Abstract
We demonstrate the design and the proof-of-concept use of a new, circular poly(methyl methacrylate)-based bioassay platform (PMMA platform), which affords for the rapid processing of 16 samples at once. The circular PMMA platform (5 cm in diameter) was coated with a silver nanoparticle film to accelerate the bioassay steps by microwave heating. A model colorimetric bioassay for biotinylated albumin (using streptavidin-labeled horse radish peroxidase) was performed on the PMMA platform coated with and without silver nanoparticles (a control experiment), and at room temperature and using microwave heating. It was shown that the simulated temperature profile of the PMMA platform during microwave heating were comparable to the real-time temperature profile during actual microwave heating of the constructed PMMA platform in a commercial microwave oven. The model colorimetric bioassay for biotinylated albumin was successfully completed in ~2 min (total assay time) using microwave heating, as compared to 90 min at room temperature (total assay time), which indicates a ~45-fold decrease in assay time. Our PMMA platform design afforded for significant reduction in non-specific interactions and low background signal as compared to non-silvered PMMA surfaces when employed in a microwave-accelerated bioassay carried out in a conventional microwave cavity.
Keywords: PMMA, Microwave-Accelerated Assays, Microwave-Induced Temperature Gradient, Silver Nanoparticles, Silver Island Films, Peroxidase, Colorimetric Assay
Introduction
The area of biosensor development has grown rapidly over the past two decades. Various technologies have been combined together to introduce a variety of biosensing devices, which can be used to detect analytes of interest from biological fluids and environmental samples.[1] Majority of the biosensors have successfully utilized enzyme-linked immunosorbent assay (ELISA)-based techniques to detect target analyte in a mixture of samples.[1] In this regard, colorimetric-based detection, which gives a visual confirmation of the presence of an analyte of interest, is the most widely used technique. Other detection methods, such as fluorescence[2-4]and chemiluminescence are routinely used due to the potential improvements in the detection limit of the bioassays compared to colorimetric detection.[5-7] Despite these latest developments, the biosensors still require long processing times especially for complex biological and environmental samples.[8-10]
The field of microwave-accelerated bioassays is a relatively new area of research where low power microwaves are used to reduce the bioassay time. Aslan et. al. successfully demonstrated the use of silver island films along with microwave heating to accelerate the assay time ~90-fold.[2] Microwave heating was also shown to have an effect on different detection methods, such as, DNA amplification applications,[11, 12] chemiluminescence[13] and fluorescence.[2] These authors also showed the use of high-throughput screening (HTS) plates in microwave-accelerated bioassays,[14] which is made of polystyrene and has a rectangular shape. However, it is well known that microwaves tend to bounce off sharp edges and non-circular surfaces. The presence of such edges on the HTS plates results in heterogeneous distribution of heat across all wells, which renders only the fraction of the 96 wells suitable for microwave-accelerated bioassays.[14] To the best of our knowledge, we are not aware of the application of microwave acceleration to electrochemical sensors.
To overcome the issue of heterogeneous heating of bioassay platforms to be used in microwave-accelerated bioassays, we have designed a circular PMMA platform, which is 5 cm in diameter (< wavelength of microwaves at 2.45 GHz ~ 12.2 cm). The PMMA platform was modified with silver nanoparticles and a 16-well silicone isolator for multiplexed processing of biological samples. PMMA has been employed in biomedical research especially in point-of-care and lab-on-a-chip systems due to its low cost of manufacturing and ease of usage. In majority of the systems, PMMA surface has been modified with amine bearing groups to enhance protein binding to the surface.[15-18] Recently, the Aslan Research Group have successfully demonstrated the use of a circular PMMA platform for crystallization of amino acids, using a method called Metal-Assisted and Microwave-Accelerated Evaporative Crystallization (MA-MAEC), which is similar to the working principle of microwave-accelerated bioassays. Both MA-MAEC and microwave-accelerated bioassay methods are based on the principle of microwave-induced temperature gradient and the accelerated mass transfer from the sample solution onto silver nanoparticle film.[19] The use of MA-MAEC technique was shown to reduce the crystallization time for amino acids and drug candidates on the circular PMMA platform by ~20 fold. In MA-MAEC, the circular PMMA platform afforded for homogenous heating by simultaneous temperature increase across all the wells. In addition, previous works from our laboratory have successfully demonstrated the use of silver nanoparticle-coated assay platforms to enhance enzymatic activity.[20, 21] Our laboratory have also demonstrated the use of several immobilization methods to attach avidin-labeled HRP enzyme at a distance of 6-8 nm from silver nanoparticles that resulted in further enhancement the HRP enzymatic activity as compared to direct enzyme immobilization onto silver nanoparticles.[21]
In this paper, we investigated the proof-of-concept use of circular PMMA platform coated with silver nanoparticles as a microwave-accelerated bioassay platform. Biotinylated albumin and streptavidin-peroxidase system was used as a model protein system, where o-phenylene diamine was used as a substrate for colorimetric detection of biotinylated albumin. The absorbance spectrum of colored product generated after the HRP enzymatic activity based on the amount of biotinylated albumin present on the PMMA platform was measured. It was found that the bioassays performed on silver nanoparticle-coated PMMA platform resulted in a larger absorbance values at 492 nm as compared to non-silvered PMMA platforms. The use of microwave power resulted in reduction of the assay time by ~45 fold on silvered PMMA platform, in addition to the reduction in the background signal.
