Abstract
We report here the quantitative detection of Vibrio cholerae toxin (CT) in isolates and stool specimens by dynamic monitoring of the full course of CT-mediated cytotoxicity in a real-time cell analysis (RTCA) system. Four cell lines, including Y-1 mouse adrenal tumor cells, Chinese hamster ovary (CHO) cells, small intestine epithelial (FHs74Int) cells, and mouse adrenal gland (PC12-Adh) cells, were evaluated for their suitability for CT-induced cytotoxicity testing. Among them, the Y-1 line was demonstrated to be the most sensitive for CT-mediated cytotoxicity, with limits of detection of 7.0 pg/ml for purified CT and 0.11 ng/ml for spiked CT in pooled negative stool specimens. No CT-mediated cytotoxicity was observed for nontoxigenic V. cholerae, non-V. cholerae species, or non-V. cholerae enterotoxins. The CT-RTCA assay was further validated with 100 stool specimens consecutively collected from patients with diarrhea and 200 V. cholerae isolates recovered from patients and the environment, in comparison to a reference using three detection methods. The CT-RTCA assay had sensitivities and specificities of 97.5% and 100.0%, respectively, for V. cholerae isolates and 90.0% and 97.2% for stool specimens. For stool specimens spiked with CT concentrations ranging from 3.5 pg/ml to 1.8 ng/ml, the inoculation-to-detection time was 1.12 ± 0.38 h, and the values were inversely correlated with CT concentrations (ρ = −1; P = 0.01). The results indicate that the CT-RTCA assay with the Y-1 cell line provides a rapid and sensitive tool for the quantitative detection of CT activities in clinical specimens.
INTRODUCTION
Vibrio cholerae is a Gram-negative, comma-shaped, bacterial pathogen causing cholera, an acute secretory diarrheal disease. Epidemic cholera is common in developing countries and affects about 100,000 people annually (1). Cholera toxin (CT) is a major virulence determinant of V. cholerae, leading to rapidly progressing dehydration, shock, metabolic acidosis, and even death within hours without adequate and appropriate therapy (2). CT is a key biomarker of V. cholerae that is used for forecasting and assessing epidemic disease, monitoring and controlling cholera outbreaks, preventing epidemics, and guiding medical personnel in providing timely treatment of patients. There has been urgent demand for the development of novel sensitive assays for the detection and identification of CT proteins.
Conventional biochemical and immunological methods for CT detection and identification can be completed only after V. cholerae is isolated. The bacterial isolation and subsequent CT detection and identification are laborious and usually require several days, resulting in significant delays in patient care and disease control (3, 4). In addition, classic CT determinations require the use of either animal methods (5, 6) or tissue culture methods (7, 8), which are also time-consuming and are subjective in the interpretation of results. Efforts have been made recently to develop more-sensitive methods, including enzyme-linked immunosorbent assays (9), latex agglutination assays (10), coagglutination assays (5), liposome-based assays (9, 11), radioimmunoassays (12), hydrogel-based immunoassays (13), monosaccharide- and antibody array-based assays (14–17), PCR-based molecular assays (10, 18–21), and biosensor-based assays (22–31). A reliable laboratory tool is desirable for clinical services and epidemiological investigation, to characterize CT activities rapidly and quantitatively.
A real-time cell analysis (RTCA) system (ACEA Biosciences, San Diego, CA) uses electric impedance sensor-based technology for dynamic real-time monitoring of the status of adherent cells in response to a broad range of physiological and pathological manipulations (32, 33). The RTCA system has been used for label-free, dynamic measurements of cytotoxicity induced by toxins and for monitoring of morphological changes resulting from cell adhesion and spreading processes (34, 35). This technology has been applied as an alternative cytotoxicity assay for the identification of Clostridium difficile toxins (36, 37). Here we developed a rapid quantitative assay for detection and identification of functional CT by using the RTCA system. The diagnostic validity of this assay was determined with a panel of V. cholerae isolates and stool specimens collected from patients with diarrhea with clinically suspected cholera.
(This study was presented in part at the 112th General Meeting of the American Society for Microbiology, San Francisco, CA, 16 to 19 June 2012.)
MATERIALS AND METHODS
Bacterial strains, toxin, and antibodies.
Reference strains used in this study, including toxigenic V. cholerae, nontoxigenic V. cholerae, non-V. cholerae species, and a panel of diarrhea-causing microbial pathogens, were obtained from the American Type Culture Collection (ATCC) (Manassas, VA) (Table 1). All of the strains were cultured with standard microbiological procedures, as described previously (38). Purified V. cholerae type Inaba 569B (catalog no. 100B), heat-labile (catalog no. 165B), Shiga (catalog no. 161), and diphtheria (catalog no. 150) toxins were purchased from List Biological Laboratories (Campbell, CA). C. difficile toxin A and B were purchased from EMD Chemicals, Inc. (Gibbstown, NJ). Cholera toxin with the maximal degree of purity (catalog no. C8052) and cholera toxin-neutralizing antibody (catalog no. C3062) were purchased from Sigma-Aldrich (St. Louis, MO).
Table 1.
