Abstract
Matrix metalloproteinases (MMPs) are a family of enzymes named for their ability to degrade proteins of the extracellular matrix. Here we describe the characterization of a rat monoclonal antibody specifically recognizing one member of this enzyme family, MMP-7. This antibody has been tested for its use in multiple assay types and was shown to be useful for direct ELISA, western blotting, immunocytochemistry and immunohistochemistry of frozen or paraffin-embedded tissues. The antibody has been evaluated for its usefulness with tissues from several different species and, by immunohistochemistry, can detect MMP-7 of human, murine, porcine and gerbil origin. Immunostaining of MMP-7 in normal tissues or benign tumors of intestinal, breast and prostatic origin indicates that this protein is normally localized luminally in glandular epithelium. The localization pattern would suggest that in normal or early stage tumors, MMP-7 is most likely not directly involved in extracellular matrix degradation. In contrast, advanced colon tumors show MMP-7 in invading cells at the advancing edge of the tumor.
Introduction
The matrix metalloproteinases (MMPs) are a family of enzymes that have been shown to play roles in multiple processes both physiological and pathological. These proteases are characterized by their initial synthesis as zymogens, the presence of a conserved PRCVGPD sequence that is involved in maintaining enzyme latency, a conserved H**GH sequence that coordinates a zinc ion at the catalytic active site, the ability to be inhibited by TIMP (tissue inhibitor of metalloproteinases) proteins, and, in many cases, the ability to degrade components of the extracellular matrix (1). Although multiple MMPs have been described and many have been shown to be important in diseases such as cancer and arthritis, there are specific functions associated with particular family members. MMP-7 (matrilysin, PUMP-1, EC 3.1.23.24) is one of the smallest members of this family possessing only the necessary domains for targeting to the secretory pathway, control of latency and catalytic activity. Most other MMPs contain these minimal domains in addition to others that contribute to substrate and inhibitor binding, intracellular activation or membrane localization. MMP-7 expression has been reported in glandular epithelium, an expression pattern distinct from many other MMPs (2). Recent evidence suggests that it plays roles in host defense mechanisms (3). Of interest from a tumor biology standpoint, MMP-7 expression has been reported in early-stage, benign tumors particularly of the GI tract and may represent an appropriate target for prevention strategies (, 5).
In order to study MMP-7 in various tumor and other tissues, we wanted to develop an antibody specific for this MMP that could be used for MMP7 detection in both human and model organisms. Here we describe the production and characterization of a rat monoclonal antibody that is useful for various assays in multiple species. We use the antibody in a range of human breast tumor specimens and show that MMP-7 protein can be detected early in the tumor development sequence, particularly in apparently normal tissue adjacent to tumor. Although MMPs are regarded as ECM modifying enzymes, a variety of other substrates have been found including growth and death factors, cytokines and adhesion proteins (6). The staining pattern of MMP-7 in differentiated glandular epithelium points toward an apical localization of this enzyme, hence it is unlikely to be involved in ECM degradation, at least in normal and benign tissues.
Materials and Methods
Immunization and hybridoma generation
The immunogen used was human recombinant MMP-7, latent form, a kind gift from Dr Harold VanWart, Syntex. The protein had been produced in CHO cells and purified using sequential ion exchange and metal chelation chromatography as described (7). A 200 μg bolus of the antigen was injected into Sprague-Dawley rats and the animals were boosted after 6 weeks. Three days following the booster injection, lymphocytes were isolated from the spleen and peripheral lymph nodes and fused with the non-secreting murine myeloma cell line Ag8.563. Successful hybridomas were detected by enzyme-linked immunosorbent assay (ELISA) screening of the supernatants. Twenty-two hybridomas were identified that gave positive results both with the ELISA screen and a western blotting screen. These were then assessed for their ability to detect human MMP-7 in paraffin-embedded formalin-fixed tissues previously shown to express MMP-7 mRNA. One hybridoma clone was selected based on this screen and was injected into nude mice to generate ascites, which was used for further characterization.