Materials and Methods
Materials and Instrumentation
PMMA discs (5 cm diameter and 0.2 cm thick) were purchased from McMaster-Carr, GA, USA. Press-to-seal 12 well silicone isolators were purchased from Electron Microscopy Services, PA, USA. Streptavidin-horse radish peroxidase, biotinylated-bovine serum albumin (b-BSA), bovine serum albumin (BSA), o-phenylene diamine HCl (OPD), sodium phosphate, citric acid, hydrogen peroxide and sulfuric acid were purchased from Sigma Aldrich, USA. Microwave experiments were performed in 900 W Frigidaire kitchen microwave (Model No-FCM09Z03KB). Absorbance was measured using Varian UV-Vis spectrophotometer. Sigma Plot version 11 software was used for statistical analysis. COMSOL Multiphysics software (version 4.3.1) with RF module was purchased from COMSOL Inc., MA, USA.
Methods. COMSOL Simulations
A 16-well PMMA platform model was designed in COMSOL and simulations were performed for a model microwave cavity of dimensions same as those of 900 W Frigidaire microwave cavity. Dimensions of silicone isolator wells and the PMMA platform were obtained from manufacturers. Initial temperature and resistance of materials of the materials was set at 22 °C and 50 Ω respectively. Duration of microwave heating was set at 20 sec and temperature profile of model was obtained and the predicted temperature of each well was obtained.
Deposition of Silver Nanoparticles on PMMA
Prior to the deposition of silver nanoparticles, PMMA discs were subjected to plasma cleaning (using Harrick Plasma-PDC 32G cleaner) for 2 min. Silver deposition was carried out immediately after plasma cleaning using an EMS 150 R sputtering instrument in argon environment. A 16-well polypropylene mask was placed on the disc to prevent silver deposition on areas other than the locations of wells. Thickness of the silver layer was set at 2 nm, which was subsequently corroborated with atomic force microscopy (AFM).
Colorimetric Bioassay Procedure
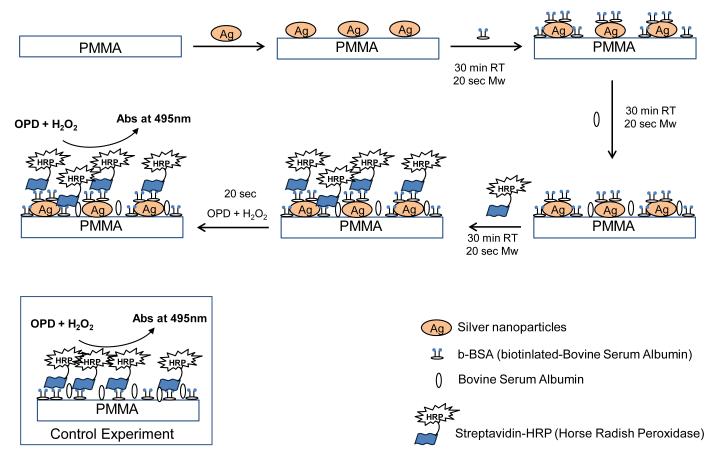
Scheme 1 depicts the steps taken in the completion of the colorimetric model bioassay. In this regard, a 30 μl solution of b-BSA (1 mg/ml solution of b-BSA prepared in PBS) was added to the wells and incubated for 30 min at room temperature or microwaved for 20 sec, followed by three times washing with deionized water. 30 μl of 5% BSA (5% w/v solution of powdered BSA prepared in PBS) was added to prevent non-specific binding and incubated for 30 min at room temperature or microwave heated for 20 sec. Wells were washed with deionized water and air dried. 30 μl of 1 mg/ml streptavidin-horse radish peroxidase solution was added and incubated at room temperature for 30 min or microwave heated for 20 sec, wells were washed and air dried. 30 μl of o-phenylene diamine HCl solution (4 mg of ophenylene diamine HCl dissolved in 10 ml of sodium phosphate-citric acid buffer of pH 5 and 4 μl of hydrogen peroxide was added one minute prior to use) was added and incubated for 2 min at room temperature or 10 sec in microwave. The reaction was stopped using 2 M sulfuric acid and absorbance spectrum of the product diaminophenazine (DAP) was measured between 400-600 nm.