Bacterial strains and toxins used in this study
| Strain or toxin | No. included | Bacterial origin | Strain or toxin no. | CT-RTCA resulta |
|---|---|---|---|---|
| Strain | 4 | Toxigenic V. cholerae | ATCC BAA-2163, 39541, 25870, and 51394b | P |
| 1 | Nontoxigenic V. cholerae | ATCC 55866 | N | |
| 1 | V. parahaemolyticus | ATCC 43996 | N | |
| 1 | V. alginolyticus | ATCC 17750 | N | |
| 1 | V. vulnificus | ATCC 27562 | N | |
| 1 | V. mimicus | ATCC 33655 | N | |
| 1 | V. fluvialis | ATCC 33812 | N | |
| 2 | Clostridium difficile | ATCC 43255 and 700057 | N | |
| 1 | Campylobacter jejuni | ATCC 33560 | N | |
| 1 | Enterobacter aerogenes | ATCC 13048 | N | |
| 1 | Clostridium perfringens | ATCC 27057 | N | |
| 1 | Escherichia coli O157:H7 | ATCC 43888 | N | |
| 1 | Listeria monocytogenes | ATCC 19111 | N | |
| 1 | Shigella flexneri | ATCC 55556 | N | |
| 1 | Salmonella enterica | ATCC 51960 | N | |
| Toxin | 2 | Cholera toxin | LBL 100Bc and SAC C8052 | P |
| 1 | Clostridium difficile toxin A | EMDC 616379 | N | |
| 1 | Clostridium difficile toxin B | EMDC 616377 | N | |
| 1 | Shiga toxin | LBL 161 | N | |
| 1 | Diphtheria toxin | LBL 150 | N | |
| 1 | Heat-labile toxin | LBL 65B | N |
P, positive; N, negative.
The four V. cholerae toxins included serotypes Ogawa serovar O1 (n = 2), Inaba serovar O1 (n = 1), and serovar O139 (n = 1). ATCC, American Type Culture Collection; SAC, Sigma-Aldrich Co.; LBL, List Biological Laboratories; EMDC, EMD Chemicals, Inc.
The cholera toxin was isolated from V. cholerae type Inaba 569B.
Cells, culture, and maintenance.
Chinese hamster ovary (CHO) cells (ATCC CCL-61), small intestine epithelial (FHs74Int) cells (ATCC CCL-241), and mouse adrenal gland (PC12-Adh) cells (ATCC CRL-1721.1) were cultured and maintained according to protocols suggested by the ATCC. Y-1 mouse adrenal tumor cells (ATCC CCL-79) were cultured and maintained in Kaighn's modification of Ham's F-12 medium (F-12K) supplemented with final concentrations of 2.5% fetal bovine serum (FBS) and 15% horse serum (F-12K growth medium). Culture flasks (75 cm2; Corning Inc., Lowell, MA) were precoated with 0.002% (wt/vol) poly-l-lysine (PLL) (P4707; Sigma-Aldrich, St. Louis, MO) for 10 min at room temperature and then were rinsed once with Dulbecco's phosphate-buffered saline (DPBS). Cells were cultured at 37°C with 5% CO2 in F-12K growth medium.
V. cholerae isolates and processing.
V. cholerae isolates were recovered from patients and environmental samples between March 1995 and October 2005, in the Zhejiang Provincial Center for Disease Control and Prevention. All isolates were thawed and subcultured in Craig's medium as described previously (39, 40), and culture supernatants were collected, filtered with 0.22-μm filters (Millex-GP; Millipore, Billerica, MA) at room temperature, diluted with DPBS with 0.1% bovine serum album (BSA) at a 1:5 ratio, and stored at 4°C prior to the RTCA assay (see below).
Clinical stool specimens.
Liquid, soft, or semisolid stool specimens were collected from patients with suspected cholera at local hospitals and the Zhejiang Provincial Center for Disease Control and Prevention between June 2004 and May 2011. Stool specimens were thawed, and 5% (wt/vol or vol/vol) suspensions were prepared in 1-ml aliquots of DPBS with 0.1% BSA. The suspensions were pelleted, and supernatants were diluted with DPBS with 0.1% bovine serum albumin (BSA), at a 1:5 ratio, prior to the RTCA assay (see below).
RTCA system.
The RTCA system (xCELLigence; ACEA Biosciences, San Diego, CA) was used for CT detection according to the manufacturer's instructions, as described previously (36). Cells were thawed, trypsinized, and resuspended in the F-12K growth medium. Cell numbers were counted and adjusted to a concentration of 1.5 × 105 cells/ml in F-12K medium. For each well of the PLL-precoated 96-well E-Plate, 100 μl of the cell suspension was added. The cell-loaded 96-well E-Plate was sealed and incubated for 16 to 24 h at 37°C in the RTCA system until ≥90% cell confluence was reached, as detected by visual examination. Two wells were used for each tested sample, in the absence and presence of CT-neutralizing antibody (5% [vol/vol] in DPBS with 0.1% BSA). The antibody was mixed with the sample at a ratio of 1:1 (vol/vol), incubated for 30 min at 37°C, and then added to the wells of the 96-well E-Plate. CT-mediated cytotoxicity was continuously monitored and recorded every 3 min for up to 25 h by the RTCA system.
The cell electrode impedance was expressed using an arbitrary unit, cell index (CI), and was analyzed by the integrated software (32–35). A normalized cell index (nCI) was calculated for each test well at a given time point, which was the last measurement time point prior to treatment (36, 41). A sample was called positive for CT when (i) the nCI value decreased from 1 to less than 0.8 (20% decrease) and (ii) the nCI decrease was completely blocked by a CT-neutralizing antibody. The toxin concentration for a sample was quantified using a calibration curve as described previously, with minor modifications (36). In brief, a series of 2-fold dilutions (from 3.5 pg/ml to 1.8 ng/ml) of purified CT were made in DPBS with 0.1% BSA. CT concentrations were derived by using the time points at which the nCI dropped by 20% and performing a nonlinear regression (36).
Cell morphological analysis.