Isotyping
Isotyping was performed using the Rat MonoAb ID/SP kit from Zymed Laboratories, San Francisco, CA and following the manufacturer's instructions. Recombinant pro-MMP7 was used as the coating antigen at a concentration of 500ng/ml.
Binding affinity assay
Dilutions covering the range 17.5 – 350 μM of either recombinant latent or active MMP-7 (both from Calbiochem, La Jolla, CA) were prepared in binding buffer (sodium bicarb pH 9.2) and 50 ul aliquots were placed in duplicate wells of a microtiter plate and incubated overnight at 4°C. Following washing with phosphate buffered saline (PBS), the plate was incubated with purified hybridoma supernatant, diluted 1:30 in PBS, for 2 hrs at room temperature and washed again with PBS. A peroxidase-labeled anti-rat IgG (Zymed), diluted 1: 1000 in PBS was then added to each well and incubated for 30 mins at room temperature. Following washing, freshly-prepared substrate (ABTS; Zymed) was added to each well and after 30 mins, absorbance of each well at 405 nm was read using a Dynex MRX microplate reader.
Baculovirus Recombinant mouse and human MMP-7
Full-length cDNA clones representing either the murine [accession number: NM010810] or human [accession number: NM002423] MMP-7 genes were ligated into the multiple cloning sites of the pVL1393 or pAcGP67 baculovirus vectors (BD Pharmingen, San Diego, CA), respectively. These were then co-transfected with BaculoGold DNA (BD Pharmingen) into Sf9 insect cells to generate recombinant baculovirus. Following viral amplification, Sf9 cells producing high levels of protein were used to provide murine and human recombinant MMP-7 protein. The Sf9 cells were routinely cultured in a serum-free defined insect cell medium (TNM-FH; BD Pharmingen) and, after 48 hrs culture, this medium (“Sf9 conditioned medium”) was collected, centrifuged to remove cellular debris, and used in assays.
Cell lines
SW480, SW620, MDA-MB-468, MDA-MB-231, A549, HT29, HCT15, HCT116 and HepG2 cells were obtained from the American Type Culture Collection (ATCC, Manassas, VA) and grown in DMEM supplemented with 10% fetal calf serum in 5% CO2 at 37°C. The human colon adenocarcinoma cell line HCA-7 was obtained from Dr Susan Kirkland, London, UK and also maintained in DMEM supplemented as above. For western analysis, conditioned media were prepared by rinsing 90% confluent cultures on 10cm plates with serum-free medium and then incubating in 4 ml serum-free OPTI-MEM (Invitrogen, Carlsbad, CA) for 24 hrs. The media were collected and clarified by centrifugation. Recombinant mouse and human MMP-7 expressed from baculovirus-infected Sf9 insect cells were harvested also by collecting serum-free conditioned media. Activation of the zymogen form (pro-MMP-7) to the active enzyme was achieved by incubation in the presence of 50 μM of 4-aminophenylmercuric acetate (APMA; Sigma, St Louis, MO) at 37°C for 2 hours.
Immunoblotting
Conditioned media samples (1 μg) were loaded on to 12% SDS-PAGE gels. Also included on these gels were supernatants from Sf9 insect cells infected with baculovirus encoding either recombinant human or mouse matrilysin. For some blots APMA-activated samples were used. Following electrophoresis, gels were transferred to nitrocellulose, blocked with 10% milk in Tris-buffered saline (TBS) and incubated with hybridoma supernatant diluted 1:10 in blocking reagent for 2 hours at room temperature or overnight at 4°C. After washing in TBS, the blots were incubated for 30 mins at room temperature with biotinylated anti-rat secondary antibody (Vector Laboratories, Burlingame, CA) diluted 1:12,000. Again after washing, the blots were incubated for 30 mins at room temperature with streptavidin-HRP (1:15,000; Vector), washed and then detected using (Western Lightening kit; Amersham Biosciences, Little Chalfont, U.K.).
