assessment of all the changes taken together.
RNA extraction and RT.
RNA was extracted from the tracheal samples of 205 dogs using the RNeasy minikit (Qiagen, Crawley, United Kingdom) as recommended by the manufacturer from a 0.5-cm2 piece of tissue. RNA was transcribed into cDNA using random hexameres (GE Healthcare, Little Chalfont, United Kingdom) and Improm II reverse transcriptase (Promega, Southampton, United Kingdom) according to the manufacturer's protocol.
Conventional PCR for GAPDH.
As an internal control, to monitor successful nucleic acid extraction, tracheal samples were tested for the presence of the glyceraldehyde-3-phosphatedehydrogenase (GAPDH) housekeeping gene as described previously (24).
CnPnV real-time PCR.
Real-time PCR was performed using Jumpstart Taq ready mix (Sigma) with final concentrations of 1 mM MgCl2 and 0.6 μM each primer, CnPnV-N forward (GACCTGTTTGAAAGGAAGCCTTATT) and CnPnV-N reverse (ACCAGAAAACAGCCCCTCAAC), and 0.2 μM probe ([6-carboxyfluorescein]CTTCCATCACTTTTGGCCTGGCCCAG[black hole quencher 1]). A standard control comprising the target 100-bp fragment of the MPV nucleocapsid gene cloned into the pGem-T Easy vector (Promega) was used. For each PCR, 4 μl of cDNA template or standard control was added to a total reaction volume of 25 μl. Samples were analyzed in triplicate. The cycling conditions were 95°C for 2 min, followed by 40 cycles of 94°C for 15 s and 60°C for 1 min, after which the plate read was taken. All reactions were performed on a DNA Engine Opticon 2 (Bio-Rad). Linear regression for each experiment was performed using the Opticon Monitor 2 software version 3.1. PCR efficiency for each run was calculated, and runs with a PCR efficiency of <90% and >110% were disregarded. Samples were considered positive for CnPnV if they yielded threshold cycle (CT) values of ≤37 cycles and were negative with CT values of >37 cycles.
ELISA.
MaxiSorp 96-well flat-bottom ELISA plates (Nunc) were coated with pneumonia virus of mice (PVM) (otherwise known as MPV) ELISA antigen or control antigen (Churchill Applied Biotech) in 0.05 M carbonate-bicarbonate buffer (pH 9.6) at 4°C overnight according to the manufacturer's instructions. Plates were washed 3× with 100 μl/well of 0.1% phosphate-buffered saline–Tween (PBST) and then blocked for 1 h at 37°C with 100 μl/well of 5% milk powder with 1% bovine serum albumin (BSA)-0.1% PBST. Canine serum samples were diluted 1:100 in blocking buffer, and 50 μl/well was dispensed in duplicate against both the PVM and control antigen, incubated for 1 h at 37°C, and then washed as described above. Secondary antibody, rabbit anti-dog IgG horseradish peroxidase (HRP) (Sigma), was diluted 1:2,500 in blocking buffer, and 50 μl was dispensed per well, incubated for 1 h at 37°C, and then washed as described above. Sigma Fast OPD (o-phenylenediamine dihydrochloride) substrate (Sigma) was prepared according to the manufacturer's instructions. Substrate was added at 100 μl/well and incubated at room temperature, in the dark, for 10 min. The reaction was stopped with 50 μl/well of 2 M H2SO4. Optical density (OD) values were measured at 490 nm with a reference of 650 nm using an automatic ELISA plate reader. The average OD value for the control antigen was subtracted from that for the PVM antigen to give the corrected OD value. Samples were regarded as positive if corrected OD values were greater than or equal to the cutoff value and negative if less than the cutoff value. The cutoff value was calculated by adding three standard deviations to the corrected OD value of a panel of 10 negative-control sera diluted 1:100. To validate the ELISA, sera obtained from the Animal Health Diagnostic Centre at Cornell University were used. The sera had been previously tested for the presence of CnPnV antibodies by virus neutralization assay (VN). The results obtained by the ELISA were consistent with those by VN.
Statistical analysis.
Data were recorded in Excel spreadsheets and imported into Intercooled Stata 9.2 (Statacorp) for analysis. Continuous variables were summarized using means (standard deviations [SD]) if normally distributed and medians (minimums, maximums) if nonnormally distributed. Categorical variables were summarized using numbers and percentages in each category and 95% confidence intervals (CI) around prevalence estimates, calculated using the Clopper-Pearson method.
Pet dog population seroprevalence was estimated overall and by both country and county within England. Age was summarized using mean and standard deviation (SD), and any association between age and seropositivity was examined using the Student t test.
Associations between seroconversion status of kenneled dogs in the serological survey and maximum respiratory score, occurrence of respiratory disease, occurrence of severe respiratory disease, age, season, and preentry status were examined using chi-square/Fisher's exact tests. Odds ratios (OR) and Wald P values for relationships with the presence/absence of seroconversion were generated using univariable logistic regression. Multivariable logistic regression was used to examine relationships between seroconversion and other factors where more than one factor was significant at the 5% level.
