Abstract
Respiratory virus infections in the elderly result in increased rates of hospitalization and death. Respiratory syncytial virus (RSV) is a leading cause of severe virus-induced respiratory disease in individuals over the age of 65. CD8 T cells play a critical role in mediating RSV clearance. While it is clear that T cell immunity declines with age, it is not clear to what extent the CD8 T cell response to RSV is altered. Using aged BALB/c mice, we demonstrated that RSV-specific CD8 T cell responses were significantly reduced in the lungs of aged mice at the peak of the T cell response and that this decrease correlated with delayed viral clearance. Despite a decrease in the overall numbers of RSV-specific CD8 T cells during acute infection, their capacity to produce effector cytokines was not impaired. Following viral clearance, the RSV-specific memory CD8 T cells were similar in total number and phenotype in young and aged mice. Furthermore, following infection with a heterologous pathogen expressing an RSV epitope, RSV-specific memory CD8 T cells exhibited similar activation and ability to provide early control of the infection in young and aged mice. These data demonstrate a decrease in the capacity of aged mice to induce a high-magnitude acute CD8 T cell response, leading to prolonged viral replication, which may contribute to the increased disease severity of RSV infection observed for aged individuals.
INTRODUCTION
Respiratory syncytial virus (RSV) infection in individuals >65 years of age is a leading cause of hospitalization and accounts for the majority of RSV-associated deaths (1). Cell-mediated immunity is critical in resolving an acute RSV infection (2, 3). Since cell-mediated immunity wanes as the immune system ages, it is important to determine the effect of age on CD8 T cell immunity following RSV infection. While it has been documented that T cell-mediated immunity to RSV is decreased in the elderly, quantification of CD8 T cells responding to specific RSV epitopes has been reported only for the peripheral blood of healthy humans (4–6). In one study, the frequency of RSV-specific memory CD8 T cells was found to be lower in elderly subjects than in young subjects (6). However, it is currently unclear whether the decreased frequency of memory CD8 T cells observed in the elderly is due to attrition of long-lived memory cells or a decreased capacity to elicit new memory cells following exposure.
Several age-associated changes to the T cell repertoire are believed to negatively impact antiviral CD8 T cell responses. Thymic involution causes a decreased output of naïve T cells into the periphery that results in a decline in T cell receptor (TCR) diversity (7). Likely in response to decreased thymic output, peripheral T cells undergo increased homeostatic proliferation, causing the overall T cell repertoire to shift from a naïve to a memory phenotype over time (8). As existing peripheral T cells age, they accumulate intrinsic defects, including impaired TCR signaling capacity and impaired responsiveness to foreign antigens, diminished effector differentiation and cytokine production, and a decreased capacity to establish a stable memory pool (9). Finally, clonal expansions of antigen-experienced memory CD8 T cells develop that could further narrow overall TCR diversity (10, 11).
In this study, we sought to assess quantitative and qualitative changes in the generation of RSV-specific CD8 T cell responses as the immune system ages by using an aged BALB/c mouse model.
We demonstrate that aged BALB/c mice mount a weak primary RSV-specific CD8 T cell response in the lung that is associated with delayed virus clearance, consistent with the critical role of CD8 T cells in terminating an acute RSV infection (2, 3). The peak magnitudes of the virus-specific CD8 T cell responses in the lung and lung airways are significantly decreased in aged BALB/c mice relative to those for young controls. In contrast, the magnitudes of the RSV-specific CD8 T cell responses in the spleen and lung-draining mediastinal lymph nodes (medLNs) are similar for young and aged mice. The abilities of RSV-specific CD8 T cells to produce effector cytokines are similar in young and aged mice. Despite differences in peak magnitude, the RSV-specific CD8 T cell responses are similar in magnitude and phenotype in the periphery, lungs, and lung airways of young and aged mice following viral clearance and into the memory phase. Furthermore, following heterologous infection with a recombinant Listeria monocytogenes strain expressing an RSV-derived CD8 T cell epitope, RSV-specific memory CD8 T cells from aged mice are able to limit the infection similarly to CD8 T cells from young mice.
MATERIALS AND METHODS
Viruses and infection of mice.
The A2 strain of RSV was a gift from Barney S. Graham (National Institutes of Health [NIH], Bethesda, MD) and was propagated on HEp-2 cells (American Type Culture Collection, Manassas, VA). BALB/cAnNCr mice were purchased from the National Cancer Institute (Bethesda, MD) and were used either between the ages of 6 and 8 weeks or at the age of >18 months. Mice were anesthetized with isoflurane and were infected intranasally (i.n.) with 2 × 106 PFU of RSV. An attenuated Listeria monocytogenes strain expressing the RSV-derived M282-90 peptide (Att LM-M282) was generated as described previously (12). An intravenous (i.v.) infection with 107 CFU of Att LM-M282 was used as a heterologous challenge. All experimental procedures were approved by the University of Iowa's Animal Care and Use Committee.
Tissue isolation and preparation.
