Abstract
Transmission of Mycobacterium tuberculosis continues at high rates among Greenland-born persons in Greenland and Denmark, with 203 and 450 notified cases per 105 population, respectively, in the year 2010. Here, we document that the predominant M. tuberculosis outbreak strain C2/1112-15 of Danish origin has been transmitted to Greenland-born persons in Denmark and subsequently to Greenland, where it is spreading at worrying rates and adding to the already heavy tuberculosis burden in this population group. It is now clear that the C2/1112-15 strain is able to gain new territories using a new population group as the “vehicle.” Thus, it might have the ability to spread even further, considering the potential clinical consequences of strain diversity such as that seen in the widely spread Beijing genotype. The introduction of the predominant M. tuberculosis outbreak strain C2/1112-15 into the Arctic circumpolar region is a worrying tendency which deserves attention. We need to monitor whether this strain already has, or will, spread to other countries.
INTRODUCTION
Together with the Faroe Islands, Denmark (DK) and Greenland (GL) constitute the Kingdom of Denmark. DK is a low-tuberculosis (TB)-burden country, whereas GL is a high-TB-burden country, with overall TB notification rates of 6.5 and 203.0 per 105 population, respectively, in the year 2010 (1, 2).
Based on 2 decades of nationwide genotyping of Mycobacterium tuberculosis complex strains from TB cases in the Danish kingdom, it has been documented that the active transmission of M. tuberculosis continues at surprisingly high rates (2–7). In DK, the transmission occurs predominantly in specific high-risk segments of the population with social problems such as homelessness and alcohol and/or drug abuse. Many Denmark-born males are infected with one specific M. tuberculosis outbreak strain called the Danish cluster 2 or mycobacterial interspersed repetitive-unit–variable-number tandem-repeat (MIRU-VNTR) 1112-15 (C2/1112-15) strain (3, 5–7). From 1992 through 2011, more than 600 Denmark-born C2/1112-15 cases were registered, and the proportion increased from 6% in 1992 to 30% in 2011 (2). The C2/1112-15 outbreak in DK has been attributed to delayed diagnosis, and it has been recognized that the situation demands increased focus on early tuberculosis diagnosis and a reduction of transmission (2, 6, 8–10).
In GL, TB remains a major health problem (1, 11, 12). During the 1990s, the overall incidence of TB doubled (13), and the proportion of children <15 years among the TB cases increased from 8% in 1990 to 25% in 1997 (13, 14). Children still account for a high proportion of new cases (1), indicating high levels of active transmission (15).
Until recently, the C2/1112-15 outbreak was considered an isolated Danish problem, as the M. tuberculosis C2/1112-15 strain has been found almost exclusively in Denmark-born persons. Now, it is recognized that the C2/1112-15 strain is being transmitted to Greenland-born persons in DK and GL at increasing rates, which introduces this strain into the Inuit community in the Arctic circumpolar region, a region already heavily burdened with TB (2, 16). We believe this to be a problem that deserves attention.
The focus of this study was to quantify and map the recent spread of the M. tuberculosis outbreak strain C2/1112-15 from Denmark-born persons to Greenland-born persons in DK and GL and point out implications for TB control.
MATERIALS AND METHODS
This was a retrospective nationwide register study based on centralized M. tuberculosis genotyping from the previous 20 years.
Tuberculosis control.
All three countries in the Danish kingdom have separate notification systems but use a common biosafety level III diagnostic facility at the International Reference Laboratory of Mycobacteriology (IRLM) in Copenhagen, Denmark. The IRLM performs all culturing, drug susceptibility testing, and molecular typing of M. tuberculosis complex strains in these countries, whereas microscopy and PCR analyses are sometimes also offered by local laboratories. The mandatory notification system is dual; physicians and the IRLM report TB cases to the national health authorities. TB diagnostics, treatment, and contact tracing are free of charge (17).