For kenneled dogs in the virological survey, associations between the presence of CnPnV in tissues and respiratory score at euthanasia, presence of respiratory disease, presence of severe respiratory disease, histology score, age, and presence of other viral agents were examined using chi-square/Fisher's exact tests, with ORs and Wald P values generated using univariable logistic regression. The Mann-Whitney U test was used to examine relationships between length of time in kennels and both PCR status and disease status.
RESULTS
Pet dog population.
The seroprevalence of CnPnV in the general pet dog population was 50.2% (314/625; 95% CI, 46.2 to 54.2%).
The overall mean age of the dogs was 7.8 years (SD, 3.7). The mean age of CnPnV-seropositive dogs (8.2 years; SD, 3.4) was significantly higher than that of seronegative dogs (7.5; SD, 3.9) (P = 0.025).
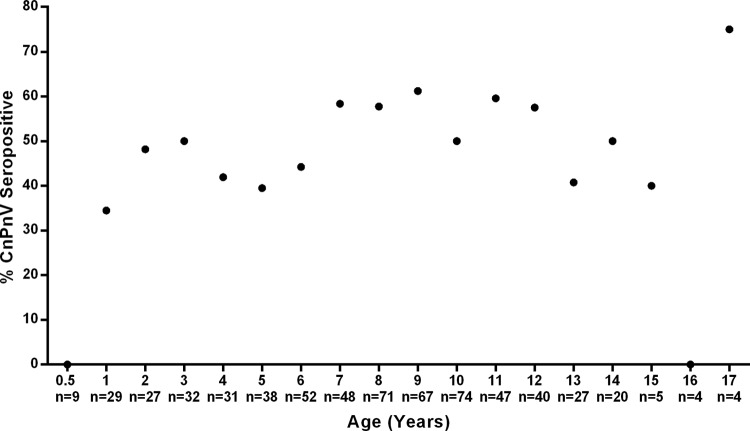
All dogs tested below the age of 6 months were seronegative, after which there was a dramatic increase in the level of seroprevalence, which reached 48% (n = 13/27; 95% CI, 28.7 to 68.1%) in dogs 2 years of age. This was followed by a continued but gradual rise in seroprevalence, plateauing at around a mean seroprevalence of 57.1% (n = 198/347; 95% CI, 51.7 to 62.3%) in dogs 7 to 12 years old, and then some age-related decline (Fig. 1).
Fig 1.
Percentage of CnPnV antibody-positive dogs by age (n = total number of dogs examined per age group).
The prevalence of CnPnV antibodies by country is shown in Table 1. In England and Scotland, the seroprevalence was slightly higher than the overall seroprevalence figure of 50.2%, while in Wales and the Republic of Ireland it was lower. The results from England were further broken down by county, and CnPnV antibodies were detected in dogs from 28 (82.4%) of the 34 counties examined (data not shown).
Table 1.
CnPnV seroprevalence by country
| Country | No. of dogs tested | No. of CnPnV positive dogs | % CnPnV positive dogs |
|---|---|---|---|
| England | 517 | 270 | 52.2 |
| Wales | 36 | 13 | 36.1 |
| Scotland (Ayrshire and Lanarkshire) | 13 | 7 | 53.9 |
| Guernsey | 6 | 2 | 33.3 |
| Northern Ireland | 8 | 4 | 50.0 |
| Republic of Ireland | 45 | 18 | 40.0 |
| Total | 625 | 314 | 50.2 |
Kennel population—serological survey. (i) Seroprevalence.
On the day of entry to the kennel, 26.0% of the 215 dogs were seropositive (n = 56; 95% CI, 20.3 to 32.5%). Of the remainder, 91.2% (n = 145) developed CnPnV antibodies by day 21, to give an overall period seroprevalence of 93.5% (n = 201; 95% CI, 90.2 to 96.8%).
(ii) Occurrence of respiratory disease.
Of the 215 dogs examined, 84.7% (n = 182) had respiratory disease at some point during the 21-day study period, and 65.6% (n = 141/215) had severe respiratory disease.
Univariable relationships between CnPnV seroconversion and disease, age, preentry status, and season are summarized in Table 2.
Table 2.
