Abstract
Disruption of the conserved motif GYxxØ in the simian immunodeficiency virus (SIV) SIVmac239 envelope (Env) cytoplasmic tail resulted in a virus (ΔGY) that exhibited a high plasma peak but uniquely failed to acutely deplete mucosal CD4+ T cells. Here, we show that ΔGY containing a flanking S727P mutation that was acquired in ΔGY-infected macaques reacquired the ability to rapidly deplete CD4+ T cells in lamina propria. This suggests that the GYxxØ motif and S727P each contribute to SIV's targeting to mucosal tissues.
TEXT
Pathogenic human immunodeficiency virus (HIV) and simian immunodeficiency virus (SIV) infections uniformly cause rapid and profound acute depletion of mucosal CD4+ T cells (1–5). Recently we described a SIVmac239 variant, known as ΔGY (6), containing a Gly-Tyr deletion of amino acids (aa) 720 to 721 in a highly conserved GYxxØ trafficking motif (where G = Gly, Y = Tyr, x = any amino acid, and Ø = an amino acid with a bulky hydrophobic side chain) in the envelope (Env) glycoprotein cytoplasmic tail (i.e., GYRPV for SIVmac239). This model was unique in demonstrating that the high acute peak viral load and acute loss of mucosal CD4+ T cells could be disassociated (6). In rhesus macaques, ΔGY exhibited an acute plasma viral RNA peak that was comparable to that of highly pathogenic parental SIVmac239 and exhibited robust replication in peripheral lymphoid tissues (e.g., tonsil, spleen, and mesenteric lymph nodes) but displayed an attenuated phenotype within the intestinal mucosa, with only patchy and transient infection of CD4+ T cells in the lamina propria (6). Consistent with this lack of gut pathology, these animals showed no evidence of microbial translocation, which is associated with a loss of epithelial barrier function and has been implicated as a driver of immune activation and disease progression (7–16). Nonetheless, even in the absence of microbial translocation, ΔGY-infected animals progressed to disease with immune activation and a gradual depletion in mucosal CD4+ T cells. Although the ΔGY mutation was maintained, disease progression was associated with several mutations in the cytoplasmic tail that flanked the ΔGY deletion, including R722G, S727P, and a 9-nucleotide deletion resulting in loss of QTH at aa 735 to 737 (6, 17). We proposed that one or more of these mutations could restore pathogenicity and/or the ability of ΔGY to target and deplete mucosal CD4+ T cells.
We examined the effects of one of these mutations, S727P, on the ability of ΔGY to target and deplete mucosal CD4+ T cells and determined its impact on disease progression. We focused on this mutation because it had previously been described in a ΔGY-infected rhesus macaque that rapidly progressed to AIDS with a high viral load (17). SIVmac239 containing both the ΔGY and the S727P mutations, designated ΔGY+S/P, was produced from transfected 293T cells. Four male Indian-origin rhesus macaques (Table 1) were inoculated intravenously with ΔGY+S/P (100 50% tissue culture infective doses [TCID50]) and compared to macaques inoculated with ΔGY or either SIVmac239 or the closely related SIVmac251, as previously described (6). Two animals were euthanized at week 4 to provide a comprehensive assessment of ΔGY+S/P infection in gut and other tissues, and two were followed through chronic infection. All animals were maintained at the Tulane National Primate Research Center in accordance with the standards of the Association for Assessment and Accreditation of Laboratory Animal Care and the Guide for the Care and Use of Laboratory Animals prepared by the National Research Council (28). The Tulane Institutional Animal Care and Use Committee approved all studies.
Table 1.
Results from individual rhesus macaques infected with ΔGY+S/Pa
| Stage of infection | Animal | Duration of infection (wk)b | Mamu type(s)c |
|---|---|---|---|
| Acute | GM76 | 4 | DRBw201, A*11, B*01 |
| GT39 | 4 | DRBw201 | |
| Chronic | GK58 | 40 | A*02, B*01 |
| GM20 | >100 | DRBw201, A*02, A*08 |
Results from four rhesus macaques infected with ΔGY+S/P are shown. (Two were euthanized at week 4 to assess pathological features during acute infection, and 2 were followed during chronic infection.) The control animals for this study have been reported previously (6) and included 8 animals infected with ΔGY and 15 animals infected with either SIVmac239 or SIVmac251.