Assessment of Cross-reactivity
Recombinant pro-MMP-7, active MMP-7 (produced in bacteria), pro-MMP-9, pro-MMP-2, pro-MMP-3 and active MMP-13 were purchased from Calbiochem. Equimolar amounts of these enzymes (1 μmole) were loaded onto a 12% SDS-PAGE gel and analyzed by western blotting using the rat monoclonal MMP-7 antibody.
Immunocytochemistry
The cell lines HCT15, HCT116 and SW620 were grown on glass chamber slides (Nunc, Rochester, NY) until 70% confluent. The cells were fixed in 10% buffered formalin for 5 minutes at room temperature, washed in TBS and incubated for 1 hour at room temperature with 10% rabbit serum in TBS. Anti-MMP-7 antibody diluted 1:100 in 10% rabbit serum was then placed on the slides for 2 hours at room temperature. After washing, the slides were incubated for 30 minutes with Alexa-fluor 568-labeled rabbit anti-rat antibody (Invitrogen) diluted 1:5000 in TBS then washed again and mounted with aqueous anti-fade mountant (Invitrogen).
Immunohistochemistry
Spontaneously occurring intestinal polyps were dissected from Min/+ mice, fixed in 10% buffered formalin, paraffin-embedded and used to generate 5 μm sections. Ventral prostates were acquired from 10-week male C57BL/6 mice that had undergone surgical castration 2 days before as previously described (8). In addition, sets of 5 μm sections of unidentified human colorectal adenoma or carcinoma tissue samples, human breast tumor tissues and normal human breast tissue from reduction mammoplasties were obtained from the Vanderbilt Tissue Acquisition Core Laboratory under IRB guidelines. Sections were dewaxed and rehydrated through a series of graded alcohols, incubated in 0.6% hydrogen peroxide for 30 minutes to remove endogenous peroxidase activity, and heated to 95°C for 3.5 minutes in 10 mM citrate buffer, pH 6.0, to reveal antigenic epitopes. Following blocking for 1 hour at room temperature in a solution containing 5% goat serum, 1% bovine serum albumin, 0.5 % Tween-20 and 0.1 M MgCl2 in 10 mM Tris-HCl, pH 7.4, the sections were incubated with a rat monoclonal antibody raised against MMP-7 diluted 1:100 in blocking solution overnight at 4°C. The slides were washed three times in TBS containing 0.05% Tween-20 before being incubated for 1 hour at room temperature with biotinylated rabbit anti-rat IgG (Vector) diluted 1:1000. After another series of washes in TBS containing 0.05% Tween-20, the sections were processed using the Vectastain Elite ABC kit (Vector) according to the manufacturer's instructions. Positive signal was detected using diaminobenzidine (Sigma) as a chromogen and the sections were counterstained with Mayer's hematoxylin (Sigma).
Results
A series of clonal hybridomas were generated by fusion of lymphocytes from an immunized Sprague-Dawley rat and the murine myeloma cell line Ag 8.563. Antibodies produced by the hybridomas were screened for their ability to recognize human MMP-7. Twenty two clones were further tested for their ability to recognize human MMP-7 protein in paraffin-embedded tissue sections. After this screen, a single clone designated 338.18 was identified and expanded. The isotype of the purified antibody was determined using a commercially available kit for rat monoclonal antibodies and was found to be IgG2a.
Since human and mouse MMP-7 protein share 70% sequence homology at the protein level (2), we assessed whether our monoclonal would recognize MMP-7 from both species. As shown in figure 1a, bands corresponding to the latent and active versions i.e. pro-MMP-7 and MMP-7, were detected by western blotting using recombinant human or mouse enzyme produced in insect cells. It was unclear from these blots if there was a binding preference for the latent or active forms. Therefore, we used a direct ELISA-type assay to analyze this. Increasing concentrations of recombinant human pro-MMP7 or active MMP-7 were used to coat duplicate wells of a microtiter plate. After incubation with the MMP-7 antibody, followed by secondary antibody and substrate, the absorbance for each well was measured and used to blot a binding curve. Fig 1b shows that the binding curves for the latent and active enzymes are almost identical, indicating similar binding affinities for each form.
