Abstract
Andes virus (ANDV) is a South American hantavirus that causes a highly lethal hantavirus pulmonary syndrome (HPS) characterized by hypoxia, thrombocytopenia, and vascular leakage leading to acute pulmonary edema. ANDV infects human pulmonary microvascular and lymphatic endothelial cells (MECs and LECs, respectively) and nonlytically enhances the permeability of interendothelial cell adherence junctions in response to vascular endothelial growth factor (VEGF). Recent findings also indicate that ANDV causes the formation of giant endothelial cells. Here, we demonstrate that hypoxic conditions alone enhance permeability and giant cell responses of ANDV-infected MECs and LECs through activation of the mTOR signaling pathway. In contrast to infection of cells with nonpathogenic Tula virus (TULV), we observed that exposure of ANDV-infected MECs and LECs to hypoxic conditions resulted in a 3- to 6-fold increase in monolayer permeability and the formation of giant cells 3× to 5× normal size. ANDV infection in combination with hypoxic conditions resulted in the enhancement of hypoxia-inducible factor 1α (HIF1α)-directed VEGF A, angiopoietin 4, and EGLN3 transcriptional responses. Constitutive mTOR signaling induces the formation of giant cells via phosphorylation of S6K, and mTOR regulates hypoxia and VEGF A-induced cellular responses. We found that S6K was hyperphosphorylated in ANDV-infected, hypoxia-treated MECs and LECs and that rapamycin treatment for 1 h inhibited mTOR signaling responses and blocked permeability and giant cell formation in ANDV-infected monolayers. These findings indicate that ANDV infection and hypoxic conditions enhance mTOR signaling responses, resulting in enhanced endothelial cell permeability and suggest a role for rapamycin in therapeutically stabilizing the endothelium of microvascular and lymphatic vessels during ANDV infection.
INTRODUCTION
Hantaviruses predominantly infect endothelial cells (ECs) and nonlytically cause diseases associated with increased vascular permeability (1–5, 112). Hantavirus pulmonary syndrome (HPS) results from the infection of hantaviruses present in North and South America, including Andes virus (ANDV), Sin Nombre virus (SNV), NY-1 virus (NY-1V), and many others (3, 6–9). HPS is characterized by thrombocytopenia, hypoxia, and acute pulmonary edema that leads to respiratory insufficiency and an associated 35 to 40% mortality rate (1, 2, 5, 10–13). ANDV is unique in both its ability to spread from person to person and cause a lethal HPS-like disease in Syrian hamsters that closely mimics human disease in rapid onset, hypoxia, and acute pulmonary edema (9, 14–17).
Extracorporeal membrane oxygenation (ECMO) of patients has dramatically reduced HPS mortality and suggests a potential role of hypoxia in HPS disease (11, 13). Hypoxia induces high-altitude pulmonary edema (HAPE) through the induction of vascular endothelial growth factor (VEGF) (18–27), and this response is transcriptionally directed by the hypoxia-inducible factor 1α (HIF1α) (26, 28, 29). Hypoxic conditions stabilize HIF-1α, which forms a transcriptionally active HIF1α/β heterodimer and directs transcription from hypoxia-responsive promoter elements (HREs) (24, 26). Hypoxia induces VEGF (9-fold), and VEGF in turn activates VEGF receptor 2 (VEGFR2) receptors on endothelial cells in an autocrine and paracrine manner (30–33). VEGFR2 signaling induces vascular permeability by directing the disassembly of interendothelial cell adherence junctions (30, 34, 35). Interestingly, VEGF further induces transcription of HIF1α, forming an HIF1α-VEGF amplification loop that enhances EC responses to hypoxia and further increases capillary permeability (26, 28, 36).
Adherens junctions (AJs) form a fluid barrier within capillaries that regulates permeability of the vascular endothelium (37). However, to permit immune cell extravasation and endothelial cell migration required for vascular repair, cells must separate without causing fluid leakage. These opposing functions are tightly regulated by redundant systems that act on unique endothelial cell receptors, junctional proteins, and signaling pathway effectors (35, 37, 38).
HPS patients are acutely hypoxic, and ANDV-infected pulmonary endothelial cells provide a means for increasing capillary permeability and causing pulmonary edema (1, 2, 5, 10–12, 39). In fact, the pulmonary edema fluid of HPS patients contains high levels of VEGF (40, 41). VEGF was originally named “vascular permeability factor” for its potent ability to induce tissue edema ∼50,000 times more effectively than histamine (38, 42). Secreted VEGF binds to receptors within 1.5 mm of its release and acts locally to induce endothelial cell division, migration, and permeability (30, 34, 37, 38). VEGFR2 is a growth factor receptor containing a C-terminal tyrosine kinase that directs the activation of several signaling pathways (24, 30, 35). Activation of the VEGFR2-Src pathway directs the disassembly of VE-cadherin from AJs and increases paracellular permeability of the endothelium, which results in edema (30). However, VEGFR2 also activates growth-inducing responses directed by mTOR (mammalian target of rapamycin), and both VEGFR2 and hypoxia-directed responses are sensitive to the inhibitory effects of rapamycin (31, 43–46).
In vitro, pulmonary microvascular endothelial cells (MECs) and human umbilical vein endothelial cells (HUVECs) are not permeabilized by hantavirus infection alone; however, the permeability of endothelial cells infected by ANDV, SNV, and NY-1V, but not nonpathogenic Tula virus (TULV) or PHV hantaviruses, is dramatically enhanced in response to VEGF (41, 47–52). Days after infection, cell-associated pathogenic hantaviruses bind inactive αvβ3 integrins which normally temper VEGFR2 responses (48, 50, 53–55). This results in both hyperphosphorylation of VEGFR2 and increases in the VEGFR2 signaling responses that direct the dissociation and internalization of VE-cadherin from adherens junctions without VE-cadherin degradation (41, 47, 48, 50–52). The internalization of VE-cadherin without degradation permits the rapid reassembly of adherens junctions and participates in the rapid ability of the endothelium to increase fluid barrier functions (34, 37). Consistent with hantavirus inactivation of αvβ3, the knocking out of β3 or inhibition of αvβ3 promotes VEGFR2-directed endothelial cell permeability (56–60). A recent report further demonstrated that ANDV infects lymphatic endothelial cells (LECs) and both enhances LEC permeability and causes the formation of giant LECs in response to VEGF (41, 47). These findings indicate that both microvascular leakage and pulmonary fluid clearance by lymphatic vessels are likely to be altered by ANDV infection of MECs and LECs.
These findings suggest that ANDV-induced edema is at least in part regulated by the activation of edemagenic VEGF signaling pathways within MECs and LECs that contribute to increased vascular permeability and edema during HPS. In this study, we investigate the role of hypoxia in directing permeability and giant cell responses of ANDV-infected MECs and LECs. Our findings demonstrate that hypoxic conditions are sufficient to induce permeability and giant cell responses in ANDV-infected MEC and LEC monolayers. ANDV infection of MECs and LECs enhanced the production of HIF1α-regulated genes and activated mTOR-directed S6 kinase (S6K) hyperphosphorylation, which controls cell size (46, 61, 62). We found that permeability, giant cell, and S6K phosphorylation responses were rapamycin sensitive (31, 62). These findings demonstrate the involvement of pathway-specific mTOR-S6K activation in the permeability and giant cell responses elicited by ANDV infection of MECs and LECs and suggest a potential role for rapamycin in regulating hypoxia-directed permeability responses following ANDV infection.