Animals were euthanized at the indicated week postinfection. Boldface text indicates euthanasia due to progressive disease. Regular text indicates euthanasia at the end of the predetermined period during acute infection. GM20 controlled infection and remained alive for >100 weeks.
Mamu alleles of infected animals are shown.
Acute plasma viral RNA for the 4 ΔGY+S/P-inoculated macaques was compared to data from 8 ΔGY- and 8 SIVmac239-infected macaques (Fig. 1A). The acute peak of infection for ΔGY+S/P occurred more rapidly (day 17) than that for ΔGY (day 21), although both were slower than SIVmac239 (day 14). Interestingly, the mean viral peak for ΔGY+S/P was higher than that for ΔGY (4.4 × 107 copies/ml versus 1.0 × 107 copies/ml, respectively; P < 0.05) but comparable to that for SIVmac239 (1.3 × 107 copies/ml; P > 0.05) (Fig. 1B).
Fig 1.
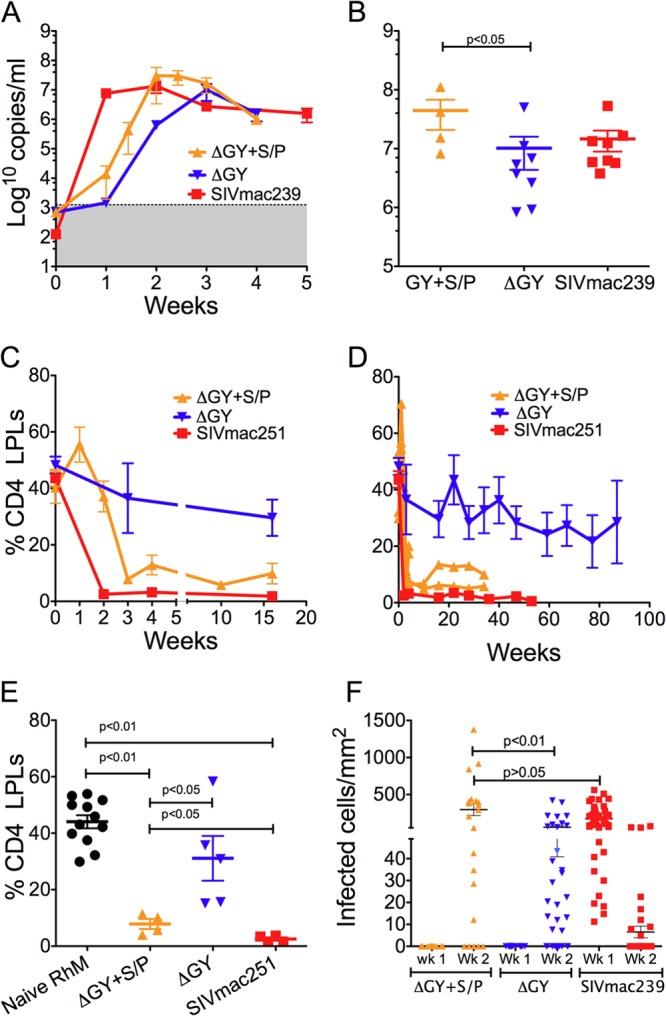
Plasma viral load and gut CD4+ T-cell infection in rhesus macaques infected with ΔGY+S/P, ΔGY, or parental SIVmac (SIVmac239 or SIVmac251). (A) Plasma viral loads (mean ± SEM) for ΔGY+S/P-, ΔGY-, and SIVmac239-infected animals are shown during acute infection. The shaded area in panel A indicates the limits of sensitivity of the viral RNA assay; (B) peak viral RNA levels are shown for individual animals (mean ± SEM). ΔGY+S/P had an earlier (A) and a higher (B) peak viremia than ΔGY. (C and D) The percentages of CD4+ T cells in the intestinal lamina propria (lamina propria lymphocytes [LPLs]), determined by flow cytometry, are shown (mean ± SEM) during acute (C) and chronic (D) infection. Values for the 2 ΔGY+S/P-infected animals followed during chronic infection are plotted individually (D). CD4+ T-cell depletion was greater and occurred more rapidly for ΔGY+S/P than for ΔGY. (E) Individual percentages of CD4+ T cells in lamina propria are shown in naive rhesus macaques (n = 12) and at the nadir of mucosal CD4 depletion for ΔGY+S/P- and ΔGY-infected animals (week 3) and for SIVmac251-infected animals (week 2; mean ± SEM). (F) The numbers of infected cells detected by in situ hybridization per mm2 of tissue surface area are shown. ΔGY+S/P infected a greater number of cells per mm2 of lamina propria at week 2 than did ΔGY, but the level of infection was similar to that of SIVmac239 at week 1 (F). The number of cells infected by SIVmac239 is reduced at week 2 due to depletion of these cells (C).