Kennel population serological survey analysis: seroconversion and seroconversion subcategory by measured variable
| Variable | Seroconversion in dogs |
ORc | 95% CI (OR) | P valuea | Total dogs |
||||
|---|---|---|---|---|---|---|---|---|---|
| Yes |
No |
||||||||
| No. | % | No. | % | No. | % | ||||
| Maximum respiratory score | |||||||||
| 1 | 13 | 39.4 | 20 | 60.6 | Ref | <0.001b | 33 | 100 | |
| 2 | 22 | 53.7 | 19 | 46.3 | 1.8 | 0.7–4.5 | 0.2 | 41 | 100 |
| 3 | 103 | 77.4 | 30 | 22.6 | 5.3 | 2.4–11.8 | <0.001 | 133 | 100 |
| 4 | 7 | 87.5 | 1 | 12.5 | 10.8 | 1.2–98.0 | 0.04 | 8 | 100 |
| Disease | |||||||||
| Yes | 132 | 72.5 | 50 | 27.5 | 4.1 | 1.9–8.8 | <0.001 | 182 | 100 |
| No | 13 | 39.4 | 20 | 60.6 | Ref | <0.001b | 33 | 100 | |
| Severe disease | |||||||||
| Yes | 110 | 78.0 | 31 | 22.0 | 4.0 | 2.2–7.2 | <0.001 | 141 | 100 |
| No | 35 | 47.3 | 39 | 52.7 | Ref | <0.001b | 74 | 100 | |
| Age (yrs) | |||||||||
| ≤1 | 53 | 84.1 | 10 | 15.9 | Ref | 0.001b | 63 | 100 | |
| 2 | 75 | 64.7 | 41 | 35.3 | 0.3 | 0.2–0.7 | 0.007 | 116 | 100 |
| ≥3 | 17 | 47.2 | 19 | 52.8 | 0.1 | 0.06–0.4 | <0.001 | 36 | 100 |
| Entry status | |||||||||
| Gift | 44 | 64.7 | 24 | 35.3 | Ref | 0.6b | 68 | 100 | |
| Stray | 101 | 68.7 | 46 | 31.3 | 1.2 | 0.6–2.2 | 0.6 | 147 | 100 |
| Season | |||||||||
| Winter | 26 | 66.7 | 13 | 33.3 | Ref | 0.6 | 39 | 100 | |
| Spring | 44 | 62.0 | 27 | 38.0 | 0.8 | 0.4–1.8 | 0.6 | 71 | 100 |
| Summer | 37 | 74.0 | 13 | 26.0 | 1.4 | 0.6–3.6 | 0.4 | 50 | 100 |
| Autumn | 38 | 69.1 | 17 | 30.9 | 1.1 | 0.5–2.7 | 0.8 | 55 | 100 |
Wald P values are from logistic regression except when otherwise noted.
Chi-square P value.
Ref, the reference against which the OR was calculated.
Seroconversion was significantly associated with the three measures of clinical disease. Overall, seroconverting dogs had 4-fold-increased odds of both respiratory disease (OR, 4.1; P < 0.001) and severe respiratory disease (OR, 4.0; P < 0.001). Dogs that seroconverted had over 5-fold-increased odds of a maximum respiratory score of 3 (P < 0.001) and over 10-fold-increased odds of a maximum score of 4 (P = 0.04) compared to dogs that did not seroconvert. Upon closer examination of the dogs that did not seroconvert, 80% (n = 56/70) had preexisting antibodies on the day of entry (PP). As the maximum respiratory score increased, so did the proportion of dogs that seroconverted, while the proportion of dogs with preexisting antibodies decreased (Fig. 2). Dogs with preexisting antibodies were significantly less likely to develop respiratory disease than those that seroconverted (P < 0.001).
Fig 2.

Comparison on the prevalence of disease by increasing respiratory score for two groups. PP, dogs that were CnPnV seropositive on the day of entry; NP, dogs that were negative for CnPnV antibodies on the day of entry but seroconverted during a 21-day stay in the kennel. Respiratory score 1, no respiratory disease, to respiratory score 4, severe respiratory disease (cough and discharge). Error bars show 95% CI.
(iii) Age.
Dogs entering the kennel were all between 0 and 4 years old. The likelihood of seroconversion decreased with age, with both 2-year-old dogs (OR, 0.3; P = 0.007) and those ages 3 years or older (OR, 0.2; P < 0.001) having reduced odds of seroconversion compared with those 1 year old or less. Further examination revealed that the proportion of dogs with preexisting antibodies on entry to the kennel increased with age, from 4.8% (3/63) in dogs 1 year or less to 44.4% (16/36) in dogs 3+ years old (Fig. 3). Multivariable logistic regression indicated that the relationships between seroconversion and either disease or severe disease was not affected by adjustment for age.
Fig 3.
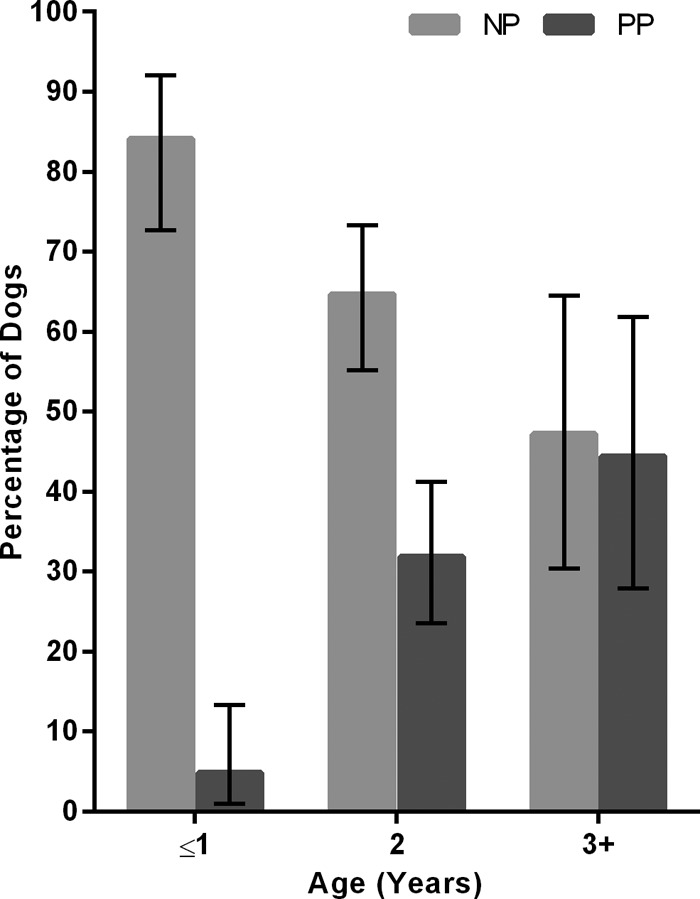
The proportion of dogs by age that seroconverted during the 21 days in the kennel (NP) or entered the kennel with preexisting antibodies to CnPnV (PP). Error bars show 95% CI.
