Abstract
Visible light irradiation of fluoresceinated concanavalin A (f-Con A) bound to the outside of resealed erythrocyte membranes caused crosslinking of as much as 50% of the membrane proteins. Crosslinking was absent in controls in which equivalent amounts of f-Con A were added to the membranes but prevented from binding by the presence of 10 mM alpha-methylmannoside. The photodamage was not accompanied by a change in the membrane permeability barrier or membrane shape. Although fluorescein bleaching accompanies the formation of protein aggregates, the amount of aggregated protein is not simply a function of the number of fluoresceins bleached. The percentage of aggregated protein decreases when the same dose of light is given in a shorter time. Although certain antioxidants and free-radical scavengers had no detected effect on the crosslinking, reducing agents such as cysteamine and reduced glutathione either blocked or reversed the protein crosslinking. The mechanism of photoinduced oxidation and the implications of these results for fluorescence studies of cell membranes are discussed.
Full text
PDF
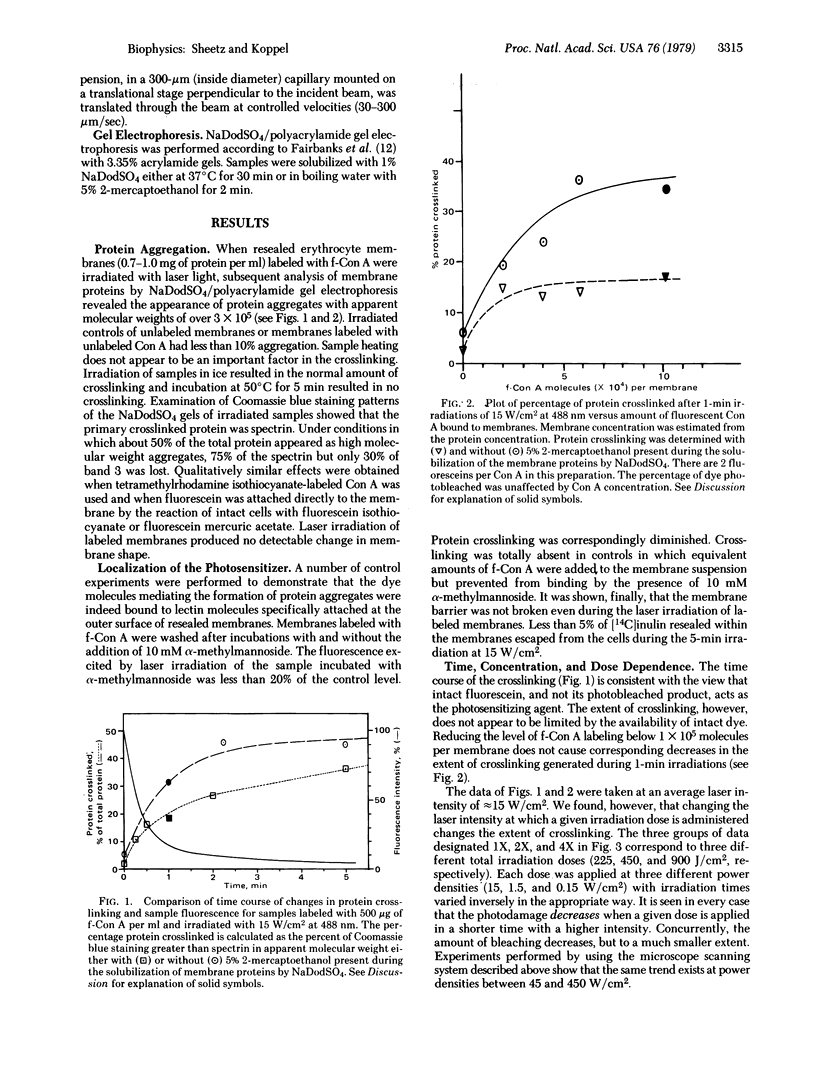
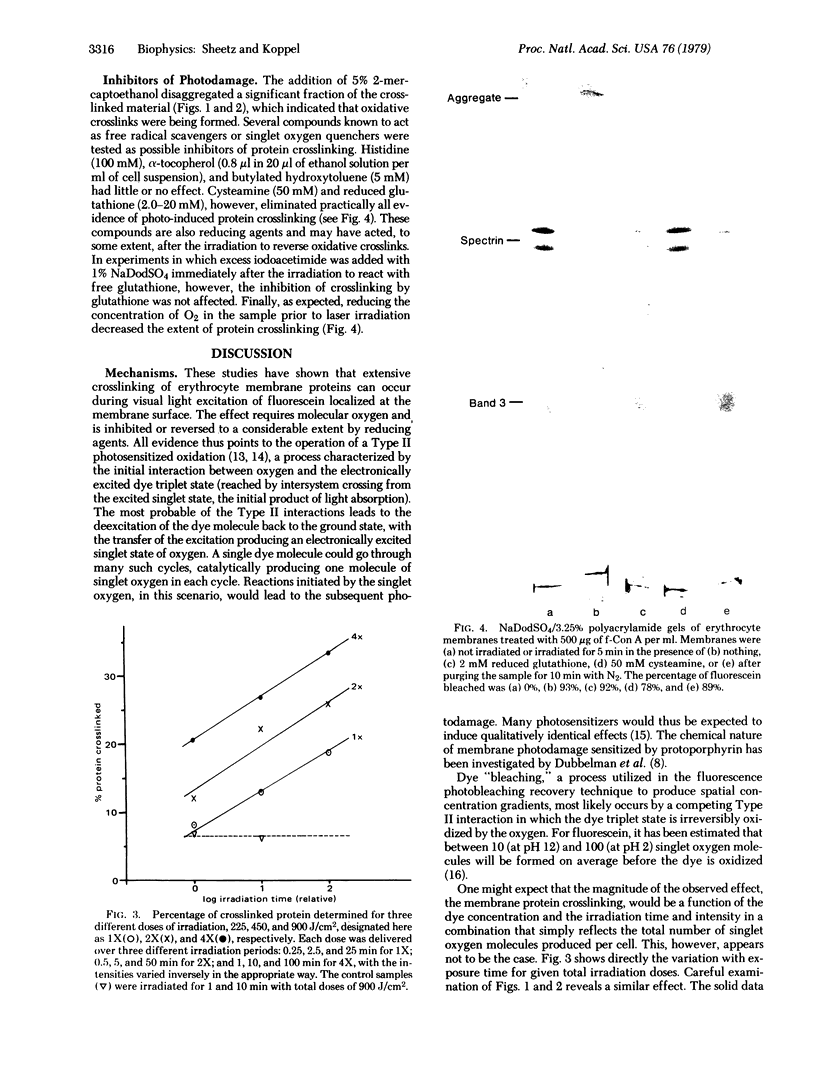

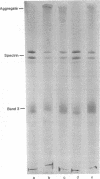
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- De Goeij A. F., Ververgaert P. H., Steveninck J. V. Photodynamic effects of protoporphyrin on the architecture of erythrocyte membranes in protoporphyria and in normal red blood cells. Clin Chim Acta. 1975 Jul 23;62(2):287–292. doi: 10.1016/0009-8981(75)90238-7. [DOI] [PubMed] [Google Scholar]
- Dubbelman T. M., de Goeij A. F., van Steveninck J. Photodynamic effects of protoporphyrin on human erythrocytes. Nature of the cross-linking of membrane proteins. Biochim Biophys Acta. 1978 Aug 4;511(2):141–151. doi: 10.1016/0005-2736(78)90309-7. [DOI] [PubMed] [Google Scholar]
- Edidin M., Zagyansky Y., Lardner T. J. Measurement of membrane protein lateral diffusion in single cells. Science. 1976 Feb 6;191(4226):466–468. doi: 10.1126/science.1246629. [DOI] [PubMed] [Google Scholar]
- Fahey P. F., Koppel D. E., Barak L. S., Wolf D. E., Elson E. L., Webb W. W. Lateral diffusion in planar lipid bilayers. Science. 1977 Jan 21;195(4275):305–306. doi: 10.1126/science.831279. [DOI] [PubMed] [Google Scholar]
- Fairbanks G., Steck T. L., Wallach D. F. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry. 1971 Jun 22;10(13):2606–2617. doi: 10.1021/bi00789a030. [DOI] [PubMed] [Google Scholar]