Figure 1.
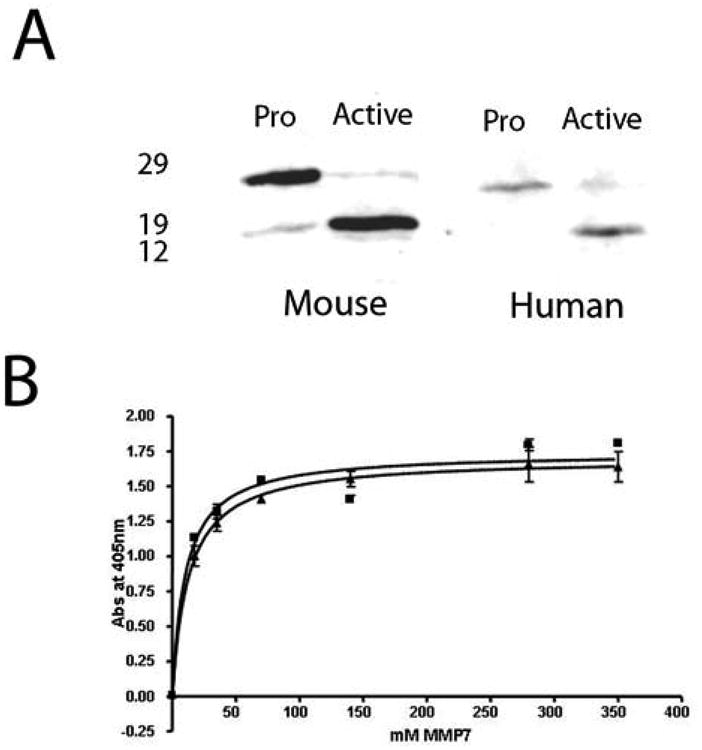
MMP-7 antibody recognizes latent (pro-) and active human and murine MMP-7. (A) Western blotting of 10 mg of baculovirus-expressed human or murine pro-MMP-7. Sizes (kDa) of molecular weight markers are shown on the left. Conversion to the active form was achieved by incubation in the presence of APMA as described in the text. (B) Binding curves for latent (squares) vs active (triangles) human MMP-7 indicate equivalent binding.
Although MMPs are not homologous, there are regions within the protein sequences of this family of enzymes that share similarity. We therefore assessed the specificity of the monoclonal antibody for MMP-7 by examining binding to equimolar amounts of recombinant MMP-2, MMP-3, MMP-7, MMP-9 and MMP-13 by western blotting. As seen in Fig 2a, the sole band obtained on this blot corresponded to MMP-7 suggesting there is no cross-reactivity between this antibody and other human members of the MMP family tested.
Figure 2.
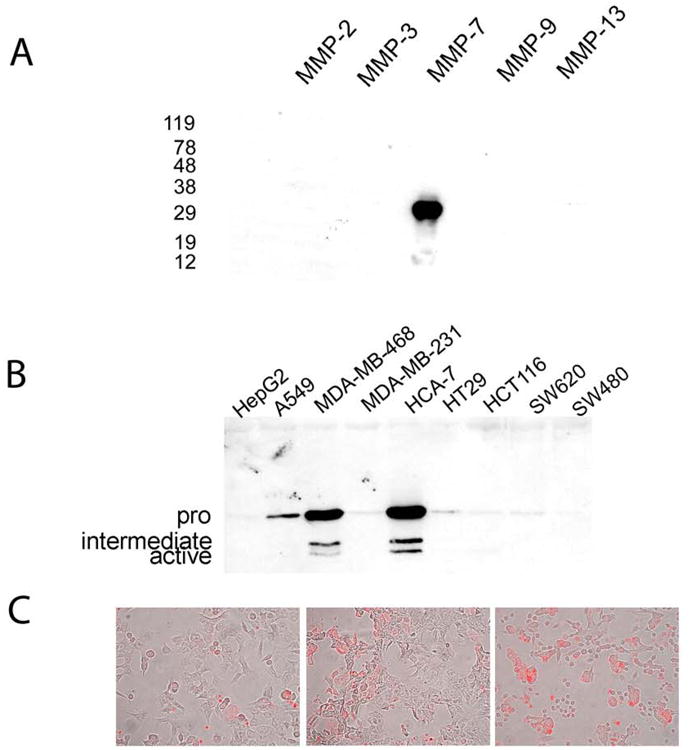
(a) Anti-MMP7 antibody specifically binds MMP-7. Western blotting of equimolar amounts (1 micro mole) of human recombinant MMP-2, MMP-3, MMP-7, MMP-9 and MMP-13 shows binding only to MMP-7. Sizes (kDa) of molecular weight markers are shown on the left. (b) MMP-7 expression in human adenocarcinoma cell lines. The cell lines used were HepG2 (hepatocellular carcinoma), A549 (lung adenocarcinoma), MDA-MB-468 (breast adenocarcinoma), MDA-MB-231 (breast adenocarcinoma), HCA-7 (colon adenocarcinoma), HT-29 (colon adenocarcinoma), HCT116 (colon adenocarcinoma), SW620 (lymph node metastasis of colon adenocarcinoma) and SW480 (colon adenocarcinoma). Bands representing the latent (30kDa), intermediate (approx 23kDa) and active (19kDa) forms of MMP-7 are evident in the strongest expressing samples MDA-MB-468 and HCA-7. Other positive samples show only the latent form. (c) Human colon adenocarcinoma cell lines display MMP-7 expression as detected by immunofluorescence (red fluorophore). Panels show from left to right HCT116, HCT-15 and SW620 cells stained for MMP-7 expression.