MATERIALS AND METHODS
Cells and virus.
Vero E6 cells (ATCC CRL-1586) were grown in Dulbecco's modified Eagle's medium (DMEM) containing 10% fetal calf serum (FCS; Sigma), penicillin (100 μg/ml), streptomycin sulfate (100 μg/ml), and amphotericin B (50 μg/ml) (Gibco). Human pulmonary microvascular endothelial cells (MECs) and human pulmonary lymphatic endothelial cells (LECs) were purchased from Cambrex, Inc., and grown in endothelial growth medium (EGM-2MV; Lonza) supplemented with endothelial cell growth factors, gentamicin (50 μg/ml), amphotericin B (50 μg/ml), and 10% FCS (Sigma). Starvation medium consists of basic growth medium (EBM-2MV) with 0.5% bovine serum albumin (BSA) but without added VEGF. Andes virus (CHI-7913) and nonpathogenic TULV (Tula/Moravia/MA 5302V/94) were determined to be mycoplasma free (Roche) and were cultivated as described previously in a biosafety level 3 (BSL3) facility (49). Viral titers were determined as previously described (53, 54). LEC and MEC monolayers were ANDV, TULV, or mock infected at a multiplicity of infection (MOI) of 0.5, and >90% of cells were infected with ANDV as determined by focus assay and immunoperoxidase staining of viral nucleocapsid proteins.
Hypoxic conditions.
MECs or LECs were infected with pathogenic ANDV or nonpathogenic TULV at an MOI of 0.5. Two days postinfection, cells were incubated overnight (18 h) in growth factor starvation medium under hypoxic conditions (1% O2 by N2 displacement, 5% CO2) in a multigas incubator (MCO-19 M; Sanyo Scientific) or under normoxic conditions (20% O2, 5% CO2). Cobalt chloride-induced hypoxia (31, 63) was used in indicated experiments by exposing cells to CoCl2 (100 μM; Sigma) in basal EBM-2MV with 0.5% bovine serum albumin for 6 h.
Immunoperoxidase staining of hantavirus-infected cells.
Rabbit polyclonal antinucleocapsid serum directed against the NY-1V nucleocapsid protein was used to detect ANDV- and TULV-infected cells, as previously described (54). Briefly, infected endothelial cell monolayers were fixed with 100% methanol and incubated with antinucleocapsid serum (1:5,000) followed by horseradish peroxidase (HRP)-conjugated goat anti-rabbit secondary antibody (1:5,000; Amersham Biosciences). Nucleocapsid protein-expressing cells were identified by staining with 3-amino-9-ethylcarbazole (0.026%) in 0.1 M sodium acetate (pH 5.2) and 0.03% H2O2, and infected foci were quantitated by microscopy (54). Cells more than 3 times normal MEC or LEC size were considered to be giant cells and quantitated by microscopy (10 fields, 1,500 cells in duplicate wells) using NIH Image.
Endothelial cell permeability assay.
A previously described permeability assay was used to assess hantavirus-induced endothelial cell permeability (48–50). Briefly, human MECs or LECs were plated on Costar Transwell plates (3-μm pores; Corning), and confluent monolayers were infected in triplicate with pathogenic ANDV or nonpathogenic TULV at an MOI of 0.5 for 2 days prior to hypoxic or normoxic treatment. Three days postinfection, fluorescein isothiocyanate (FITC)-dextran (40 kDa, 0.5 mg/ml; Sigma) was added to the upper chamber, and the lower chamber was monitored for the presence of FITC-dextran 2 h later using a BioTek FLx800 fluorimeter (490-nm excitation, 530-nm emission) (48–50). The fold change in FITC-dextran fluorescence intensity over mock-treated controls was used as a measure of MEC and LEC monolayer paracellular permeability. Where indicated, 2 days postinfection, cells were grown overnight in growth factor starvation medium and subsequently VEGF A (100 ng/ml) stimulated 1 h prior to the addition of FITC-dextran. Rapamycin (20 ng/ml) was added 1 h prior to VEGF A addition under normoxic conditions or 1 h prior to FITC-dextran addition (hypoxic or normoxic conditions). Monolayers were equivalently infected (∼80%), as assayed by immunoperoxidase staining of MEC or LEC monolayers (54). Analysis of virus present in the supernatants of ANDV-infected ECs 3 days postinfection that were rapamycin treated (1 to 24 h) or untreated revealed no difference in viral titers (4.5 × 104/ml).
qRT-PCR.
Total cellular RNA was extracted from mock-, ANDV- or TULV-infected MECs and LECs using RNeasy (Qiagen) (64, 65). Total RNA (1 μg) was reverse transcribed using Transcriptor first strand cDNA synthesis (Roche) and oligo(dT)18 primers (25°C for 10 min, 55°C for 30 min, and 85°C for 5 min). The primers for quantitative reverse transcription-PCR (qRT-PCR) (Table 1) were operon designed and used to amplify human glyceraldehyde-3-phosphate dehydrogenase (GAPDH), VEGF A, angiopoietin 4 (ANG4), and EGLN3 in duplicate using Sybr green on an Applied Biosystems 7300 under the following thermocycling parameters: 50°C for 2 min, 95°C for 10 min, 95°C for 15 s, and 60°C for 1 min for 40 cycles (65). mRNA levels were normalized to GAPDH mRNA levels, and the fold changes in mRNA levels were compared between ANDV- and mock-infected cells under hypoxic and normoxic conditions (64, 65). The fold change in VEGF A, ANG4, and EGLN3 mRNA levels was determined using the threshold cycle (2−ΔCT) method and plotted (±standard error of the mean [SEM]) using GraphPad Prism 5 software (65).
Table 1.
Real-time PCR primers used in this study
| Gene product | Name | Primer sequences | GenBank accession no. |
|---|---|---|---|
| Vascular endothelial growth factor A | VEGF A | 5′-TGCAGATTATGCGGATCAAACC-3′ | AF022375 |
| 5′-TGCATTCACATTTGTTGTGCTGTAG-3′ | |||
| Angiopoietin-like 4 | ANGLT4 | 5′-AGACACAACTCAAGGCTCAG-3′ | NM_139314 |
| 5′-CTCATGGTCTAGGTGCTTGTG-3′ | |||
| egl nine homolog 3 (Caenorhabditis elegans) | EGLN3 | 5′-ATTCATAGCAGATGTGGAGCC-3′ | NM_022073 |
| 5′-TCAGCATCAAAGTACCAGACAG-3′ | |||
| Glyceraldehyde-3-phosphate dehydrogenase | GAPDH | 5′-GGAAGCTCACTGGCATGGC-3′ | NM_001256799 |
| 5′-TAGACGGCAGGTCAGGTCCA-3′ |
Western blot.