Scheme 1.
Schematic depiction of the model colorimetric bioassay carried out on our PMMA platform coated with silver nanoparticles.
Results and Discussion
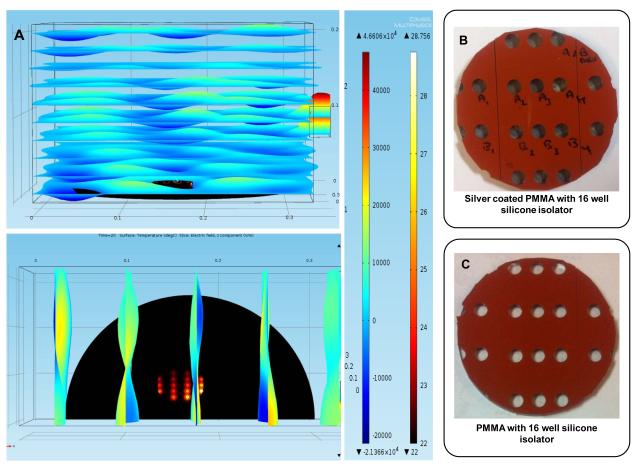
Figure 1A shows a simulated temperature profile and electric field (E2z) distribution in a commercially available microwave cavity containing the PMMA platform (top- and side-view) after microwave heating for 20 sec at 900 W at 2.45 GHz. Since the heating pattern and the electric field propagation in a commercial microwave cavity are symmetric through median of the cavity, these simulations were performed for one-half of the microwave cavity. In addition, since each of the bioassay step in the microwave-accelerated bioassays takes 20 sec, these simulations were carried out for only 20 sec. It is important to note that the total assay time, which includes all of the steps is longer than the time for the completion of each step. Figure 1A predicts that 20 sec of microwave heating results in a temperature increase by a maximum of 6.7 °C inside the microwave cavity and an increase of 2-3 °C (to ~25 °C) among all wells in the PMMA platform. These predictions imply that our PMMA platform design is successful in achieving homogeneous heating of all wells. Simulations also predict a higher temperature for wells closer to the port (on the right side) compared to those away from it, which is due to the heterogeneous nature of microwaves generated by a commercially available source. Figure 1B and 1C show the real-color photographs of the PMMA platform with and without silver nanoparticles film (dark spots inside the wells), respectively. The PMMA platform was constructed by using two 12 well silicone isolators: one of the silicone isolators was attached along the center of the PMMA platform and the other was divided into two halves and placed on either side of the first one. Any extensions of silicone isolator outside the PMMA platform were removed to avoid reflection electric field of the microwaves. Design and location of wells on the platform was optimized using theoretical calculations (i.e., COMSOL software) to achieve homogeneous heat distribution across the PMMA platform. It is important to note that the silicone isolators used in the PMMA platform do not absorb microwaves due to their low heat conductivity values, which that helps in preventing excessive heating of well surfaces and their contents.
Figure 1.
(A) COMSOL simulation of 16-well PMMA platform for 20 sec and 900 W power. (Top) XY-plane view (Bottom) XZ-plane view. The scale bars are for temperature on the right and for z-component of the electric field (E2z) on the left. Real color photograph of 16 well PMMA platform with (B) and without silver coating (C).