Bronchoalveolar lavage (BAL) fluid was harvested from mice by cannulation of the trachea and 3 successive washes with 1 ml of RPMI 1640 (Gibco, Grand Island, NY) supplemented with 10 U/ml penicillin G, 10 μg/ml streptomycin sulfate, 2 mM l-glutamine (Gibco), 0.1 mM nonessential amino acids (Gibco), 1 mM sodium pyruvate (Gibco), 10 mM HEPES (Gibco), 5 × 10−5 M 2-mercaptoethanol (Sigma-Aldrich, St. Louis, MO), and 10% fetal calf serum (FCS; Atlanta Biologicals, Lawrenceville, GA). After collection of the BAL fluid, lungs were perfused with 5 ml phosphate-buffered saline (PBS) via the right ventricle of the heart, removed, cut into small pieces, and subsequently digested in 4 ml Hanks balanced salt solution (HBSS) with CaCl2 and MgCl2 (Invitrogen, Grand Island, NY) supplemented with 125 U/ml collagenase (Invitrogen) and 60 U/ml DNase I (Sigma-Aldrich) for 30 min at 37°C. Mediastinal lymph nodes were similarly digested in 1 ml collagenase and DNase I. Single-cell suspensions were prepared by pressing lung tissue through a wire mesh screen (Cellector; Bellco Glass, Vineland, NJ). Spleens and lymph nodes were pressed between the frosted ends of glass slides (Surgipath, Richmond, IL).
Cell surface staining.
Single-cell suspensions (1 × 106 to 2 × 106 cells) were plated in 96-well round-bottom plates (Corning, Corning, NY). Cells were blocked with a monoclonal antibody (MAb) against FcγRII/III (clone 93) and were stained with MAbs specific for CD4 (RM4.5), CD8 (53-6.7), CD25 (PC61), CD44 (IM7), CD62L (MEL-14), CD69 (H1.2F3), Thy1.2 (53-2.1), CD107a (1D4B), CD127 (A7R34), and KLRG1 (2F1). All antibodies were obtained from eBioscience (San Diego, CA). Cells were stained for 20 min at 4°C, washed twice with cold fluorescence-activated cell sorter (FACS) buffer (PBS, 2% FCS, and 0.02% sodium azide), and subsequently fixed with FACS lysing solution (BD Biosciences). For staining of major histocompatibility complex class I tetramers, cells were stained with optimized concentrations of allophycocyanin (APC)-conjugated RSV Kd/M282-90 tetramers (obtained from the National Institutes of Health Tetramer Core Facility, Atlanta, GA) for 30 min at 4°C prior to extracellular staining. Samples were run on a FACSCanto flow cytometer (BD Biosciences), and data were analyzed using FlowJo software (TreeStar, Ashland, OR).
Ex vivo peptide stimulation and intracellular staining.
Single-cell suspensions were plated in 96-well round-bottom plates (Corning) for 5 h at 37°C with or without 1 μM M282-90, M2127-235, or F85-93 peptide in the presence of 10 μg/ml brefeldin A (Sigma-Aldrich). Cells were subsequently stained for cell surface CD8 and Thy1.2 and were fixed with FACS lysing solution. Fixed cells were washed with permeabilization buffer (FACS buffer containing 0.5% saponin; Sigma-Aldrich) and were subsequently stained with antibodies against gamma interferon (IFN-γ) (XMG1.2; eBioscience) and tumor necrosis factor alpha (TNF-α) (MP6-XT22; eBioscience). Cells were washed once with permeabilization buffer and again with FACS buffer prior to analysis by flow cytometry.
Plaque and colony assays.
Lungs were harvested from RSV-infected mice on days 4, 7, and 12 postinfection (p.i.) and were processed for plaque assays on Vero cells as described previously (13). On day 2 following Att LM-M282-90 infection, the spleens were harvested and were processed to determine the CFU as described previously (14).
Data analysis.
Graphical analysis was performed using Prism software (GraphPad Software, San Diego, CA). Statistical analyses were performed using InStat software (GraphPad Software).
RESULTS
Increased RSV-induced disease and delayed virus clearance in aged mice.
RSV infection causes substantially increased morbidity and mortality in elderly individuals (15). Therefore, we compared the severity of RSV-induced disease in young (6- to 8-week-old) and aged (>18-month-old) BALB/c mice by monitoring weight loss and airway resistance (expressed as enhanced pause [Penh]) daily following infection. Aged animals exhibited significantly more weight loss and more-sustained airway resistance than young mice following acute RSV infection (Fig. 1A and B). In addition, previous studies have shown that RSV clearance is delayed in aged animals relative to that in young animals (4, 5). To confirm these findings, we examined the kinetics of RSV replication and clearance from the lungs of young and aged BALB/c mice. Peak virus titers (day 4 p.i.) in the lungs were similar for young and aged mice (Fig. 1C). Whereas the virus level was below the limit of detection in young mice by day 7 p.i., high levels of virus were still present in the lungs of aged mice at that time. However, aged mice cleared virus from the lungs by day 12 p.i. These data suggest that increased and sustained viral replication in the lungs of aged mice may contribute to the increased and prolonged disease severity observed following RSV infection. Moreover, since CD8 T cells are responsible for resolving an acute RSV infection (2), these results suggest that CD8 T cell-mediated immunity may be compromised in aged mice.