Western blots were performed as previously described (6, 66). Briefly, MECs or LECs were infected with ANDV or TULV and grown under normoxic or hypoxic conditions as described above. Cells were lysed in buffer containing 1% NP-40 (150 mM NaCl, 40 mM Tris-Cl, 2 mM EDTA, 10 nM sodium fluoride, 2.5 mM sodium pyrophosphate, 2 mM sodium orthovanadate, 10 mM β-glycerophosphate) with protease inhibitor cocktail (Sigma). Total protein levels were determined by a bicinchoninic acid assay (Pierce), and 20 μg of protein was resolved by SDS-polyacrylamide (10%) gel electrophoresis. Proteins were transferred to nitrocellulose, blocked in 2% bovine serum albumin, and incubated with anti-p70S6 rabbit polyclonal antibody (Cell Signaling), anti β-tubulin monoclonal antibody (Santa Cruz Biotechnology), and anti-nucleocapsid protein rabbit serum (as described above) and detected using HRP-conjugated anti-mouse and anti-rabbit secondary antibodies and ECL enhanced chemiluminescence analysis (Amersham).
Statistical analysis.
Results were derived from two to five independent experiments and are presented as means ± standard errors of the means (SEM), with P values of <0.01 and <0.001 considered significant. Multiple group comparisons were made by one-way analysis of variance (ANOVA). Two-way comparisons were performed by two-tailed, unpaired Student's t test. All analyses were performed using GraphPad Prism software version 5.0.
RESULTS
Hypoxia and ANDV infection synergistically enhance MEC and LEC permeability.
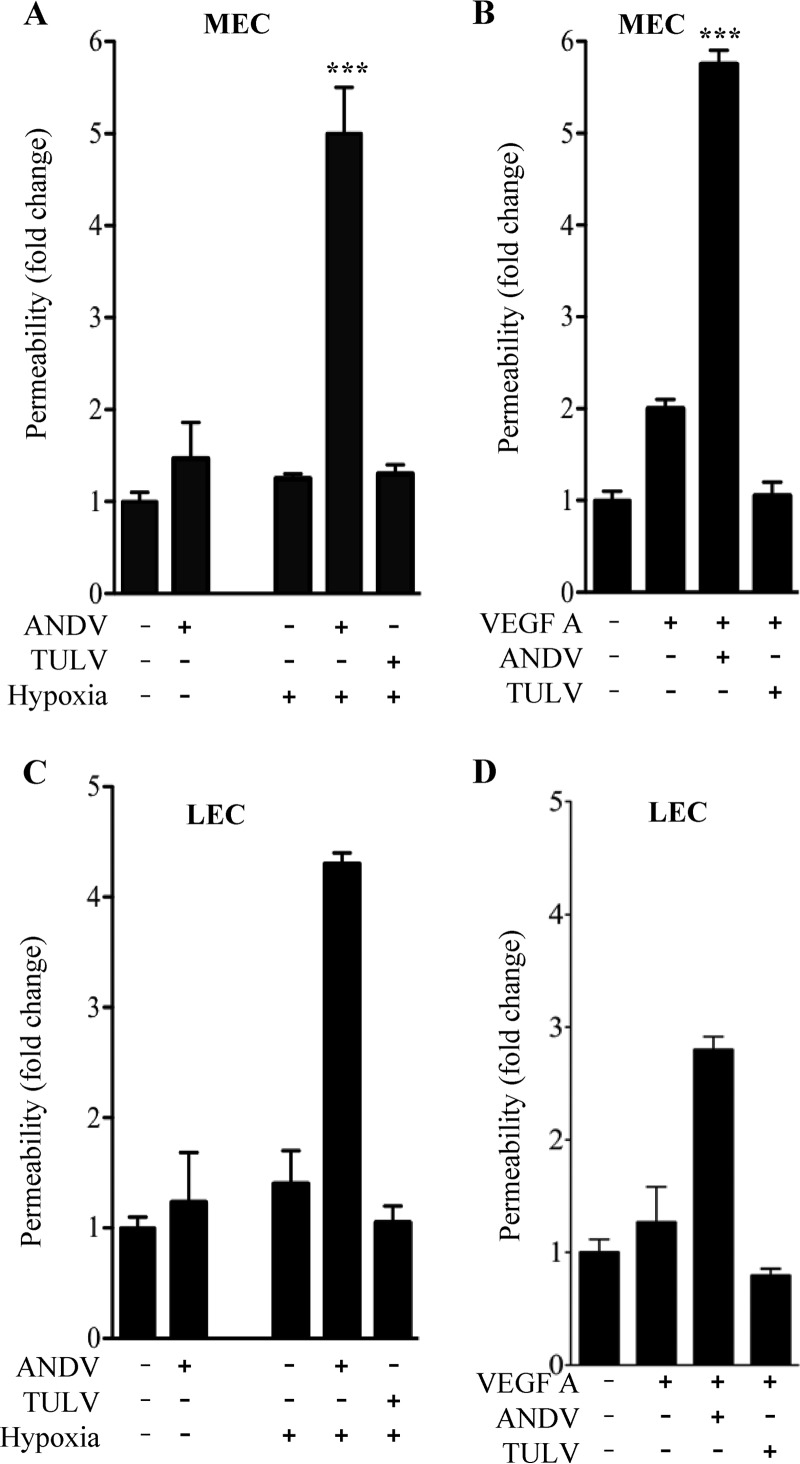
ANDV infects the endothelial cell lining of capillaries and results in patient hypoxia and acute pulmonary edema leading to respiratory distress (1, 2, 5–7, 9, 10, 12). Hypoxia causes high-altitude-induced pulmonary edema by inducing the permeabilizing effector VEGF, and thus hypoxia may also be a potential edemagenic factor during ANDV infection of human endothelial cells (18, 20, 26, 38, 42). To address this, we evaluated responses of human pulmonary MECs and LECs grown under normoxic and hypoxic conditions following infection by ANDV (HPS) and TULV (nonpathogenic). Two days postinfection, ECs were kept under normoxic conditions or incubated under hypoxic conditions for 18 h and deprived of VEGF overnight. The permeability of normoxic and hypoxic MEC and LEC monolayers was assayed by adding FITC-dextran to the upper chamber and monitoring levels in the lower chamber of confluent EC monolayers. As previously reported (47, 50–52), we observed little change in the permeability of ANDV-infected MECs or LECs alone but observed a dramatic increase in the permeability of hypoxia-treated ANDV-infected MECs and LECs (Fig. 1A and C). In contrast, neither TULV infection nor hypoxia treatment of TULV-infected cells resulted in an increase in MEC or LEC permeability (Fig. 1A and C). In comparison with hypoxia-induced MEC and LEC permeability, we analyzed the effect of VEGF addition to MECs and LECs and found that VEGF similarly induced permeability of ANDV- but not TULV-infected MECs and LECs (Fig. 1B and D). These findings indicate that similar to VEGF, hypoxia uniquely directs the hyperpermeability of ANDV-infected pulmonary MECs and LECs.
Fig 1.
Hypoxia induces hyperpermeability of ANDV-Infected ECs. ECs were grown in CO2 (5%) incubators that regulate O2 levels by nitrogen displacement, resulting in normoxic (20% O2) or hypoxic (1% O2) conditions for cell growth. MECs (A and B) and LECs (C and D) were plated on vitronectin-coated Transwell inserts and grown under normoxic conditions (47, 49–52). Confluent EC monolayers were mock infected or infected with ANDV or TULV at an MOI of 0.5. Two days postinfection, ECs were growth factor starved overnight and incubated under normoxic conditions (control) or hypoxic conditions for 18 h (A and C). In parallel experiments, MECs and LECs (B and D) were stimulated with VEGF under normoxic conditions. Changes in monolayer permeability were assessed by adding FITC-dextran (40 kDa) to the upper chamber and monitoring its presence in lower chambers (A, B, C, and D) (47, 49–52). The data represent results from four independent experiments. Results are expressed as the fold increase in monolayer permeability over normoxic controls (**, P < 0.01; ***, P < 0.001).