The Y-1 cells were viewed at a time point at which a significant CI change was detected during the continuous monitoring by the RTCA system. The real-time status of the Y-1 cells in the 96-well microplate incubated with purified CT was monitored with an inverted phase-contrast microscope and photographed with an integrated digital camera (Nikon Eclipse TE 2000-U system with Nikon ACT-1 version 2.62; Nikon Corp., Japan). To visualize the cell morphology at the time point with the minimum nCI value, the F-12K culture medium was completely removed, and cells were stained using a Reastain Quick-Diff kit (Reagena International Oy Ltd., Toivala, Finland), according to the manufacturer's instructions. After the wells were air-dried at room temperature, the cells were viewed with a Cellvista system (Roche Applied Sciences, Indianapolis, IN).
Real-time PCR.
For reference strains and clinical isolates, 1 ml of alkaline peptone water medium (BD, Sparks, MD) containing 105 to 106 colonies of V. cholerae was prepared and nucleic acids were extracted by using a Genomic-tip 20/G kit (Qiagen, Inc., Valencia, CA), according to the manufacturer's instructions. For stool specimens, nucleic acids were extracted from 200 μl of 5% (wt/vol or vol/vol) stool in DPBS by using a QIAamp DNA stool minikit (Qiagen, Inc.), according to the manufacturer's instructions. The extracted DNA was resuspended in 200 μl of elution buffer, and 5 μl was used for nucleic acid amplification. A Primer Design quantification real-time PCR kit, purchased from Primer Design Ltd. (Southampton, United Kingdom), was used to detect the cholera toxin B gene according to the manufacturer's instructions (42). A calibration curve was established by plotting cycle threshold (CT) versus gene copy number using 7500 FAST sequence detection system version 1.4 software (Life Technologies, Foster City, CA).
Reversed passive latex agglutination.
Detection of CT by a V. cholerae enterotoxin and E. coli heat-labile enterotoxin reversed passive latex agglutination (VET-RPLA) kit (Oxoid, Remel, Lenexa, KS) was performed in triplicate, according to the manufacturer's instructions. Briefly, 25-μl aliquots of seven processed stool or isolate supernatants diluted 2-fold were added to a 96-well microtiter plate. After 25-μl samples of sensitized latex and latex control were added, the 96-well microtiter plate was incubated for 20 h at room temperature, with a lid, and results were analyzed according to the manufacturer's instructions (10, 18). Positive, negative, and blank controls were included in each run.
Data analysis.
A combination reference standard was defined as two or more matching results among the real-time PCR, VET-RPLA, and CT-RTCA results. The diagnostic sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) of the CT-RTCA assay were determined. The Spearman correlation was calculated to evaluate the relationship between the CT concentration and the inoculation-to-detection time (IDT). Odds ratios (ORs), 95% confidence intervals, and r, ρ, and P values were calculated by using SPSS version 13.0 software (SPSS Inc., Chicago, IL), and P values of ≤0.05 were considered statistically significant.
RESULTS
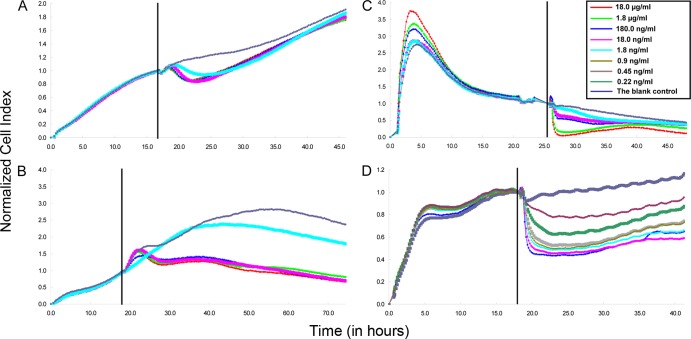
Four cell lines, i.e., CHO, FHs74Int, PC12-Adh, and Y-1, were tested for cytotoxicity induced by CT. When cells entered the relatively stable CI phase, a series of CT concentrations ranging from 0.22 ng/ml to 18.0 μg/ml were tested. No concentration- or time-dependent responses of CT were observed in the CHO and PC12-Adh lines (Fig. 1A and B). In contrast, the nCI curves of the FHs74Int and Y-1 lines showed distinct dose-response relationships (Fig. 1C and D). After seeding, the cell growth pattern of the FHs74Int line was unstable, showing a rapid increase in CI during the first 5 h followed by a gradual decrease in the nCI curve. For Y-1 cells, the nCI curve of the blank control remained stable, while the nCI responses showed dose- and time-dependent decreases after CT treatment (Fig. 1D). The CT-mediated cytotoxicity in the RTCA assay was confirmed by neutralization of the toxic effects by CT-neutralizing antibodies. These data revealed that the Y-1 line was suitable for monitoring of quantitative CT-mediated cytotoxicity in the RTCA system.
Fig 1.
Responses of four cell lines to CT. Each curve is representative of triplicate results. The growth curves corresponding to four cell lines, a Chinese hamster ovary cell line (A), a mouse adrenal gland cell line (B), a small intestine epithelial cell line (C), and the Y-1 mouse adrenal tumor cell line (D), were monitored in real time. The cellular changes were induced by serial dilutions of purified CT. The vertical lines indicate the time points at which the sample/toxin was added (t = 0).