We next compared the impact of ΔGY+S/P on mucosal CD4+ T cells during acute and chronic infection to those of ΔGY- and SIVmac251-infected controls. Flow cytometry was performed on serial jejunal biopsy specimens to measure CD4+ T-cell populations over time in lamina propria (Fig. 1C and D). In marked contrast to ΔGY-infected animals, ΔGY+S/P-infected animals exhibited a rapid and profound depletion of mucosal CD4+ T cells by day 21 (7.9% for ΔGY+S/P versus 36.5% for ΔGY; P < 0.05) (Fig. 1C). For the two ΔGY+S/P-infected macaques followed through chronic infection, this difference was sustained for up to 34 weeks, at which time these animals showed 6 and 10% CD4+ T cells, in contrast to a mean of 32.7% for 4 chronic ΔGY-infected animals (Fig. 1D). Depletion of CD4+ T cells was clearly evident in animals infected with ΔGY+S/P compared to naive animals (P < 0.01) and animals infected with ΔGY at a similar time postinfection (P < 0.05) (Fig. 1E); however, this reduction was less than for SIVmac251 (i.e., 3.2% at week 4 and 1.3% at week 36; P < 0.05) (Fig. 1D and E). These findings indicate that the S727P mutation significantly restores, at least in part, the ability of ΔGY to deplete mucosal CD4+ T cells.
To further examine the ability of ΔGY+S/P to infect mucosal CD4+ T cells, we performed SIV RNA in situ hybridization and multilabel confocal microscopy on intestinal tissues, as described previously (18, 19). The intestinal tissues examined included serial jejunal biopsy specimens collected during acute infection from animals inoculated with ΔGY (n = 6), ΔGY+S/P (n = 4), and SIVmac239 (n = 6).
SIV-infected cells were quantified in lamina propria at the peak of mucosal infection for ΔGY+S/P, ΔGY (week 2), and SIVmac239 (week 1) (Fig. 1F). SIVmac239-infected animals showed a high level of infection (mean ± standard error of the mean [SEM], 450 ± 10 cells/mm2) at week 1, which declined to 10 ± 4 cells/mm2 at week 2 due to their rapid depletion (Fig. 1C). While no infected cells were seen for ΔGY+S/P or ΔGY at week 1, by week 2, ΔGY+S/P had infected a significantly greater number of cells than ΔGY (295.5 ± 80.4 versus 57.9 ± 16.8, respectively; P < 0.01), which was similar to SIVmac239-infected animals at week 1 (P > 0.05) (Fig. 1F). Thus, although delayed, the frequency of lamina propria CD4+ T-cell infection was similar for ΔGY+S/P and SIVmac239. This observation appears consistent with the plasma viral RNA for ΔGY+S/P, which showed delayed kinetics (Fig. 1A) but a comparable peak viremia (Fig. 1B). In addition, SIV-infected cells were more diffusely distributed throughout the jejunum in SIVmac239- and ΔGY+S/P-infected animals (Fig. 2A and B) than in ΔGY-infected animals, where infection was sparse and more focally distributed (Fig. 2C) (6).