Since 1992, nearly all (94%) culture-verified new and recurrent M. tuberculosis complex cases diagnosed in the Danish kingdom have been genotyped. The results have been stored in a laboratory register at the IRLM together with the laboratory findings and case-related information (17). From 1992 through 2006, isolates were genotyped by the gold standard IS6110 restriction fragment length polymorphism (RFLP) method (18), and from 1 January 2004 to the end of the study period, isolates were genotyped by the new gold standard, the 24-locus-based mycobacterial interspersed repetitive-unit–variable-number tandem-repeat (MIRU-VNTR) method (19, 20). PCR amplification of the 24 MIRU-VNTR loci was performed with the MIRU-VNTR genotyping kit (Genoscreen, Lille, France) as described in the manufacturer's manual, and DNA profiles were imported into the MIRU-VNTRplus web application (see http://www.miru-vntrplus.org/MIRU/index.faces) and named (21, 22).
Cohort and analysis.
In this study, we analyzed the genotyping results from all M. tuberculosis culture-positive TB cases in DK and GL from 1992 through 2011, including only one strain per case. The cases were genotyped as soon as the cultures were available. The genotyping results, laboratory findings, and available epidemiologically relevant information were linked at the case level. Based on their country of birth, the cases were categorized as Denmark-born or Greenland-born, with the vast majority of the Greenland-born people being Inuit. For any case without this information in the Civil Registration System, the country of birth was categorized as “other.” Epidemiological linkage information has not been collected or registered on a routine basis in the Danish kingdom and was thus available for only a few cases. Information on social factors has also not been registered systematically, but we retrieved data on living conditions from the Construction and Housing Register based on the case address information.
For the years 2005 and 2006, both IS6110 RFLP and MIRU-VNTR typing were performed. This overlapping period allowed us to identify the C2/1112-15 strain, originally discovered by the IS6110 RFLP method (5), by MIRU-VNTR typing as well. For 92.2% (153/166) of the cases genotyped by both methods, cluster 2 corresponded to one specific MIRU-VNTR genotype designated 1112-15. Therefore, MIRU-VNTR 1112-15 was considered to be identical to C2 and referred to as C2/1112-15. The MIRU-VNTR pattern 1112-15 can be seen at http://www.miru-vntrplus.org/MIRU/nomenclature.faces (enter “1112-15” in “single query”) (21, 22).
A TB case was classified as clustered, and thus potentially part of an active transmission chain, if the RFLP and/or MIRU-VNTR type was 100% identical to the type of at least one other case in the same country (DK or GL) during the study period.
The cases included in GL were categorized according to genotype and stratified by sex, age, disease characteristics, and country of origin (Table 1). Furthermore, the categorized cases were stratified by the year of genotyping (Table 2). The C2/1112-15 cases in DK were categorized according to ethnicity and stratified by the year of genotyping from 1992 through 2011 (Fig. 1). The mean age (MA) and standard deviation (SD) at the time of genotyping were calculated (Table 1). The incidence rate (IR) of the genotyped M. tuberculosis culture-positive new/reinfection cases in GL per 100,000 population per year was calculated based on population data retrieved from Statistics Greenland (Table 2) (see http://bank.stat.gl/Dialog/varval.asp?ma=BEDSAT1&ti=Befolkning+og+befolkningstilv%E6kst+1901%2D2012+efter+tid%2C+art+og+f%F8dested&path=./Database/Gr%F8nland/Befolkning/Folketal/&lang=4 [population 1901-2012]). The probability of equal distribution of all cases in GL by time (1992 to 2001 versus 2002 to 2011), genotype (clustered versus nonclustered), sex, age (20-year age groups), and disease localization (pulmonary versus nonpulmonary) was tested, as was smear positivity (positive versus negative) in clustered versus nonclustered cases, using the chi-square test (23).
Table 1.
