Abstract
The envelope (E) protein of dengue virus (DENV) is the major target of neutralizing antibodies (Abs) and vaccine development. Previous studies of human dengue-immune sera reported that a significant proportion of anti-E Abs, known as group-reactive (GR) Abs, were cross-reactive to all four DENV serotypes and to one or more other flaviviruses. Based on studies of mouse anti-E monoclonal antibodies (MAbs), GR MAbs were nonneutralizing or weakly neutralizing compared with type-specific MAbs; a GR response was thus not regarded as important for vaccine strategy. We investigated the epitopes, binding avidities, and neutralization potencies of 32 human GR anti-E MAbs. In addition to fusion loop (FL) residues in E protein domain II, human GR MAbs recognized an epitope involving both FL and bc loop residues in domain II. The neutralization potencies and binding avidities of GR MAbs derived from secondary DENV infection were stronger than those derived from primary infection. GR MAbs derived from primary DENV infection primarily blocked attachment, whereas those derived from secondary infection blocked DENV postattachment. Analysis of the repertoire of anti-E MAbs derived from patients with primary DENV infection revealed that the majority were GR, low-avidity, and weakly neutralizing MAbs, whereas those from secondary infection were primarily GR, high-avidity, and potently neutralizing MAbs. Our findings suggest that the weakly neutralizing GR anti-E Abs generated from primary DENV infection become potently neutralizing MAbs against the four serotypes after secondary infection. The observation that the dengue immune status of the host affects the quality of the cross-reactive Abs generated has implications for new strategies for DENV vaccination.
INTRODUCTION
The four serotypes of dengue virus (DENV) are the leading cause of arboviral diseases in humans in tropical and subtropical regions (1, 2). More than 2.5 billion people in over 100 countries are estimated to be at risk of infection, and 50 to 100 million DENV infections occur every year worldwide (1, 2). After DENV infection, most individuals are asymptomatic or present with a self-limited illness known as dengue fever, but some may develop severe and potentially life-threatening diseases, known as dengue hemorrhagic fever/dengue shock syndrome. Despite considerable efforts to develop vaccines, with several being tested in clinical trials, there is no licensed dengue vaccine currently available (1, 3).
DENV belongs to the genus Flavivirus in the family Flaviviridae. It is a positive-sense, single-stranded RNA virus with a genome of approximately 10.6 kb. The genome contains a single open reading frame, which encodes a polyprotein which is cleaved by cellular and viral proteases into three structural proteins (the capsid, precursor membrane [prM], and envelope [E] proteins) and seven nonstructural proteins (4). After binding to its cellular receptor, DENV enters the cell through receptor-mediated endocytosis (5, 6). In the low-pH environment of endosomes, the E protein undergoes a series of conformational changes leading to fusion of the viral membrane to the endosomal membrane and subsequent virus entry (6, 7).
The E protein is present as 90 “head-to-tail” homodimers on the surfaces of mature virions (4, 6, 8). The E protein participates in virus entry and is the major target of neutralizing as well as enhancing antibodies (Abs) (9). In the presence of cross-reactive nonneutralizing Abs or a suboptimal concentration of neutralizing Abs, DENV replicates to higher titers in human Fcγ receptor-bearing cells in vitro, a phenomenon known as antibody-dependent enhancement (9, 10). The ectodomain of E protein contains three domains (7). Domain I is located in the center, domain II contains a fusion loop (FL) at the tip and is involved in membrane fusion and dimerization of E protein, and domain III is believed to participate in receptor binding (4, 6, 11).
In the genus Flavivirus, there are several serocomplexes, including the DENV serocomplex, Japanese encephalitis virus serocomplex, and tick-borne encephalitis virus serocomplex (4). Anti-E Abs that recognize members from different serocomplexes, members within a serocomplex, and a single member are called flavivirus group-reactive (GR), complex-reactive (CR), and type-specific (TS) Abs, respectively (12). Previous studies of mouse anti-E monoclonal antibodies (MAbs) against DENV have reported different epitopes and neutralizing potencies for different categories of MAbs. The epitopes of GR MAbs were mapped primarily to the highly conserved residues in the FL of domain II; the epitopes of CR and TS MAbs were found in domain III (13–16). Some interdomain epitopes have also been reported (17–19). Since most mouse GR MAbs were nonneutralizing or weakly neutralizing compared to several potently neutralizing TS MAbs (14, 15), the GR response was regarded as useless for vaccination strategies.
Studies of polyclonal human sera after DENV infection have shown that a significant proportion of anti-E Abs are GR Abs and recognize FL residues in domain II (13, 19–21). Recent studies of human anti-E MAbs against DENV identified a few epitopes of TS MAbs (22–24), but the epitopes of human GR MAbs remain largely unknown (25–27). In this study, we investigated the epitopes, binding avidities, and neutralization potencies of 32 human GR anti-E MAbs. We found two main epitopes recognized by human GR MAbs, with one containing FL residues only and the other containing both FL residues and residues in the bc loop of domain II. Interestingly, the neutralization potencies and binding avidities of GR MAbs derived from patients with secondary DENV infection were stronger than those of GR MAbs derived from primary infections. Analysis of the repertoire of anti-E MAbs derived from four individuals revealed predominantly low-avidity and weakly neutralizing GR MAbs from patients with primary DENV infection and predominantly high-avidity and potently neutralizing GR MAbs from patients with secondary infection. These findings suggest that the weakly neutralizing GR anti-E Abs generated from primary DENV infection become potently neutralizing Abs after secondary infection and have implications for alternative vaccine strategies against DENV.
MATERIALS AND METHODS
Human MAbs and binding specificity.
With the approval of the Institutional Review Board of the University of Hawaii at Manoa (approval CHS#17568), 28 human anti-E MAbs derived from four patients with primary DENV infection (by each of the four DENV serotypes) and 23 anti-E MAbs derived from four patients with secondary infection were included in this study (Table 1). The MAbs from patients DVG, DVD, DVC, and DVB were generated by a B-cell immortalization protocol as described previously (22, 25) and were provided by the Pediatric Dengue Vaccine Initiative. The MAbs from patients 749, 751, 753, and 750 were generated at the Imperial College of London by a previously described reverse transcription-PCR (RT-PCR) and cloning protocol for antibody-secreting cells derived from peripheral blood mononuclear cells (PBMCs) (28), in compliance with the guidelines of the Institutional Ethical Committee, and were shipped to the University of Hawaii at Manoa in August 2010 for analysis. Binding specificity was determined by dot blot assay (29) or Western blot analysis (19, 20).
Table 1.
Patients and origins of group-reactive anti-E human MAbs in this study
| Patient ID | Type of infectiona | Serotypea | Time of samplingb | No. of anti-E MAbs (specificity)c | No. of GR anti-E MAbs studied |
|---|---|---|---|---|---|
| DVG | Primary | D1 | 1 mo | 5 (4 GR, 0 CR, 1 TS) | 4 |
| DVD | Primary | D4 | 1 mo | 12 (6 GR, 2 CR, 4 TS) | 6 |
| DVCd | Primary | D2 | 8 yr | 10 (1 GR, 4 CR, 5 TS) | 1 |
| DVBe | Primary | D3 | 1 yr | 1 (1 GR, 0 CR, 0 TS) | 1 |
| 749 | Secondary | D1 | 4 days | 4 (4 GR, 0 CR, 0 TS) | 4 |
| 751 | Secondary | D1 | 4 days | 10 (9 GR, 1 CR, 0 TS) | 9 |
| 753 | Secondary | D1 | 5 days | 6 (5 GR, 1 CR, 0 TS) | 5 |
| 750 | Secondary | D1 | 5 days | 3 (2 GR, 1 CR, 0 TS) | 2 |
Primary or secondary DENV infection and the infecting serotype of patients were determined as reported previously (22, 29). D, DENV.
Time from the onset of illness to collection of blood to prepare MAbs.
Number of anti-E MAbs with known binding specificity and epitopes. GR, group reactive; CR, complex reactive; TS, type specific.
Ten MAbs were derived from patient DVC by use of a recombinant domain III selective subcloning method as described previously (22). Only one GR anti-E MAb was identified.
Sixteen MAbs were derived from patient DVB (1 GR anti-E MAb, 10 anti-prM MAbs, and 5 unknown MAbs). Only one GR anti-E MAb was identified due to the subcloning process, as described previously (22).
Epitope mapping by dot blot assay.
Epitopes of human MAbs were determined by a dot blot assay using lysates derived from 293T cells transfected with a wild-type (WT) DENV1 prM/E construct or each of the 67 predicted surface-exposed E alanine mutants and hybridization with each MAb or mixed MAbs (a pool of mouse MAbs, including GR, CR, and TS MAbs) under nonreducing conditions (1% NP-40 buffer) (19). The intensities of E protein dots of the WT and mutants were analyzed by ImageQuant (GE Healthcare, United Kingdom). The recognition index (RI) of a MAb for a mutant E protein was determined as follows: RI = (intensity of mutant E dot/intensity of WT E dot) (recognized by a MAb)/(intensity of mutant E dot/intensity of WT E dot) (recognized by mixed MAbs) (19, 20).
VLP-capture ELISA.
Candidate epitope residues identified by dot blot assay were verified by a capture enzyme-linked immunosorbent assay (ELISA) using WT and mutant virus-like particles (VLPs), as described previously (13, 19). Briefly, a 96-well plate was coated with rabbit anti-DENV sera at 4°C overnight, followed by blocking with 1% bovine serum albumin (BSA) in 1× phosphate-buffered saline (PBS) for 1 h and addition of VLPs, anti-E MAb, and anti-mouse IgG conjugated with horseradish peroxidase (HRP) at 37°C for 1 h, with tetramethylbenzidine (TMB) substrate and stop solution added at the final step (13, 19). The optical density at 450 nm (OD450) was read with a reference wavelength of 650 nm. Binding curves, maximum binding (Bmax), and relative binding (% of Bmax) were determined by a nonlinear regression analysis, using GraphPad Prism 5.0 (GraphPad Inc., CA) (19).