Fig 2.
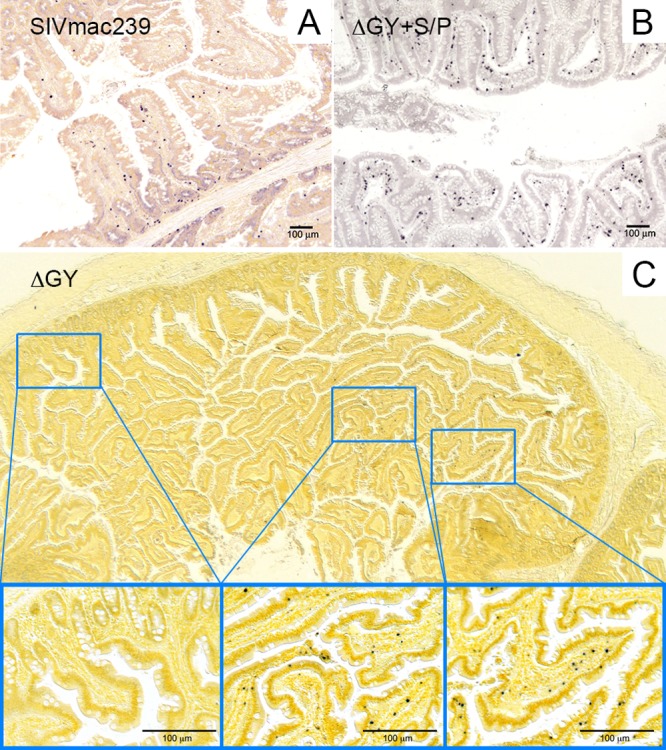
SIV RNA in situ hybridization of jejunal tissues at the peak of mucosal infection. In situ hybridization of SIV RNA is shown for SIVmac239 infection at week 1 (A) and for ΔGY+S/P infection at week 2 (B). Infection for both viruses is diffuse and distributed throughout lamina propria. (C) Jejunum from a ΔGY-infected animal at week 2 with magnified insets demonstrating the sparse multifocal pattern of infection with focal areas of intense infection and large areas of no infection.
We then characterized the immunophenotype of ΔGY+S/P-infected cells in jejunal biopsy specimens using multilabel confocal microscopy, as described previously (18, 19). Similar to ΔGY infection, during the acute infection ΔGY+S/P-infected cells were predominantly CD3+ and CD68−, indicating that they were T cells and not macrophages (data not shown). We then used immunohistochemistry and a specific memory T-cell marker, OPD4 (anti-CD45RO) that was shown to label only CD4+ T cells (20). For both ΔGY and ΔGY+S/P, the majority of infected cells were CD45RO+, indicating that memory CD4+ T cells were the predominant target of infection (Fig. 3). However, as shown in Fig. 3, a strikingly higher density and more diffuse distribution of infected cells were found with ΔGY+S/P than with ΔGY. These findings are consistent with flow cytometry and morphometric quantification of infected cells in the gastrointestinal mucosa (Fig. 1C to F), indicating that acquisition of the S727P mutation resulted in a marked gain of function in the extent of CD4+ T-cell infection and depletion in the intestine by ΔGY (1, 2).
Fig 3.
Analysis of SIV-infected cells in jejunal biopsy specimens from ΔGY- and ΔGY+S/P-infected animals at week 2 postinfection by double-label confocal microscopy for CD45RO/OPD4 (green) and SIV in situ hybridization (red). Differential interference contrast images (DIC) of the tissue are shown in gray. Merged images show that infected cells for both viruses are memory CD4 cells expressing CD45RO, although their frequency is greater for ΔGY+S/P infection. The individual channels are shown on the left.