To examine the expression of MMP-7 in human tumor cell lines, supernatants from various lines were collected after incubating confluent cultures in serum-free medium for 48 hours. Immediately after harvesting, supernatants were clarified by centrifugation. Using equal protein levels, these supernatants were loaded on 12% SDS—PAGE gels, transferred to nitrocellulose and examined by immunoblotting with the MMP-7 antibody. As can be seen in Fig 2b, human adenocarcinomas from a variety of tissues showed expression of MMP-7 although there were some notable exceptions. The liver carcinoma cell line HepG2, the breast carcinoma cell line MDA-MB-231 and the colon carcinoma cell line SW480 were all negative, however 4 out of 5 colon tumor lines (HCA-7, HT29, HCT116 and SW620), a lung adenocarcinoma (A549) and a breast adenocarcinoma (MDA-MB-468) all had detectable expression of MMP-7 albeit at varying levels. Expression of MMP7 in the colon tumor lines SW620, HCT15 and HCT116 was also demonstrated by immunofluorescence as shown in Fig 2c.
Mice deficient in MMP-7 have been described (9). In mice, constitutive expression of MMP-7 can be detected in the Paneth cells of the small intestine (2). Since the original purpose of developing the monoclonal was to obtain an antibody suitable for immuno-histochemistry of paraffin-embedded tissues, we next analyzed MMP-7 protein expression in sections from formalin-fixed paraffin-embedded intestinal tissue from wild-type and MMP-7-null mice. Immunohistochemical staining revealed no reactivity in MMP-7-null tissues while the protein was present in the Paneth cells of wildtype mice as expected (Fig 3a, c) (10). In situ hybridization with a probe to murine MMP-7 was used to verify the staining (Fig 3b, d). Expression of MMP-7 mRNA has been documented in the murine ventral prostate after induction of involution following androgen ablation (11). Immunostaining of MMP-7 using the rat monoclonal antibody confirmed protein expression and revealed that it was localized to the luminal side of involuting murine prostates (Fig 3e,f). In addition, tissue sections from both pig and gerbil models of pancreatic and gastric disease respectively, indicated that MMP-7 could be specifically detected in tissues from these species (H.C. Crawford unpublished data).
Figure 3.