Epitope analysis by structure-based modeling.
The program UCSF Chimera (http://www.cgl.ucsf.edu/chimera/) was used to determine the locations of candidate residues and distances between them (on the same or an adjacent monomer) on E-E dimers or on virus particles (19).
Binding avidity by virion-capture ELISA.
Virion-capture ELISA was performed in the same way as VLP-capture ELISA (13, 19), except that virions derived from ultracentrifugation of culture supernatants of infected Vero cells were used as antigen. The dissociation constant (Kd) was determined by the program GraphPad Prism 5.0. DENV1 virion-capture ELISA was performed for all GR MAbs, and DENV4 virion-capture ELISA was performed for the repertoire of MAbs (4 TS and 6 GR MAbs) from a DENV4 case. A linear relationship was found between the Kd of 6 GR MAbs determined by DENV4 virion-capture ELISA and that determined by DENV1 virion-capture ELISA (correlation coefficient [r] = 0.83; P = 0.029 by the two-tailed Spearman correlation test) (data not shown), supporting the use of the Kd determined by DENV1 virion-capture ELISA to compare binding avidities among GR MAbs.
Competitive virion-capture ELISA.
A previously described mouse GR MAb (E53) recognizing FL (30) was prepared at 0.002 μg/ml (corresponding to its mean Kd value for four DENV serotypes), mixed with or without each human GR MAb (at 0.25 μg/ml, corresponding to the mean Kd for GR MAbs to DENV1), and subjected to virion-capture ELISA using DENV1 (Hawaii strain), DENV2 (NGC strain), DENV3 (H87 strain), or DENV4 (H241 strain), derived from ultracentrifugation of culture supernatants of infected Vero cells, with anti-mouse IgG as a secondary Ab (31). Inhibition was determined as follows: % inhibition = (1 − OD450 of E53 alone/OD450 of E53 plus human GR MAb) × 100.
FRNT and mechanism of neutralization.
The neutralization potency of each MAb was determined by the concentration that gave 50% inhibition in a focus reduction neutralization test (FRNT50 value) (13). Fourfold serial dilutions of each MAb were incubated with 50 focus-forming units (FFU) of each serotype and added to Vero cell monolayers grown in a 96-well plate. The four serotypes included DENV1 (Hawaii strain), DENV2 (NGC strain), DENV3 (CH53489), and DENV4 (H241 strain) grown in Vero cells. Foci were counted by use of a CTL immunospot analyzer 3 to 4 days later. The FRNT50 titers were determined by a nonlinear regression analysis (GraphPad Prism 5.0). For the mechanism of neutralization, MAbs were added at pre- and postattachment steps to study the mechanism of neutralization as described previously (13, 18). The relative amount of infection was determined as follows: % infection = number of foci in the presence of MAb/number of foci in the absence of MAb.
Relative binding activity after low-pH treatment.
A flat-bottom 96-well plate was coated with DENV1 virions at 4°C overnight, followed by blocking with 1% BSA in 1× PBS for 1 h and the addition of anti-E MAb at pH 7.4 and 37°C for 2 h; the concentration near maximum binding was used because of the requirement of higher occupancy to reach neutralization by FL MAbs (14). After washing twice with 1× PBS and once with the indicated buffer at pH 7.4 (blocking buffer) or pH 5.0 (150 mM NaCl and 50 mM MES [morpholineethanesulfonic acid]), followed by incubation with pH 7.4 or pH 5.0 buffer at room temperature for 30 min (18) and washing with 1× PBS twice, ELISA was completed by the regular capture ELISA protocol (13, 19). The relative binding activity after low-pH treatment was determined as follows: % binding activity = OD450 at pH 5.0/OD450 at pH 7.4.
Statistical analysis.
The two-tailed Mann-Whitney test was used to determine the differences in Kd, FRNT50, and relative binding activity after low-pH treatment between two groups, and the two-tailed Spearman correlation test was used to determine the relationship between Kd and FRNT50 by GraphPad Prism 5.0.
RESULTS
Epitope residues containing FL only or both FL and the bc loop of domain II are recognized by GR human anti-E MAbs.
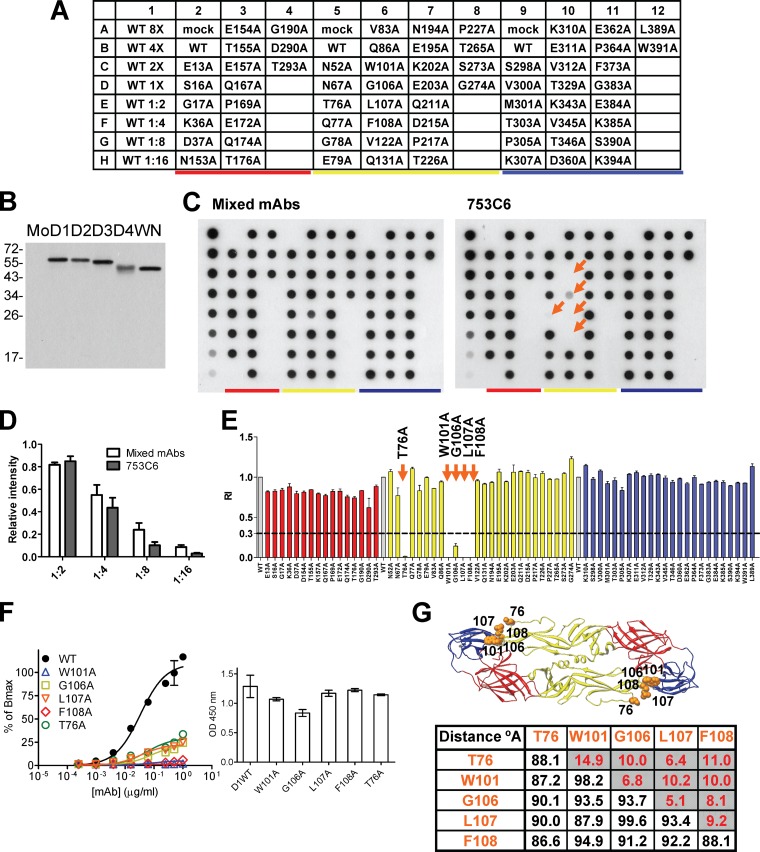
The origins of the 12 and 20 human GR anti-E MAbs derived from primary and secondary DENV infections, respectively, are summarized in Table 1. We employed a previously described dot blot assay, VLP-capture ELISA, and structure-based analysis to investigate the epitopes of these MAbs. As shown in Fig. 1B, the GR MAb 753C6 can recognize the E proteins of four DENV serotypes and West Nile virus (WNV), another flavivirus. The intensity of each mutant dot recognized by mixed MAbs was generally comparable to that of the WT dot, suggesting that comparable amounts of E protein were dotted on the membrane (Fig. 1C). Compared with the binding to the WT dot, the E-binding activity of 753C6 was greatly reduced by four alanine mutations in the FL of domain II (W101A, G106A, L107A, and F108A) and a mutation in the bc loop of domain II (T76A) (RI ≤ 0.3), suggesting that these five residues were possibly the epitope of 753C6 (Fig. 1C and E). This was further verified by VLP-capture ELISA, in which the binding of 753C6 to mutant VLPs of these five mutants was greatly reduced compared to that of the WT (Fig. 1F). Structure-based analysis revealed that the distances between these residues from the same monomer were less than 30 Å, suggesting that these five residues (four in the FL and one in the bc loop) likely form an epitope (Fig. 1G).
Fig 1.
An epitope involving both FL and bc loop residues is recognized by GR human MAb 753C6. (A) Layout of dot blot assay. (B) Binding specificity by Western blotting (19). Mo, mock; D1, DENV1; D2, DENV2; D3, DENV3; D4, DENV4; WN, WNV. (C) Dot blot assay using lysates of 293T cells transfected with WT pCB-D1 or each of the 67 alanine E mutants (19). Each membrane was probed with MAb 753C6 or mixed MAbs. The dots containing mutations in domains I, II, and III are underlined by red, yellow, and blue, respectively. Twofold dilutions of WT lysates were dotted on row 1 to assess the exposure of each membrane. Arrows indicate mutants of epitope residues which showed severe reductions (RI ≤ 0.3) in binding. Data for one representative experiment of two are shown. (D) The relative intensities of WT dots in row 1 showed a linear decrease from 1:2 to 1:16 dilutions for membranes probed with mixed MAbs (open bars) or MAb 753C6 (closed bars). (E) The intensity of each dot was quantified to determine the RI (19, 20). Data are means with standard deviations for two experiments. (F) Capture ELISA was performed using WT or mutant VLPs (13, 19). Data are means with standard deviations for duplicates from one representative experiment of two. The bar graph shows that comparable amounts of WT and mutant VLPs were added, based on recognition of E by pooled human dengue-immune sera. (G) Structure-based analysis was performed to determine the locations of and distances (Å) between epitope residues from the same (shaded) or adjacent monomers.
For another GR MAb, 749B4 (Table 2), the binding activity was greatly reduced (RI ≤ 0.3) for three FL loop mutants (W101A, L107A, and F108A mutants) compared with that to the WT dot (data not shown). These three residues as an epitope were further verified by VLP-capture ELISA and supported by structure-based analysis. Table 2 summarizes the epitopes of 32 GR human MAbs. There were two types of epitope, namely, one containing FL residues only and one containing both FL residues and residues in the bc loop of domain II; this epitope has never been reported for human MAbs against DENV E protein thus far.