ANDV infection enhances hypoxia-directed HIF1α transcriptional responses.
Hypoxia reportedly stabilizes the formation of HIF1α transcriptional complexes that transcriptionally induce VEGF and additional hypoxia-responsive factors and stress regulators (26, 67, 68). We evaluated the role of ANDV infection on selected hypoxia-induced factors (VEGF A, angiopoietin 4, and EGLN3) (28) using qRT-PCR. We infected MECs and LECs with ANDV and 2 days postinfection subjected the cells to hypoxic or normoxic conditions for 18 h. MEC and LEC RNA levels were evaluated and compared to the levels of GAPDH mRNAs that were not hypoxia regulated. Consistent with hypoxia literature (28), we found that hypoxic conditions alone directed an increase in VEGF A, ANG4, and EGLN3 mRNAs within cells (Fig. 2). However, we observed that ANDV infection dramatically increased VEGF A, ANG4, and EGLN3 mRNA levels within hypoxic MECs and LECs. These findings demonstrate that hypoxia and HIF1α enhanced responses of ANDV-infected MECs and LECs that result in increased expression of VEGF A and are consistent with the increased permeability of ANDV-infected endothelial cells in response to hypoxic conditions.
Fig 2.
ANDV enhances induction of hypoxia-induced genes. MECs and LECs were ANDV or mock infected and exposed to normoxic or hypoxic conditions for 18 h as in Fig. 1 (47, 49–52). Total cellular RNA was extracted, and human VEGF A, angiopoietin 4 (ANG4), and EGLN3 transcript levels were determined by qRT-PCR of duplicate samples (Table 1) (64, 65). mRNA levels were normalized to GAPDH mRNA levels, and the fold increase in mRNA levels over mock-infected controls was calculated. The means and standard errors from two independent experiments are shown.
Hypoxia and ANDV infection enhance the formation of giant MECs and LECs.
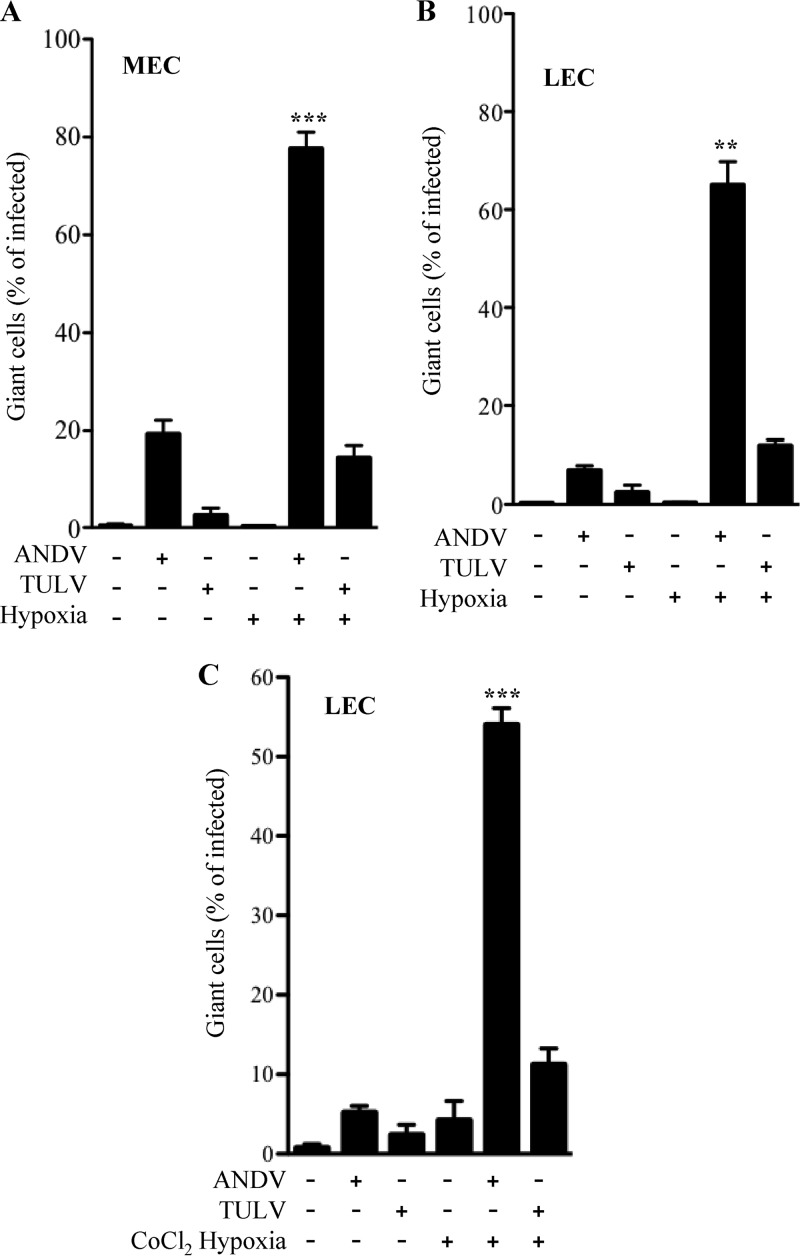
The ability of ANDV infection to cause the formation of giant LECs in response to VEGF was previously reported and suggested to be regulated by rapamycin-sensitive mTOR signaling responses (41). Combined with the results presented above, these findings suggested that hypoxic conditions might be sufficient to increase the size of ANDV-infected MECs and LECs. We infected MECs and LECs with ANDV or TULV as in Fig. 1 and assayed the formation of giant cells in infected monolayers under normoxic and hypoxic conditions. We found that ANDV infection of pulmonary MECs resulted in nearly 20% giant MECs under normoxic conditions (Fig. 3A), while ∼8% of ANDV-infected LECs were giant cells (Fig. 3B). In contrast 2 to 3% of TULV-infected MECs or LECs had increased diameters (Fig. 3A and B). However, following 18 h of hypoxia, there was a dramatic increase in the number of ANDV-infected giant cells, which encompassed 80% or 70% of MECs or LECs, respectively. The number of TULV-infected giant cells also increased to 10 to 15% following hypoxia, although there was no increase in the number of uninfected giant cells under normoxic or hypoxic conditions. We similarly induced hypoxia using CoCl2 (63) and found a similar enhancement of giant LEC responses following ANDV infection (∼55% giant cells) (Fig. 3C). These findings indicate that ANDV infection combined with hypoxic conditions has a dramatic effect on MEC and LEC cell size and monolayer permeability.
Fig 3.
ANDV and hypoxia induce giant cell responses. MECs (A) and LECs (B and C) were infected with ANDV or TULV or mock infected under normoxic or hypoxic conditions as in Fig. 1 (47, 49–52). LECs were infected as described above and 3 days postinfection treated with CoCl2 (100 mM) for 6 h to induce hypoxia under normoxic incubator conditions (C). Cells were methanol fixed, immunoperoxidase stained for N protein, and visualized by light microscopy. Hantavirus-infected giant cells (∼3× normal size) were measured and quantified using NIH Image and expressed as a percentage of total infected cells (47, 49–52). The data represent results from six independent experiments (**, P < 0.01, and ***, P < 0.001, versus ANDV- or TULV-infected levels).