Characteristics of Mycobacterium tuberculosis-positive cases in Greenland during the 20 years from 1992 through 2011, according to genotypea
| Characteristic | C2/1112-15 strain | Other, clustered | Nonclustered | Total |
|---|---|---|---|---|
| All cases (n [%]) | 36 (3.9) | 786 (84.8) | 105 (11.3) | 927 (100.0) |
| Sex (n [%]) | ||||
| Male | 21 (4.0) | 442 (85.0) | 57 (11.0) | 520 (100.0) |
| Female | 15 (3.7) | 344 (84.5) | 48 (11.8) | 407 (100.0) |
| Age (n [%]) | ||||
| All ages | 36 (100.0) | 786 (100.0) | 105 (100.0) | 927 (100.0) |
| 0–19 yr | 1 (2.8) | 172 (21.9) | 16 (15.2) | 189 (100.0) |
| 20–39 yr | 11 (30.6) | 337 (42.9) | 28 (26.7) | 376 (100.0) |
| 40–59 yr | 23 (63.9) | 216 (27.5) | 36 (34.3) | 275 (100.0) |
| 60–79 yr | 1 (2.8) | 57 (7.3) | 24 (22.9) | 82 (100.0) |
| ≥80 yr | 0 | 4 (0.5) | 1 (1.0) | 5 (100.0) |
| Mean age (SD) | 43 (10.8) | 34 (16.9) | 43 (19.6) | 35 (17.4) |
| Disease characteristics (n [%]) | ||||
| All locations | 36 (100.0) | 786 (100.0) | 105 (100.0) | 927 (100.0) |
| Pulmonary | 32 (88.9) | 738 (93.9) | 95 (90.5) | 865 (93.3) |
| Sputum smear positive | 20 (62.5) | 398 (53.9) | 41 (43.2) | 459 (53.1) |
| Nonpulmonaryb | 4 (11.1) | 48 (6.1) | 10 (9.5) | 62 (6.7) |
| Country of originc | ||||
| Greenland | 36 (3.9) | 778 (84.7) | 104 (11.3) | 918 (100.0) |
| Denmark | 0 | 6 (85.7) | 1 (14.3) | 7 (100.0) |
| Other | 0 | 2 (100.0) | 0 | 2 (100.0) |
Analyzed by the IS6110 RFLP or MIRU-VNTR method (see Materials and Methods).
Nonpulmonary only.
Country of origin was categorized as Denmark, Greenland, or other (see Materials and Methods).
Table 2.
Temporal distribution of Mycobacterium tuberculosis-positive cases in Greenland during the 20 years from 1992 through 2011, according to genotypea
| Yr | No. (%) of M. tuberculosis-positive cases categorized as: |
Total no. (%) | Total IRb | ||
|---|---|---|---|---|---|
| C2/1112-15 strain | Other, clustered | Nonclustered | |||
| 1992 | 0 | 22 (81.5) | 5 (18.5) | 27 (100.0) | 47.6 |
| 1993 | 0 | 20 (87.0) | 3 (13.0) | 23 (100.0) | 40.1 |
| 1994 | 0 | 28 (87.5) | 4 (12.5) | 32 (100.0) | 56.6 |
| 1995 | 0 | 29 (100.0) | 0 | 29 (100.0) | 50.8 |
| 1996 | 0 | 47 (85.5) | 8 (14.5) | 55 (100.0) | 95.5 |
| 1997 | 0 | 44 (84.6) | 8 (15.4) | 52 (100.0) | 88.5 |
| 1998 | 0 | 35 (79.5) | 9 (20.5) | 44 (100.0) | 74.2 |
| 1999 | 0 | 40 (87.0) | 6 (13.0) | 46 (100.0) | 76.9 |
| 2000 | 0 | 29 (85.3) | 5 (14.7) | 34 (100.0) | 60.4 |
| 2001 | 1 (1.7) | 55 (91.7) | 4 (6.7) | 60 (100.0) | 106.5 |
| 2002 | 0 | 39 (97.5) | 1 (2.5) | 40 (100.0) | 71.0 |
| 2003 | 1 (2.6) | 34 (87.2) | 4 (10.3) | 39 (100.0) | 69.2 |
| 2004 | 1 (2.2) | 37 (80.4) | 8 (17.4) | 46 (100.0) | 81.6 |
| 2005 | 8 (11.0) | 61 (83.6) | 4 (5.5) | 73 (100.0) | 129.5 |
| 2006 | 3 (5.7) | 45 (84.9) | 5 (9.4) | 53 (100.0) | 94.0 |
| 2007 | 7 (15.6) | 34 (75.6) | 4 (8.9) | 45 (100.0) | 79.9 |
| 2008 | 3 (6.8) | 38 (86.4) | 3 (6.8) | 44 (100.0) | 76.4 |
| 2009 | 5 (10.0) | 37 (74.0) | 8 (16.0) | 50 (100.0) | 86.8 |
| 2010 | 3 (3.8) | 66 (82.5) | 11 (13.8) | 80 (100.0) | 138.8 |
| 2011 | 4 (7.3) | 46 (83.6) | 5 (9.1) | 55 (100.0) | 95.4 |
| Total | 36 (3.9) | 786 (84.8) | 105 (11.3) | 927 (100.0) | 81.3 |
Analyzed by the IS6110 RFLP or MIRU-VNTR method (see Materials and Methods).