Table 2.
Epitopes of group-reactive anti-E human MAbs in this study
| MAb | Epitopea |
|
|---|---|---|
| FL residues only | FL and bc loop residues | |
| MAbs derived from primary DENV infection | ||
| DVD19.4 | 101, 106, 108 | |
| DVD19.13 | 101, 106, 108 | |
| DVD23.3 | 101, 106 | |
| DVD23.4 | 101, 106 | |
| DVB64.31 | 101, 106, 107, 108 | |
| DVC23.13 | 101, 108 | |
| DVG6.3 | 101, 108 | |
| DVG7.5 | 101, 108 | |
| DVG1.3 | 107 | |
| DVG12.2 | 107, 108, 76 | |
| DVD26.3 | 101, 107, 108, 78 | |
| DVD26.11 | 101, 107, 108, 78 | |
| MAbs derived from secondary DENV infection | ||
| 749B4 | 101, 107, 108 | |
| 749B12 | 101, 107, 108 | |
| 753C1 | 101, 107, 108 | |
| 751A1 | 101, 107, 108 | |
| 751B11 | 101, 106, 108 | |
| 751B12 | 101, 106, 108 | |
| 750D7 | 101, 106, 108 | |
| 749B2 | 101, 106, 107, 108 | |
| 751C4 | 101, 106, 107, 108 | |
| 751A2 | 101, 106, 107, 108 | |
| 751B3 | 101, 106, 107, 108 | |
| 749B6 | 101, 108 | |
| 753A1 | 101 | |
| 750C9 | 101 | |
| 751B6 | 101, 108, 78 | |
| 753C6 | 101, 106, 107, 108, 76 | |
| 751C10 | 101, 106, 107, 108, 76, 78 | |
| 753C8 | 101, 106, 107, 108, 76 | |
| 753C12 | 101, 107, 108, 76 | |
| 751A12 | 101, 107, 108, 76 | |
Epitopes were identified by a previously described dot blot assay, VLP-capture ELISA, and structure-based analysis (19).
Stronger neutralization potencies of GR anti-E MAbs derived from secondary DENV infection than those of MAbs derived from primary infection.
We next examined the neutralization potencies of these 32 MAbs by FRNT (13). As shown in Table 3, the concentrations that gave 50% inhibition in FRNT (FRNT50) for most MAbs derived from patients with primary DENV infection were ≥1 μg/ml against four DENV serotypes, whereas the FRNT50 titers of MAbs derived from patients with secondary infection were generally lower than 1 μg/ml. The FRNT50 concentrations of MAbs derived from secondary DENV infection were significantly lower than those derived from primary infection (P < 0.0001, P = 0.0005, P = 0.004, and P = 0.01 for DENV1, DENV2, DENV3, and DENV4, respectively, by the two-tailed Mann-Whitney test), indicating that the GR anti-E MAbs derived from secondary DENV infection were more potent neutralizing MAbs than those derived from primary infection.
Table 3.
Neutralization potencies and binding avidities of group-reactive human anti-E MAbs in this study
| MAb | Patient ID, immune statusa | Neutralization potency (FRNT50 value) (μg/ml) |
Kd (μg/ml)b | |||
|---|---|---|---|---|---|---|
| D1 | D2 | D3 | D4 | |||
| DVG1.3 | DVG, primary D1 | 1 | 0.25 | >1 | 1 | 0.021 ± 0.002 |
| DVG6.3 | DVG, primary D1 | >1 | 1 | >1 | >1 | 0.397 ± 0.078 |
| DVG7.5 | DVG, primary D1 | 1 | 1 | >1 | >1 | 0.777 ± 0.048 |
| DVG12.2 | DVG, primary D1 | >1 | >1 | >1 | 1 | 0.684 ± 0.052 |
| DVD19.4 | DVD, primary D4 | >1 | 1 | >1 | 0.25 | 0.199 ± 0.027 |
| DVD19.13 | DVD, primary D4 | 1 | 0.25 | >1 | 1 | 0.296 ± 0.037 |
| DVD23.3 | DVD, primary D4 | 1 | 1 | >1 | 0.25 | 0.207 ± 0.019 |
| DVD23.4 | DVD, primary D4 | >1 | 0.25 | 1 | 1 | 0.234 ± 0.024 |
| DVD26.3 | DVD, primary D4 | >1 | 1 | >1 | 1 | 0.509 ± 0.056 |
| DVD26.11 | DVD, primary D4 | >1 | >1 | >1 | >1 | 1.878 ± 0.178 |
| DVC23.13 | DVC, primary D2 | 1 | 1 | 1 | 1 | 0.065 ± 0.005 |
| DVB64.31 | DVB, primary D3 | 1 | >1 | >1 | >1 | 0.360 ± 0.030 |
| 749B2 | 749, secondary D1 | 1 | 0.25 | 1 | 0.25 | 0.052 ± 0.005 |
| 749B4 | 749, secondary D1 | 0.063 | 0.25 | >1 | 0.016 | 0.043 ± 0.003 |
| 749B6 | 749, secondary D1 | 1 | 1 | >1 | >1 | 0.032 ± 0.002 |
| 749B12 | 749, secondary D1 | 0.063 | 0.25 | 1 | 0.016 | 0.034 ± 0.003 |
| 751A1 | 751, secondary D1 | 0.25 | 0.25 | 0.25 | 0.25 | 0.047 ± 0.003 |
| 751A2 | 751, secondary D1 | 0.25 | 0.25 | 1 | 1 | 0.097 ± 0.005 |
| 751A12 | 751, secondary D1 | 1 | 1 | >1 | 1 | 0.052 ± 0.007 |
| 751B3 | 751, secondary D1 | 1 | 0.25 | 1 | 1 | 0.105 ± 0.009 |
| 751B6 | 751, secondary D1 | 0.063 | 0.063 | 0.83 | 1 | 0.025 ± 0.002 |
| 751B11 | 751, secondary D1 | 0.25 | 0.25 | 1 | 0.25 | 0.013 ± 0.001 |
| 751B12 | 751, secondary D1 | 1 | 0.25 | 1 | 0.25 | 0.022 ± 0.001 |
| 751C4 | 751, secondary D1 | 0.25 | 0.063 | 0.31 | 0.25 | 0.011 ± 0.001 |
| 751C10 | 751, secondary D1 | 0.063 | 0.063 | 1 | 0.063 | 0.019 ± 0.003 |
| 753A1 | 753, secondary D1 | 1 | 0.25 | >1 | 1 | 0.130 ± 0.010 |
| 753C1 | 753, secondary D1 | 0.25 | 0.063 | 0.25 | 0.25 | 0.015 ± 0.001 |
| 753C6 | 753, secondary D1 | 0.063 | 0.063 | 1 | 0.25 | 0.024 ± 0.002 |
| 753C8 | 753, secondary D1 | 1 | 0.25 | 1 | 1 | 0.063 ± 0.004 |
| 753C12 | 753, secondary D1 | 0.25 | 0.063 | 0.25 | 0.063 | 0.035 ± 0.003 |
| 750C9 | 750, secondary D1 | 0.25 | 1 | >1 | 1 | 0.296 ± 0.030 |
| 750D7 | 750, secondary D1 | 1 | 0.25 | >1 | 1 | 0.070 ± 0.006 |
Higher binding avidities of GR anti-E MAbs derived from secondary DENV infection than those of MAbs derived from primary infection.
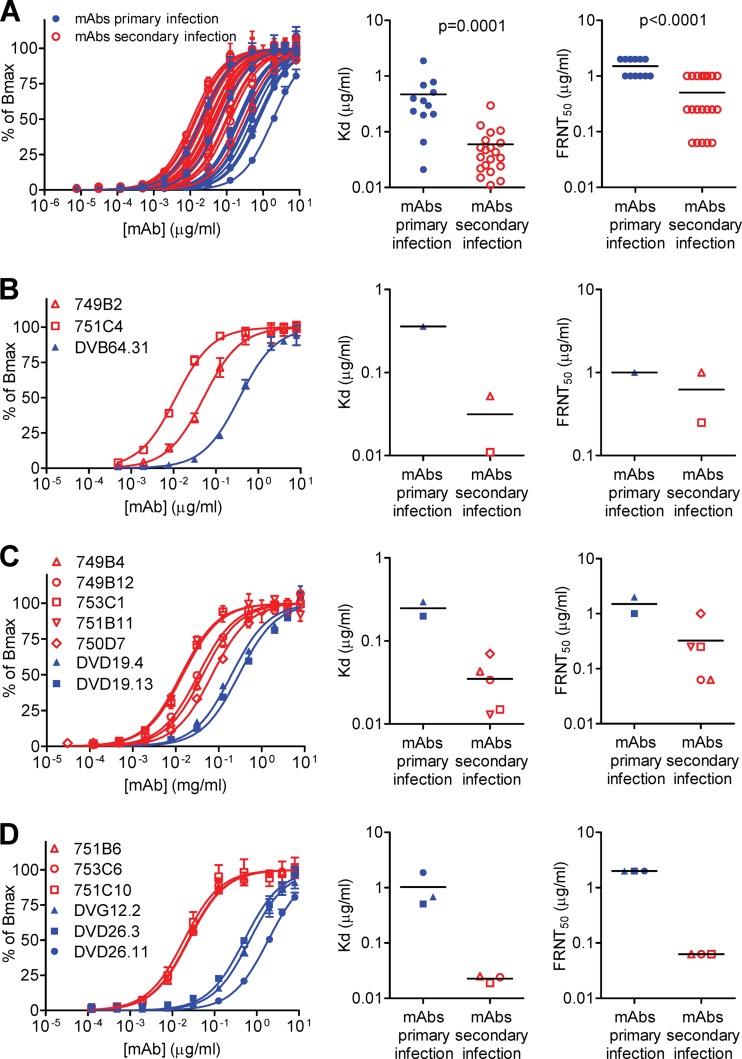
Since the 32 GR MAbs recognized the E proteins of four DENV serotypes similarly well (data not shown), we examined the binding avidities of these MAbs by using a DENV1 virion-capture ELISA and also determined the dissociation constant (Kd). As shown in Fig. 2A, the Kd values of MAbs derived from patients with secondary DENV infection were significantly lower than those of MAbs derived from patients with primary infection (P = 0.0001 by the two-tailed Mann-Whitney test), indicating that GR anti-E MAbs derived from secondary DENV infection had higher binding avidities than those derived from primary infection.