To determine the analytical specificity of the CT-RTCA assay, a panel of diarrhea-related pathogens and toxins were tested. Y-1 cell morphological changes detected by the RTCA assay were observed solely in toxigenic V. cholerae strains. No cell morphological changes were detected in nontoxigenic V. cholerae, Vibrio parahaemolyticus, Vibrio alginolyticus, Vibrio vulnificus, Vibrio mimicus, or Vibrio fluvialis or other diarrhea-related bacterial pathogens (Table 1). Enterotoxins originating from Escherichia coli or Corynebacterium diphtheriae and C. difficile toxins A and B (Table 1) induced no morphological changes in Y-1 cells at concentrations of 90 ng/ml. E. coli heat-labile toxin induced morphological changes in Y-1 cells; however, the cytotoxic effect was not neutralized by CT-neutralizing antibody (data not shown).
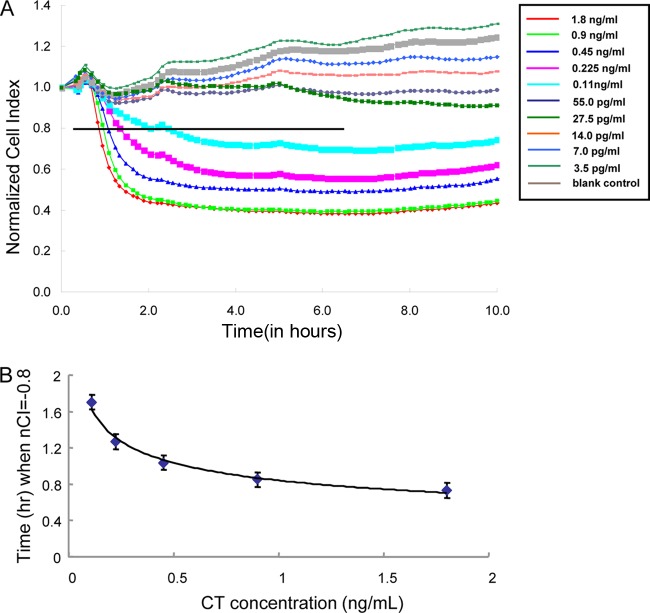
The analytical sensitivity of CT detection in the RTCA system was determined using purified CT diluted in DPBS with 0.1% BSA and in 5% pooled CT-negative stool specimens. CT concentrations ranged from 3.5 pg/ml to 1.8 ng/ml, with each toxin concentration being tested in triplicate (Fig. 2A). When a 20% decrease in the nCI was used as the cutoff value, the limits of detection were 7.0 pg/ml for toxin diluted in DPBS-BSA and 0.11 ng/ml for toxin diluted in pooled negative stool samples. The IDT correlated significantly with toxin concentrations, resulting in dose- and time-dependent curves (Fig. 2A). Fast cell death kinetics (reduction of nCI) were observed in the samples with high CT concentrations, indicating a nonlinear relationship between IDT and CT concentrations. Among positive concentrations ranging from 0.11 to 18.0 ng/ml spiked in pooled negative stool specimens, the average IDT was 1.12 h, with a standard deviation of 0.38 h. The CT concentration (y) was derived by using the equation y = 0.8387x−0.3045, where x is the IDT in hours (R2 = 0.986, P = 0.001) (Fig. 2B).
Fig 2.
Analytical sensitivity of CT diluted with pooled negative stool specimens, as detected by the RTCA assay using Y-1 cells. (A) Time- and concentration-dependent responses of the Y-1 line to CT, as monitored continuously with the RTCA system. The individual curves displayed are representative of triplicate results. The coefficient of variation of the nCI values is less than 5%. The y axis indicates the time point at which the sample/toxin was added (t = 0), and the x axis indicates the monitoring times. The horizontal line indicates the nCI 20% cutoff value. (B) Dependency of the time for a 20% decrease in nCI (y axis) in a well containing CT on the toxin concentration (x axis), as determined by nonlinear regression.
We further assessed the dynamic morphological changes of the Y-1 line in response to five CT concentrations, i.e., 0.11, 0.22, 0.45, 0.9, and 1.8 ng/ml, with inverted phase-contrast microscopy, using rounding of Y-1 cells as the indication of CT-mediated cytotoxicity. When 1.8 ng/ml and 0.9 ng/ml concentrations of purified CT were inoculated, cells started to change morphology at approximately 1 h and 1.25 h, respectively, and 95% of cells became rounded at 2.25 h. Limited numbers of cells were rounded at 2.25 h with 0.45 ng/ml, and no cell morphological changes appeared for CT at 0.22 to 0.11 ng/ml or the blank control (data not shown). These results indicated that the reduction of nCI detected by the RTCA system occurred earlier and was more sensitive than the CT-induced morphological changes.
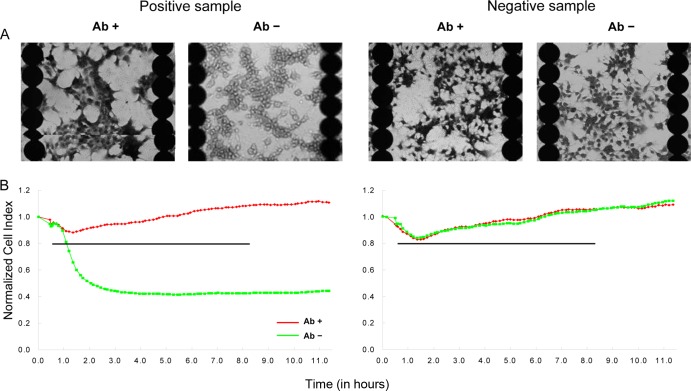
A total of 200 V. cholerae isolates and 100 clinical stool specimens were used to determine the validity of the CT-RTCA assay for CT detection (Table 2). The representative positive and negative results determined according to the aforementioned criteria are shown in Fig. 3. The CT-RTCA assay detected CT in 77 (38.5%) isolates and in 29 (29.0%) stool specimens. The means and standard deviations of IDT for CT-positive isolates and stool specimens determined by the CT-RTCA assay were 0.53 ± 0.25 h and 0.89 ± 0.51 h, respectively, which corresponded to toxin concentrations of 41.22 ± 96.21 ng/ml and 19.90 ± 64.10 ng/ml, respectively, based on the conversion formula described above.