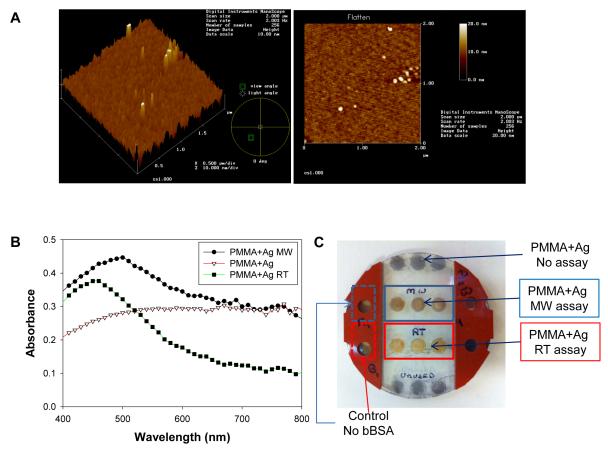
Figure 2A shows AFM images of 2 nm silver nanoparticle film coated on the PMMA platform. The AFM images show a homogeneous coating of silver nanoparticle film on the PMMA surface. The presence of silver nanoparticles on the PMMA platform was also verified by UV-Visible spectroscopy, which was performed for wavelengths between 400 nm to 800 nm. As shown in Figure 2B (PMMA+Ag), there is an increase in the overall absorbance in the wavelength range > 450 nm, which is similar to those of semi-continuous metal films on glass slides.[20] We note that silver nanoparticles were only deposited onto selected regions of the PMMA platform corresponding to the location of the wells. Since the silvered regions are separated by silicon isolators and the diameter of the wells (2 mm) is significantly less than the wavelength of microwaves at 2.45 GHz (12.2 cm), no sparking of the silvered surfaces during microwave heating is expected. Our aim was to use silver nanoparticle films in a homogeneous manner, as afforded by the film thickness of 2 nm. In this regard, we did not attempt to vary the thickness of the silver nanoparticle films. It is also important to note that other metals (i.e., gold, aluminum, copper, zinc, etc.) can be used with the circular PMMA platforms due their larger thermal conductivity values (larger than PMMA and water), which also afford for the generation of microwave-induced temperature gradient.
Figure 2.
Atomic force microscopy images of the PMMA platform coated with 2 nm silver nanoparticle film. (B) UV-Vis absorbance of blank PMMA and silver nanoparticle coated PMMA platform before and after the completion of the bioassay at room temperature (RT) and microwave heating (MW). (C) Real-color photograph of the silvered PMMA platform after the model bioassay was completed. The silicon isolator in the middle was removed to visually demonstrate the color of the wells.
Since the colorimetric model bioassay used in this study is based on the generation of a colored product as a result of enzymatic activity due to the presence of biotinylated albumin on the PMMA platform modified with silver nanoparticles, it is important to comment on the physical appearance of the PMMA platform after the model bioassays are completed. In this regard, the absorption spectrum of silvered PMMA platform (Figure 2B: PMMA+Ag MW assay) shows that there was no detachment of silver nanoparticles from the silvered PMMA platform at the end of the microwave-accelerated bioassay. However, there was an increase in absorbance at 492 nm (where the main peak for the colored product occurs), which is attributed to the slight attachment of the colored product to silver nanoparticles. Subsequently, one can conclude that the absorbance spectrum for the colored product generated as a result of the microwave-accelerated bioassay do not contain silver nanoparticles. On the other hand, Figure 2C shows that a greater extent of the colored product (yellow) appears to be attached to the silvered PMMA platform for the bioassay run at room temperature (PMMA+Ag RT assay). In addition, Figure 2B shows that the absorbance spectrum of the silver nanoparticles measured after the completion of the room temperature bioassay (PMMA+Ag RT) shows a decrease in the absorbance values >600 nm, as compared to silver nanoparticles before the start of the bioassay. Although not completely elucidated at this time, these observations (for PMMA+Ag RT) can be partially attributed to the change in the dielectric constant of the nanoparticles due to the attachment of the colored product and the loss of silver nanoparticles during the bioassay steps (Figure 2C). Nonetheless, the absorption spectrum of the colored product measured from the PMMA platform after the completion of enzymatic reactions at room temperature and microwave heating are identical to those provided by the vendor. This implies that any loss of silver nanoparticles from the PMMA platform that occurred during the completion of the bioassay at room temperature is not expected to affect the results of the bioassays. In addition, we also note that the colored product did not attach to blank PMMA platform (Figure S1, Supporting Information) due to the hydrophobic nature of the PMMA platform (contact angle of water is >60°, data not shown). We remind the reader that our main goal was to demonstrate the successful proof-of-concept use of circular PMMA platform in a microwave-accelerated bioassay scheme for the detection of biotinylated albumin.