(iv) Preentry housing status.
Of the 215 dogs entering the kennel, 31.6% (n = 68) were gifts, and the remainder were strays. The likelihood of being seropositive on entry to kennels or of seroconverting while in kennels did not differ significantly between gifts and strays (P = 0.8 or P = 0.6, respectively).
(v) Seasonality.
Disease was prevalent in the kennel throughout the 3-year study period, and there was no significant relationship between season and occurrence of respiratory disease (P = 0.4), severe respiratory disease (P = 0.4), or maximum respiratory score (P = 0.5). Occurrences of seroconversions to CnPnV also took place throughout the year. No significant seasonal trends associated with occurrences of seroconversion were observed (P = 0.6).
Kennel population—virological survey. (i) PCR results and disease measures.
Of the 205 dogs for which tracheal tissues had been examined, 14.2% (n = 29; 95% CI, 90.7 to 19.7) were positive for CnPnV. Overall, the prevalence of respiratory disease at the time of euthanasia was 65.4% (n = 143; 95% CI, 58.4 to 71.9%), and the prevalence of severe respiratory disease was 47.8% (n = 98; 95% CI, 40.8 to 54.9).
Relationships between CnPnV PCR status and other factors are summarized in Table 3. There was no significant variation in respiratory score (P = 0.6) or difference in the prevalence of either disease (P = 1.0) or severe disease (P = 0.7) between CnPnV-positive and -negative dogs.
Table 3.
Kennel population virological survey analysis: detection of CnPnV by measured variable
| Variable | CnPnV PCR (analysis of tracheal tissues) |
ORc | 95% CI (OR) | P valuea | |||
|---|---|---|---|---|---|---|---|
| Positive |
Negative |
||||||
| No. | % | No. | % | ||||
| Respiratory score at euthanasia | |||||||
| 1 | 10 | 34.5 | 61 | 34.7 | Ref | 0.6b | |
| 2 | 6 | 20.7 | 30 | 17.0 | 1.2 | 0.4–3.7 | 0.7 |
| 3 | 12 | 41.4 | 62 | 35.2 | 1.2 | 0.5–2.9 | 0.7 |
| 4 | 0 | 0 | 9 | 5.1 | |||
| 5 | 1 | 3.4 | 14 | 8.0 | 0.4 | 0.05–3.7 | 0.4 |
| Disease | |||||||
| Yes | 19 | 65.5 | 115 | 65.3 | 1.0 | 0.4–2.3 | 1.0 |
| No | 10 | 34.5 | 61 | 34.7 | Ref | ||
| Severe disease | |||||||
| Yes | 13 | 44.8 | 85 | 48.3 | 0.9 | 0.4–1.9 | 0.7 |
| No | 16 | 55.1 | 91 | 51.7 | Ref | ||
| Histology score | |||||||
| 0 | 4 | 13.8 | 13 | 7.4 | Ref | 0.3b | |
| 1 | 15 | 51.7 | 100 | 56.8 | 0.5 | 0.1–1.7 | 0.2 |
| 2 | 9 | 31.0 | 41 | 23.3 | 0.7 | 0.2–2.7 | 0.6 |
| 3 | 1 | 3.4 | 22 | 12.5 | 0.1 | 0.01–1.5 | 0.1 |
| Histological changes | |||||||
| Yes | 25 | 86.2 | 163 | 92.6 | 2.0 | 0.6–6.6 | |
| No | 4 | 13.8 | 13 | 7.4 | Ref | 0.2 | |
| Age (yrs) | |||||||
| 1 | 3 | 10.7 | 21 | 11.9 | Ref | 0.1b | |
| 2 | 12 | 42.9 | 105 | 59.7 | 0.8 | 0.2–3.1 | 0.7 |
| 3 | 12 | 42.9 | 39 | 22.2 | 2.1 | 0.5–8.5 | 0.3 |
| 4 | 1 | 3.6 | 11 | 6.3 | 0.6 | 0.06–6.7 | 0.7 |
| Time in kennel (days) | |||||||
| 0–7 | 1 | 5.9 | 16 | 94.1 | Ref | 0.001b | |
| 8–14 | 26 | 24.5 | 80 | 75.5 | 5.2 | 0.6–41.1 | 0.2 |
| 15–21 | 0 | 0 | 32 | 100 | |||
| 22–28 | 1 | 4.6 | 21 | 95.4 | 0.8 | 0.04–13.1 | 0.8 |
| >28 | 1 | 3.6 | 27 | 96.4 | 0.6 | 0.03–10.1 | 0.7 |
| Total | 29 | 100 | 176 | 100 | |||
Wald P values are from logistic regression except when otherwise noted.
Chi-square P value.