Table 2.
Sensitivities, specificities, and predictive values for CT detection by CT-RTCA, VET-RPLA, and real-time PCR assays
| Type | Assay | No. of isolates/stool specimensa |
Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | |||
|---|---|---|---|---|---|---|---|---|---|
| S+ T+ | S+ T− | S− T+ | S− T− | ||||||
| Strain | CT-RTCA | 77 | 2 | 0 | 121 | 97.5 | 100.0 | 100.0 | 98.4 |
| VET-RPLA | 61 | 18 | 0 | 121 | 77.2 | 100 | 100.0 | 87.1 | |
| Real-time PCR | 79 | 0 | 22 | 99 | 100.0 | 81.8 | 78.2 | 100.0 | |
| Stool | CT-RTCA | 27 | 3 | 2 | 68 | 90.0 | 97.2 | 93.1 | 95.8 |
| VET-RPLA | 17 | 13 | 1 | 69 | 56.7 | 98.6 | 94.4 | 84.1 | |
| Real-time PCR | 30 | 0 | 6 | 64 | 100.0 | 91.4 | 83.3 | 100.0 | |
| Total | CT-RTCA | 104 | 5 | 2 | 189 | 95.4 | 99.0 | 98.1 | 97.4 |
| VET-RPLA | 78 | 31 | 1 | 190 | 71.6 | 99.5 | 98.7 | 86.0 | |
| Real-time PCR | 109 | 0 | 28 | 163 | 100.0 | 85.3 | 79.6 | 100.0 | |
S, standard; T, test (CT-RTCA, VET-RPLA or real-time PCR assay); +, positive; −, negative; PPV, positive predictive value; NPV, negative predictive value.
Fig 3.
Representative CT-RTCA results for isolates and clinical specimens. (A) Stained images of Y-1 cells seeded on 96-well E-Plates. (B) Real-time monitoring of the cytotoxic effects of CT on Y-1 cells by using the RTCA system. The specimens were tested by mixing them with neutralizing antibody (Ab+) or not (Ab−). The y axis indicates the time point at which the sample/toxin (t = 0) was added, and the x axis indicates the monitoring times. The horizontal line indicates the nCI 20% cutoff value.
In comparison with the combined reference standard, the sensitivities, specificities, PPVs, and NPVs of the CT-RTCA assay were 97.5%, 100.0%, 100.0%, and 98.4% for V. cholerae isolates and 90.0%, 97.2%, 93.1%, and 95.8% for stool specimens, respectively. Among both V. cholerae isolates and stool specimens, the sensitivity (71.6%; OR, 8.27 [95% confidence interval, 3.07 to 22.23]; χ2 = 20.80, P < 0.01) and NPV (86.0%; OR, 6.17 [95% confidence interval, 2.35 to 16.20]; χ2 = 15.68, P < 0.01) of the CT-RTCA assay were significantly higher than those of the VET-RPLA but lower than those of real-time PCR (100%; Fisher's exact test, P = 0.06 to 0.065). In contrast, the specificity (85.3%; OR, 16.23 [95% confidence interval, 3.81 to 69.19]; χ2 = 22.61, P < 0.01) and PPV (79.6%; OR, 13.36 [95% confidence interval, 3.10 to 57.49]; χ2 = 17.33, P < 0.0001) were significantly higher than those of real-time PCR (Table 2).
DISCUSSION
Advances in biotechnology over the past decades have led to the development of genomic and proteomic methods for the detection and characterization of pathogenic toxins (31). In this study, we report for the first time the use of real-time, dynamic, CT-mediated cytotoxicity monitoring of cell lines for detecting and identifying CT activities. Among the four cell lines being used for CT characterization, we revealed that the Y-1 cell line was the most sensitive for detection of CT-mediated cytotoxicity.
The differences in clinical manifestations caused by CT-producing and non-CT-producing V. cholerae are substantial; therefore, development of a laboratory tool to detect and to identify CT quickly and accurately is desirable for controlling and preventing V. cholerae-related epidemic diseases (43, 44). The CT-RTCA system had a sensitivity of 97.5% and a specificity of 100.0% when V. cholerae isolates were tested. Mostly important, the assay can be used directly on stool specimens, with a slightly decreased sensitivity of 90.0% and a specificity of 97.2%. When the assay is applied in clinical diagnosis and/or epidemiological investigations, confluent Y-1 cells can be prepared in advance to decrease the test turnaround time. In our study, most of the stool specimens containing CT were detected by the CT-RTCA assay within 2 h after inoculation. With a cell culture facility, the CT-RTCA system represents a rapid, sensitive, and easy-to-use tool for functional detection of cholera toxin in a real-time manner. A new integrated portable system is being developed to facilitate implementation of the CT-RTCA assay in routine clinical laboratories without cell culture facilities.