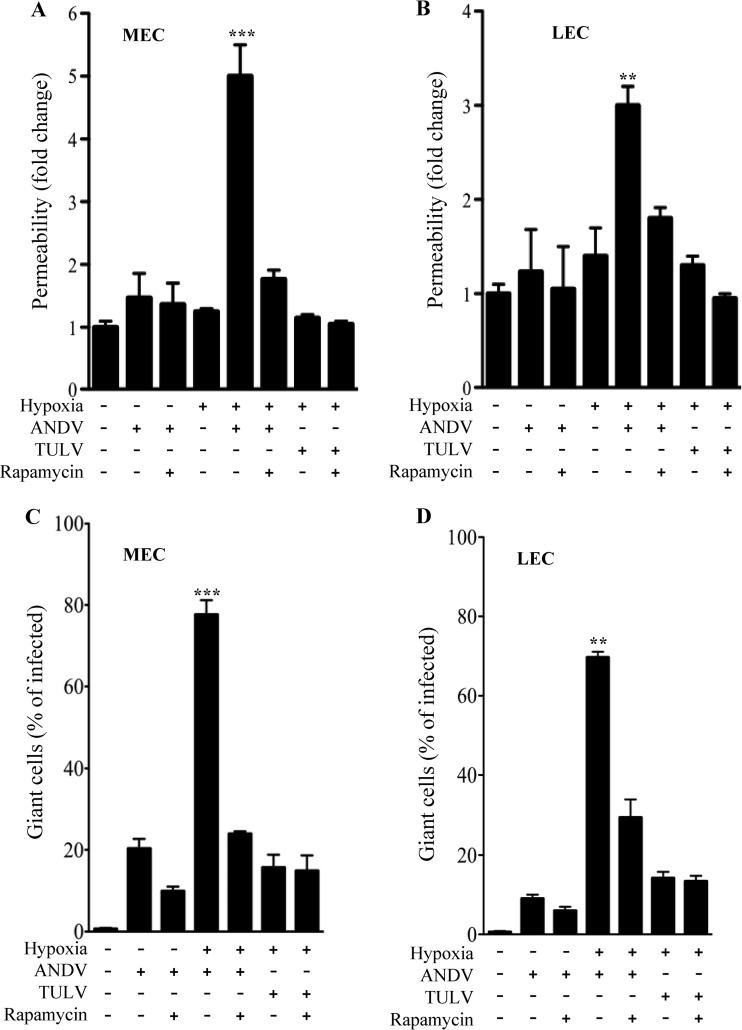
Rapamycin inhibits permeability and giant cell responses.
Constitutive mTOR activation results in the formation of giant cells, and mTOR signaling responses control HIF1α- and VEGF-directed permeability (26, 31, 45, 61, 67, 69). Rapamycin binding to FKBP12 regulates cell size and hypoxia-induced cellular responses by blocking mTOR signaling directed by TORC1 complexes (62). Here we determined whether permeability and giant cell responses induced by ANDV infection and hypoxia are mTOR dependent and blocked by rapamycin. Two days postinfection with ANDV and TULV, MECs and LECs were grown under normoxic or hypoxic conditions for 18 h and treated with rapamycin for 1 h prior to evaluation of permeability or giant cell responses. Analysis of virus present in the supernatants of rapamycin-treated or untreated ECs revealed no change in viral titers resulting from 1 to 24 h of rapamycin treatment. Figure 4 indicates that both ANDV-induced permeability and giant cell formation in response to hypoxia are dramatically inhibited by rapamycin. These findings indicate that synergistic effects of hypoxia and ANDV infection are directed by mTOR activation and inhibited by rapamycin.
Fig 4.
Hypoxia- and ANDV-induced permeability and giant cell responses are mTOR dependent. MECs (A and C) or LECs (B and D) were infected with ANDV or TULV or mock infected as described in the legend to Fig. 1 (47, 49–52). Two days postinfection, MECs and LECs were grown under normoxic or hypoxic conditions for 18 h and treated with rapamycin (20 ng/ml) for 1 h prior to evaluation of permeability (A and B) or giant cell responses (C and D) as in Fig. 2 and 3. The data represent results of four independent experiments (**, P < 0.01, and ***, P < 0.001, versus ANDV- or TULV-infected levels).
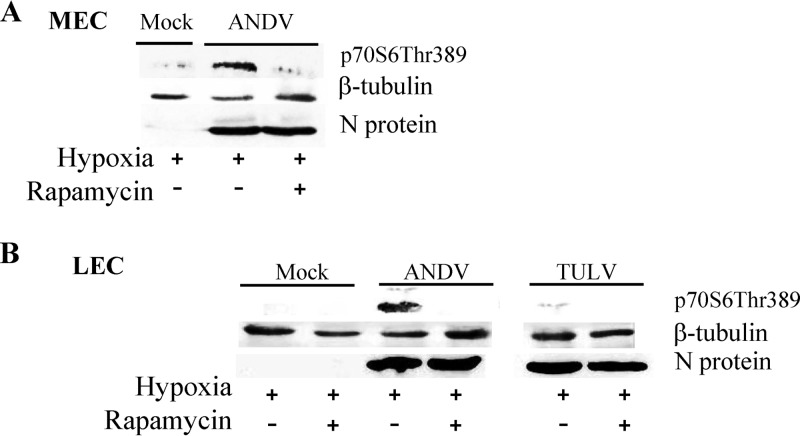
ANDV enhances mTOR-directed S6K phosphorylation.
Cell size is determined by mTOR-directed phosphorylation of p70S6K, and constitutive activation of this signaling pathway in tuberous sclerosis protein 1 or 2 (TSC1/TSC2) genetic mutations results in the formation of giant cells (62, 70, 71). MECs or LECs that were ANDV infected for 2 days were hypoxia treated as described above and evaluated by Western blotting for the presence of expressed viral N protein, phospho-S6K, and β-tubulin. Figure 5A demonstrates that ANDV infection in combination with hypoxic conditions increased pS6K levels in MECs and that the addition of rapamycin 1 h prior to cell lysis abolished ANDV-directed S6K phosphorylation. Experiments performed in LECs infected with ANDV or TULV indicate that TULV infection did not increase S6K phosphorylation under hypoxic conditions. In contrast, hypoxic conditions and ANDV infection dramatically enhanced S6K phosphorylation, and this response was blocked by the mTOR (TORC1) inhibitor rapamycin (Fig. 5B). Collectively, these findings indicate that in the presence of hypoxia, ANDV directs the pathway-specific activation of mTOR-S6K signaling responses that contribute to the increased size of infected pulmonary MECs and LECs. The role of mTOR in hypoxia-directed responses during ANDV infection suggests that rapamycin has the potential to reduce lymphatic and vascular endothelial cell permeability responses that are likely to contribute to hypoxic edematous responses elicited in HPS patients.
Fig 5.