Fig 1.

Increased disease severity and delayed RSV clearance in the lungs of aged mice. Young and aged mice were infected with RSV. (A and B) Disease severity was monitored by weight loss, expressed as a percentage of starting weight (A), and airway resistance (Penh) (B). Data were analyzed by two-way analysis of variance with repeated measures and Bonferroni's posttest. *, P < 0.05; ***, P < 0.001. (C) Viral burdens in the lungs were assessed via plaque assay at days 4, 7, and 12 p.i. Data are cumulative from two separate experiments (n, 8 to 9 mice/group).
Diminished RSV-specific CD8 T cell response in aged mice.
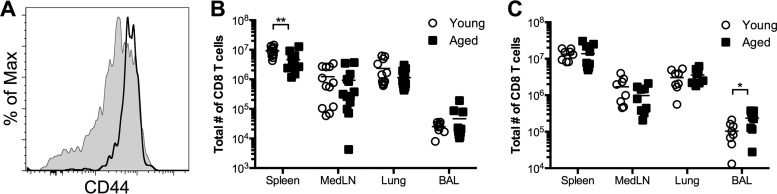
Given that viral clearance was delayed in aged mice and that CD8 T cells are critical for clearing an acute infection (2, 3), we wanted to determine the impact of age on the magnitude and kinetics of the primary RSV-specific CD8 T cell response. The proportion of T cells with a naïve phenotype (CD44lo) decreases with age as cells with a memory phenotype (CD44hi) continually accumulate (16–18). In agreement with these findings, the majority of CD8 T cells from the peripheral blood of aged mice exhibited a CD44hi memory phenotype, in contrast to those of naïve young mice (Fig. 2A). Following RSV infection, there were similar numbers of total CD8 T cells in the lung-draining medLNs and lung parenchyma at days 8 and 12 p.i. (Fig. 2B and C). However, the total number of CD8 T cells in the lung airways (isolated via bronchoalveolar lavage [BAL]) was increased in aged mice at day 12 p.i. (Fig. 2C). The sustained presence of CD8 T cells in the airways of aged mice may be a consequence of the prolonged viral replication in aged mice relative to that in young mice.
Fig 2.
The total CD8 T cell population has a memory-like phenotype in aged mice. (A) Peripheral blood CD8 T cells from uninfected aged mice exhibit a memory phenotype. Representative histograms show CD44 expression by CD8 T cells from young (shaded histogram) and aged (black line) mice. (B and C) Total numbers of CD8 T cells on days 8 (B) and 12 (C) following RSV infection. Data are cumulative from two separate experiments (n, 8 to 13 mice/group). Data were analyzed by two-way analysis of variance and Bonferroni's posttest. *, P < 0.05; **, P < 0.01.
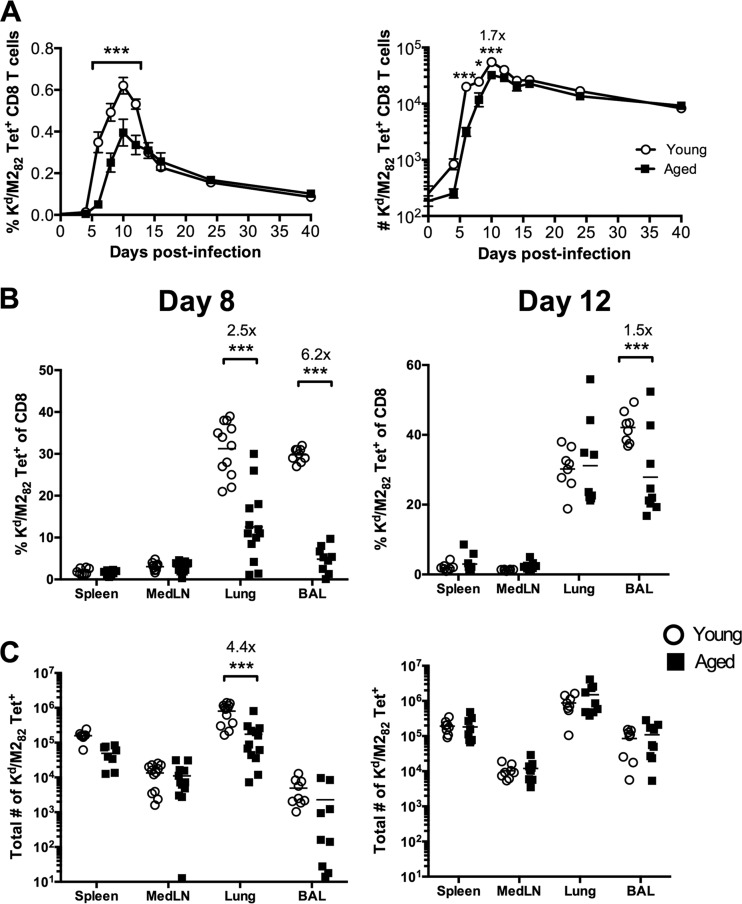
We next examined the RSV-specific CD8 T cell response to the immunodominant H-2Kd-restricted M282-90 epitope. Both the frequency and the total number of M282-specific CD8 T cells within the peripheral blood on days 6, 8, and 10 p.i. were significantly higher in young mice than in aged mice (Fig. 3A). However, by day 12 p.i., there was no difference in the total number of M282-specific CD8 T cells in the peripheral blood between naïve and aged mice. In the lungs, the frequency and total number of M282-specific CD8 T cells in young mice were significantly higher than those in aged mice on day 8 p.i. (Fig. 3B and C). However, by day 12 p.i., this discrepancy in the frequency and total number of M282-specific CD8 T cells in the lungs between young and aged mice was resolved (Fig. 3B and C). Furthermore, the frequency of M282-specific CD8 T cells in the lung airways was higher in young mice on days 8 and 12 p.i., but due to the increased total number of CD8 T cells in the lung airways of aged mice, the total numbers of M282-specific cells were similar (Fig. 3B and C). These data suggest that the magnitude of the virus-specific CD8 T cell response differs early after RSV infection but is similarly sustained in young and aged mice following viral clearance due to differential contraction of the CD8 T cells.