Given the marked acute depletion of mucosal CD4+ T cells in ΔGY+S/P infection, which was similar to pathogenic SIVmac239 and SIVmac251, the long-term follow-up of these animals to assess disease outcome was of particular interest. One animal (GK58) developed a viral set point above 5 × 105 RNA copies/ml, similar to SIVmac239-infected animals, with declining peripheral CD4+ T cells, increased monocyte turnover, thrombocytopenia, and progression to clinical AIDS within 1 year (see Fig. S1A to C in the supplemental material). However, the other (GM20) developed a viral set point of <1,000 RNA copies/ml, retained normal numbers of CD4+ T cells, and maintained monocyte turnover at preinfection levels (see Fig. S1A to C). The survival of the two ΔGY+S/P-infected rhesus macaques fell between those observed for ΔGY and SIVmac239 (see Fig. S1D) (6). Although the numbers of animals are small, these findings suggest that while the ΔGY+S/P mutation is able to restore, at least in part, the ability of the ΔGY mutant to cause depletion of mucosal CD4+ T cells, it may not be sufficient to restore virulence for all infected animals.
Single genome amplification (SGA) analysis, as described previously (6), was performed to assess the stability of the S727P mutation and possible evolution of the ΔGY+S/P Env. Plasma samples were collected for SGA analysis at weeks 8 and 34 from GK58, although only at week 8 for GM20, due to its low viral set point during chronic infection. The ΔGY and S727P mutations were maintained in all amplicons (see Fig. S1E in the supplemental material). An R751G consensus mutation was also observed in both animals by week 8, as has been reported for SIVmac239 (21) and ΔGY (6) in rhesus macaques, reflecting an apparent optimizing effect of this mutation on SIVmac239-based viruses in vivo (6, 21). For GK58, additional mutations were also acquired by week 34 in the context of a high viral set point and progression to AIDS. Interestingly, one of these changes was a L786Y at the transmembrane C terminus in 7/9 amplicons, which resulted in a new YxxØ consensus sequence (YTLL) (see Fig. S1E). Possible functional effects of this new motif are under investigation.
The S727P mutation was first described in a ΔGY-infected rhesus macaque that developed a high viral load and rapidly progressed to AIDS, in contrast to 2 other ΔGY-infected animals that controlled viral replication (17). Furthermore, recently we described the appearance of the S727P mutation associated with disease progression in 3 chronically ΔGY-infected rhesus macaques (6). Notably, the acquisition of a proline at this position does not create a recognizable trafficking signal. Indeed, preliminary findings have confirmed that neither an endocytosis nor a basolateral sorting signal is conferred by this change (M. Marsh and Scott Lawrence, University College London, personal communication). These results suggest that S727P could be compensating for additional functions of the GYxxØ trafficking signal. Given that ΔGY infection in vivo is remarkable for its selectively attenuated replication in mucosal but not peripheral CD4+ T cells (6) and the results of the present study that S727P partially restores this effect, taken together, these data indicate that there are strong selection pressures in vivo to maintain the ability to target and deplete mucosal CD4+ T cells. Of interest, an S727P mutation was also reported in a pathological variant of the attenuated SIVmac239 derivative, ΔNef, that emerged in vivo (21). This change was not required for an interaction of Env with rhesus tetherin, a function that was gained through more distal mutations in the Env cytoplasmic tail (22). The role of the S727P mutation in pathogenicity of this virus is unknown.
While the ability of SIV (1, 23) and HIV-1 (4, 24) to selectively target and deplete gut CD4+ T cells is well recognized, the mechanism that underlies this effect is unknown. For HIV-1, the binding of gp120 to the integrin alpha-4/beta-7, expressed on gut CD4+ T cells, has been proposed as one explanation (25, 26), although this finding is controversial based on findings for transmitted/founder HIV-1 Env proteins (27). Our findings for SIVmac indicate that the GYxxØ trafficking signal (aa 720 to 723), which is highly conserved in HIV-1 (aa 711 to 714; HXB numbering) and all nonhuman primate lentiviruses, contributes to mucosal CD4+ T-cell infection and depletion during acute infection. Further studies of the role of S727P in reestablishing this function, as well as quantitative studies of cell types infected and viral loads in multiple tissues when this trafficking signal is disrupted, could provide insights as to the underlying mechanism.