ANDV and hypoxia enhance mTOR-directed S6K phosphorylation. MECs (A) or LECs (B) were infected with ANDV and TULV for 2 days under normoxic conditions and subsequently grown under normoxic or hypoxic conditions as in Fig. 1. Three days postinfection, cells were treated with rapamycin (20 ng/ml) for 1 h or left untreated. Cells were lysed with 1% NP-40, and total protein was analyzed by Western blotting using anti-p70S6-Thr389 rabbit polyclonal antibody (Cell Signaling), anti-β-tubulin monoclonal antibody (Santa Cruz Biotechnology), and antinucleocapsid rabbit serum as previously described (65, 111).
DISCUSSION
HPS is a highly lethal disease resulting in acute rapidly progressive pulmonary edema and shock (1, 2, 5, 13). Hypoxia, thrombocytopenia, and vascular permeability are hallmark findings of hantavirus patients and contribute to acute pulmonary edema in HPS (1, 2, 5). HPS patients often seek medical attention as a result of the sudden onset of respiratory distress resulting from pulmonary edema that accumulates by as much as 1.0 liter per hour (2, 11–13). Potential pathogenic mechanisms accounting for the high rate of pulmonary fluid accumulation have yet to be demonstrated (13, 18, 22, 41, 47, 48, 72–76) but appear be a consequence of the noncytolytic hantavirus infection of endothelial cells. Although, MECs and LECs are not permeabilized by hantavirus infection alone (47, 49–52), hantavirus infection of the endothelium provides a means for the virus to alter EC responses that normally regulate capillary leakage and pulmonary fluid clearance. In vitro data indicate that ANDV infection of human pulmonary MECs and LECs alters VEGF A-mTOR signaling responses within ECs (40, 41, 47, 49–52, 65), and recent studies suggest that bradykinin may contribute to hantavirus-directed vascular permeability (75, 76).
Interestingly, hypoxia is linked to ANDV dysregulation of normal endothelial cell functions through effects on bradykinin, VEGF, and thrombocytopenia, all of which regulate vascular permeability (18, 20, 21, 30, 44, 45, 66, 67, 77–85). Hypoxic conditions cause high-altitude-induced pulmonary edema (HAPE) (18–25, 27), and the ability of hypoxia alone to induce edema and thrombocytopenia suggests that hypoxic conditions may play a critical role in the HPS disease process (11, 13). Interestingly, HAPE results from hypoxia induction of VEGF A (18–20, 28, 33), a potent vascular permeability factor that amplifies cellular responses to hypoxia and is a known cause of edema (38). HPS patients are clearly hypoxic (1, 2, 5, 13), and consistent with this finding, a recent report indicates that HPS patient pulmonary edema fluid contains elevated VEGF A levels (40).
Hantavirus-infected primary human MECs and LECs are hyperresponsive to the permeabilizing effects of VEGF (41, 47, 49, 50, 52, 86). The increased permeability of ANDV-infected EC monolayers to VEGF is a progressive event that occurs days after infection and at a time when cell-associated pathogenic hantaviruses accumulate on the surface of ECs (48, 87). Cell association of ANDV is mediated by inactive αvβ3 integrin conformers on the EC surface, which normally temper VEGFR2-directed permeability responses (48). Thus, the ability of hypoxia to induce VEGF suggests that edema following ANDV infection of MECs and LECs might be directed by hypoxic conditions present in HPS patients.
In this study, we determined whether the presence of hypoxic conditions during ANDV infection is sufficient to enhance the permeability of MEC and LEC monolayers. We demonstrated that both ANDV and nonpathogenic TULV infect primary pulmonary MECs and LECs. However, only ANDV enhanced MEC and LEC permeability in response to hypoxic conditions. We further observed that hypoxia-responsive genes, including those coding for VEGF A, angiopoietin 4, and EGLN3, which are HIF1α transcription factor responsive (28), were dramatically induced by combining ANDV infection with hypoxic conditions. These findings demonstrate that ANDV enhances hypoxia-directed MEC and LEC responses resulting in VEGF induction, as well as by amplifying the EGLN3 feedback loop, which directs HIF1α hydroxylation and degradation (28). Collectively, these findings suggest that hypoxia is likely to induce MEC responses that contribute to capillary permeability within HPS patients (1, 2, 18, 26, 33, 40, 41, 47, 50–52, 63, 75, 76, 78). Although the roles of LECs and lymphatic vessels in HPS have not been defined in HPS patients or animal models, there is also a compelling rationale for hypoxic changes to ANDV-infected LECs to impede fluid clearance functions of pulmonary lymphatic vessels (88).
A previous report demonstrated that ANDV infection of LECs resulted in the formation of giant cells 4× to 5× larger than normal LECs (47). Here we found that hypoxic conditions directed by low O2 levels or CoCl2 treatment of MECs or LECs dramatically induced giant cell formation in ANDV- but not TULV-infected cells. Giant cell formation is directed by mTOR signaling responses via TORC1-directed phosphorylation of S6K (62). Interestingly, we found that hypoxia-directed permeability and giant cell responses of ANDV-infected MECs and LECs were inhibited by rapamycin, which blocks TORC1 activation (62). Consistent with this, we found that phosphorylated p70S6K levels were enhanced in ANDV-infected MECs and LECs in response to hypoxia. Furthermore, rapamycin abolished S6K phosphorylation, demonstrating that MEC and LEC responses following ANDV infection occurred via mTOR (TORC1)-directed signaling pathway activation (62). These findings indicate that hypoxia activates mTOR signaling responses within ANDV- but not TULV-infected MECs and LECs. However, it is not clear how ANDV or TULV infections contribute to the differential regulation of mTOR signaling responses or why ANDV selectively enhances monolayer permeability and giant cell formation in hypoxic ECs.
VEGFR2 responses activate mTOR, while rapamycin inhibits mTOR-directed giant cell and VEGF A-directed permeability responses in mice and hamsters (43–46, 89–91). Tuberous sclerosis complex proteins (TSC1 and TSC2) are upstream negative regulators of mTOR activation, and giant cells are caused by mutations in TSC1/TSC2 that result in constitutive activation of mTOR and S6K (43, 45, 46, 61, 92). Hypoxic conditions induce VEGF A, but paradoxically, hypoxia also induces the expression of REDD1, which stabilizes TSC1/TSC2 complexes and inhibits mTOR activation (31, 61, 69, 89). Hypoxia and ANDV infections result in the constitutive activation of mTOR and pS6K, which are associated with giant cell formation and increased permeability responses (45, 61, 62, 69). Thus, patient hypoxia is likely to drive an HIF-1α-VEGF amplification loop and uniquely activates mTOR, resulting in increased capillary permeability and giant cell formation during ANDV infection of MECs and LECs (26). Our findings suggest that ANDV infection may alter hypoxia-directed REDD1 induction, stability, or TSC1/TSC2 regulation, which normally inhibits mTOR activation (45, 61, 62, 69). A suggested role for the hantavirus N protein in altered P-body-directed microRNA responses (65, 93–95) may contribute to this pathway activation, since microRNA levels altered by hantavirus infection regulate pathways that activate TORC1 and stabilize HIF1α (93, 94, 96). Alternatively, ANDV-altered hypoxic responses could be the result of ANDV effects on a variety of pathways that impact mTOR (31, 61, 69, 89).