Fig 3.
Aged mice exhibit a diminished CD8 T cell response following acute RSV infection. (A) Frequency of M282 tetramer-positive CD8 T cells, expressed as a percentage of total peripheral blood cells (left), and total number of M282 tetramer-positive CD8 T cells per ml of peripheral blood (right) in young and aged mice at the indicated time points following RSV infection. (B and C) Frequency (B) and total numbers (C) of M282 tetramer-positive CD8 T cells at day 8 (left) and day 12 (right) p.i. in young and aged mice. Cumulative data from two to three separate experiments are shown (n, 8 to 13 mice/group). Data were analyzed by two-way analysis of variance and Bonferroni's posttest. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
Cytokine production by RSV-specific CD8 T cells in aged mice.
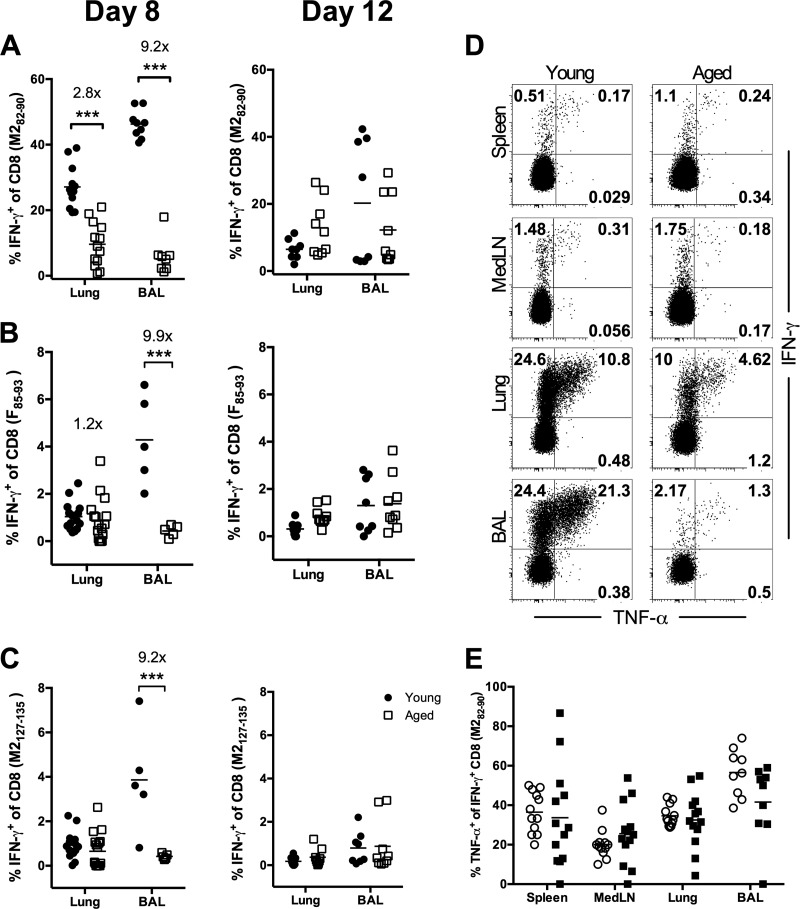
As individuals age, naïve T cells accumulate defects that inhibit their capacity to proliferate and acquire effector functions (19–21). To examine the effector functions of RSV-specific CD8 T cells generated in aged animals, cells were stimulated ex vivo with the dominant M282-90 peptide or the subdominant F85-93 or M2127-135 peptide and were assessed for IFN-γ and TNF-α production. While the responses in the spleens and medLNs of young and aged mice were similar, there were profound decreases in both the frequency and the total number of RSV-specific IFN-γ-producing CD8 T cells in the lung parenchyma and airways of aged mice at day 8 p.i. (Fig. 4A to D; also data not shown). Still, similar frequencies of M282-specific IFN-γ+ CD8 T cells were capable of coproducing TNF-α in all tissues examined at day 8 p.i. (Fig. 4E). However, by day 12 p.i. the frequency and total number of IFN-γ-producing CD8 T cells in response to RSV peptide stimulation in the lung and lung airways were similar for young and aged mice (Fig. 4 and data not shown). Together, these data demonstrate that although RSV-specific CD8 T cell responses are initially significantly reduced in aged mice, the RSV-specific CD8 T cells retain their capacity to produce multiple effector cytokines.