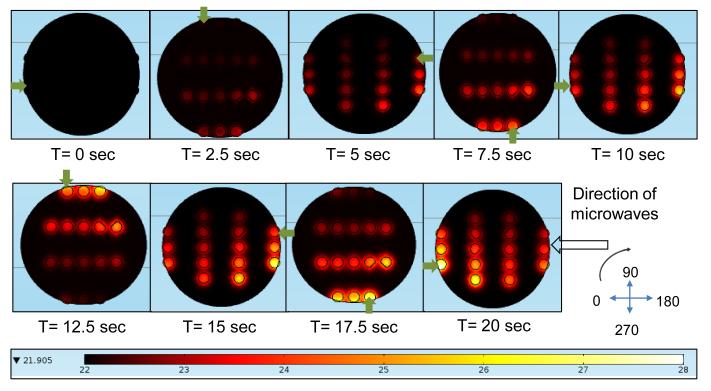
To minimize the heterogeneous heating of the PMMA platform, the PMMA platform is allowed to rotate in the commercially available microwave cavity. In order to study the impact of rotation of PMMA platform, first, a simulation of PMMA platform at various orientations within the electric field was conducted. We observed the actual amount of time taken for one complete rotation in the microwave oven as 10 sec, and based on that observed time; the orientation of the PMMA platform was set at intervals of 2.5 sec. Figure 3 shows the temperature profile of 16 wells on the PMMA platform at different orientations within the microwave cavity. Initial parameters for the temperature and power used were set at 22 °C and 900 W, respectively. In order to better understand the temperature trend of wells, one well was selected and tracked throughout the simulation. As shown in the Figure 3, the change in orientation of the PMMA platform results in a variation of temperature of the wells. This can potentially result in a variation of the extent of protein binding between the wells during microwave heating. Subsequently, in our model colorimetric bioassays, the PMMA platform is allowed to make 2 complete rotations (20 sec) in a commercially available microwave oven, which significantly reduces the effect of heterogeneous heating inside the cavity. One can also employ microwave devices operating in mono-mode to generate homogeneous heating of samples in microwave-accelerated bioassays. However, microwave devices operating in mono-mode typically cost around ~$20,000. In this regard, the use of a ~$50 microwave cavity originally designed for home use with our circular PMMA platform is well-justified; mainly due to the reduction of the effect of heterogeneous heating inside the cavity by the rotation of the samples.
Figure 3.
Simulated temperature profile in PMMA platform with silicon isolator wells with 30 ml water drop during microwave heating for 20 sec at 900 W power. PMMA platform is observed at different orientations every 2.5 sec. Green arrow indicates the well under observation.
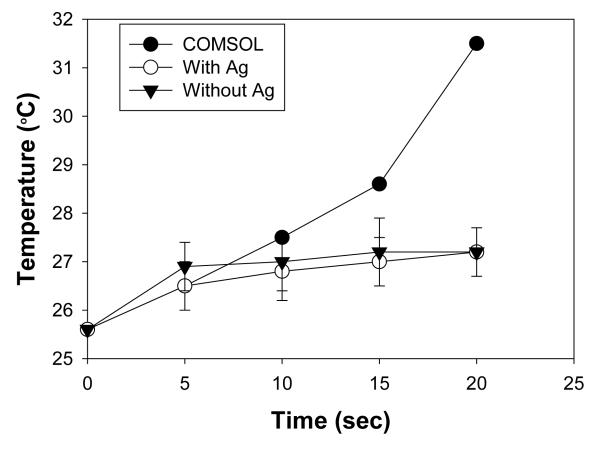
Figure 4 shows the increase in temperature of the selected well at the observed time intervals during the actual microwave heating of the PMMA platform in a microwave cavity and from theoretical simulations. Theoretical calculations predict a linear trend of increase in the temperature of the selected well from the initial temperature of 25.4 °C to 30.4 °C after 20 sec of microwave heating. A single well with and without silver nanoparticle film coating at same location used in theoretical simulations was selected for real time monitoring in a 900 W microwave oven. Using an infrared thermal gun, temperature of the water sample inside the well was measured for 20 sec of microwave heating at every 5 sec intervals. After 20 sec of microwave heating, the temperature of the sample was increased form an initial temperature of 25.3 °C and 25.8 °C to 27.4 °C and 26.9 °C for the PMMA platform with silver coating and without silver coating, respectively. For the same microwave heating time and power parameters, the observed temperature increases for the sample in the actual experiment are less than the temperature increase predicted by theoretical calculations. This can be attributed to the methodology used for the theoretical calculations, where microwave heating is assumed to be linear process and loss of energy is negligible during calculations. However, due to the heterogeneous nature of the microwaves, microwave energy is lost at a greater extent in a commercially available microwave cavity used in this study. Subsequently, the predicted temperature values slightly differ from the experimental values, which strongly demonstrate the need for experimental data for temperature for microwave-accelerated processes. It is also important to note that the temperature predicted by COMSOL software and the experimental temperature data indicates that application of microwave heating for a short period of time can be used without damaging the biological molecules, which are typically denatured at > 37 °C.