One report suggested that an mTOR inhibitor reduced ANDV replication but not RNA synthesis when added before or during initial infection of cells (97). However, our findings indicate that rapamycin inhibited EC permeability and giant cell formation when added for 1 to 24 h, 3 days after infection, but that rapamycin had no effect on viral titers following addition. Rather than an effect on hantavirus replication, our findings suggest the specific and rapid ability of rapamycin to inhibit hantavirus permeability and giant cell responses. These findings are consistent with an effect of rapamycin on mTOR signaling responses that regulate cell size, cell cycle, and permeability responses of ECs (61, 62, 89). Although the role of giant cell formation in ANDV-directed permeability responses remains unclear, findings that both processes are rapamycin regulated suggest a connection that is consistent with reports that rapamycin is a negative effector of hypoxia- or VEGF A-induced permeabilization responses (45, 61, 62, 69).
Prior studies of hantavirus-infected endothelial cells have demonstrated the hyperresponsiveness of MECs and LECs to the addition of VEGF (47–52, 65). In several studies, the addition of VEGF to pathogenic hantavirus-infected ECs, previously grown in the absence of VEGF, has been shown to transiently increase the internalization of VE-cadherin from adherens junctions (47–52, 65). This occurs without VE-cadherin degradation in response to VEGF addition (50–52) or, as presented here, in response to hypoxia. In fact, just as VEGF stimulation induces permeability via VE-cadherin internalization from adherens junctions, internalized VE-cadherin is not degraded but rapidly returned to adherens junctions to stabilize and reduce the permeability of the endothelium (30, 34, 35, 37, 49, 52). One study suggests that hantavirus-infected ECs transiently degraded VE-cadherin at a single specific time point (98). Consistent with our findings, another study of infected HUVECs demonstrated no change in VE-cadherin degradation in cells grown persistently in VEGF but didn't assess VE-cadherin internalization or VEGF responsiveness (75). Interestingly, this paper demonstrates a role for kallikrein-directed bradykinin responses to increase the permeability of hantavirus-infected ECs (75). Although not evaluated by the study, bradykinin and VEGF synergistically increase VEGFR2 phosphorylation (83, 85), and bradykinin secreted from smooth muscle cells induces the expression of VEGF (99). In fact, hypoxia itself induces bradykinin type 2 receptors on endothelial cells (82), fostering the potential interrelationship between VEGF, bradykinin, and hypoxia-induced responses (41, 47–52, 75, 76, 82, 85) in hantavirus-directed permeability.
Hypoxia directs a number of additional cellular responses that act on endothelial cell and platelet functions and which may participate in vascular leakage during ANDV infection (82, 94, 100). Hypoxia increases endothelial NO synthase (eNOS), which is responsible for lymphatic vessel contraction and fluid clearance functions (101, 102). Although tumor necrosis factor alpha (TNF-α) alone does not permeabilize hantavirus-infected cells (103), hypoxia also synergistically induces VEGF in response to transforming growth factor β (TGF-β) or TNF-α stimulation, suggesting a potential role for these factors under hypoxic conditions in vivo (104). Prolonged hypoxia also impairs active sodium transport across the alveolar epithelium by inhibiting the Na+-K+-ATPase and directing ubiquitin proteasome-directed degradation of the Na+ pump (72). Several additional mechanisms may also participate in hypoxia-induced pulmonary edema during hantavirus infection that include responses of T cells (105), induced cytokines (106), and recently reported findings of kalikrein-directed bradykinin permeability responses (75, 76) that may also enhance VEGFR2 signaling responses (82, 83, 85, 99).
Explanations for acute thrombocytopenia during hantavirus infections have yet to be defined. However, hypoxia causes thrombocytopenia in mice, and hypoxia induces the production of the platelet inhibitor prostacyclin (66, 78–80, 107, 108), which renders platelets quiescent (109, 110). A prior report demonstrates that platelets bind the surface of hantavirus-infected cells via interactions with cell-associated virus (48). This occurs days after infection and via the binding of quiescent platelets to the infected EC surface (48). This is intriguing since quiescent platelets are not normally adherent to ECs unless activated and adherence of platelets to the endothelial cell lining of capillaries could both sequester platelets and alter normal gas exchange, causing or exacerbating hypoxia (78, 79, 107, 109, 110). These findings suggest a potential mechanism for hypoxia to render platelets quiescent and contribute to thrombocytopenia by distributing platelets to the surface of the hantavirus-infected endothelium without platelet aggregation or coagulation.
Hypoxia impacts a host of systems that regulate vascular permeability and pulmonary fluid clearance functions. Patient hypoxia is likely to drive an HIF1α-VEGF amplification loop that constitutively activates mTOR and results in increase capillary permeability, as well as additional hypoxia-directed responses of immune and epithelial cells that contribute to HPS (13, 26, 41, 51, 76, 86). At present, there is little understanding of how these systems impact vascular functions and coordinately dysregulate pulmonary responses in order to cause acute pulmonary edema. However, varied hypoxia-dependent responses may be keys to therapeutically resolving HPS at late stages of infection when viral replication and entry inhibitors are ineffective.
ACKNOWLEDGMENTS
We thank Nadine Dalrymple for helpful discussions and critical review of the manuscript.
This work was supported by National Institutes of Health grants R01AI47873, PO1AI055621, R21AI1080984, and U54AI57158 (Northeast Biodefense Center [director, W. I. Lipkin]).