Fig 4.
RSV-specific CD8 T cells generated in aged mice retain the ability to produce cytokines. Cells from the lung parenchyma and BAL fluid were isolated at days 8 and 12 p.i., stimulated ex vivo with a peptide, and subsequently stained for intracellular IFN-γ and TNF-α. (A to C) Frequencies of IFN-γ-producing CD8 T cells isolated from RSV-infected young and aged mice following stimulation with the M282-90 (A), F85-93 (B), or M2127-135 (C) peptide on days 8 (left) and 12 (right) p.i. (D and E) Representative flow plots (D) and cumulative frequencies (E) of TNF-α+, IFN-γ-producing M282-specific CD8 T cells in young and aged mice on day 8 p.i. Data are representative of two to three separate experiments (n, 8 to 17 mice/group). Data were analyzed by two-way analysis of variance and Bonferroni's posttest. ***, P < 0.001.
RSV-specific memory CD8 T cells in aged mice.
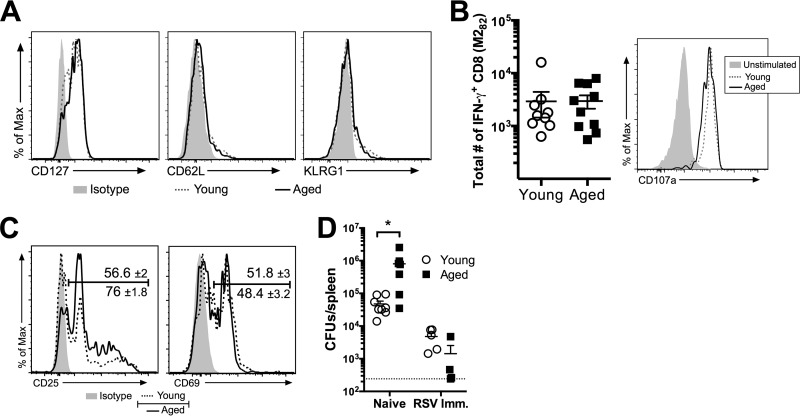
Given that the frequencies and total numbers of M282 tetramer-positive CD8 T cells within the peripheral blood were similar following viral clearance through day 40 p.i. (Fig. 3A), we next examined the RSV-specific CD8 T cells in young and aged mice at a memory time point (>30 days p.i.). The phenotypes of the M282 tetramer-positive memory CD8 T cells, based on the expression of the well-defined memory CD8 T cell markers CD127, CD62L, and KLRG1, were similar for young and aged mice (Fig. 5A). In addition, the capacities of the M282-specific memory CD8 T cells to produce IFN-γ and to express CD107a following restimulation in vitro with the M282-90 peptide were similar for young and aged mice (Fig. 5B). We next assessed the abilities of the M282-specific memory CD8 T cells in young and aged mice to respond to a heterologous infection with an attenuated Listeria monocytogenes strain expressing the M282-90 peptide (Att LM-M282). On day 2 following i.v. challenge with 107 CFU of Att LM-M282, the M282 tetramer-positive CD8 T cells expressed similar levels of CD69 (Fig. 5C). However, aged mice had a significantly (P <0.01) higher frequency of M282 tetramer-positive CD8 T cells expressing CD25 than young mice (Fig. 5C). The bacterial burdens in the spleens of previously naïve aged control mice were significantly higher than those in young controls (Fig. 5D). However, the levels of bacteria in young and aged RSV-immune mice were similar. These data indicate that the M282-specific memory CD8 T cells have similar phenotypes and functional capacities during maintenance and following a heterologous infection.
Fig 5.