Figure 4.
Temperature trend of selected well in COMSOL simulations and experimental temperature trend of 30 ml of water in a well on PMMA platform coated with and without silver nanoparticles during microwave heating at 900 W power.
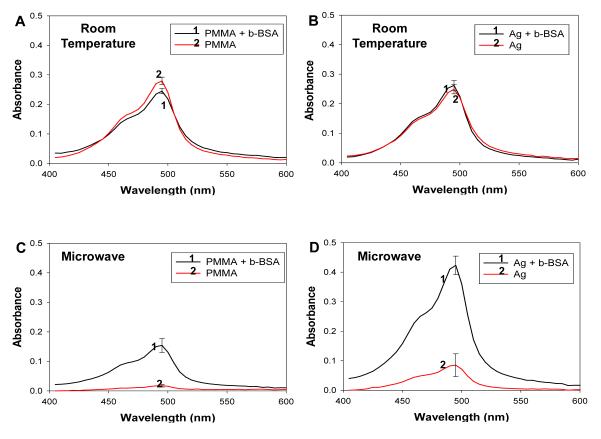
Subsequently, we have carried out a series of model colorimetric bioassays with appropriate controls to demonstrate the proof-of-concept use of our PMMA platform in microwave-accelerated bioassays for the detection of biotinylated albumin. Figure 5 shows the absorption spectrum for the colored product generated after the enzymatic activity on the PMMA platform with and without silver coating at room temperature and after microwave heating. At room temperature, we observed a significant amount of non-specific binding of streptavidin-HRP onto PMMA surface (Figure 5A) and silvered PMMA surface (Figure 5B), which resulted in a high background on both surfaces (no b-BSA). In the case of microwave heating, we observed a dramatic reduction of non-specific binding on PMMA platform without (Figure 5C) and with silver coating (Figure 5D). Absorbance value at 492 nm for the colored product measured from the microwave-accelerated bioassays was higher as compared to that measured from the bioassays carried out at room temperature. This is partially attributed to the attachment of colored product to the silver nanoparticles on the PMMA platform used for room temperature bioassay. One would expect the absorbance values for the colored product shown in Figures 5B and 5D to be similar, since the same amount of proteins/enzyme is present on the surfaces.[22] For the silvered PMMA platform (Figure 5D), the use of microwave heating resulted in the reduction of non-specific binding of avidin-HRP, which was also observed previously for fluorophore-labeled avidin used in identical bioassay scheme.[2, 23, 24] These observations can be attributed to the reduced time of incubation of samples using microwaves, which does not allow non-specific binding of streptavidin-HRP to the PMMA surface.[2, 14, 25] It is important to note that the quantity of proteins on all surfaces was expected to be similar, based on the previous results obtained by Grell et. al.[22]
Figure 5.
Absorbance spectrum of diaminophenazine generated after the completion of the HRP activity on the PMMA platform at room temperature and using microwave heating.