In conclusion, we found that a single amino acid mutation (S727P), which developed in ΔGY in vivo in two independent experiments (6, 17), largely restored the ability of this virus to infect and rapidly deplete mucosal CD4+ T cells. In contrast to animals infected with ΔGY, animals infected with ΔGY+S/P had a higher and earlier acute peak of plasma viremia and exhibited a more rapid and sustained depletion of gut mucosal CD4+ T cells than ΔGY. Furthermore, using in situ hybridization, we found greater numbers of ΔGY+S/P-infected cells in intestinal tissues and showed that these cells were more diffusely distributed in the lamina propria than ΔGY. Collectively, these findings (i.e., the attenuated infection and depletion of mucosal CD4+ T cells in ΔGY-infected animals being compensated for by S727P) strongly implicate the importance of a domain in the proximal Env cytoplasmic tail that modulates SIV mucosal tropism and pathogenesis. Further in vivo and in vitro studies of ΔGY and related mutants will help to identify the basis for this effect.
Supplementary Material
ACKNOWLEDGMENTS
We thank Julie Bruhn and Calvin Lanclos for flow cytometry support, Cecily Midkiff and Faith Schiro for technical assistance, Robin Rodriguez for image preparation, Maury Duplantis for tissue collection, and Xiaolei Wang for providing us with technical assistance with the immunohistochemistry staining. We also thank Susan Westmoreland (New England Primate Research Center, Harvard Medical School, Southborough, MA) for providing information on historical time-matched control samples.
This work was supported by National Institutes of Health grants RR000164/P51OD011104 (TNPRC) and RR000168 (NEPRC), RO1 AI074362 (J.A.H.), RO1 AI097059 (M.J.K.), AI045008 (Penn CFAR), and RR021309 (T32) and was supported in part with federal funds from the National Cancer Institute, National Institutes of Health, under contract HHSN261200800001E.
The authors have no financial conflicts of interest.
Footnotes
Published ahead of print 11 September 2013
Supplemental material for this article may be found at http://dx.doi.org/10.1128/JVI.02126-13.
REFERENCES
- 1.Veazey RS, DeMaria M, Chalifoux LV, Shvetz DE, Pauley DR, Knight HL, Rosenzweig M, Johnson RP, Desrosiers RC, Lackner AA. 1998. The gastrointestinal tract as a major site of CD4+ T cell depletion and viral replication in SIV infection. Science 280:427–431 [DOI] [PubMed] [Google Scholar]
- 2.Li Q, Duan L, Estes JD, Ma ZM, Rourke T, Wang Y, Reilly C, Carlis J, Miller CJ, Haase AT. 2005. Peak SIV replication in resting memory CD4+ T cells depletes gut lamina propria CD4+ T cells. Nature 434:1148–1152 [DOI] [PubMed] [Google Scholar]
- 3.Brenchley JM, Price DA, Douek DC. 2006. HIV disease: fallout from a mucosal catastrophe? Nat. Immunol. 7:235–239 [DOI] [PubMed] [Google Scholar]
- 4.Mehandru S, Poles MA, Tenner-Racz K, Horowitz A, Hurley A, Hogan C, Boden D, Racz P, Markowitz M. 2004. Primary HIV-1 infection is associated with preferential depletion of CD4+ T lymphocytes from effector sites in the gastrointestinal tract. J. Exp. Med. 200:761–770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guadalupe M, Reay E, Sankaran S, Prindiville T, Flamm J, McNeil A, Dandekar S. 2003. Severe CD4+ T-cell depletion in gut lymphoid tissue during primary human immunodeficiency virus type 1 infection and substantial delay in restoration following highly active antiretroviral therapy. J. Virol. 77:11708–11717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Breed MW, Jordan AP, Aye PP, Lichtveld CF, Midkiff C, Schiro F, Haggarty BS, Sugimoto C, Alvarez X, Sandler NG, Douek DC, Kuroda MJ, Pahar B, Piatak M, Jr, Lifson JD, Keele BF, Hoxie JA, Lackner AA. 2013. Loss of a tyrosine-dependent trafficking motif in the simian immunodeficiency virus envelope cytoplasmic tail spares mucosal CD4 cells but does not prevent disease progression. J. Virol. 