Aged RSV-specific memory CD8 T cells have a phenotype and function similar to that of young memory cells. (A) At day 40 p.i., peripheral blood cells were isolated for the determination of CD127, CD62L, and KLRG1 expression on M282 tetramer-positive CD8 T cells in young and aged RSV-infected mice. (B) At day 48 p.i., peripheral blood cells were restimulated ex vivo with the M282-90 peptide in order to determine the total number of IFN-γ-producing cells per ml of blood and the expression of CD107a by IFN-γ+ CD8 T cells in young and aged mice. (C and D) At day 50 following RSV infection (day 2 post-Att LM-M282 challenge), CD25 and CD69 expression on M282 tetramer-positive CD8 T cells (C) and the total CFU in the spleen (D) were determined for young and aged mice. Data are representative of two separate experiments (n, 4 to 9 mice/group). (C) Histograms are concatenated in FlowJo showing equal representation of events per mouse from one independent experiment (n, 3 to 5 mice/group). The mean gate frequencies ± standard errors of the means are shown. Data were analyzed by a Student t test (B and C) or by two-way analysis of variance with Bonferroni's posttest (D). *, P < 0.05. Imm., immune.
DISCUSSION
As individuals age, both extrinsic and intrinsic alterations to the immune system negatively impact adaptive immunity to pathogens. Because infection with respiratory viruses is a major cause of morbidity and mortality in the elderly, understanding how age affects the generation of T cell responses is important to vaccine design. While there is evidence that the magnitude of virus-specific CD8 T cell responses decreases with age, few studies have directly examined the effect of age on RSV-specific CD8 T cell responses, and in none of these cases was the effect of age on de novo generation of virus-specific CD8 T cell immunity directly addressed (4–6, 22–24). Although indirect tests have shown decreased cytolytic activity and IFN-γ production by RSV-specific CD8 T cells isolated from aged animals, it is not clear if this represents diminished effector function, a decrease in the magnitude of the response, or both (4, 24). In this study, we sought to determine the impact of aging on the generation of an RSV-specific CD8 T cell response in the well-characterized BALB/c mouse model.
We demonstrate profound decreases in the sizes of both dominant M282-90 and subdominant F85-93 and M2127-135 RSV-specific CD8 T cell responses in the lung parenchyma and airways at the peak of the T cell response on day 8 p.i. (Fig. 3 and 4). However, this difference was no longer present at day 12 p.i., suggesting that the rate of expansion, as well as the rate of contraction, of RSV-specific CD8 T cells is lower in aged mice than in young mice. This could be caused by a variety of T cell-intrinsic factors that can contribute to the development of “holes” in the CD8 T cell repertoire. First, over time, the T cell repertoire shifts from a naïve (CD44lo) to a memory (CD44hi) phenotype, likely due to increased homeostatic proliferation and recurring exposure to commensal and foreign antigens. Regardless of the cause, naturally arising CD44hi CD8 T cells in uninfected aged mice respond less well to antigenic challenge than naïve CD44lo cells in aged mice, and both subsets in aged mice respond less well than naïve CD8 T cells from young mice (25). Second, clonal expansions of memory cells arise over time and can represent a large fraction of the total CD8 T cell pool (26, 27). These clonal expansions reduce the diversity of naïve CD8 T cells that can recognize and respond to a foreign antigen. Third, the absolute number of antigen-specific precursors decreases with age, and the surviving precursors are largely of a more differentiated CD44hi memory phenotype (25, 28). All three of these factors likely combine to negatively impact the magnitude and contraction of the RSV-specific T cell response in aged hosts. However, we observed similar numbers of RSV-specific CD8 T cells subsequent to viral clearance in young and aged mice (Fig. 3 and 4). In addition, the phenotypes and functional capacities of the RSV-specific memory CD8 T cells were similar in young and aged mice (Fig. 5). These data suggest that the decreased total magnitude of the RSV-specific response at the peak of the acute T cell response may be due either to a diminished capacity of the innate immune response to activate naïve T cells or to an intrinsic deficiency in the proliferative capacity of the naïve T cells.
Interestingly, the decrease in the M282-specific response was less profound in secondary lymphoid tissues, and no difference was observed in the medLNs. This observation could suggest diminished trafficking to and/or retention of effector CD8 T cells in the lungs and airways. However, we did not observe a biased distribution of RSV-specific CD8 T cells in the periphery, nor did we observe decreased expression of chemokine receptors or homing molecules, such as CXCR3, CCR5, CD11a, CD18, CD29, CD49a, and CD49d, that are required for entry into inflamed lung tissue in aged mice (Fig. 3 and data not shown). Age-dependent changes in the immune system can also have extrinsic effects on the activation of pathogen-specific T cells. A recent study found diminished migration of respiratory dendritic cells (DCs) to the draining lymph nodes in response to infection with viruses, including RSV (29). This defect was traced to increased basal levels of prostaglandin D2 (PGD2) in the lungs, which correlated with lower levels of CCR7 expression on respiratory DCs; CCR7 has been implicated in the trafficking of DCs to draining lymph nodes (29, 30). Disruption of PGD2 signaling by blocking its binding with its receptor, the D prostanoid 1 receptor, in aged mice dramatically increased the migration of respiratory DCs to draining LNs, resulting in a concomitant increase in the magnitude of the virus-specific CD8 T cell response. While RSV-specific T cells were not examined in this blocking experiment, the diminished trafficking of respiratory DCs to draining LNs after RSV infection is also consistent with the decreased peak magnitude of RSV-specific T cell responses reported in this article.