Ref, the reference against which the OR was calculated.
Overall, 91.7% (188/205) of dogs had histological changes in their respiratory tissues. There was no significant difference in the proportions of CnPnV-positive dogs (86.2%; 25/29) and CnPnV-negative dogs (92.6%; 163/176) with histological changes (P = 0.2).
Euthanized dogs were between 1 and 4 years old, with a mean age of 2.3 years. There was no significant difference in age between CnPnV-positive and -negative dogs (P = 0.1).
The number of days the 205 dogs had been in the kennel ranged from 3 to 211 days, with a median stay of 12 days. CnPnV-positive dogs had been kenneled for significantly less time (median, 9 days; range, 7 to 37 days) than CnPnV-negative dogs (median, 14 days; range, 3 to 211 days) (P < 0.001).
Dogs with respiratory disease had been kenneled for significantly longer (median, 13 days; range, 6 to 202 days) than dogs with no respiratory disease (median, 9 days; range, 3 to 211 days) (P = 0.008). Dogs with severe respiratory disease had also been kenneled for significantly longer (median, 13.5 days; range, 7 to 56 days) than dogs without severe respiratory disease (median, 10 days; range, 3 to 211 days) (P = 0.009).
However, when grouped into weeklong time periods, the majority of CnPnV-positive dogs (51.7%; 106/205) had been housed in the kennel for 8 to 14 days. In that same time period, there was an 18-fold increase in the prevalence of respiratory disease, compared to the time period of 0 to 7 days (P < 0.001) (Fig. 4).
Fig 4.
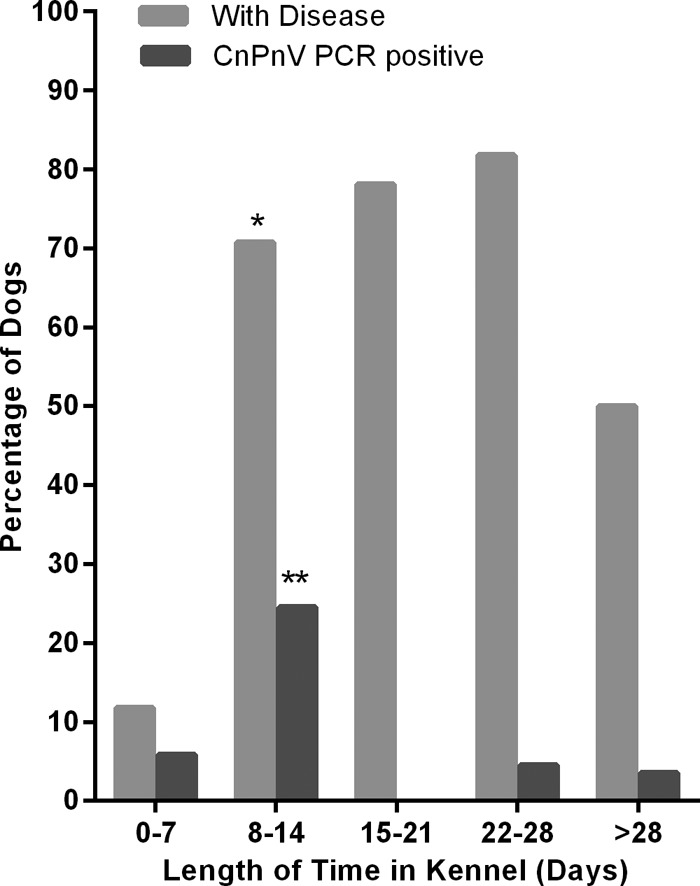
Summary of the proportion of dogs with respiratory disease (respiratory scores 2 to 5) and the proportion of CnPnV RNA-positive dogs versus time in the kennel. *, statistically significant (P < 0.000) compared to incidence of disease for 0 to 7 days; **, tracheal samples tested from dogs euthanized during this time point are 5.2 times more likely to be positive for CnPnV by PCR compared to those from dogs kenneled for 0 to 7days (P < 0.118).
Coinfections in CnPnV-positive dogs.
Data relating to viral coinfections were available for 23 of the 29 CnPnV-positive dogs, of which coinfections were detected in 16 (69.5%) animals. The most frequently detected were canine respiratory coronavirus (56%; n = 9/16) and canine parainfluenza virus (50%; n = 8/16). No clear differences were observed between the average respiratory score, histology score, and length of stay for dogs with or without coinfections.
DISCUSSION
This is the first study to provide evidence that CnPnV is present in the dog population of the United Kingdom and the Republic of Ireland and to establish a strong relationship between CnPnV and respiratory disease in kenneled dogs.
Two different populations were investigated in this retrospective study: the pet dog population, which represents pet dogs in the United Kingdom and Republic of Ireland, and the kenneled dog population, which represents a high-risk group for CIRD. CIRD was endemic in the kennel studied and could not be controlled through the use of recommended vaccines, suggesting that additional agents, not included in the vaccines, were contributing to the disease.