87:1528–1543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brenchley JM, Price DA, Schacker TW, Asher TE, Silvestri G, Rao S, Kazzaz Z, Bornstein E, Lambotte O, Altmann D, Blazar BR, Rodriguez B, Teixeira-Johnson L, Landay A, Martin JN, Hecht FM, Picker LJ, Lederman MM, Deeks SG, Douek DC. 2006. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat. Med. 12:1365–1371 [DOI] [PubMed] [Google Scholar]
- 8.Douek D. 2007. HIV disease progression: immune activation, microbes, and a leaky gut. Top. HIV Med. 15:114–117 [PubMed] [Google Scholar]
- 9.Estes JD, Harris LD, Klatt NR, Tabb B, Pittaluga S, Paiardini M, Barclay GR, Smedley J, Pung R, Oliveira KM, Hirsch VM, Silvestri G, Douek DC, Miller CJ, Haase AT, Lifson J, Brenchley JM. 2010. Damaged intestinal epithelial integrity linked to microbial translocation in pathogenic simian immunodeficiency virus infections. PLoS Pathog. 6:e1001052. 10.1371/journal.ppat.1001052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brenchley JM, Silvestri G, Douek DC. 2010. Nonprogressive and progressive primate immunodeficiency lentivirus infections. Immunity 32:737–742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pandrea I, Gautam R, Ribeiro RM, Brenchley JM, Butler IF, Pattison M, Rasmussen T, Marx PA, Silvestri G, Lackner AA, Perelson AS, Douek DC, Veazey RS, Apetrei C. 2007. Acute loss of intestinal CD4+ T cells in not predictive of simian immunodeficiency virus virulence. J. Immunol. 179:3035–3046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gordon SN, Klatt NR, Bosinger SE, Brenchley JM, Milush JM, Engram JC, Dunham RM, Paiardini M, Klucking S, Danesh A, Strobert EA, Apetrei C, Pandrea IV, Kelvin D, Douek DC, Staprans SI, Sodora DL, Silvestri G. 2007. Severe depletion of mucosal CD4+ T cells in AIDS-free simian immunodeficiency virus-infected sooty mangabeys. J. Immunol. 179:3026–3034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bosinger SE, Sodora DL, Silvestri G. 2011. Generalized immune activation and innate immune responses in simian immunodeficiency virus infection. Curr. Opin. HIV AIDS 6:411–418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Paiardini M, Cervasi B, Reyes-Aviles E, Micci L, Ortiz AM, Chahroudi A, Vinton C, Gordon SN, Bosinger SE, Francella N, Hallberg PL, Cramer E, Schlub T, Chan ML, Riddick NE, Collman RG, Apetrei C, Pandrea I, Else J, Munch J, Kirchhoff F, Davenport MP, Brenchley JM, Silvestri G. 2011. Low levels of SIV infection in sooty mangabey central memory CD T cells are associated with limited CCR5 expression. Nat. Med. 17:830–836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pandrea I, Gaufin T, Brenchley JM, Gautam R, Monjure C, Gautam A, Coleman C, Lackner AA, Ribeiro RM, Douek DC, Apetrei C. 2008. Cutting edge: experimentally-induced immune activation in natural hosts of simian immunodeficiency virus induces significant increases in viral replication and CD4+ T cell depletion. J. Immunol. 181:6687–6691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chahroudi A, Bosinger SE, Vanderford TH, Paiardini M, Silvestri G. 2012. Natural SIV hosts: showing AIDS the door. Science 335:1188–1193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fultz PN, Vance PJ, Endres MJ, Tao B, Dvorin JD, Davis IC, Lifson JD, Montefiori DC, Marsh M, Malim MH, Hoxie JA. 2001. In vivo attenuation of simian immunodeficiency virus by disruption of a tyrosine-dependent sorting signal in the envelope glycoprotein cytoplasmic tail. J. Virol. 