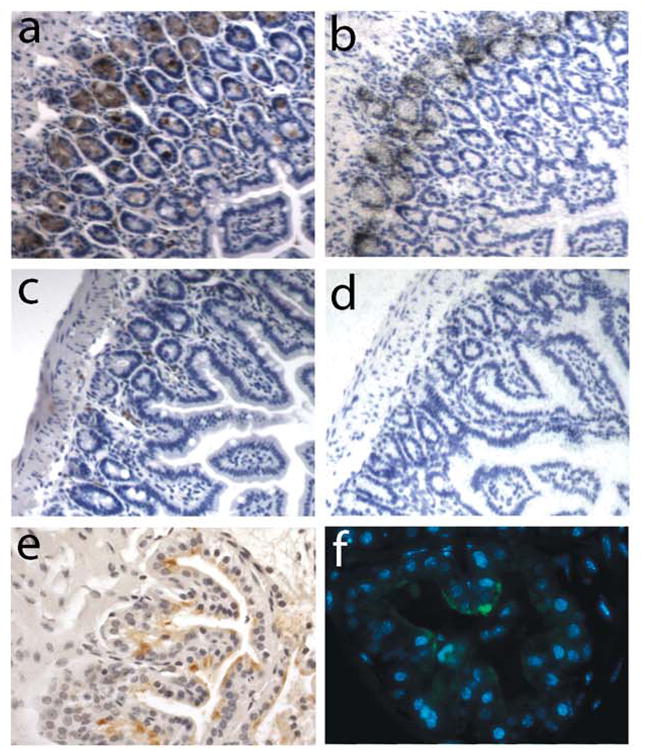
Expression of MMP-7 in murine tissue sections.(a,b) Section of small intestine from a wild-type C57bl/6 mouse showing immunohistochemical (a) and in situ hybridization (b) detection of MMP-7 expressed in the Paneth cells. (c, d) Sections of small intestine from a MMP-7-/- mouse demonstrating no signal by immunohistochemisty (c) or in situ hybridization (d) in Paneth cells. (e,f) Sections of ventral prostate from male C57bl/6 mice harvested 2-days post androgen ablation. Immunoperoxidase (e) and immunofluorescence (green fluorophore) (f) signals demonstrate localization of the induced MMP-7 protein to the luminal surface of the glands.
We have been especially interested in determining the expression of MMP-7 protein in human tumors at various stages of tumor progression. Analysis of human breast tissue specimens showed that MMP-7 expression could be found in normal-appearing ducts that were adjacent to areas of tumor (Fig 4a). However, normal breast tissue obtained from reduction mammoplasties showed no MMP-7 protein positivity (Fig 4b) suggesting that MMP-7 expression may be a marker of early neoplastic changes that are not detectable as histological abnormalities. Alternatively, MMP-7 may be induced in response to signals produced by adjacent tumor cells. Interestingly, as in the murine prostate, MMP-7 protein in normal-appearing human breast ductal epithelium was localized to the luminal side of the glands (Fig 4a). This was also the case in murine and human intestinal tissue where MMP-7 could be detected focally in adenomas (Fig 4 c,d). In more advanced intestinal tumors that had lost cell polarity, MMP7 protein was observed throughout expressing cells (Fig 4 e,f).
Figure 4.