The ability of effector T cells to produce multiple cytokines has been associated with enhanced antiviral immunity (31). We did not find statistically significant differences in the quantitative or qualitative (i.e., mean fluorescence intensity) abilities of effector and RSV-specific memory CD8 T cells to produce IFN-γ or to coproduce TNF-α following ex vivo peptide stimulation (Fig. 4 and 5; also data not shown). While a loss of function has been reported for CD4 T cells, CD8 T cells in aged mice expand poorly but often retain their capacity to produce effector cytokines (18, 29). Although there could be age-dependent differences in cytokine production by CD8 T cells in vivo, future research should focus primarily on enhancing the priming and proliferation of the surviving pathogen-specific CD8 T cell precursors.
In conclusion, this study demonstrates that aged mice mount diminished RSV-specific CD8 T cell responses at the peak magnitude of the acute T cell response. In contrast, the generation and maintenance of memory CD8 T cells are similar for young and aged mice. Furthermore, young and aged memory CD8 T cells exhibit similar capacities to become activated and to provide initial control of a subsequent infection. Our results suggest that immunization strategies using live attenuated pathogens may be problematic in aged hosts due to a diminished capacity to mount a primary T cell response. However, once generated, memory T cells in aged hosts appear to function similarly to those in their younger counterparts.
ACKNOWLEDGMENTS
We thank Cory Knudson, Allison Christiaansen, and Stacey Hartwig for technical assistance and Pete Lauer (Aduro Biotech, Inc., Berkeley, CA) for the Listeria monocytogenes vectors.
This work was supported by NIH grant R56 AI106776 (to J.T.H. and S.M.V.), NIH grant R01 AI063520 (to S.M.V.), NIH grant T32 AI007511 (to R.B.F.), and NIH grant T32 AI748518 (to K.A.W.).
Footnotes
Published ahead of print 18 September 2013
REFERENCES
- 1.Thompson WW, Shay DK, Weintraub E, Brammer L, Cox N, Anderson LJ, Fukuda K. 2003. Mortality associated with influenza and respiratory syncytial virus in the United States. JAMA 289:179–186 [DOI] [PubMed] [Google Scholar]
- 2.Graham BS, Bunton LA, Wright PF, Karzon DT. 1991. Role of T lymphocyte subsets in the pathogenesis of primary infection and rechallenge with respiratory syncytial virus in mice. J. Clin. Invest. 88:1026–1033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Olson MR, Varga SM. 2008. Pulmonary immunity and immunopathology: lessons from respiratory syncytial virus. Expert Rev. Vaccines 7:1239–1255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu B, Kimura Y. 2007. Local immune response to respiratory syncytial virus infection is diminished in senescence-accelerated mice. J. Gen. Virol. 88:2552–2558 [DOI] [PubMed] [Google Scholar]
- 5.Boukhvalova MS, Yim KC, Kuhn KH, Hemming JP, Prince GA, Porter DD, Blanco JC. 2007. Age-related differences in pulmonary cytokine response to respiratory syncytial virus infection: modulation by anti-inflammatory and antiviral treatment. J. Infect. Dis. 195:511–518 [DOI] [PubMed] [Google Scholar]
- 6.de Bree GJ, Heidema J, van Leeuwen EM, van Bleek GM, Jonkers RE, Jansen HM, van Lier RA, Out TA. 2005. Respiratory syncytial virus-specific CD8+ memory T cell responses in elderly persons. J. Infect. Dis. 191:1710–1718 [DOI] [PubMed] [Google Scholar]
- 7.Lynch HE, Goldberg GL, Chidgey A, Van den Brink MR, Boyd R, Sempowski GD. 2009. Thymic involution and immune reconstitution. Trends Immunol. 30:366–373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Linton PJ, Dorshkind K. 2004. Age-related changes in lymphocyte development and function. Nat. Immunol. 5:133–139 [DOI] [PubMed] [Google Scholar]
- 9.Maue AC, Yager EJ, Swain SL, Woodland DL, Blackman MA, Haynes L. 2009. T-cell immunosenescence: lessons learned from mouse models of aging. Trends Immunol. 30:301–305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ahmed M, Lanzer KG, Yager EJ, Adams PS, Johnson LL, Blackman MA. 2009. Clonal expansions and loss of receptor diversity in the naive CD8 T cell repertoire of aged mice. J. Immunol. 182:784–792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kohlmeier JE, Connor LM, Roberts AD, Cookenham T, Martin K, Woodland DL. 2010. Nonmalignant clonal expansions of memory CD8+ T cells that arise with age vary in their capacity to mount recall responses to infection. J. Immunol. 185:3456–3462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brockstedt DG, Giedlin MA, Leong ML, Bahjat KS, Gao Y, Luckett W, Liu W, Cook DN, Portnoy DA, Dubensky TW., Jr 2004. Listeria-based cancer vaccines that segregate immunogenicity from toxicity. Proc. Natl. Acad. Sci. U. S. A. 101:13832–13837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Olson MR, Varga SM. 2007. CD8 T cells inhibit respiratory syncytial virus (RSV) vaccine-enhanced disease. J. Immunol. 179:5415–5424 [DOI] [PubMed] [Google Scholar]