75:278–291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vinet-Oliphant H, Alvarez X, Buza E, Borda JT, Mohan M, Aye PP, Tuluc F, Douglas SD, Lackner AA. 2010. Neurokinin-1 receptor (NK1-R) expression in the brains of SIV-infected rhesus macaques: implications for substance P in NK1-R immune cell trafficking into the CNS. Am. J. Pathol. 177:1286–1297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang X, Rasmussen T, Pahar B, Poonia B, Alvarez X, Lackner AA, Veazey RS. 2007. Massive infection and loss of CD4+ T cells occurs in the intestinal tract of neonatal rhesus macaques in acute SIV infection. Blood 109:1174–1181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang X, Pahar B, Rasmussen T, Alvarez X, Dufour J, Rasmussen K, Lackner AA, Veazey RS. 2008. Differential cross-reactivity of monoclonal antibody OPD4 (anti-CD45RO) in macaques. Dev. Comp. Immunol. 32:859–868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alexander L, Illyinskii PO, Lang SM, Means RE, Lifson J, Mansfield K, Desrosiers RC. 2003. Determinants of increased replicative capacity of serially passaged simian immunodeficiency virus with nef deleted in rhesus monkeys. J. Virol. 77:6823–6835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Serra-Moreno R, Jia B, Breed M, Alvarez X, Evans DT. 2011. Compensatory changes in the cytoplasmic tail of gp41 confer resistance to tetherin/BST-2 in a pathogenic nef-deleted SIV. Cell Host Microbe 9:46–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mattapallil JJ, Douek DC, Hill B, Nishimura Y, Martin M, Roederer M. 2005. Massive infection and loss of memory CD4+ T cells in multiple tissues during acute SIV infection. Nature 434:1093–1097 [DOI] [PubMed] [Google Scholar]
- 24.Brenchley JM, Schacker TW, Ruff LE, Price DA, Taylor JH, Beilman GJ, Nguyen PL, Khoruts A, Larson M, Haase AT, Douek DC. 2004. CD4+ T cell depletion during all stages of HIV disease occurs predominantly in the gastrointestinal tract. J. Exp. Med. 200:749–759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cicala C, Martinelli E, McNally JP, Goode DJ, Gopaul R, Hiatt J, Jelicic K, Kottilil S, Macleod K, O'Shea A, Patel N, Van Ryk D, Wei D, Pascuccio M, Yi L, McKinnon L, Izulla P, Kimani J, Kaul R, Fauci AS, Arthos J. 2009. The integrin alpha4beta7 forms a complex with cell-surface CD4 and defines a T-cell subset that is highly susceptible to infection by HIV-1. Proc. Natl. Acad. Sci. U. S. A. 106:20877–20882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nawaz F, Cicala C, Van Ryk D, Block KE, Jelicic K, McNally JP, Ogundare O, Pascuccio M, Patel N, Wei D, Fauci AS, Arthos J. 2011. The genotype of early-transmitting HIV gp120s promotes α4β7-reactivity, revealing α4β7+/CD4+ T cells as key targets in mucosal transmission. PLoS Pathog. 7:e1001301. 10.1371/journal.ppat.1001301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Parrish NF, Wilen CB, Banks LB, Iyer SS, Pfaff JM, Salazar-Gonzalez JF, Salazar MG, Decker JM, Parrish EH, Berg A, Hopper J, Hora B, Kumar A, Mahlokozera T, Yuan S, Coleman C, Vermeulen M, Ding H, Ochsenbauer C, Tilton JC, Permar SR, Kappes JC, Betts MR, Busch MP, Gao F, Montefiori D, Haynes BF, Shaw GM, Hahn BH, Doms RW. 2012. Transmitted/founder and chronic subtype C HIV-1 use CD4 and CCR5 receptors with equal efficiency and are not inhibited by blocking the integrin alpha4beta7. PLoS Pathog. 8:e1002686. 10.1371/journal.ppat.1002686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.National Research Council 2011. Guide for the care and use of laboratory animals, 8th ed. National Academies Press, Washington, DC: http://www.nap.edu/catalog.php?record_id=12910 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