IR, incidence rate for genotyped Mycobacterium tuberculosis culture-positive new/reinfection cases in Greenland per 100,000 population per year.
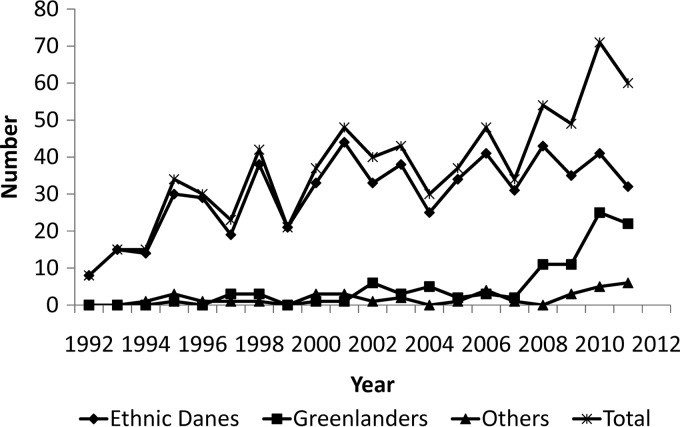
Fig 1.
Cases with the Mycobacterium tuberculosis C2/1112-15 genotype in Denmark categorized according to country of birth (see Materials and Methods) in ethnic Danes, Greenlanders, and others and according to total nationalities, by year of genotyping from 1992 through 2011.
RESULTS
The cohort consisted of 7,755 genotyped M. tuberculosis-positive TB cases, with 927 cases in GL and 6,828 cases in DK. During the 20-year study period, this accounted for 76.5% (7,755/10,140) of all notified TB cases, 64.2% (927/1,443) in GL, and 78.5% (6,828/8,697) in DK. Also, it accounted for 93.9% (7,755/8,259) of all the culture-verified cases in the two countries. Thus, 6.1% of all the culture-verified M. tuberculosis cases had no valid genotype or had not yet been genotyped, and 23.5% of the notified cases were culture negative or had no specimen sent for culture (mainly the cases based on clinical diagnoses alone, e.g., children). Of the 927 genotyped M. tuberculosis-positive TB cases in GL (Table 1), 99.0% were Greenlandic, 56.1% were male, and 93.3% had pulmonary TB, and 53.1% of the pulmonary cases were sputum smear positive. The MA was 35 years (SD, 17.4 years).
Of the 927 genotyped M. tuberculosis-positive TB cases in GL (Table 2), 88.7% had clustered strains and 11.3% had nonclustered strains. The C2/1112-15 strain accounted for 3.9% of the cases (Table 2), with the first case observed in Nuuk, the capital of GL, in the year 2001. The other clustered and nonclustered cases accounted for 84.8% and 11.3% of all cases, respectively (Table 2). There were no significant changes in the distribution of the clustered versus nonclustered cases during the first and last 10-year periods (chi-square, 1.8; P = 0.18), and in a comparison of the clustered and nonclustered cases, there were no significant differences in sex (chi-square, 0.2; P = 0.69) or disease location (chi-square, 1.5; P = 0.22). However, there were significantly more clustered sputum smear-positive pulmonary cases (chi-square, 4.2; P = 0.04). Also, there were more nonclustered pulmonary and nonpulmonary cases in the older age groups (chi-square, 34.3; P < 0.01). Based on living addresses, the vast majority of the C2/1112-15 cases in GL (86.1%) were located in Nuuk in the Sermersooq municipality, with a high concentration of the cases in one specific huge block of flats that house approximately 1% of the Greenlandic population, many of whom are unemployed, are poor, and/or abuse alcohol.