Antibodies to CnPnV were prevalent in pet dogs throughout the United Kingdom and the Republic of Ireland, with an overall seroprevalence of 50% (95% CI, 46.3 to 54.1%). The detection of seropositive dogs indicates widespread exposure to CnPnV throughout the United Kingdom and Republic of Ireland and increased with age. Increases in seroprevalence during the first few years of life are likely to be associated with increased socialization and mixing with other dogs and is similar to the age-related pattern previously observed for other CIRD-associated viral agents, including canine respiratory coronavirus, for which antibodies were also detected in this same cohort of dogs (25).
It should be acknowledged that since the pet dog samples used in this study were submitted for clinical investigation, they cannot be considered to represent a true cross-section of the pet dog population as a whole. Small sample numbers or a bias in sampling from a particular town, city, or practice within a region might also account for some of the variations in regional seroprevalence that were observed.
Within the kennel population, the prevalence of antibodies in dogs entering the kennel was 26% (95% CI, 20.3 to 32.4%), consistent with the overall seroprevalence of CnPnV in the surrounding London area for the pet dog cohort. The significant increase in seroprevalence observed in dogs after a 21-day stay in the kennel demonstrated not only the presence of CnPnV in the kennel population but also its rapid transmission to immunologically naive dogs.
There was no difference in the serological status of the dogs entering the kennel when comparing those that were either gifts to the kennel or stray prior to entry. Furthermore, there was no discernible difference in the proportion of dogs that seroconverted to CnPnV when comparing gifted or stray dogs. The gifted or stray status of the dog prior to entry to the kennel is therefore not an indicator of either preexposure to or susceptibility to CnPnV within the kennel.
There was a strong association between length of stay and the occurrence of respiratory disease during the first 28 days in the kennel. The observation that the large majority of the CnPnV-positive dogs (by RT-PCR) had been housed for 8 to 14 days at the time of euthanasia supported serological evidence of a rapid transmission of CnPnV to dogs shortly after entry to the kennel and coincided with a time in which there was a significant increase in the risk of developing respiratory disease compared to the risk for those kenneled for 1 to 7 days. As expected, clinical disease in CnPnV-positive dogs was associated with mild to moderate histological changes in the respiratory tissues. The majority of CnPnV-positive dogs displayed clinical signs of respiratory disease; however, for those that did not, it is possible that some dogs may have become infected with CnPnV but remained asymptomatic, or, given the time scale of infection, it is entirely possible that CnPnV could have been detected in these dogs during the early stages of infection, before clinical signs of disease were apparent.
Importantly, dogs with preexisting antibodies to CnPnV on the day of entry were significantly less likely to develop respiratory disease than those that entered with no detectable CnPnV antibodies. The significance increased when occurrences of severe signs of clinical disease were compared to the occurrence of no to mild disease. The presence of antibodies to CnPnV, therefore, appeared to have a significantly protective effect against respiratory disease in this population, suggesting a causative role for CnPnV in the pathogenesis of CIRD and a potential benefit in vaccinating against CnPnV as part of a wider disease prevention strategy.
The above-described findings indicate that CnPnV was strongly associated with respiratory disease during the early stages of CIRD onset. However, a defined role for CnPnV in the pathogenesis of CIRD remains to be determined. Other Pneumovirinae have been reported to act both as primary agents of disease and within associated disease complexes and have formed the basis of several reviews (26–28). Indeed, following an experimental infection of BALB/c mice with CnPnV, viral antigen was detected primarily in the epithelial cells lining the bronchioles, and its presence was associated with inflammation similar to that observed with murine pneumovirus (23). As with other Pneumovirinae, CnPnV may have the potential to be a primary agent of disease. However, within the disease complex, it is likely that CnPnV also acts to predispose dogs to secondary viral or bacterial infections, leading to a prolonged or more severe clinical disease profile. Similar roles have been proposed for other CIRD viral agents, such as canine respiratory coronavirus (CRCoV) (29–31) and canine parainfluenza virus (CPIV) (32–34), and for bovine RSV in bovine respiratory disease complex (16, 28).
A number of dogs in this study that had preexisting CnPnV antibodies on the day of entry to kennels developed signs of mild respiratory disease; this suggests that other infectious agents are involved in the pathogenesis of disease or that antibodies to CnPnV may not always protect against CnPnV infection. Previously, CRCoV was shown to be associated with the early development of CIRD within this cohort (4). Mycoplasma cynos and Streptococcus equi subsp. zooepidemicus have also been detected in these kenneled dogs during the later stages of disease (weeks 3 to 4) (35, 36). Studies to determine the prevalence of other viral and bacterial pathogens are under way.
While it is likely that many of the cases of disease in dogs with existing CnPnV immunity are attributable to other infectious agents, it should also be considered that antibodies to CnPnV may not always be protective against subsequent CnPnV infections. For RSV and indeed other Pneumovirinae species, considerable antigenic variation occurs in the heavily glycosylated attachment glycoprotein (G). This variation, brought about by differences in sequence and glycosylation, has been shown to impact significantly on the binding and neutralizing capabilities of antibodies that may allow for repeated infections throughout life (reviewed in reference 37). In a recent study examining the G protein sequences of several U.S. CnPnV isolates, the variation observed led to the designation of A and B subtypes of the virus (38). It will be necessary to carry out a detailed assessment of the degree of both genetic and antigenic variation associated with CnPnV strains and to establish whether there is a link between subtype and clinical outcome.