In addition to detection and characterization, the RTCA system may provide quantification of CT existing in samples. Our data indicated that the IDT correlated significantly with toxin concentrations, resulting in dose- and time-dependent curves. The correlation between time and CT concentrations was not linear, as the fastest cell death kinetics (reduction of nCI) were observed in the samples with the highest CT concentrations, while much slower kinetics were observed in specimens with lower CT concentrations (Fig. 2B). CT concentrations can be derived from a formula based on the IDT values by using nonlinear regression. In a previous study, a similar formula was used to estimate C. difficile toxin concentrations in stool specimens, to assess disease severity and therapeutic responses (36). To our knowledge, there is no evidence to support the utility of quantitative measurements of CT for prediction of clinical courses or outcomes or for clinical decision-making when treating patients with cholera. A large clinical study is being designed to explore whether the CT concentrations derived from IDT values for stool specimens can be used as clinical monitoring markers.
Y-1 cell morphological changes observed with an inverted phase-contrast microscope were compared with the nCI reductions detected by the RTCA system. Rapid steep nCI reductions appeared in parallel with fast morphological changes observed by microscopy at the high CT concentration. However, for CT concentrations lower than 0.45 ng/ml, no visible cell morphological changes occurred until 2 h after inoculation. These results demonstrated that CT-induced cytotoxic events were initiated earlier than visible cell morphological changes, suggesting that the RTCA system is a quicker and more sensitive tool for detection of functional CT in given specimens.
The RTCA system provides a real-time and continuous tool to monitor dynamic changes in toxin-mediated cytotoxicity. It is a very different approach than conventional endpoint cytotoxicity assays and immunological tests. The RTCA assay is based on a dynamic process of interactions between living cells and pathogenic toxins in real time (32); therefore, it might be used to study different pathogenic mechanisms for individual toxins, resulting in different outputs (2). We noted that both C. difficile toxin and cholera toxin resulted in cell rounding, but different nCI curves were produced. The C. difficile toxin, an actin-modifying adenine dinucleotide protein-ribosyltransferase, not only impairs the structure of the actin cytoskeleton in epithelial cells but also promotes the adherence of pathogens by changing the formation of microtubule-based protrusions on the surface of the epithelial cells (45). From previous studies (36), when the C. difficile toxin was inoculated into HS27 cells in the absence of neutralizing antibodies, the nCI decreased gradually from 1.0 to 0. When the cellular responses were monitored continuously for more than 40 h, the nCI remained constantly at its lowest level. These results demonstrated that the HS27 cells were killed as a result of the presence of the C. difficile toxin. In contrast, cholera toxin only provokes loss of water and electrolytes in epithelial cells, disrupting both cell attachment and cell-cell contact, while it does not cause cell death (46). At the concentration of 0.11 ng/ml, the nCI returned to 0.8 by approximately 11 h after treatment and continued increasing slowly (data not shown).
Three technical modifications were used in the RTCA system to enhance the detection of CT-mediated cytotoxicity. First, we used PLL to coat tissue culture flasks, which improved cell adherence and CI values. PLL is commonly used to facilitate cell attachment, spreading, and growth in cell culture (33, 47). Y-1 cells spread evenly on the PLL-coated surface, and the cell growth indicated by CI values was much quicker than that on uncoated plates (data not shown). Second, we shortened the monitoring interval to every 3 min for CT assessment and recording in the RTCA system. Consequently, the IDT was shortened, especially for samples with higher CT concentrations. Finally, we optimized the cell concentration for inoculation in each well, in order to shorten the time to the stable-CI phase.
In summary, we report the use of the RTCA system for quantitative detection of functional V. cholerae toxin by real-time monitoring of CT-mediated cytotoxicity. The RTCA assay is demonstrated to be a powerful reliable tool as a potential alternative for the diagnosis of cholera, because of the reasonably high throughput, efficient data acquisition rate, and satisfactory stability, reproducibility, and ease of handling. The assay reported here might be applied to monitor epidemic trends and therapeutic responses. In addition, this system can be further adapted to screen a panel of diarrhea-related toxins by using mixed sensitive cell lines.
ACKNOWLEDGMENTS
This work was supported in part by a program of the Zhejiang Leading Team of Science and Technology Innovation, a research grant from the Zhejiang Provincial Program for the Cultivation of High-Level Innovative Health Talents, and a research contract between the Memorial Sloan-Kettering Cancer Center and ACEA Biosciences.
We thank Jieying Wu, Jenny Zhu, Yan Zou, and Jeff Irelan for their technical, statistical, and editorial assistance.