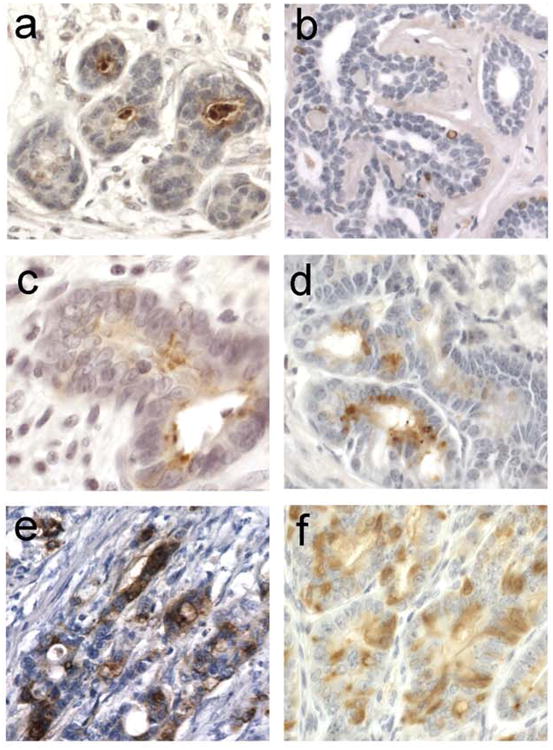
MMP-7 expression in neoplastic tissue. (a) Positive signal in the lumens of mammary ducts from a patient with ductal carcinoma in situ. (b) Breast tissue from a non-diseased patient (who underwent reduction mammoplasty) shows no MMP-7 expression in the ducts. (c) Human colonic adenoma shows MMP-7 expression in glandular lumen. (d) Murine intestinal adenoma from a 6-week ApcMin/+ mouse also shows lumenal staining. (e) Invasive human colonic adenocarcinoma shows MMP-7 is highly expressed in tumor cells, with no apparent discrete cellular localization. (f) Large murine adenoma from a 17-week ApcMin/+ mouse shows focal expression of MMP-7.
Discussion
As a member of the matrix metalloproteinase family of enzymes, MMP-7 has been assumed to be particularly involved in degradation of extracellular matrix proteins. Studies using MMP-7-deficient mice have instead shown that biologically relevant substrates in vivo include proteins of the tumor necrosis factor (TNF) family (8, 12, 13, 14), bactericidal defensins (10) and the adhesion molecule E-cadherin (15). Using the antibody described here, we have shown that the localization of MMP-7 protein in early tumors would support the concept of non-matrix substrates being more relevant for this enzyme. The MMP-7 protein is predominantly found in the apical/luminal side of glandular epithelium away from the basement membrane. Of course histochemical approaches for secreted proteins are inherently difficult to interpret as most of the protein stained is within cells and therefore not clearly associated with a particular direction of secretion. Nevertheless, based on clear differences in staining patterns as tumors progress as well as the apparent filling up of glandular lumens with positive staining secretions (e.g. Fig 4a), we are confident that MMP-7 is primarily apically localized.
As we tested the antibody for its usefulness in different assays, we examined tissue lysates by western blotting from wildtype and MMP-7-null animals. Surprisingly, a band of similar molecular weight to MMP-7 was detected in all lysates irrespective of the genotype of the donor animal (data not shown) Closer detection revealed fainter bands representing both pro- and active MMP-7 only in the lysates from wildtype animals. Although the non-specific band is not seen in all tissues (eg prostate), its presence leads us to recommend that this antibody is not routinely used for western analysis of murine tissue lysates. Other applications for which this antibody was also tested but proved not to be useful were immunoprecipitation and neutralization of enzyme activity
In summary, we have generated a rat monoclonal antibody with specificity for MMP-7. This antibody recognizes MMP-7 from multiple species and can detect both pro- or latent MMP-7 as well as the active form. We have used this antibody in direct ELISA, western blotting, immunocytochemistry and immunohistochemistry.
Acknowledgments
The authors wish to thank Dr Rebecca Shattuck-Brandt for preparation of the baculovirus MMP-7 constructs, Dr Susan Kirkland for kindly providing the HCA-7 cells and Dr Mary Ann Accavitti and the Hybridoma Core Facility at UAB for assistance with initial hybridoma generation and screening. This work was supported by NIH R01s CA60867 and CA84360 awarded to LMM. JRC was supported by NIH grant AR36457. We also gratefully acknowledge the Hybridoma Core Facility at UAB and Arthritis Center P30, NIH/NIAMS AR48311.