The average IR for the genotyped M. tuberculosis culture-positive TB cases in GL during the 20-year study period was 81.3, with the lowest IRs from 1992 through 1995 (47.6 to 50.8) and higher IRs during the rest of the period (up to 138.8 in 2010) (Table 2).
DISCUSSION
This study documents that the M. tuberculosis outbreak strain C2/1112-15 can no longer be considered a Danish problem only, as it is no longer confined to Denmark-born persons. An increasing number of Greenland-born persons, especially those in DK and now also those in GL, are infected with the M. tuberculosis strain C2/1112-15. During the first 10 years of the study (1992 through 2001), C2/1112-15 infection occurred only sporadically among Greenland-born persons in DK, and there were no cases in Greenland. Since then, among Greenland-born persons in DK with TB, the number of C2/1112-15 cases has increased significantly, reaching 37% in 2011. At the same time, the relative proportion of C2/1112-15 cases among Denmark-born persons has decreased from 100% in the early 1990s to 53% in 2011. Today, the Greenland-born minority in DK accounts for more than one-third of all C2/1112-15 cases in the Danish kingdom, although the C2/1112-15 genotype still accounts for no less than 30% of all clustered Denmark-born cases (2).
In DK, the C2/1112-15 outbreak among Denmark-born persons has been associated with specific high-risk segments of the population struggling with social problems, and transmission has been attributed to delayed diagnosis (2, 5–7). A large number of C2/1112-15 patients are male and have smear-positive (contagious) pulmonary TB (6, 7, 24). Focusing on the 99 Greenland-born C2/1112-15 cases in DK identified in this study, we found that 29% had no permanent living address or were living in shelters/institutions, and a further 55% lived in social housing at the time of diagnosis. Thus, at least 84% of all Greenlandic C2/1112-15 patients in DK lived under potentially difficult social conditions. During the last 2 decades covered by this study, other strains of M. tuberculosis have been introduced into the same vulnerable segment of the population without causing large outbreaks like the C2/1112-15 strain did. Thus, it is tempting to speculate that C2/1112-15 is more virulent than other strains, as was reported for the Beijing strains (25), but the present study does not enable any conclusions on virulence. Fortunately, nearly all C2/1112-15 strains are susceptible to first-line antituberculous drugs.
GL has some of the highest rates of pulmonary TB in the world (26). In this study, 93% of all the genotyped M. tuberculosis culture-positive TB patients in GL had pulmonary TB, among whom 53% were sputum smear positive. The proportion of the sputum smear-positive cases was significantly higher among the clustered pulmonary cases than the nonclustered pulmonary cases, and the proportion of elderly persons was significantly higher among the nonclustered cases than the clustered cases. Both findings indicate high rates of active transmission in the younger age groups in GL. It is striking that no less than 89% of all genotyped M. tuberculosis culture-positive cases in GL during these 20 years were clustered, even though higher clustering rates are to be expected in geographically isolated populations. The very high cluster frequency can also indicate high rates of active transmission in GL (27). In comparison, the overall cluster frequencies in DK and in The Netherlands are 56% and 45%, respectively (7, 28).
From the limited epidemiological linkage information, it is known that the first C2/1112-15 case observed in GL (in Nuuk in 2001) has family relations in DK and that this person lived in the Danish town Aalborg from August 1998 through November 1999. Aalborg has the second highest concentration of C2/1112-15 cases among Greenland-born persons in DK. Thus, it is possible that this patient was infected in DK and subsequently developed TB in GL approximately 2 years later, but the exact route of transmission cannot be proven.
The present resurgence of TB in DK and GL, especially the M. tuberculosis outbreak strain C2/1112-15 among Greenland-born persons in DK and GL, demands an increased focus on early tuberculosis diagnosis and control of transmission. Improved action will require prioritizing the prevention and control of tuberculosis both politically and economically (2). The health authorities in DK and GL have already taken some initiatives, but despite these efforts, the TB incidence is reaching new heights in GL, and the overall number of new TB cases is increasing in DK. It is obvious that the actions taken until now are not sufficient. It is important to eliminate the factors that continue to fuel the TB epidemic. Social factors such as housing, alcohol consumption, and nutrition have been identified as important obstacles, as well as the late detection of active cases, causing increased active transmission (29, 30). In GL, microepidemics occurring in small isolated settlements primarily affect young adults and children (13), and the widely scattered population living in small communities can be difficult to reach (11). The new European Union standards for tuberculosis care have emphasized that all persons presenting with signs, symptoms, a history, or risk factors compatible with TB should be evaluated for TB and that those suspected of having pulmonary TB should have at least two sputum specimens submitted for microscopic examination, culture, and drug susceptibility testing (DST) in a quality-assured laboratory (31). In addition, all molecular diagnostic results must be confirmed by culture-based DST (31). Fortunately, HIV infection plays no role in the present resurgence of TB in DK and GL (30, 32).