- 14.Badovinac VP, Harty JT. 2000. Adaptive immunity and enhanced CD8+ T cell response to Listeria monocytogenes in the absence of perforin and IFN-γ. J. Immunol. 164:6444–6452 [DOI] [PubMed] [Google Scholar]
- 15.Falsey AR, Hennessey PA, Formica MA, Cox C, Walsh EE. 2005. Respiratory syncytial virus infection in elderly and high-risk adults. N. Engl. J. Med. 352:1749–1759 [DOI] [PubMed] [Google Scholar]
- 16.Lerner A, Yamada T, Miller RA. 1989. Pgp-1hi T lymphocytes accumulate with age in mice and respond poorly to concanavalin A. Eur. J. Immunol. 19:977–982 [DOI] [PubMed] [Google Scholar]
- 17.Ernst DN, Weigle WO, Noonan DJ, McQuitty DN, Hobbs MV. 1993. The age-associated increase in IFN-γ synthesis by mouse CD8+ T cells correlates with shifts in the frequencies of cell subsets defined by membrane CD44, CD45RB, 3G11, and MEL-14 expression. J. Immunol. 151:575–587 [PubMed] [Google Scholar]
- 18.Fulton RB, Varga SM. 2009. Effects of aging on the adaptive immune response to respiratory virus infections. Aging Health 5:775. 10.2217/ahe.09.69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Roberts AD, Ely KH, Woodland DL. 2005. Differential contributions of central and effector memory T cells to recall responses. J. Exp. Med. 202:123–133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kapasi ZF, Murali-Krishna K, McRae ML, Ahmed R. 2002. Defective generation but normal maintenance of memory T cells in old mice. Eur. J. Immunol. 32:1567–1573 [DOI] [PubMed] [Google Scholar]
- 21.Haynes L, Eaton SM, Burns EM, Randall TD, Swain SL. 2003. CD4 T cell memory derived from young naive cells functions well into old age, but memory generated from aged naive cells functions poorly. Proc. Natl. Acad. Sci. U. S. A. 100:15053–15058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Looney RJ, Falsey AR, Walsh E, Campbell D. 2002. Effect of aging on cytokine production in response to respiratory syncytial virus infection. J. Infect. Dis. 185:682–685 [DOI] [PubMed] [Google Scholar]
- 23.Lee FE, Walsh EE, Falsey AR, Liu N, Liu D, Divekar A, Snyder-Cappione JE, Mosmann TR. 2005. The balance between influenza- and RSV-specific CD4 T cells secreting IL-10 or IFNγ in young and healthy-elderly subjects. Mech. Ageing Dev. 126:1223–1229 [DOI] [PubMed] [Google Scholar]
- 24.Zhang Y, Wang Y, Gilmore X, Xu K, Wyde PR, Mbawuike IN. 2002. An aged mouse model for RSV infection and diminished CD8+ CTL responses. Exp. Biol. Med. (Maywood) 227:133–140 [DOI] [PubMed] [Google Scholar]
- 25.Decman V, Laidlaw BJ, Doering TA, Leng J, Ertl HC, Goldstein DR, Wherry EJ. 2012. Defective CD8 T cell responses in aged mice are due to quantitative and qualitative changes in virus-specific precursors. J. Immunol. 188:1933–1941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hingorani R, Choi IH, Akolkar P, Gulwani-Akolkar B, Pergolizzi R, Silver J, Gregersen PK. 1993. Clonal predominance of T cell receptors within the CD8+ CD45RO+ subset in normal human subjects. J. Immunol. 151:5762–5769 [PubMed] [Google Scholar]
- 27.Callahan JE, Kappler JW, Marrack P. 1993. Unexpected expansions of CD8-bearing cells in old mice. J. Immunol. 151:6657–6669 [PubMed] [Google Scholar]
- 28.Rudd BD, Venturi V, Li G, Samadder P, Ertelt JM, Way SS, Davenport MP, Nikolich-Zugich J. 2011. Nonrandom attrition of the naive CD8+ T-cell pool with aging governed by T-cell receptor:pMHC interactions. Proc. Natl. Acad. Sci. U. S. A. 108:13694–13699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhao J, Legge K, Perlman S. 2011. Age-related increases in PGD2 expression impair respiratory DC migration, resulting in diminished T cell responses upon respiratory virus infection in mice. J. Clin. Invest. 121:4921–4930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Randolph GJ, Ochando J, Partida-Sanchez S. 2008. Migration of dendritic cell subsets and their precursors. Annu. Rev. Immunol. 26:293–316 [DOI] [PubMed] [Google Scholar]
- 31.Seder RA, Darrah PA, Roederer M. 2008. T-cell quality in memory and protection: implications for vaccine design. Nat. Rev. Immunol. 8:247–258 [DOI] [PubMed] [Google Scholar]