References
- 1.Visse R, Nagase H. Matrix metalloproteinases and tissue inhibitors of metalloproteinases: structure, function and biochemistry. Circ Res. 2003;92:827–839. doi: 10.1161/01.RES.0000070112.80711.3D. [DOI] [PubMed] [Google Scholar]
- 2.Wilson CL, Matrisian LM. Matrilysin: an epithelial matrix metalloproteinase with potentially novel functions. Int J Biochem Cell Biol. 1996;28:123–36. doi: 10.1016/1357-2725(95)00121-2. [DOI] [PubMed] [Google Scholar]
- 3.Parks WC, Wilson CL, López-Boado YS. Matrix metalloproteinases as modulators of inflammation and innate immunity. Nature Revs Immunol. 2004;4:617–629. doi: 10.1038/nri1418. [DOI] [PubMed] [Google Scholar]
- 4.Wagenaar-Miller RA, Gorden L, Matrisian LM. Cancer Metastas Revs. 2004;23:119–35. doi: 10.1023/a:1025819214508. [DOI] [PubMed] [Google Scholar]
- 5.Hawk ET, Umar A, Viner JL. Colorectal cancer chemoprevention - an overview of the science. Gastroenterology. 2004;126:1423–1447. doi: 10.1053/j.gastro.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 6.Lynch CC, Matrisian LM. Matrix metalloproteinases in tumor-host cell communication. Differentiation. 2002;70:561–73. doi: 10.1046/j.1432-0436.2002.700909.x. [DOI] [PubMed] [Google Scholar]
- 7.Barnett J, et al. purification, and characterization of human matrilysin (PUMP) from recombinant Chinese hamster ovary cells. Prot Express Purif. 1994;5:27–36. doi: 10.1006/prep.1994.1004. [DOI] [PubMed] [Google Scholar]
- 8.Powell WC, Fingleton B, Wilson CL, Boothby M, Matrisian LM. The metalloproteinase matrilysin proteolytically generates active soluble Fas ligand and potentiates epithelial cell apoptosis. Curr Biol. 1999;9:1441–7. doi: 10.1016/s0960-9822(00)80113-x. [DOI] [PubMed] [Google Scholar]
- 9.Wilson CL, Heppner KJ, Labosky PA, Hogan BL, Matrisian LM. Intestinal tumorigenesis is suppressed in mice lacking the metalloproteinase matrilysin. Proc Natl Acad Sci USA. 1997;94:1402–7. doi: 10.1073/pnas.94.4.1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wilson CL, et al. Regulation of intestinal alpha-defensin activation by the metalloproteinase matrilysin in innate host defense. Science. 1999;286:113–7. doi: 10.1126/science.286.5437.113. [DOI] [PubMed] [Google Scholar]
- 11.Powell WC, Domann FE, Jr, Mitchen JM, Matrisian LM, Nagle RB, Bowden GT. Matrilysin expression in the involuting rat ventral prostate. Prostate. 1996;29:159–68. doi: 10.1002/1097-0045(199609)29:3<159::aid-pros2990290304>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 12.Crawford HC, Scoggins CR, Washington MK, Matrisian LM, Leach SD. Matrix metalloproteinase-7 is expressed by pancreatic cancer precursors and regulates acinar-to-ductal metaplasia in exocrine pancreas. J Clin Invest. 2002;109:1437–44. doi: 10.1172/JCI15051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lynch CC, et al. MMP-7 promotes prostate cancer-induced ostolysis via the solubilization of RANKL. Cancer Cell. 2005;7:485–496. doi: 10.1016/j.ccr.2005.04.013. [DOI] [PubMed] [Google Scholar]
- 14.Haro H, Crawford HC, Fingleton B, Shinomiya K, Spengler DM, Matrisian LM. Matrix metalloproteinase-7-dependent release of tumor necrosis factor- alpha in a model of herniated disc resorption. J Clin Invest. 2000;105:143–50. doi: 10.1172/JCI7091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McGuire JK, Li Q, Parks WC. Matrilysin (Matrix metalloproteinase-7) mediates E-cadherin ectodomain shedding in injured lung epithelium. Am J Pathol. 2003;162:1831–1843. doi: 10.1016/S0002-9440(10)64318-0. [DOI] [PMC free article] [PubMed] [Google Scholar]