In conclusion, Greenland-born persons in DK and GL currently have significant TB problems (4, 13, 29, 33). Therefore, it is worrying that the predominant M. tuberculosis outbreak strain C2/1112-15 of Danish origin is spreading among Greenland-born persons in DK and GL. From this study, it is clear that the C2/1112-15 strain is able to spread to new territories using the Greenland-born population as a vehicle. Through the Greenland-born population in DK and subsequently in GL, the C2/1112-15 strain has been introduced into the Arctic circumpolar region and, in view of the potential clinical consequences of strain diversity, has the potential to spread even further, as seen with the widely spread Beijing genotype (34). If TB control is prioritized politically and economically, the histories in both DK and GL show that the problems can be overcome. In the 1940s, DK was leading the international WHO campaign against TB because of the successful Danish TB campaign (2), and as recently as the 1980s, GL had a more acceptable TB incidence of 22 per 105 population (13).
ACKNOWLEDGMENTS
We thank the staff at the IRLM for carefully and persistently performing genotyping for 20 years. We also thank Russell Hays for English proofreading.
Footnotes
Published ahead of print 25 September 2013
REFERENCES
- 1.TB Group, Board of Health and Prevention, Department of Health 2011. National TB strategy [2012-2016]. Government of Greenland, Nuuk, Greenland: (In Danish.) [Google Scholar]
- 2.Lillebæk T, Andersen AB, Seersholm NJ, Thomsen VO. 2012. Continued problems with tuberculosis among Danes and Greenlanders in Denmark and the need for reinforced control: a systematic review. Ugeskr. Laeger 174:2696–2701 (In Danish.) [PubMed] [Google Scholar]
- 3.Yang ZH, de Haas PE, Wachmann CH, van Soolingen D, van Embden JD, Andersen AB. 1995. Molecular epidemiology of tuberculosis in Denmark in 1992. J. Clin. Microbiol. 33:2077–2081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yang ZH, de Haas PE, van Soolingen D, van Embden JD, Andersen AB. 1994. Restriction fragment length polymorphism Mycobacterium tuberculosis strains isolated from Greenland during 1992: evidence of tuberculosis transmission between Greenland and Denmark. J. Clin. Microbiol. 32:3018–3025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bauer J, Yang Z, Poulsen S, Andersen AB. 1998. Results from 5 years of nationwide DNA fingerprinting of Mycobacterium tuberculosis complex isolates in a country with a low incidence of M. tuberculosis infection. J. Clin. Microbiol. 36:305–308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lillebaek T, Dirksen A, Kok-Jensen A, Andersen AB. 2004. A dominant Mycobacterium tuberculosis strain emerging in Denmark. Int. J. Tuberc. Lung Dis. 8:1001–1006 [PubMed] [Google Scholar]
- 7.Kamper-Jørgensen Z, Andersen AB, Kok-Jensen A, Bygbjerg IC, Andersen PH, Thomsen VO, Kamper-Jørgensen M, Lillebaek MT. 2012. Clustered tuberculosis in a low burden country: nationwide genotyping through 15 years. J. Clin. Microbiol. 50:2660–2667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lillebaek T, Thomsen VO. 2005. Worrying trend in the spread of tuberculosis among Danish men. Ugeskr. Laeger 167:388–391 (In Danish.) [PubMed] [Google Scholar]
- 9.Kok-Jensen A. 2005. Tuberculosis among Danish men. Ugeskr. Laeger 167:373 (In Danish.) [PubMed] [Google Scholar]