Fig 2.
Binding avidities and neutralization potencies of GR human anti-E MAbs. (A) Binding curves and Kd values for 12 and 20 GR human anti-E MAbs derived from primary and secondary DENV infections, respectively, were determined by a DENV1 virion-capture ELISA. The FRNT50 values against DENV1 are shown on the right. The two-tailed Mann-Whitney test was used to compare the two groups. (B to D) Binding curves, Kd values, and FRNT50 values against DENV1 for three GR human MAbs recognizing the same epitope involving four FL residues (W101, G106, L107, and F108) (B), seven GR human MAbs recognizing three FL residues (three residues among W101, F108, G106, and L107) as an epitope (C), and six GR human MAbs recognizing both FL residues and bc loop residues as an epitope (D). Data are means (with standard deviations for binding curves) for duplicates from one representative experiment of two. Blue closed symbols, MAbs derived from primary DENV infections; red open symbols, MAbs derived from secondary DENV infections.
To exclude the possibility that the different epitopes (either FL residues only or both FL and bc loop residues) may account for the differences in binding avidity and neutralizing potency, we first compared the binding curves of three MAbs recognizing the same epitope involving four FL residues (W101, G106, L107, and F108). As shown in Fig. 2B, the Kd values of the two MAbs (749B2 and 751C4) derived from secondary DENV infection were lower than that of the MAb (DVB64.31) derived from primary infection. Consistent with this, the mean FRNT50 concentration of these two MAbs was lower than that of the MAb derived from primary DENV infection (Fig. 2B and Table 3). We next compared the binding curves of seven MAbs recognizing similar epitopes involving three FL residues (three residues among W101, F108, G106, and L107) (Table 2). As shown in Fig. 2C, the Kd values of the five MAbs derived from secondary DENV infection were lower than those of the two MAbs derived from primary infection; this is consistent with the stronger neutralization potencies of these five MAbs (Fig. 2C and Table 3). A similar trend was observed for six MAbs recognizing epitopes involving both FL and bc loop residues, in that the binding avidities and neutralization potencies of three MAbs derived from secondary DENV infection were greater than those of three MAbs derived from primary infection (Fig. 2D and Table 3). Taken together, these findings suggest that the higher binding avidities of GR MAbs derived from secondary DENV infection, recognizing either FL residues or both FL and bc loop residues, may account for their stronger neutralization potencies.
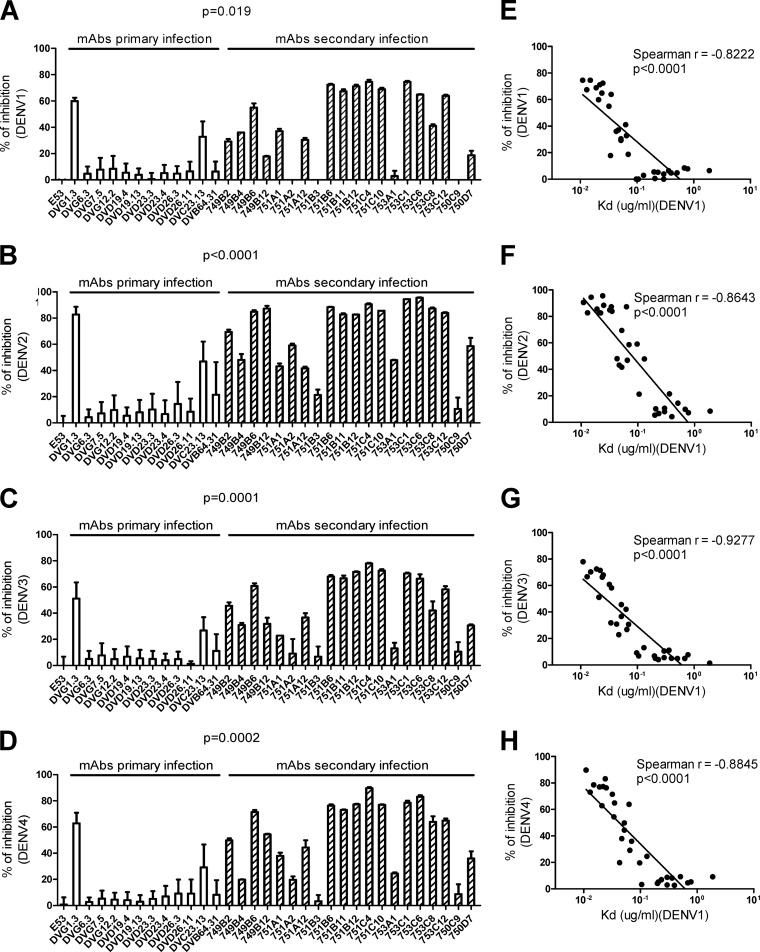
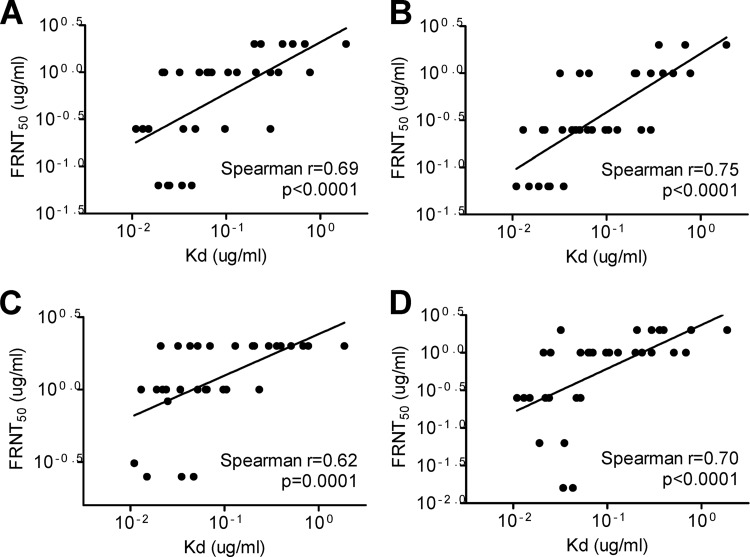
To assess the binding avidities of these GR MAbs for each of the four DENV serotypes by a different assay, we employed a previously described competitive virion-capture ELISA in which a known concentration of each human GR MAb competed with a mouse GR MAb (E53) recognizing FL (30, 31). We found that the % inhibition in DENV1, DENV2, DENV3, and DENV4 competitive virion-capture ELISAs by GR MAbs derived from secondary infection was significantly greater than that by GR MAbs derived from primary infection (P = 0.019, P < 0.0001, P = 0.0001, and P = 0.0002 for DENV1, DENV2, DENV3, and DENV4 competitive virion-capture ELISAs, respectively, by the two-tailed Mann-Whitney test) (Fig. 3A to D), suggesting higher binding avidities to all four serotypes for GR MAbs derived from secondary infection. Notably, a linear relationship was found between the Kd values and FRNT50 titers against the four serotypes (P < 0.0001, P < 0.0001, P = 0.0001, and P < 0.0001 for DENV1, DENV2, DENV3, and DENV4, respectively, by the two-tailed Spearman correlation test) (Fig. 4). These findings suggest that the higher binding avidities of GR MAbs derived from secondary DENV infection may account for the stronger neutralization potencies against four DENV serotypes than those of MAbs derived from primary infection (Table 3).
Fig 3.
Percent inhibition in competitive virion-capture ELISAs of four DENV serotypes by GR human anti-E MAbs. A mouse GR MAb (E53) recognizing FL (30) was prepared at 0.002 μg/ml (corresponding to its mean Kd for the four DENV serotypes), mixed with or without each human GR MAb (at 0.25 μg/ml, corresponding to the mean Kd for GR MAbs to DENV1), and subjected to competitive capture ELISA using DENV1 (A), DENV2 (B), DENV3 (C), or DENV4 (D) virions, with anti-mouse IgG as the secondary Ab (31). Inhibition was determined as follows: % inhibition = (1 − OD450 of E53 alone/OD450 of E53 plus human GR MAb) × 100. Data are means with standard deviations for two independent experiments (each in duplicate). The two-tailed Mann-Whitney test was used to compare the two groups. A linear relationship was found between the Kd determined by DENV1 virion-capture ELISA and the % inhibition in DENV1 (E), DENV2 (F), DENV3 (G), and DENV4 (H) competitive virion-capture ELISAs. The two-tailed Spearman correlation test was performed using GraphPad Prism 5.0.
Fig 4.
Relationship between Kd and FRNT50 values for GR MAbs against four DENV serotypes. The binding curves and Kd values for 32 GR human anti-E MAbs were determined by a DENV1 virion-capture ELISA. Neutralization potencies were determined by the FRNT50 values against DENV1 (A), DENV2 (B), DENV3 (C), and DENV4 (D). The two-tailed Spearman correlation test was performed using GraphPad Prism 5.0.