In summary, CnPnV has an increasingly international presence and a growing wealth of evidence which suggests a role in the pathogenesis of the widespread and complex problem of CIRD. However, further studies are required to establish the true global presence of CnPnV and its role in CIRD pathogenesis. Changes in the way traditional kennel cough is perceived, as being far more complex and multifactorial in etiology than previously thought, has led to the detection of a number of novel viruses, such as CRCoV and CnPnV. Elucidating the involvement of these viruses in CIRD could reveal new ways of treating and managing this disease.
ACKNOWLEDGMENTS
This work was supported by a grant awarded to Judy A. Mitchell by the Petplan Charitable Trust for the analysis of the archived samples.
We thank Simon Priestnall and Kerstin Erles at the Royal Veterinary College for their contribution to the sample archives used in this study.
Footnotes
Published ahead of print 2 October 2013
REFERENCES
- 1.Erles K, Brownlie J. 2005. Investigation into the causes of canine infectious respiratory disease: antibody responses to canine respiratory coronavirus and canine herpesvirus in two kennelled dog populations. Arch. Virol. 150:1493–1504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Erles K, Dubovi EJ, Brooks HW, Brownlie J. 2004. Longitudinal study of viruses associated with canine infectious respiratory disease. J. Clin. Microbiol. 42:4524–4529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Appel MJ, Binn LN. 1987. Canine infectious tracheobronchitis short review: kennel cough, p 201–211 In Virus infections of carnivores. Elsevier Science Publishing Co, New York, NY. [Google Scholar]
- 4.Erles K, Toomey C, Brooks HW, Brownlie J. 2003. Detection of a group 2 coronavirus in dogs with canine infectious respiratory disease. Virology 310:216–223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kapoor A, Mehta N, Dubovi EJ, Simmonds P, Govindasamy L, Medina JL, Street C, Shields S, Lipkin WI. 2012. Characterization of novel canine bocaviruses and their association with respiratory disease. J. Gen. Virol. 93:341–346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kapoor A, Simmonds P, Gerold G, Qaisar N, Jain K, Henriquez JA, Firth C, Hirschberg DL, Rice CM, Shields S, Lipkin WI. 2011. Characterization of a canine homolog of hepatitis C virus. Proc. Natl. Acad. Sci. U. S. A. 108:11608–11613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Crawford PC, Dubovi EJ, Castleman WL, Stephenson I, Gibbs EP, Chen L, Smith C, Hill RC, Ferro P, Pompey J, Bright RA, Medina MJ, Johnson CM, Olsen CW, Cox NJ, Klimov AI, Katz JM, Donis RO. 2005. Transmission of equine influenza virus to dogs. Science 310:482–485 [DOI] [PubMed] [Google Scholar]
- 8.Renshaw RW, Zylich NC, Laverack MA, Glaser AL, Dubovi EJ. 2010. Pneumovirus in dogs with acute respiratory disease. Emerg. Infect. Dis. 16:993–995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Renshaw R, Laverack M, Zylich N, Glaser A, Dubovi E. 2011. Genomic analysis of a pneumovirus isolated from dogs with acute respiratory disease. Vet. Microbiol. 150:88–95 [DOI] [PubMed] [Google Scholar]
- 10.International Committee on Taxonomy of Viruses, King AMQ 2012. Virus taxonomy: classification and nomenclature of viruses: ninth report of the International Committee on Taxonomy of Viruses. Elsevier Academic Press, London, United Kingdom [Google Scholar]
- 11.Hall CB, Weinberg GA, Iwane MK, Blumkin AK, Edwards KM, Staat MA, Auinger P, Griffin MR, Poehling KA, Erdman D, Grijalva CG, Zhu Y, Szilagyi P. 2009. The burden of respiratory syncytial virus infection in young children. N. Engl. J. Med. 360:588–598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nair H, Nokes DJ, Gessner BD, Dherani M, Madhi SA, Singleton RJ, O'Brien KL, Roca A, Wright PF, Bruce N, Chandran A, Theodoratou E, Sutanto A, Sedyaningsih ER, Ngama M, Munywoki PK, Kartasasmita C, Simoes EA, Rudan I, Weber MW, Campbell H. 2010. Global burden of acute lower respiratory infections due to respiratory syncytial virus in young children: a systematic review and meta-analysis. Lancet 375:1545–1555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dowell SF, Anderson LJ, Gary HE, Jr, Erdman DD, Plouffe JF, File TM, Jr, Marston BJ, Breiman RF. 1996. Respiratory syncytial virus is an important cause of community-acquired lower respiratory infection among hospitalized adults. J. Infect. Dis. 174:456–462 [DOI] [PubMed] [Google Scholar]
- 14.Falsey AR. 2007. Respiratory syncytial virus infection in adults. Sem. Respir. Crit. Care Med. 28:171–181 [DOI] [PubMed] [Google Scholar]
- 15.Falsey AR, Hennessey PA, Formica MA, Cox C, Walsh EE. 2005. Respiratory syncytial virus infection in elderly and high-risk adults. N. Engl. J. Med. 352:1749–1759 [DOI] [PubMed] [Google Scholar]