- 10.Leutscher P, Madsen G, Erlandsen M, Veirum J, Ladefoged K, Thomsen V, Wejse C, Hilberg O. 2012. Demographic and clinical characteristics in relation to patient and health system delays in a tuberculosis low-incidence country. Scand. J. Infect. Dis. 44:29–36 [DOI] [PubMed] [Google Scholar]
- 11.de Colombani P, Thomsen VO, Wilcke JT. 2011. Tuberculosis control in Greenland: report on a country visit 30 April–6 May 2010. WHO Regional Office for Europe, Copenhagen, Denmark [Google Scholar]
- 12.Jones J, Gastellu-Etchegorry M, Stenz FK, Baudon C, Bloem SJ, Bondonneau M, Cohuet S, Diggle R, Ewing RW, Gerstenbluth I, Grangeon JP, Kumar Alla K, Lajoinie G, Tromp M, Tumahai T, Yvon JF, Swaan CM, Gossner CM. 2011. Epidemiology, surveillance and control of infectious diseases in the European overseas countries and territories. Euro Surveill. 16:19923 http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=19923. [PubMed] [Google Scholar]
- 13.Søborg C, Søborg B, Pouelsen S, Pallisgaard G, Thybo S, Bauer J. 2001. Doubling of the tuberculosis incidence in Greenland over an 8-year period (1990-1997). Int. J. Tuberc. Lung Dis. 5:257–265 [PubMed] [Google Scholar]
- 14.Søborg B, Andersen AB, Melbye M, Wohlfahrt J, Andersson M, Biggar RJ, Ladefoged K, Thomsen VO, Koch A. 2011. Risk factors for Mycobacterium tuberculosis infection among children in Greenland. Bull. World Health Organ. 89:741–748, 748A–748E [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.European Centre for Disease Prevention and Control 2010. Progressing towards TB elimination. European Centre for Disease Prevention and Control, Stockholm, Sweden [Google Scholar]
- 16.Kamper-Jørgensen Z, Andersen AB, Kok-Jensen A, Kamper-Jørgensen M, Bygbjerg IC, Andersen PH, Thomsen VO, Lillebaek T. 2012. Migrant tuberculosis: the extent of transmission in a low burden country. BMC Infect. Dis. 12:60. 10.1186/1471-2334-12-60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kamper-Joergensen Z. 2011. Tuberculosis in Denmark: molecular epidemiology and application in control. Ph.D. thesis Statens Serum Institut and University of Copenhagen, Copenhagen, Denmark: (In Danish.) [Google Scholar]
- 18.van Embden JD, Cave MD, Crawford JT, Dale JW, Eisenach KD, Gicquel B, Hermans P, Martin C, McAdam R, Shinnick TM, Small PM. 1993. Strain identification of Mycobacterium tuberculosis by DNA fingerprinting: recommendations for a standardized methodology. J. Clin. Microbiol. 31:406–409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Supply P, Allix C, Lesjean S, Cardoso-Oelemann M, Rusch-Gerdes S, Willery E, Savine E, de Haas P, van Deutekom H, Roring S, Bifani P, Kurepina N, Kreiswirth B, Sola C, Rastogi N, Vatin V, Gutierrez MC, Fauville M, Niemann S, Skuce R, Kremer K, Locht C, van Soolingen D. 2006. Proposal for standardization of optimized mycobacterial interspersed repetitive unit-variable-number tandem repeat typing of Mycobacterium tuberculosis. J. Clin. Microbiol. 44:4498–4510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Oelemann MC, Diel R, Vatin V, Haas W, Rusch-Gerdes S, Locht C, Niemann S, Supply P. 2007. Assessment of an optimized mycobacterial interspersed repetitive-unit-variable-number tandem-repeat typing system combined with spoligotyping for population-based molecular epidemiology studies of tuberculosis. J. Clin. Microbiol. 45:691–697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Weniger T, Krawczyk J, Supply P, Niemann S, Harmsen D. 2010. MIRU-VNTRplus: a web tool for polyphasic genotyping of Mycobacterium tuberculosis complex bacteria. Nucleic Acids Res. 38:W326–W331. 