Mechanism of neutralization.
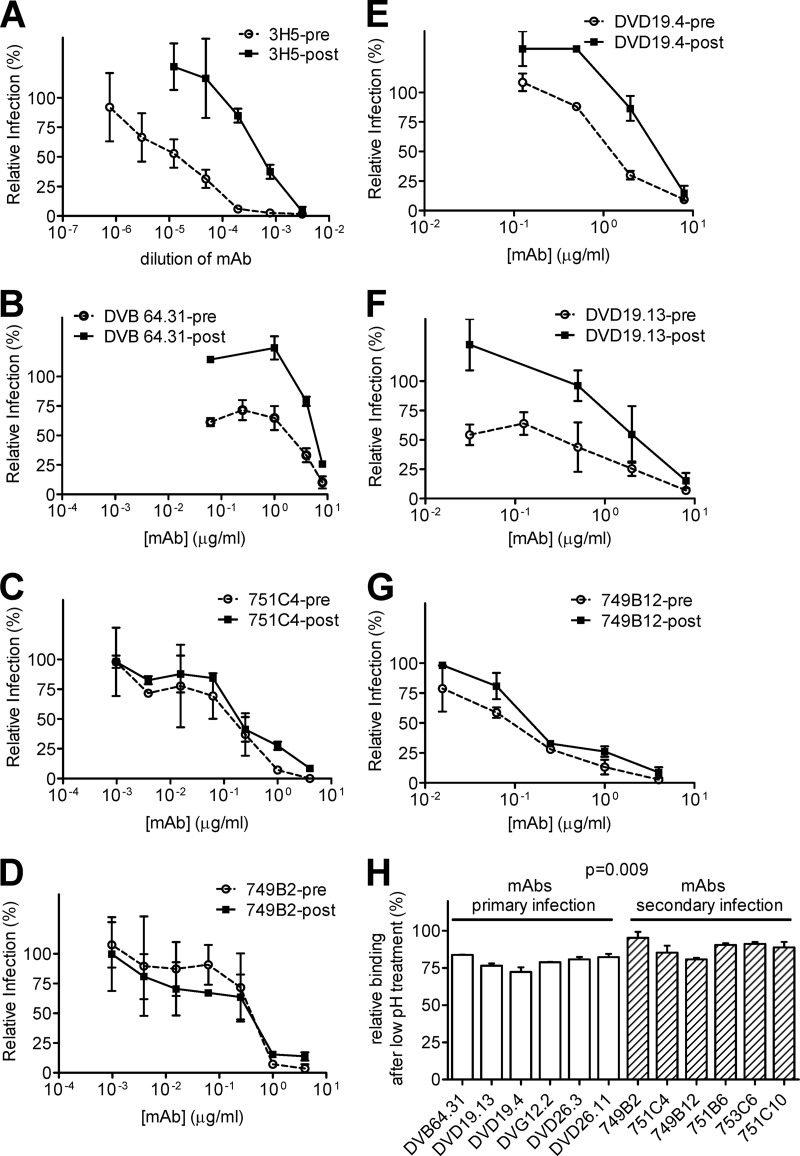
To investigate if the mechanisms of neutralization by GR MAbs derived from primary and secondary DENV infections are different, we performed a previously described pre- and postattachment neutralization assay for 3 GR MAbs recognizing the same FL epitope (residues W101, G106, L107, and F108) (Table 2) (11, 18). As shown in Fig. 5B, the GR MAb derived from primary DENV infection, DVB64.31, had greater inhibition in the preattachment assay than in the postattachment assay, suggesting that it neutralizes DENV mainly by blocking attachment. The pattern was similar to that of a control mouse MAb, 3H5, as reported previously (Fig. 5A) (11). In contrast, two GR MAbs (751C4 and 749B2) derived from secondary DENV infection showed similar inhibition both preattachment and postattachment, suggesting that at least part of their neutralization was at the postattachment step (Fig. 5C and D) (18). This observation was further verified by another 3 GR MAbs (DVD19.4 and DVD19.13, derived from primary DENV infection, and 749B12, derived from secondary infection) recognizing a similar FL epitope (three residues among W101, F108, G106, and L107) (Fig. 5E to G).
Fig 5.
Mechanism of neutralization. (A to G) Pre- and postattachment neutralization assays against DENV2 were performed as described previously (11, 18) for 3 GR MAbs recognizing the same FL epitope (residues W101, G106, L107, and F108), including DVB64.31 (B), derived from primary DENV infection, and 751C4 (C) and 749B2 (D), derived from secondary infection, and for 3 GR MAbs recognizing similar FL epitopes (three residues among W101, F108, G106, and L107), including DVD19.4 (E) and DVD19.13 (F), derived from primary infection, and 749B12 (G), derived from secondary infection. (A) A previously reported mouse MAb, 3H5, served as a control (11). The relative amount of infection was determined as follows: % infection = number of foci in the presence of MAb/number of foci in the absence of MAb. (H) DENV virion-capture ELISA was performed at pH 7.4, followed by incubation at pH 5.0 for 12 GR MAbs. The relative binding activity after low-pH treatment was determined as follows: % binding activity = OD450 at pH 5.0/OD450 at pH 7.4. The two-tailed Mann-Whitney test was used to compare the two groups. Data are means with standard deviations for three independent experiments (each in duplicate).
The possibility that GR MAbs derived from secondary DENV infection, which have high binding avidities at neutral pH, continue to bind virions tightly in the low-pH environment in the endosome compared to those derived from primary infection, and thus can block the postattachment step, was further examined by a virion-capture ELISA performed at neutral pH followed by low-pH incubation, which mimics the steps of entry from attachment at neutral pH to fusion at low pH. As shown in Fig. 5H, the relative binding activities after low-pH treatment of 6 GR MAbs derived from secondary DENV infection were significantly higher than those of 6 GR MAbs derived from primary infection (P = 0.009 by the two-tailed Mann-Whitney test), suggesting that the stronger binding activity of GR MAbs derived from secondary infection after low-pH treatment may account for their ability to neutralize DENV at the postattachment step in the endosome.
Repertoire of anti-E MAbs derived from patients with primary and secondary DENV infections.
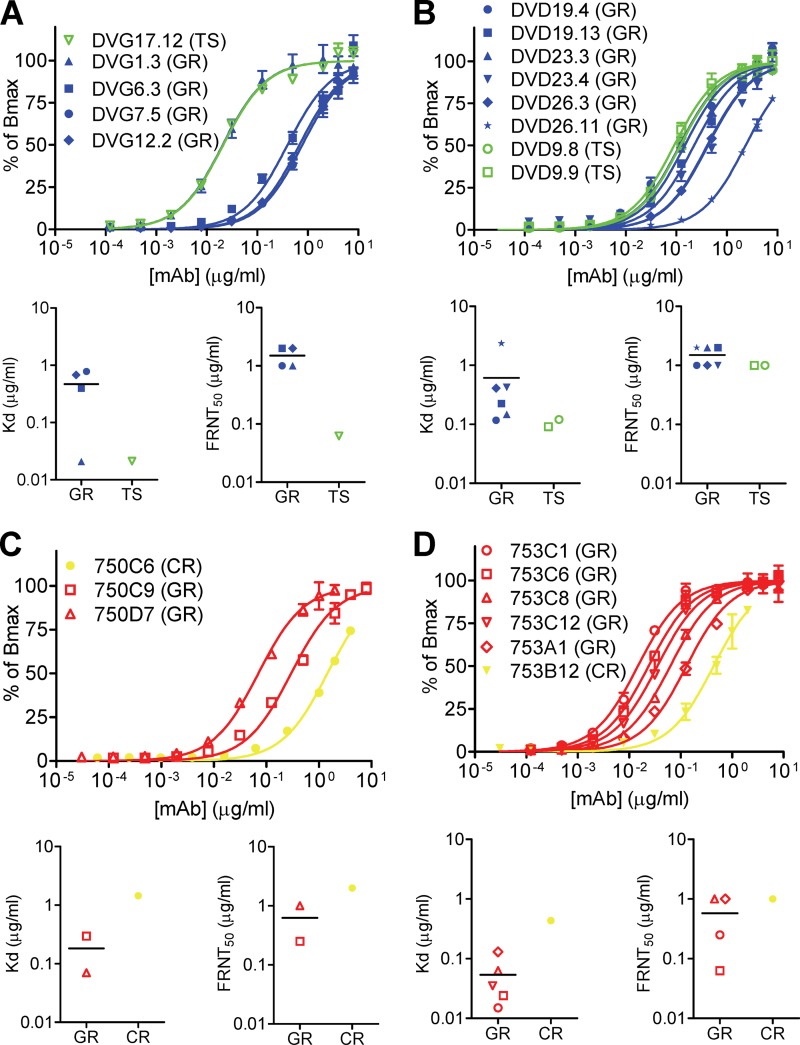
Previous studies of polyclonal human sera after DENV infection revealed that a significant proportion of anti-E Abs were GR Abs, whereas a minor proportion were TS Abs (13, 19–21). To better understand the anti-E Ab responses after primary DENV infection, we examined the repertoire of anti-E MAbs (including TS, CR, and GR MAbs) derived from two patients with primary infection, namely, DVG and DVD (Table 1). The five MAbs derived from DVG, a patient with primary DENV1 infection, included four GR MAbs and one DENV1-TS MAb. As shown in Fig. 6A, the TS MAb had a higher binding avidity to DENV1 than three of the four GR MAbs. Consistent with this, the TS MAb had stronger neutralization potency against DENV1 than the four GR MAbs. The 12 MAbs derived from DVD, a patient with primary DENV4 infection, included 6 GR MAbs, 2 CR MAbs, and 4 DENV4-TS MAbs. Because of the presence of DENV4-TS MAbs in this case, DENV4 virion-capture ELISA and FRNT against DENV4 were performed and compared. The mean Kd value of the TS MAbs was lower than that of the GR MAbs (Fig. 6B). Similarly, the mean FRNT50 concentration of TS MAbs was lower than that of the GR MAbs.