- 16.Paccaud MF, Jacquier C. 1970. A respiratory syncytial virus of bovine origin. Arch. Gesamte Virusforsch. 30:327–342 [DOI] [PubMed] [Google Scholar]
- 17.Evermann JF, Liggitt HD, Parish SM, Ward AC, LeaMaster BR. 1985. Properties of a respiratory syncytial virus isolated from a sheep with rhinitis. Am. J. Vet. Res. 46:947–951 [PubMed] [Google Scholar]
- 18.Lehmkuhl HD, Smith MH, Cutlip RC. 1980. Morphogenesis and structure of caprine respiratory syncytial virus. Arch. Virol. 65:269–276 [DOI] [PubMed] [Google Scholar]
- 19.Brodersen BW. 2010. Bovine respiratory syncytial virus. Vet. Clin. North Am. Food Anim. Pract. 26:323–333 [DOI] [PubMed] [Google Scholar]
- 20.Kraft V, Meyer B. 1990. Seromonitoring in small laboratory animal colonies. A five year survey: 1984–1988. Z. Versuchstierkd. 33:29–35 [PubMed] [Google Scholar]
- 21.Horsfall FL, Curnen EC. 1946. Studies on pneumonia virus of mice (Pvm): II. Immunological evidence of latent infection with the virus in numerous mammalian species. J. Exp. Med. 83:43–64 [PubMed] [Google Scholar]
- 22.Kaplan C, Healing TD, Evans N, Healing L, Prior A. 1980. Evidence of infection by viruses in small British field rodents. J. Hyg. 84:285–294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Percopo CM, Dubovi EJ, Renshaw RW, Dyer KD, Domachowske JB, Rosenberg HF. 2011. Canine pneumovirus replicates in mouse lung tissue and elicits inflammatory pathology. Virology 416:26–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Grone A, Weckmann MT, Capen CC, Rosol TJ. 1996. Canine glyceraldehyde-3-phosphate dehydrogenase complementary DNA: polymerase chain reaction amplification, cloning, partial sequence analysis, and use as loading control in ribonuclease protection assays. Am. J. Vet. Res. 57:254–257 [PubMed] [Google Scholar]
- 25.Priestnall SL, Brownlie J, Dubovi EJ, Erles K. 2006. Serological prevalence of canine respiratory coronavirus. Vet. Microbiol. 115:43–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dyer KD, Garcia-Crespo KE, Glineur S, Domachowske JB, Rosenberg HF. 2012. The pneumonia virus of mice (PVM) model of acute respiratory infection. Viruses 4:3494–3510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Easton AJ, Domachowske JB, Rosenberg HF. 2004. Animal pneumoviruses: molecular genetics and pathogenesis. Clin. Microbiol. Rev. 17:390–412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sarmiento-Silva RE, Nakamura-Lopez Y, Vaughan G. 2012. Epidemiology, molecular epidemiology and evolution of bovine respiratory syncytial virus. Viruses 4:3452–3467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Priestnall SL, Mitchell JA, Brooks HW, Brownlie J, Erles K. 2009. Quantification of mRNA encoding cytokines and chemokines and assessment of ciliary function in canine tracheal epithelium during infection with canine respiratory coronavirus (CRCoV). Vet. Immunol. Immunopathol. 127:38–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mitchell JA, Brooks HW, Szladovits B, Erles K, Gibbons R, Shields S, Brownlie J. 2013. Tropism and pathological findings associated with canine respiratory coronavirus (CRCoV). Vet. Microbiol. 162:582–594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Erles K, Brownlie J. 2008. Canine respiratory coronavirus: an emerging pathogen in the canine infectious respiratory disease complex. Vet. Clin. North Am. Small Anim. Pract. 38:815–825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ellis JA, Krakowka GS. 2012. A review of canine parainfluenza virus infection in dogs. J. Am. Vet. Med. Assoc. 240:273–284 [DOI] [PubMed] [Google Scholar]
- 33.Appel MJ, Percy DH. 1970. SV-5-like parainfluenza virus in dogs. J. Am. Vet. Med. Assoc. 156:1778–1781 [PubMed] [Google Scholar]
- 34.Binn LN, Lazar EC, Rogul M, Shepler VM, Swango LJ, Claypoole T, Hubbard DW, Asbill SG, Alexander AD. 1968. Upper respiratory disease in military dogs: bacterial, mycoplasma, and viral studies. Am. J. Vet. Res. 29:1809–1815 [PubMed] [Google Scholar]
- 35.Chalker VJ, Owen WM, Paterson C, Barker E, Brooks H, Rycroft AN, Brownlie J. 2004. Mycoplasmas associated with canine infectious respiratory disease. Microbiology 150:3491–3497 [DOI] [PubMed] [Google Scholar]
- 36.Chalker VJ, Brooks HW, Brownlie J. 2003. The association of Streptococcus equi subsp. zooepidemicus with canine infectious respiratory disease. Vet. Microbiol. 95:149–156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sullender WM. 2000. Respiratory syncytial virus genetic and antigenic diversity. Clin. Microbiol. Rev. 13:1–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Glineur SF, Renshaw RW, Percopo CM, Dyer KD, Dubovi EJ, Domachowske JB, Rosenberg HF. 2013. Novel pneumoviruses (PnVs): evolution and inflammatory pathology. Virology 443:257–264 [DOI] [PMC free article] [PubMed] [Google Scholar]