Footnotes
Published ahead of print 18 September 2013
REFERENCES
- 1.Harris JB, LaRocque RC, Qadri F, Ryan ET, Calderwood SB. 2012. Cholera. Lancet 379:2466–2476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sears CL, Kaper JB. 1996. Enteric bacterial toxins: mechanisms of action and linkage to intestinal secretion. Microbiol. Rev. 60:167–215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chaicumpa W, Srimanote P, Sakolvaree Y, Kalampaheti T, Chongsa-Nguan M, Tapchaisri P, Eampokalap B, Moolasart P, Nair GB, Echeverria P. 1998. Rapid diagnosis of cholera caused by Vibrio cholerae O139. J. Clin. Microbiol. 36:3595–3600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rabbani GH, Greenough WB., III 1999. Food as a vehicle of transmission of cholera. J. Diarrhoeal Dis. Res. 17:1–9 [PubMed] [Google Scholar]
- 5.Ronnberg B, Wadstrom T. 1983. Rapid detection by a coagglutination test of heat-labile enterotoxin in cell lysates from blood agar-grown Escherichia coli. J. Clin. Microbiol. 17:1021–1025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Spangler BD. 1992. Structure and function of cholera toxin and the related Escherichia coli heat-labile enterotoxin. Microbiol. Rev. 56:622–647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Donta ST, Moon HW, Whipp SC. 1974. Detection of heat-labile Escherichia coli enterotoxin with the use of adrenal cells in tissue culture. Science 183:334–336 [DOI] [PubMed] [Google Scholar]
- 8.Said B, Scotland SM, Rowe B. 1994. The use of gene probes, immunoassays and tissue culture for the detection of toxin in Vibrio cholerae non-O1. J. Med. Microbiol. 40:31–36 [DOI] [PubMed] [Google Scholar]
- 9.Edwards KA, March JC. 2007. GM1-functionalized liposomes in a microtiter plate assay for cholera toxin in Vibrio cholerae culture samples. Anal. Biochem. 368:39–48 [DOI] [PubMed] [Google Scholar]
- 10.Honma Y, Higa N, Tsuji T, Iwanaga M. 1995. Comparison of a reversed passive latex agglutination and a polymerase chain reaction for identification of cholera toxin producing Vibrio cholerae O1. Microbiol. Immunol. 39:59–61 [DOI] [PubMed] [Google Scholar]
- 11.Ahn-Yoon S, DeCory TR, Baeumner AJ, Durst RA. 2003. Ganglioside-liposome immunoassay for the ultrasensitive detection of cholera toxin. Anal. Chem. 75:2256–2261 [DOI] [PubMed] [Google Scholar]
- 12.Hejtmancik KE, Peterson JW, Markel DE, Kurosky A. 1977. Radioimmunoassay for the antigenic determinants of cholera toxin and its components. Infect. Immun. 17:621–628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Charles PT, Velez F, Soto CM, Goldman ER, Martin BD, Ray RI, Taitt CR. 2006. A galactose polyacrylate-based hydrogel scaffold for the detection of cholera toxin and staphylococcal enterotoxin B in a sandwich immunoassay format. Anal. Chim. Acta 578:2–10 [DOI] [PubMed] [Google Scholar]
- 14.Ngundi MM, Taitt CR, McMurry SA, Kahne D, Ligler FS. 2006. Detection of bacterial toxins with monosaccharide arrays. Biosens. Bioelectron. 21:1195–1201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rucker VC, Havenstrite KL, Herr AE. 2005. Antibody microarrays for native toxin detection. Anal. Biochem. 339:262–270 [DOI] [PubMed] [Google Scholar]
- 16.Ligler FS, Taitt CR, Shriver-Lake LC, Sapsford KE, Shubin Y, Golden JP. 2003. Array biosensor for detection of toxins. Anal. Bioanal. Chem. 377:469–477 [DOI] [PubMed] [Google Scholar]
- 17.Lian W, Wu D, Lim DV, Jin S. 2010. Sensitive detection of multiplex toxins using antibody microarray. Anal. Biochem. 401:271–279 [DOI] [PubMed] [Google Scholar]
- 18.Evers DL, He J, Mason JT, O'Leary TJ. 2010. The liposome PCR assay is more sensitive than the Vibrio cholerae enterotoxin and Escherichia coli heat-labile enterotoxin reversed passive latex agglutination test at detecting cholera toxin in feces and water. J. Clin. Microbiol. 48:4620–4622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fields PI, Popovic T, Wachsmuth K, Olsvik O. 1992. Use of polymerase chain reaction for detection of toxigenic Vibrio cholerae O1 strains from the Latin American cholera epidemic. J. Clin. Microbiol. 30:2118–2121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huang J, Zhu Y, Wen H, Zhang J, Huang S, Niu J, Li Q. 2009. Quadruplex real-time PCR assay for detection and identification of Vibrio cholerae O1 and O139 strains and determination of their toxigenic potential. Appl. Environ. Microbiol. 75:6981–6985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Blackstone GM, Nordstrom JL, Bowen MD, Meyer RF, Imbro P, DePaola A. 2007. Use of a real time PCR assay for detection of the ctxA gene of Vibrio cholerae in an environmental survey of Mobile Bay. J. Microbiol. Methods 68:254–259 [DOI] [PubMed] [Google Scholar]
- 22.Zayats M, Raitman OA, Chegel VI, Kharitonov AB, Willner I. 2002. Probing antigen-antibody binding processes by impedance measurements on ion-sensitive field-effect transistor devices and complementary surface plasmon resonance analyses: development of cholera toxin sensors. Anal. Chem. 74:4763–4773 [DOI] [PubMed] [Google Scholar]
- 23.Taitt CR, Anderson GP, Lingerfelt BM, Feldstein MJ, Ligler FS. 2002. Nine-analyte detection using an array-based biosensor. Anal. Chem. 74:6114–6120 [DOI] [PubMed] [Google Scholar]
- 24.Alfonta L, Willner I, Throckmorton DJ, Singh AK. 2001. Electrochemical and quartz crystal microbalance detection of the cholera toxin employing horseradish peroxidase and GM1-functionalized liposomes. Anal. Chem. 73:5287–5295 [DOI] [PubMed] [Google Scholar]