Fig 6.
Binding avidities and neutralization potencies of anti-E MAbs from patients with primary and secondary DENV infections. (A to D) Binding curves, Kd values, and FRNT50 values for the repertoire of anti-E MAbs derived from two patients with primary DENV infection, DVG (A) and DVD (B), and two patients with secondary infection, 750 (C) and 753 (D). DENV1 virion-capture ELISA and FRNT against DENV1 were performed, except for DVD (a patient with primary DE NV4 infection), for whom DENV4 virion-capture ELISA and FRNT against DENV4 were performed. Data are means (with standard deviations for binding curves) for duplicates from one representative experiment of two. Green open symbols, TS MAbs; blue closed symbols, GR MAbs; yellow closed symbols, CR MAbs; red open symbols, GR MAbs.
For comparison, we examined the repertoire of anti-E MAbs derived from two patients with secondary DENV infection, namely, patients 750 and 753 (Table 1). For the three MAbs derived from patient 750, a patient with secondary DENV1 infection, the binding avidities and neutralization potencies of two GR MAbs were higher than those of the CR MAb (Fig. 6C). For the six MAbs derived from patient 753, also a patient with secondary DENV1 infection, the mean Kd and FRNT50 concentration of five GR MAbs were lower than those of the CR MAb (Fig. 6D).
DISCUSSION
In this study, we investigated the epitopes, neutralization potencies, and binding avidities of GR human anti-E MAbs derived from primary and secondary DENV infections. Based on previous studies of mouse anti-E MAbs against DENV, most GR MAbs were weakly neutralizing and thus not regarded as important responses for dengue vaccine strategies (14–16). However, when we compared GR human anti-E MAbs derived from different origins, we found that GR MAbs derived from patients with secondary DENV infection had stronger neutralization potencies and higher binding avidities than those of MAbs derived from patients with primary infection, suggesting that the weakly neutralizing GR anti-E Abs generated from primary DENV infection become potently neutralizing Abs after secondary DENV infection. This was further supported by analysis of the repertoire of anti-E MAbs derived from two patients with primary DENV infection and two patients with secondary DENV infection. To our knowledge, this is the first report showing that the dengue immune status of the host (primary versus secondary DENV infection) may affect the quality of cross-reactive Abs generated. These findings not only add to our understanding of human anti-E Abs after natural DENV infection but also have implications for alternative strategies for dengue vaccine development.
Previously, Beltramello et al. characterized the neutralizing and enhancing activities of 51 human anti-E MAbs derived from five dengue cases (25). Of the 27 MAbs derived from two patients with secondary DENV infection, 22 recognized domain I/II, cross-reacted to four DENV serotypes, and were presumably GR MAbs. Of the 24 MAbs derived from three patients with primary DENV infection, most were TS or CR MAbs, and only 3 were presumably GR MAbs, based on their binding to domain I/II and cross-reactivity. Due to the small number of GR MAbs derived from primary DENV infection in the data set, a statistical analysis to compare the neutralization potencies of GR MAbs derived from primary and secondary DENV infections was not possible. As shown in Table 1, we studied all available 12 and 20 GR MAbs derived from primary and secondary DENV infections, respectively, excluding the possibility of selection bias in this study. Recent studies reported that temperature can affect the structure and neutralization of DENV (32–34) and that DENV strains grown in different cell types have different N-linked glycans (35). In this study, different binding assays and an FRNT assay (except for pre- and postattachment assays in the mechanistic study) were all performed at 37°C, and the viruses used were all derived from Vero cells grown at 37°C. Therefore, it is unlikely that the correlation between Kd and FRNT50 (Fig. 4) observed will be affected by variations such as the temperature and sources of virus in these assays.
We and others reported previously that a significant proportion of anti-E Abs in polyclonal human sera after DENV infection were GR Abs and primarily recognized the FL of domain II, whereas only a minor proportion were TS Abs and recognized domain III (13, 19–21). In agreement with this, our analysis of the repertoire of anti-E MAbs derived from two patients with primary DENV infection revealed that the majority were GR MAbs and a minor proportion were TS or CR MAbs. Moreover, the binding avidities of TS MAbs were generally greater than those of GR MAbs in these patients (Fig. 6A and B). The epitopes recognized by GR anti-E MAbs involved either FL residues only or both FL and bc loop residues; these residues were highly conserved by different flaviviruses and absolutely conserved by the four DENV serotypes. It is conceivable that during secondary DENV infection, memory B cells recognizing these highly conserved FL and/or bc loop residues expand greatly and generate high-avidity GR Abs through affinity maturation (36). In agreement with this interpretation, studies of polyclonal human sera after DENV infection, which contained a significant proportion of GR anti-E Abs, revealed that the avidity of serum anti-DENV Abs from secondary infection was higher than that of Abs from primary infection (37, 38). In addition, recent studies reported that cross-reactive memory B cells or plasma cells, as well as serum avidity, increased greatly during acute secondary DENV infection (38–40).
Investigation of the mechanism of neutralization by pre- and postattachment assays revealed that GR MAbs derived from primary DENV infection neutralize DENV mainly at the attachment step, whereas GR MAbs derived from secondary infection can also neutralize DENV at the postattachment step, probably by blocking membrane fusion in the endosome. A previous study of mouse MAbs reported that domain III MAbs (such as 3H5) primarily block virus at the attachment step and domain I-II MAbs (such as GR MAb 4G2) also block attachment, though to a lesser extent (11). Another study demonstrated that a potent domain III MAb against WNV, E16, can block virus at the postattachment steps (18, 30). Since 3H5 and E16 recognized similar epitope residues in the lateral ridge of domain III, the observations that 3H5 blocks attachment and E16 can block postattachment steps suggest that a subtle difference in epitope or other factors, such as avidity, may account for different mechanisms of neutralization. In this regard, our findings suggest that the stronger binding activity after low-pH treatment for GR MAbs derived from secondary DENV infection than for those derived from primary infection may contribute to the ability to neutralize DENV at the postattachment step.
After primary DENV infection, individuals developed life-long protection against the infecting serotype, which has been shown to correlate with the appearance of TS neutralizing Abs against that serotype (41, 42). After secondary DENV infection, individuals developed not only homotypic neutralizing Abs against the serotypes to which they had previously been exposed but also heterotypic neutralizing Abs against the serotypes to which they had not yet been exposed (9). Such heterotypic neutralizing Abs are believed to contribute to the very small numbers of hospitalization cases for humans after a third or fourth DENV infection (43) and to the low rate of viremia after a third DENV infection in monkeys (44, 45). Our finding that the weakly neutralizing GR anti-E Abs generated from primary DENV infection become potently neutralizing Abs after secondary infection suggests that such GR anti-E Abs, present as a predominant population, may contribute to the heterotypic neutralizing activities against the serotypes to which they have not yet been exposed. The possibility that some CR anti-E Abs generated after secondary DENV infection also contribute to such heterotypic neutralizing activities cannot be ruled out completely, though they constituted a minor population and very few human CR MAbs are available for characterization thus far. In this regard, our analysis of 4 CR anti-E MAbs which recognized an epitope in the domain II central interface (residues Q211, D215, and/or P217; highly conserved in DENV serocomplex viruses) revealed that the binding avidities and neutralizing potencies of those derived from secondary DENV infection were stronger than those of MAbs derived from primary infection (data not shown). This provides more evidence supporting the hypothesis that the immune status of the host (primary versus secondary DENV infection) affects the quality of cross-reactive Abs generated.
There are several limitations of this study. First, the time of blood sampling to prepare MAbs in this study was either the acute phase (4 to 5 days), for GR MAbs derived from secondary DENV infection, or convalescent phase (1 month), for most (10 of 12 MAbs) of the GR MAbs derived from primary infection (Table 1). Whether the observations of higher binding avidities and neutralizing potencies of GR MAbs derived from secondary DENV infection than those of GR MAbs from primary infection hold true at later time points after DENV infection remains to be investigated. In this regard, it is worth noting that in the study of Beltramello et al., several potently neutralizing GR MAbs derived from secondary DENV infection were prepared at 7 to 17 months postinfection (25). Second, the DENV serotypes of primary infections were different. For the 12 GR MAbs derived from primary infection, 4 were from DENV1 infection, 6 from DENV4 infection, and 1 each from DENV2 and DENV3 infections (Table 1). For the 20 GR MAbs derived from secondary infections, the serotypes of the primary infections were not known. Whether the sequence of primary and secondary DENV infection affects the quality of GR MAbs generated remains to be investigated. Third, the donors were different, and the sample size was small. Fourth, the methodologies for generating these MAbs were different. Future studies with a larger sample size and using the same methodology to generate MAbs from the same subjects following their own sequential primary or secondary infection are needed to validate these observations.