- Foote C. S. Mechanisms of photosensitized oxidation. There are several different types of photosensitized oxidation which may be important in biological systems. Science. 1968 Nov 29;162(3857):963–970. doi: 10.1126/science.162.3857.963. [DOI] [PubMed] [Google Scholar]
- Jacobson K., Hou Y., Wojcieszyn J. Evidence for lack of damage during photobleaching measurements of the lateral mobility of cell surface components. Exp Cell Res. 1978 Oct 1;116(1):179–189. doi: 10.1016/0014-4827(78)90074-5. [DOI] [PubMed] [Google Scholar]
- Jacobson K., Wu E., Poste G. Measurement of the translational mobility of concanavalin A in glycerol-saline solutions and on the cell surface by fluorescence recovery after photobleaching. Biochim Biophys Acta. 1976 Apr 16;433(1):215–222. doi: 10.1016/0005-2736(76)90189-9. [DOI] [PubMed] [Google Scholar]
- Koppel D. E., Axelrod D., Schlessinger J., Elson E. L., Webb W. W. Dynamics of fluorescence marker concentration as a probe of mobility. Biophys J. 1976 Nov;16(11):1315–1329. doi: 10.1016/S0006-3495(76)85776-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepock J. R., Thompson J. E., Kruuv J. Photoinduced crosslinking of membrane proteins by fluorescein isothiocyanate. Biochem Biophys Res Commun. 1978 Nov 14;85(1):344–350. doi: 10.1016/s0006-291x(78)80048-5. [DOI] [PubMed] [Google Scholar]
- Magde D., Elson E. L., Webb W. W. Fluorescence correlation spectroscopy. II. An experimental realization. Biopolymers. 1974 Jan;13(1):29–61. doi: 10.1002/bip.1974.360130103. [DOI] [PubMed] [Google Scholar]
- Matheson I. B., Etheridge R. D., Kratowich N. R., Lee J. The quenching of singlet oxygen by amino acids and proteins. Photochem Photobiol. 1975 Mar;21(3):165–171. doi: 10.1111/j.1751-1097.1975.tb06647.x. [DOI] [PubMed] [Google Scholar]
- Nigg E., Kessler M., Cherry R. J. Labeling of human erythrocyte membranes with eosin probes used for protein diffusion measurements: inhibition of anion transport and photo-oxidative inactivation of acetylcholinesterase. Biochim Biophys Acta. 1979 Jan 19;550(2):328–340. doi: 10.1016/0005-2736(79)90219-0. [DOI] [PubMed] [Google Scholar]
- Schlessinger J., Koppel D. E., Axelrod D., Jacobson K., Webb W. W., Elson E. L. Lateral transport on cell membranes: mobility of concanavalin A receptors on myoblasts. Proc Natl Acad Sci U S A. 1976 Jul;73(7):2409–2413. doi: 10.1073/pnas.73.7.2409. [DOI] [PMC free article] [PubMed] [Google Scholar]