It is important to note that the circular PMMA platform offers the following advances for microwave-accelerated bioassays: 1) circular shape for homogenous microwave heating, 2) smaller size than microwave wavelength for effective microwave heating and 3) silver nanoparticle films for microwave-induced temperature gradient, which is the crux of microwave-accelerated bioassays. In addition, when compared to a traditional HTS plate with sharp edges, our circular PMMA platform is well suited for microwave heating due to the absence of sharp edges, resulting homogeneous heat distribution across wells. These results imply that the principle of microwave-accelerated bioassays can be applied to further enhance the speed and sensitivity of any colorimetric-based ELISA for the rapid detection of analyte of interest present in very low concentrations. In addition, we note that the main purpose of this study was to demonstrate the proof-of-principle use of the circular PMMA platform. In this regard, we are currently working on the in vitro detection of glial fibrillary acidic protein using the circular PMMA platform in a microwave-accelerated bioassay scheme, where we will provide results related to the level of sensitivity that microwave-accelerated bioassays can afford. These results will be reported in due course.
Conclusions
We have demonstrated the proof-of-concept use of our 16-well PMMA platform for the colorimetric detection of biotinylated albumin. The use of silver nanoparticle films along with short bursts of microwave energy results in the acceleration of bioassay steps as well as the reduction of non-specific binding of proteins. Temperature increases predicted by theoretical calculations and the observed temperature in our PMMA platform are significantly lower than the cut off temperature of 37 °C for denaturation of most proteins used in immunoassays, which is critically important in the successful use of our PMMA platform in bioassays. Our circular PMMA platform can be implemented for other on-demand and point-of-care systems that require a rapid and simple method of qualitative and quantitative analysis for a substance of interest.
Supplementary Material
Acknowledgement
The project described was supported by Award Number 5-K25EB007565-05 from the National Institute of Biomedical Imaging and Bioengineering. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Biomedical Imaging and Bioengineering or the National Institutes of Health.
References
- 1.Heeger PS, Heeger AJ. Making sense of polymer-based biosensors. Proc Natl Acad Sci U S A. 1999;96(22):12219–21. doi: 10.1073/pnas.96.22.12219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aslan K, Geddes CD. Microwave-accelerated metal-enhanced fluorescence: platform technology for ultrafast and ultrabright assays. Anal Chem. 2005;77(24):8057–67. doi: 10.1021/ac0516077. [DOI] [PubMed] [Google Scholar]
- 3.Aslan K, et al. Metal-enhanced fluorescence-based RNA sensing. J Am Chem Soc. 2006;128(13):4206–7. doi: 10.1021/ja0601179. [DOI] [PubMed] [Google Scholar]
- 4.Malicka J, Gryczynski I, Lakowicz JR. DNA hybridization assays using metal-enhanced fluorescence. Biochem Biophys Res Commun. 2003;306(1):213–8. doi: 10.1016/S0006-291X(03)00935-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cooke RF, Arthington JD. Concentrations of haptoglobin in bovine plasma determined by ELISA or a colorimetric method based on peroxidase activity. J Anim Physiol Anim Nutr (Berl) 2012 doi: 10.1111/j.1439-0396.2012.01298.x. [DOI] [PubMed] [Google Scholar]
- 6.Chen S, et al. Plasmon-enhanced colorimetric ELISA with single molecule sensitivity. Nano Lett. 2011;11(4):1826–30. doi: 10.1021/nl2006092. [DOI] [PubMed] [Google Scholar]
- 7.Grund S, et al. Comparison of hemagglutination inhibition assay, an ELISA-based micro-neutralization assay and colorimetric microneutralization assay to detect antibody responses to vaccination against influenza A H1N1 2009 virus. J Virol Methods. 2011;171(2):369–73. doi: 10.1016/j.jviromet.2010.11.024. [DOI] [PubMed] [Google Scholar]
- 8.Dixit CK, Kaushik A. Nano-structured arrays for multiplex analyses and Lab-on-a-Chip applications. Biochem Biophys Res Commun. 2012;419(2):316–20. doi: 10.1016/j.bbrc.2012.02.018. [DOI] [PubMed] [Google Scholar]