Because of cocirculation of multiple DENV serotypes commonly seen in areas where dengue is endemic and the association of increased disease severity in persons during secondary DENV infection, the goal of current dengue vaccines is to attain balanced neutralizing Ab and T cell responses against all four DENV serotypes (1, 3, 46). Although several tetravalent live-attenuated dengue vaccines have moved to phase II or III clinical trials, a challenge of such an approach is the difficulty in simultaneously achieving balanced TS neutralizing Abs against all four DENV serotypes, due to dominant viremia by one or two serotypes resulting from interserotype interference (3, 46–48). Other approaches, such as epitope-modified vaccines presenting the potently neutralizing epitopes recognized by TS Abs and eliminating or masking the poorly neutralizing epitopes in the tetravalent format, have been proposed and reported recently (49–51). Our finding that secondary DENV infection greatly improves the quality of cross-reactive anti-E Abs generated suggests that an alternative approach of sequential heterotypic immunization (with nonreplicating dengue candidate vaccines) to mimic sequential natural infection might elicit potently neutralizing Abs against all four DENV serotypes. It should be noted that a recent study reporting no efficacy for a tetravalent dengue vaccine against DENV2 in a phase IIb clinical trial raised concerns about the relevance of the in vitro neutralization assay and the importance of T cell immunity in protection (48, 52). Although the in vitro neutralization assay has been used as a surrogate of protective immunity in vaccine studies (reviewed in reference 3), several studies failed to show perfect correlation between neutralization and protection in mouse and nonhuman primate models (31, 53, 54). In contrast to previous studies showing T cell responses associated with severe dengue disease in humans (55, 56), a recent study of T cell responses against DENV suggested a protective role of CD8 T cells (57). These highlight the need to explore different functional assays of Abs and T cells to understand the immune correlates of protection and the application of better assays to vaccine studies.
ACKNOWLEDGMENTS
We thank Gwong-Jen Chang at the Centers for Disease Control and Prevention at Fort Collins for kindly providing rabbit sera for capture ELISA and Saguna Verma at the University of Hawaii at Manoa for kindly providing lysates of WNV-infected cells.
This work was supported by a cooperative research agreement from the International Vaccine Institute/Pediatric Dengue Vaccine Initiative and by grant P20GM103516 from the National Institute of General Medical Sciences, NIH.
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Published ahead of print 11 September 2013
REFERENCES
- 1.Guzman MG, Halstead SB, Artsob H, Buchy P, Farrar J, Gubler DJ, Hunsperger E, Kroeger A, Margolis HS, Martínez E, Nathan MB, Pelegrino JL, Simmons C, Yoksan S, Peeling RW. 2010. Dengue: a continuing global threat. Nat. Rev. Microbiol. 12:S7–S16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization 2009. Dengue hemorrhagic fever: diagnosis, treatment, prevention and control, 3rd ed. World Health Organization, Geneva, Switzerland [Google Scholar]
- 3.Murphy BR, Whitehead SS. 2011. Immune response to dengue virus and prospects for a vaccine. Annu. Rev. Immunol. 29:587–619 [DOI] [PubMed] [Google Scholar]
- 4.Lindenbach BD, Thiel HJ, Rice CM. 2007. Flaviviridae: the viruses and their replication, p 1101–1152 In Knipe DM, Howley PM, Griffin DE, Lamb RA, Martin MA, Roizman B, Straus SE. (ed), Fields virology, 5th ed. Lippincott Williams & Wilkins, Philadelphia, PA [Google Scholar]
- 5.Stiasny K, Heinz FX. 2006. Flavivirus membrane fusion. J. Gen. Virol. 87:2755–2766 [DOI] [PubMed] [Google Scholar]
- 6.Randolph VB, Stollar V. 1990. Low pH-induced cell fusion in flavivirus-infected Aedes albopictus cell cultures. J. Gen. Virol. 71:1845–1850 [DOI] [PubMed] [Google Scholar]
- 7.Modis Y, Ogata S, Clements D, Harrison SC. 2004. Structure of the dengue virus envelope protein after membrane fusion. Nature 427:313–319 [DOI] [PubMed] [Google Scholar]
- 8.Kuhn RJ, Zhang W, Rossmann MG, Pletnev SV, Corver J, Lenches E, Jones CT, Mukhopadhyay S, Chipman PR, Strauss EG, Baker TS, Strauss JH. 2002. Structure of dengue virus: implications for flavivirus organization, maturation, and fusion. Cell 108:717–725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Halstead SB. 1988. Pathogenesis of dengue: challenges to molecular biology. Science 239:476–481 [DOI] [PubMed] [Google Scholar]
- 10.Halstead SB, O'Rourke EJ. 1977. Antibody-enhanced dengue virus infection in primate leukocytes. Nature 265:739–741 [DOI] [PubMed] [Google Scholar]
- 11.Crill WD, Roehrig JT. 2001. Monoclonal antibodies that bind to domain III of dengue virus E glycoprotein are the most efficient blockers of virus adsorption to Vero cells. J. Virol. 75:7769–7773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Calisher CH, Karabatsos N, Dalrymple JM, Shope RE, Porterfield JS, Westaway EG, Brandt WE. 1989. Antigenic relationships between flaviviruses as determined by cross-neutralization tests with polyclonal antisera. J. Gen. Virol. 70:37–43 [DOI] [PubMed] [Google Scholar]
- 13.Crill WD, Hughes HR, Delorey MJ, Chang GJ. 2009. Humoral immune responses of dengue fever patients using epitope-specific serotype-2 virus-like particle antigens. PLoS One 4:e4991. 10.1371/journal.pone.0004991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gromowski GD, Barrett AD. 2007. Characterization of an antigenic site that contains a dominant, type-specific neutralization determinant on the envelope protein domain III (ED3) of dengue 2 virus. Virology 366:349–360 [DOI] [PubMed] [Google Scholar]
- 15.Sukupolvi-Petty S, Austin K, Purtha WE, Oliphant T, Nybakken GE, Schlesinger JJ, Roehrig JT, Gromowski GD, Barrett AD, Fremont DH, Diamond MS. 2007. Type and subcomplex-specific neutralizing antibodies against domain III of dengue virus type 2 envelope protein recognize adjacent epitopes. J. Virol. 81:12816–12826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shrestha B, Brien JD, Sukupolvi-Petty S, Austin K, Edeling MA, Kim T, O'Brien KM, Nelson CA, Johnson S, Fremont DH, Diamond MS. 2010. The development of therapeutic antibodies that neutralize homologous and heterologous genotypes of dengue virus type 1. PLoS Pathog. 6:e1000823. 10.1371/journal.ppat.1000823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goncalvez AP, Chien CH, Tubthong K, Gorshkova I, Roll C, Donau O, Schuck P, Yoksan S, Wang SD, Purcell RH, Lai CJ. 2008. Humanized monoclonal antibodies derived from chimpanzee Fabs protect against Japanese encephalitis virus in vitro and in vivo. J. Virol. 82:7009–7021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vogt MR, Moesker B, Goudsmit J, Jongeneelen M, Austin SK, Oliphant T, Nelson S, Pierson TC, Wilschut J, Throsby M, Diamond MS. 2009. Human monoclonal antibodies against West Nile virus induced by natural infection neutralize at a postattachment step. J. Virol. 83:6494–6507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lin HE, Tsai WY, Liu IJ, Li PC, Liao MY, Tsai JJ, Wu YC, Lai CY, Lu CH, Huang JH, Chang GJ, Wu HC, Wang WK. 2012. Analysis of epitopes on dengue virus envelope protein recognized by monoclonal antibodies and polyclonal human sera by a high-throughput assay. PLoS Negl. Trop. Dis. 6:e1447. 10.1371/journal.pntd.0001447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lai CY, Tsai WY, Lin SR, Kao CL, Hu SP, King CC, Wu HC, Wang WK. 2008. Antibodies to envelope glycoprotein of dengue virus during the natural course of infection are predominantly cross-reactive and recognize epitopes containing highly conserved residues at the fusion loop of domain II. J. Virol. 82:6631–6643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wahala WM, Kraus AA, Haymore LB, Accavitti-Loper MA, de Silva AM. 2009. Dengue virus neutralization by human immune sera: role of envelope protein domain III-reactive antibody. Virology 392:103–113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.de Alwis R, Beltramello M, Messer WB, Sukupolvi-Petty S, Wahala WM, Kraus A, Olivarez NP, Pham Q, Brian J, Tsai WY, Wang WK, Halstead S, Kliks S, Diamond MS, Baric R, Lanzavecchia A, Sallusto F, de Silva AM. 2011. In-depth analysis of the antibody response of individuals exposed to primary dengue virus infection. PLoS Negl. Trop. Dis. 5:e1188. 10.1371/journal.pntd.0001188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.de Alwis R, Smith SA, Olivarez NP, Messer WB, Huynh JP, Wahala WM, White LJ, Diamond MS, Baric RS, Crowe JE, Jr, de Silva AM. 2012. Identification of human neutralizing antibodies that bind to complex epitopes on dengue virions. Proc. Natl. Acad. Sci. U. S. A. 109:7439–7444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Teoh EP, Kukkaro PP, Teo EW, Lim AP, Tan TT, Yip A, Schul W, Aung M, Kostyuchenko VA, Leo YS, Chan SH, Smith KG, Chan AH, Zou G, Ooi EE, Kemeny DM, Tan GK, Ng JK, Ng ML, Alonso S, Fisher D, Shi PY, Hanson BJ, Lok SM, MacAry PA. 2012. The structural basis for serotype-specific neutralization of dengue virus by a human antibody. Sci. Transl. Med. 4:139ra83. 10.1126/scitranslmed.3003888 [DOI] [PubMed] [Google Scholar]