- 25.Phillips KS, Han JH, Martinez M, Wang Z, Carter D, Cheng Q. 2006. Nanoscale glassification of gold substrates for surface plasmon resonance analysis of protein toxins with supported lipid membranes. Anal. Chem. 78:596–603 [DOI] [PubMed] [Google Scholar]
- 26.Viswanathan S, Wu LC, Huang MR, Ho JA. 2006. Electrochemical immunosensor for cholera toxin using liposomes and poly(3,4-ethylenedioxythiophene)-coated carbon nanotubes. Anal. Chem. 78:1115–1121 [DOI] [PubMed] [Google Scholar]
- 27.Chen H, Zheng Y, Jiang JH, Wu HL, Shen GL, Yu RQ. 2008. An ultrasensitive chemiluminescence biosensor for cholera toxin based on ganglioside-functionalized supported lipid membrane and liposome. Biosens. Bioelectron. 24:684–689 [DOI] [PubMed] [Google Scholar]
- 28.Cheng Q, Zhu S, Song J, Zhang N. 2004. Functional lipid microstructures immobilized on a gold electrode for voltammetric biosensing of cholera toxin. Analyst 129:309–314 [DOI] [PubMed] [Google Scholar]
- 29.Kuramitz H, Miyagaki S, Ueno E, Hata N, Taguchi S, Sugawara K. 2011. Binding assay for cholera toxin based on sequestration electrochemistry using lactose labeled with an electroactive compound. Analyst 136:2373–2378 [DOI] [PubMed] [Google Scholar]
- 30.Ngundi MM, Taitt CR, Ligler FS. 2006. Simultaneous determination of kinetic parameters for the binding of cholera toxin to immobilized sialic acid and monoclonal antibody using an array biosensor. Biosens. Bioelectron. 22:124–130 [DOI] [PubMed] [Google Scholar]
- 31.Chiriaco MS, Primiceri E, D'Amone E, Ionescu RE, Rinaldi R, Maruccio G. 2011. EIS microfluidic chips for flow immunoassay and ultrasensitive cholera toxin detection. Lab Chip 11:658–663 [DOI] [PubMed] [Google Scholar]
- 32.Xing JZ, Zhu L, Jackson JA, Gabos S, Sun XJ, Wang XB, Xu X. 2005. Dynamic monitoring of cytotoxicity on microelectronic sensors. Chem. Res. Toxicol. 18:154–161 [DOI] [PubMed] [Google Scholar]
- 33.Atienza JM, Zhu J, Wang X, Xu X, Abassi Y. 2005. Dynamic monitoring of cell adhesion and spreading on microelectronic sensor arrays. J. Biomol. Screen. 10:795–805 [DOI] [PubMed] [Google Scholar]
- 34.Yu N, Atienza JM, Bernard J, Blanc S, Zhu J, Wang X, Xu X, Abassi YA. 2006. Real-time monitoring of morphological changes in living cells by electronic cell sensor arrays: an approach to study G protein-coupled receptors. Anal. Chem. 78:35–43 [DOI] [PubMed] [Google Scholar]
- 35.Solly K, Wang X, Xu X, Strulovici B, Zheng W. 2004. Application of real-time cell electronic sensing (RT-CES) technology to cell-based assays. Assay Drug Dev. Technol. 2:363–372 [DOI] [PubMed] [Google Scholar]
- 36.Ryder AB, Huang Y, Li H, Zheng M, Wang X, Stratton CW, Xu X, Tang YW. 2010. Assessment of Clostridium difficile infections by quantitative detection of tcdB toxin by use of a real-time cell analysis system. J. Clin. Microbiol. 48:4129–4134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.He X, Wang J, Steele J, Sun X, Nie W, Tzipori S, Feng H. 2009. An ultrasensitive rapid immunocytotoxicity assay for detecting Clostridium difficile toxins. J. Microbiol. Methods 78:97–100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brooks GF, Carroll KC, Butel JS, Morse SA. 2007. Jawetz, Melnick & Adelberg's medical microbiology, 24th ed. McGraw-Hill, Columbus, OH [Google Scholar]
- 39.Almeida RJ, Hickman-Brenner FW, Sowers EG, Puhr ND, Farmer JJ, III, Wachsmuth IK. 1990. Comparison of a latex agglutination assay and an enzyme-linked immunosorbent assay for detecting cholera toxin. J. Clin. Microbiol. 28:128–130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yam WC, Lung ML, Ng MH. 1992. Evaluation and optimization of a latex agglutination assay for detection of cholera toxin and Escherichia coli heat-labile toxin. J. Clin. Microbiol. 30:2518–2520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Atienzar FA, Tilmant K, Gerets HH, Toussaint G, Speeckaert S, Hanon E, Depelchin O, Dhalluin S. 2011. The use of real-time cell analyzer technology in drug discovery: defining optimal cell culture conditions and assay reproducibility with different adherent cellular models. J. Biomol. Screen. 16:575–587 [DOI] [PubMed] [Google Scholar]
- 42.Dick MH, Guillerm M, Moussy F, Chaignat CL. 2012. Review of two decades of cholera diagnostics: how far have we really come? PLoS Negl. Trop. Dis. 6:e1845. 10.1371/journal.pntd.0001845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Levine MM, Kaper JB, Black RE, Clements ML. 1983. New knowledge on pathogenesis of bacterial enteric infections as applied to vaccine development. Microbiol. Rev. 47:510–550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Levine MM, Kaper JB, Herrington D, Losonsky G, Morris JG, Clements ML, Black RE, Tall B, Hall R. 1988. Volunteer studies of deletion mutants of Vibrio cholerae O1 prepared by recombinant techniques. Infect. Immun. 56:161–167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bacci S, Molbak K, Kjeldsen MK, Olsen KE. 2011. Binary toxin and death after Clostridium difficile infection. Emerg. Infect. Dis. 17:976–982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vanden Broeck D, Horvath C, De Wolf MJ. 2007. Vibrio cholerae: cholera toxin. Int. J. Biochem. Cell Biol. 39:1771–1775 [DOI] [PubMed] [Google Scholar]
- 47.Cherbas L, Cherbas P. 2010. Treatment of surfaces with poly-l-lysine for Drosophila cell cultivation. Cold Spring Harb. Protoc. 2010:pdb.prot5001. 10.1101/pdb.prot5001 [DOI] [PubMed] [Google Scholar]