10.1093/nar/gkq351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Allix-Béguec C, Harmsen D, Weniger T, Supply P, Niemann S. 2008. Evaluation and strategy for use of MIRU-VNTRplus, a multifunctional database for online analysis of genotyping data and phylogenetic identification of Mycobacterium tuberculosis complex isolates. J. Clin. Microbiol. 46:2692–2699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Preacher KJ. 2001. Calculation for the chi-square test: an interactive calculation tool for chi-square tests of goodness of fit and independence. Vanderbilt University, Nashville, TN: http://quantpsy.org/chisq/chisq.htm [Google Scholar]
- 24.Poulsen S, Ronne T, Kok-Jensen A, Bauer JO, Miorner H. 1999. Epidemiology of tuberculosis in Denmark 1972-1996. Ugeskr. Laeger 161:3452–3457 (In Danish.) [PubMed] [Google Scholar]
- 25.Hanekom M, Gey van Pittius NC, McEvoy C, Victor TC, van Helden PD, Warren RM. 2011. Mycobacterium tuberculosis Beijing genotype: a template for success. Tuberculosis 91:510–523 [DOI] [PubMed] [Google Scholar]
- 26.Zhang X, Andersen AB, Lillebaek T, Kamper-Jorgensen Z, Thomsen VO, Ladefoged K, Marrs CF, Zhang L, Yang Z. 2011. Effect of sex, age, and race on the clinical presentation of tuberculosis: a 15-year population-based study. Am. J. Trop. Med. Hyg. 85:285–290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Glynn JR, Bauer J, de Boer AS, Borgdorff MW, Fine PE, Godfrey-Faussett P, Vynnycky E. 1999. Interpreting DNA fingerprint clusters of Mycobacterium tuberculosis. European Concerted Action on Molecular Epidemiology and Control of Tuberculosis. Int. J. Tuberc. Lung Dis. 3:1055–1060 [PubMed] [Google Scholar]
- 28.Lambregts-van Weezenbeek CS, Sebek MM, van Gerven PJ, De Vries G, Verver S, Kalisvaart NA, van Soolingen D. 2003. Tuberculosis contact investigation and DNA fingerprint surveillance in The Netherlands: 6 years' experience with nation-wide cluster feedback and cluster monitoring. Int. J. Tuberc. Lung Dis. 7:S463–S470 [PubMed] [Google Scholar]
- 29.Thomsen VO, Lillebaek T, Stenz F. 2004. Tuberculosis in Greenland—current situation and future challenges. Int. J. Circumpolar Health 63(Suppl 2):225–229 [DOI] [PubMed] [Google Scholar]
- 30.Ladefoged K, Rendal T, Skifte T, Andersson M, Soborg B, Koch A. 2011. Risk factors for tuberculosis in Greenland: case-control study. Int. J. Tuberc. Lung Dis. 15:44–49 [PubMed] [Google Scholar]
- 31.Migliori GB, Zellweger JP, Abubakar I, Ibraim E, Caminero JA, De Vries G, D'Ambrosio L, Centis R, Sotgiu G, Menegale O, Kliiman K, Aksamit T, Cirillo DM, Danilovits M, Dara M, Dheda K, Dinh-Xuan AT, Kluge H, Lange C, Leimane V, Loddenkemper R, Nicod LP, Raviglione MC, Spanevello A, Thomsen VO, Villar M, Wanlin M, Wedzicha JA, Zumla A, Blasi F, Huitric E, Sandgren A, Manissero D. 2012. European union standards for tuberculosis care. Eur. Respir. J. 39:807–819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Taarnhøj GA, Engsig FN, Ravn P, Johansen IS, Larsen CS, Roge B, Andersen AB, Obel N. 2011. Incidence, risk factors and mortality of tuberculosis in Danish HIV patients 1995-2007. BMC Pulm. Med. 11:26. 10.1186/1471-2466-11-26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Grzybowski S, Styblo K, Dorken E. 1976. Tuberculosis in Eskimos. Tubercle 57:S1–S58 [DOI] [PubMed] [Google Scholar]
- 34.Nicol MP, Wilkinson RJ. 2008. The clinical consequences of strain diversity in Mycobacterium tuberculosis. Trans. R. Soc. Trop. Med. Hyg. 102:955–965 [DOI] [PubMed] [Google Scholar]