- 25.Beltramello M, Williams KL, Simmons CP, Macagno A, Simonelli L, Quyen NT, Sukupolvi-Petty S, Navarro-Sanchez E, Young PR, de Silva AM, Rey RA, Varani L, Whitehead SS, Diamond MS, Harris E, Lanzavecchia A, Sallusto F. 2010. The human immune response to dengue virus is dominated by highly cross-reactive antibodies endowed with neutralizing and enhancing activity. Cell Host Microbe 8:271–283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schieffelin JS, Costin JM, Nicholson CO, Orgeron NM, Fontaine KA, Isern S, Michael SF, Robinson JE. 2010. Neutralizing and non-neutralizing monoclonal antibodies against dengue virus E protein derived from a naturally infected patient. Virol. J. 7:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Smith SA, Zhou Y, Olivarez NP, Broadwater AH, de Silva AM, Crowe JE., Jr 2012. Persistence of circulating memory B cell clones with potential for dengue virus disease enhancement for decades following infection. J. Virol. 86:2665–2675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Smith K, Garman L, Wrammert J, Zheng NY, Capra JD, Ahmed R, Wilson PC. 2009. Rapid generation of fully human monoclonal antibodies specific to a vaccinating antigen. Nat. Protoc. 4:372–384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dejnirattisai W, Jumnainsong A, Onsirisakul N, Fitton P, Vasanawathana S, Limpitikul W, Puttikhunt C, Edwards C, Duangchinda T, Supasa S, Chawansuntati K, Malasit P, Mongkolsapaya J, Screaton C. 2010. Cross-reacting antibodies enhance dengue virus infection in humans. Science 328:745–748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thompson BS, Moesker B, Smit JM, Wilschut J, Diamond MS, Fremont DH. 2009. A therapeutic antibody against West Nile virus neutralizes infection by blocking fusion within endosomes. PLoS Pathog. 5:e1000453. 10.1371/journal.ppat.1000453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Williams KL, Sukupolvi-Petty S, Beltramello M, Johnson S, Sallusto F, Lanzavecchia A, Diamond MS, Harris E. 2013. Therapeutic efficacy of antibodies lacking FcγR against lethal dengue virus infection is due to neutralizing potency and blocking of enhancing antibodies. PLoS Pathog. 9:e1003157. 10.1371/journal.ppat.1003157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fibriansah G, Ng TS, Kostyuchenko VA, Lee J, Lee S, Wang J, Lok SM. 2013. Structural changes in dengue virus when exposed to a temperature of 37°C. J. Virol. 87:7585–7592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang X, Sheng J, Plevka P, Kuhn RJ, Diamond MS, Rossmann MG. 2013. Dengue structure differs at the temperatures of its human and mosquito hosts. Proc. Natl. Acad. Sci. U. S. A. 110:6795–6799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dowd KA, Jost CA, Durbin AP, Whitehead SS, Pierson TC. 2011. A dynamic landscape for antibody binding modulates antibody-mediated neutralization of West Nile virus. PLoS Pathog. 7:e1002111. 10.1371/journal.ppat.1002111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hacker K, White L, de Silva AM. 2009. N-linked glycans on dengue viruses grown in mammalian and insect cells. J. Gen. Virol. 90:2097–2106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yoshida T, Mei H, Dörner T, Hiepe F, Radbruch A, Fillatreau S, Hoyer BF. 2010. Memory B and memory plasma cells. Immunol. Rev. 237:117–139 [DOI] [PubMed] [Google Scholar]
- 37.de Souza VA, Tateno AF, Oliveira RR, Domingues RB, Araújo ES, Kuster GW, Pannuti CS. 2007. Sensitivity and specificity of three ELISA-based assays for discriminating primary from secondary acute dengue virus infection. J. Clin. Virol. 39:230–233 [DOI] [PubMed] [Google Scholar]
- 38.Zompi S, Montoya M, Pohl MO, Balmaseda A, Harris E. 2012. Dominant cross-reactive B cell response during secondary acute dengue virus infection in humans. PLoS Negl. Trop. Dis. 6:e1568. 10.1371/journal.pntd.0001568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mathew A, West K, Kalayanarooj S, Gibbons RV, Srikiatkhachorn A, Green S, Libraty D, Jaiswal S, Rothman AL. 2011. B-cell responses during primary and secondary dengue virus infections in humans. J. Infect. Dis. 204:1514–1522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wrammert J, Onlamoon N, Akondy RS, Perng GC, Polsrila K, Chandele A, Kwissa M, Pulendran B, Wilson PC, Wittawatmongkol O, Yoksan S, Angkasekwinai N, Pattanapanyasat K, Chokephaibulkit K, Ahmed R. 2012. Rapid and massive virus-specific plasmablast responses during acute dengue virus infection in humans. J. Virol. 86:2911–2918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sabin AB. 1952. Research on dengue during World War II. Am. J. Trop. Med. Hyg. 1:30–50 [DOI] [PubMed] [Google Scholar]
- 42.Halstead SB. 1974. Etiologies of the experimental dengues of Siler and Simmons. Am. J. Trop. Med. Hyg. 23:974–982 [DOI] [PubMed] [Google Scholar]
- 43.Gibbons RV, Kalanarooj S, Jarman RG, Nisalak A, Vaughn DW, Endy TP, Mammen MP, Jr, Srikiatkhachorn A. 2007. Analysis of repeat hospital admissions for dengue to estimate the frequency of third or fourth dengue infections resulting in admissions and dengue hemorrhagic fever, and serotype sequences. Am. J. Trop. Med. Hyg. 77:910–913 [PubMed] [Google Scholar]
- 44.Halstead SB, Shotwell H, Casals J. 1973. Studies on the pathogenesis of dengue infection in monkeys. I. Clinical laboratory responses to primary infection. J. Infect. Dis. 128:7–14 [DOI] [PubMed] [Google Scholar]
- 45.Scherer WF, Breakenridge FA, Dickerman RW. 1972. Cross-protection studies and search for subclinical disease in new world monkeys infected sequentially with different immunologic types of dengue viruses. Am. J. Epidemiol. 95:67–79 [DOI] [PubMed] [Google Scholar]
- 46.Webster DP, Farrar J, Rowland-Jones JS. 2009. Progress towards a dengue vaccine. Lancet Infect. Dis. 9:678–687 [DOI] [PubMed] [Google Scholar]
- 47.Guy B, Barban V, Mantel N, Aguirre M, Gulia S, Pontvianne J, Jourdier TM, Ramirez L, Gregoire V, Charnay C, Burdin N, Dumas R, Lang J. 2009. Evaluation of interferences between dengue vaccine serotypes in a monkey model. Am. J. Trop. Med. Hyg. 80:302–311 [PubMed] [Google Scholar]
- 48.Sabchareon A, Wallace D, Sirivichayakul C, Limkittikul K, Chanthavanich P, Suvannadabba S, Jiwariyavej V, Dulyachai W, Pengsaa K, Wartel TA, Moureau A, Saville M, Bouckenooghe A, Viviani S, Tornieporth NG, Lang J. 2012. Protective efficacy of the recombinant, live-attenuated, CYD tetravalent dengue vaccine in Thai schoolchildren: a randomised, controlled phase 2b trial. Lancet 380:1559–1567 [DOI] [PubMed] [Google Scholar]
- 49.Diamond MS, Pierson TC, Fremont DH. 2008. The structural immunology of antibody protection against West Nile virus. Immunol. Rev. 225:212–225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Crill WD, Hughes HR, Trainor NB, Davis BS, Whitney MT, Chang GJ. 2012. Sculpting humoral immunity through dengue vaccination to enhance protective immunity. Front. Immunol. 3:334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hughes HR, Crill WD, Chang GJ. 2012. Manipulation of immunodominant dengue virus E protein epitopes reduces potential antibody-dependent enhancement. Virol. J. 9:115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nature Publishing Group 2012. Defeating dengue: a challenge for a vaccine. Nat. Med. 18:1622–1623 [DOI] [PubMed] [Google Scholar]
- 53.Brien JD, Austin SK, Sukupolvi-Petty S, O'Brien KM, Johnson S, Fremont DH, Diamond MS. 2010. Genotype-specific neutralization and protection by antibodies against dengue virus type 3. J. Virol. 84:10630–10643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.White LJ, Sariol CA, Mattocks MD, Wahala MPBW, Yingsiwaphat V, Collier ML, Whitley J, Mikkelsen R, Rodriguez IV, Martinez MI, de Silva A, Johnston RE. 2013. An alphavirus vector-based tetravalent dengue vaccine induces a rapid and protective immune response in macaques that differs qualitatively from immunity induced by live virus infection. J. Virol. 87:3409–3424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mongkolsapaya J, Dejnirattisai W, Xu XN, Vasanawathana S, Tangthawornchaikul N, Chairunsri A, Sawasdivorn S, Duangchinda T, Dong T, Rowland-Jones S, Yenchitsomanus PT, McMichael A, Malasit P, Screaton G. 2003. Original antigenic sin and apoptosis in the pathogenesis of dengue hemorrhagic fever. Nat. Med. 9:921–927 [DOI] [PubMed] [Google Scholar]
- 56.Duangchinda T, Dejnirattisai W, Vasanawathana S, Limpitikul W, Tangthawornchaikul N, Malasit P, Mongkolsapaya J, Screaton G. 2010. Immunodominant T-cell responses to dengue virus NS3 are associated with DHF. Proc. Natl. Acad. Sci. U. S. A. 107:16922–16927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Weiskopf D, Angelo MA, de Azeredo EL, Sidney J, Greenbaum JA, Fernando AN, Broadwater A, Kolla RV, De Silva AD, de Silva AM, Mattia KA, Doranz BJ, Grey HM, Shresta S, Peters B, Sette A. 2013. Comprehensive analysis of dengue virus-specific responses supports an HLA-linked protective role for CD8+ T cells. Proc. Natl. Acad. Sci. U. S. A. 110:E2046–E2053 [DOI] [PMC free article] [PubMed] [Google Scholar]