- 9.Lee MF, et al. One-tube loop-mediated isothermal amplification combined with restriction endonuclease digestion and ELISA for colorimetric detection of resistance to isoniazid, ethambutol and streptomycin in Mycobacterium tuberculosis isolates. J Microbiol Methods. 2010;83(1):53–8. doi: 10.1016/j.mimet.2010.07.018. [DOI] [PubMed] [Google Scholar]
- 10.Jablonski JE, et al. Determination of protein levels in soy and peanut oils by colorimetric assay and ELISA. J AOAC Int. 2010;93(1):213–20. [PubMed] [Google Scholar]
- 11.Kempitiya A, et al. Localized microwave heating in microwells for parallel DNA amplification applications. Applied Physics Letters. 2009;94(6):064106–3. [Google Scholar]
- 12.Shaw KJ, et al. Rapid PCR amplification using a microfluidic device with integrated microwave heating and air impingement cooling. Lab on a Chip. 2010;10(13):1725–1728. doi: 10.1039/c000357n. [DOI] [PubMed] [Google Scholar]
- 13.Chowdhury MH, et al. Metal-enhanced chemiluminescence: Radiating plasmons generated from chemically induced electronic excited states. Appl Phys Lett. 2006;88(17):173104. doi: 10.1063/1.2195776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aslan K, Holley P, Geddes CD. Microwave-Accelerated Metal-Enhanced Fluorescence (MAMEF) with silver colloids in 96-well plates: Application to ultra fast and sensitive immunoassays, High Throughput Screening and drug discovery. J Immunol Methods. 2006;312(1-2):137–47. doi: 10.1016/j.jim.2006.03.009. [DOI] [PubMed] [Google Scholar]
- 15.Dixit CK, et al. Development of a high sensitivity rapid sandwich ELISA procedure and its comparison with the conventional approach. Anal Chem. 2010;82(16):7049–52. doi: 10.1021/ac101339q. [DOI] [PubMed] [Google Scholar]
- 16.Bai Y, et al. Surface modification for enhancing antibody binding on polymer-based microfluidic device for enzyme-linked immunosorbent assay. Langmuir. 2006;22(22):9458–67. doi: 10.1021/la061123l. [DOI] [PubMed] [Google Scholar]
- 17.Fixe F, et al. Functionalization of poly(methyl methacrylate) (PMMA) as a substrate for DNA microarrays. Nucleic Acids Res. 2004;32(1):e9. doi: 10.1093/nar/gng157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fixe F, et al. One-step immobilization of aminated and thiolated DNA onto poly(methylmethacrylate) (PMMA) substrates. Lab Chip. 2004;4(3):191–5. doi: 10.1039/b316616c. [DOI] [PubMed] [Google Scholar]
- 19.Alabanza A, Mohammed M, Aslan K. Crystallization of L-alanine in the Presence of Additives on a Circular PMMA Platform Designed for Metal-Assisted and Microwave-Accelerated Evaporative Crystallization. CrystEngComm. 2012 doi: 10.1039/C2CE26363G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Abel B, et al. Plasmon-Enhanced Enzymatic Reactions: A Study of Nanoparticle-Enzyme Distance- and Nanoparticle Loading-Dependent Enzymatic Activity. Nano Biomed Eng. 2011;3(3):184–191. doi: 10.5101/nbe.v3i3.p184-191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Abel B, Aslan K. Plasmon-Enhanced Enzymatic Reactions 2:Optimization of Enzyme Activity by Surface Modification of Silver Island Films with Biotin-Poly (Ethylene-glycol)-Amine. Nano Biomed Eng. 2012;4(1):23–28. doi: 10.5101/nbe.v4i1.p23-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grell TA, et al. Quantitative Comparison of Protein Surface Coverage on Glass Slides and Silver Island Films in Metal-Enhanced Fluorescence-based Biosensing Applications. Nano Biomed Eng. 2010;2(3):165–170. doi: 10.5101/nbe.v2i3.p165-170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Aslan K, Geddes CD. Microwave-accelerated metal-enhanced fluorescence (MAMEF): application to ultra fast and sensitive clinical assays. J Fluoresc. 2006;16(1):3–8. doi: 10.1007/s10895-005-0026-z. [DOI] [PubMed] [Google Scholar]
- 24.Aslan K, et al. Microwave-accelerated metal-enhanced fluorescence: application to detection of genomic and exosporium anthrax DNA in <30 seconds. Analyst. 2007;132(11):1130–8. doi: 10.1039/b707876e. [DOI] [PubMed] [Google Scholar]
- 25.Aslan K, Grell TA. Rapid and sensitive detection of troponin I in human whole blood samples by using silver nanoparticle films and microwave heating. Clin Chem. 2011;57(5):746–52. doi: 10.1373/clinchem.2010.159889. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.