Footnotes
Published ahead of print 25 September 2013
REFERENCES
- 1.Duchin JS, Koster FT, Peters CJ, Simpson GL, Tempest B, Zaki SR, Ksiazek TG, Rollin PE, Nichol S, Umland ET, Moolenaar RL, Reef SE, Nolte KB, Gallaher MM, Butler JC, Breiman RF, Hantavirus Study Group 1994. Hantavirus pulmonary syndrome: a clinical description of 17 patients with a newly recognized disease. N. Engl. J. Med. 330:949–955 [DOI] [PubMed] [Google Scholar]
- 2.Nolte KB, Feddersen RM, Foucar K, Zaki SR, Koster FT, Madar D, Merlin TL, McFeeley PJ, Umland ET, Zumwalt RE. 1995. Hantavirus pulmonary syndrome in the United States: a pathological description of a disease caused by a new agent. Hum. Pathol. 26:110–120 [DOI] [PubMed] [Google Scholar]
- 3.Schmaljohn C. 2001. Bunyaviridae and their replication, p 1581–1602 In Knipe DM, Howley PM, Griffin DE, Lamb RA, Martin MA, Roizman B, Straus SE. (ed), Fields virology, 4th ed, vol 2 Lippincott Williams & Wilkins, Philadelphia, PA [Google Scholar]
- 4.Schmaljohn C, Hjelle B. 1997. Hantaviruses: a global disease problem. Emerg. Infect. Dis. 3:95–104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zaki S, Greer P, Coffield L, Goldsmith C, Nolte K, Foucar K, Feddersen R, Zumwalt R, Miller G, Rollin P, Ksiazek T, Nichol S, Peters C. 1995. Hantavirus pulmonary syndrome: pathogenesis of an emerging infectious disease. Am. J. Pathol. 146:552–579 [PMC free article] [PubMed] [Google Scholar]
- 6.Enria D, Padula P, Segura EL, Pini N, Edelstein A, Posse CR, Weissenbacher MC. 1996. Hantavirus pulmonary syndrome in Argentina. Possibility of person to person transmission. Medicina 56:709–711 [PubMed] [Google Scholar]
- 7.Lopez N, Padula P, Rossi C, Lazaro ME, Franze-Fernandez MT. 1996. Genetic identification of a new hantavirus causing severe pulmonary syndrome in Argentina. Virology 220:223–226 [DOI] [PubMed] [Google Scholar]
- 8.Nichol ST, Spiropoulou CF, Morzunov S, Rollin PE, Ksiazek TG, Feldmann H, Sanchez A, Childs J, Zaki S, Peters CJ. 1993. Genetic identification of a hantavirus associated with an outbreak of acute respiratory illness. Science 262:914–917 [DOI] [PubMed] [Google Scholar]
- 9.Padula PJ, Edelstein A, Miguel SD, Lopez NM, Rossi CM, Rabinovich RD. 1998. Hantavirus pulmonary syndrome outbreak in Argentina: molecular evidence for person-to-person transmission of Andes virus. Virology 241:323–330 [DOI] [PubMed] [Google Scholar]
- 10.Bustamante EA, Levy H, Simpson SQ. 1997. Pleural fluid characteristics in hantavirus pulmonary syndrome. Chest 112:1133–1136 [DOI] [PubMed] [Google Scholar]
- 11.Chang B, Crowley M, Campen M, Koster F. 2007. Hantavirus cardiopulmonary syndrome. Semin. Respir. Crit. Care Med. 28:193–200 [DOI] [PubMed] [Google Scholar]
- 12.Hallin GW, Simpson SQ, Crowell RE, James DS, Koster FT, Mertz GJ, Levy H. 1996. Cardiopulmonary manifestations of hantavirus pulmonary syndrome. Crit. Care Med. 24:252–258 [DOI] [PubMed] [Google Scholar]
- 13.Koster F, Mackow E. 2012. Pathogenesis of the hantavirus pulmonary syndrome. Future Virol. 7:41–51 [Google Scholar]
- 14.Hammerbeck CD, Hooper JW. 2011. T cells are not required for pathogenesis in the Syrian hamster model of hantavirus pulmonary syndrome. J. Virol. 85:9929–9944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hooper JW, Larsen T, Custer DM, Schmaljohn CS. 2001. A lethal disease model for hantavirus pulmonary syndrome. Virology 289:6–14 [DOI] [PubMed] [Google Scholar]
- 16.Safronetz D, Ebihara H, Feldmann H, Hooper JW. 2012. The Syrian hamster model of hantavirus pulmonary syndrome. Antiviral Res. 95:282–292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wahl-Jensen V, Chapman J, Asher L, Fisher R, Zimmerman M, Larsen T, Hooper JW. 2007. Temporal analysis of Andes virus and Sin Nombre virus infections of Syrian hamsters. J. Virol. 81:7449–7462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Berger MM, Hesse C, Dehnert C, Siedler H, Kleinbongard P, Bardenheuer HJ, Kelm M, Bartsch P, Haefeli WE. 2005. Hypoxia impairs systemic endothelial function in individuals prone to high-altitude pulmonary edema. Am. J. Respir. Crit. Care Med. 172:763–767 [DOI] [PubMed] [Google Scholar]
- 19.Christou H, Yoshida A, Arthur V, Morita T, Kourembanas S. 1998. Increased vascular endothelial growth factor production in the lungs of rats with hypoxia-induced pulmonary hypertension. Am. J. Respir. Cell Mol. Biol. 18:768–776 [DOI] [PubMed] [Google Scholar]
- 20.Dehler M, Zessin E, Bartsch P, Mairbaurl H. 2006. Hypoxia causes permeability oedema in the constant-pressure perfused rat lung. Eur. Respir. J. 27:600–606 [DOI] [PubMed] [Google Scholar]
- 21.Hanaoka M, Droma Y, Naramoto A, Honda T, Kobayashi T, Kubo K. 2003. Vascular endothelial growth factor in patients with high-altitude pulmonary edema. J. Appl. Physiol. 94:1836–1840 [DOI] [PubMed] [Google Scholar]
- 22.Hopkins SR, Garg J, Bolar DS, Balouch J, Levin DL. 2005. Pulmonary blood flow heterogeneity during hypoxia and high-altitude pulmonary edema. Am. J. Respir. Crit. Care Med. 171:83–87 [DOI] [PubMed] [Google Scholar]
- 23.Kaner RJ, Crystal RG. 2004. Pathogenesis of high altitude pulmonary edema: does alveolar epithelial lining fluid vascular endothelial growth factor exacerbate capillary leak? High Alt. Med. Biol. 5:399–409 [DOI] [PubMed] [Google Scholar]
- 24.Mukhopadhyay D, Tsiokas L, Zhou XM, Foster D, Brugge JS, Sukhatme VP. 1995. Hypoxic induction of human vascular endothelial growth factor expression through c-Src activation. Nature 375:577–581 [DOI] [PubMed] [Google Scholar]
- 25.Scherrer U, Rexhaj E, Jayet PY, Allemann Y, Sartori C. 2010. New insights in the pathogenesis of high-altitude pulmonary edema. Prog. Cardiovasc. Dis. 52:485–492 [DOI] [PubMed] [Google Scholar]
- 26.Tang N, Wang L, Esko J, Giordano FJ, Huang Y, Gerber HP, Ferrara N, Johnson RS. 2004. Loss of HIF-1alpha in endothelial cells disrupts a hypoxia-driven VEGF autocrine loop necessary for tumorigenesis. Cancer Cell 6:485–495 [DOI] [PubMed] [Google Scholar]
- 27.Voelkel NF. 2002. High-altitude pulmonary edema. N. Engl. J. Med. 346:1606–1607 [DOI] [PubMed] [Google Scholar]
- 28.Manalo DJ, Rowan A, Lavoie T, Natarajan L, Kelly BD, Ye SQ, Garcia JG, Semenza GL. 2005. Transcriptional regulation of vascular endothelial cell responses to hypoxia by HIF-1. Blood 105:659–669 [DOI] [PubMed] [Google Scholar]
- 29.Minet E, Arnould T, Michel G, Roland I, Mottet D, Raes M, Remacle J, Michiels C. 2000. ERK activation upon hypoxia: involvement in HIF-1 activation. FEBS Lett. 468:53–58 [DOI] [PubMed] [Google Scholar]
- 30.Gavard J, Gutkind JS. 2006. VEGF controls endothelial-cell permeability by promoting the beta-arrestin-dependent endocytosis of VE-cadherin. Nat. Cell Biol. 8:1223–1234 [DOI] [PubMed] [Google Scholar]
- 31.Hudson CC, Liu M, Chiang GG, Otterness DM, Loomis DC, Kaper F, Giaccia AJ, Abraham RT. 2002. Regulation of hypoxia-inducible factor 1alpha expression and function by the mammalian target of rapamycin. Mol. Cell. Biol. 22:7004–7014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lampugnani MG, Orsenigo F, Gagliani MC, Tacchetti C, Dejana E. 2006. Vascular endothelial cadherin controls VEGFR-2 internalization and signaling from intracellular compartments. J. Cell Biol. 174:593–604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pham I, Uchida T, Planes C, Ware LB, Kaner R, Matthay MA, Clerici C. 2002. Hypoxia upregulates VEGF expression in alveolar epithelial cells in vitro and in vivo. Am. J. Physiol. Lung Cell. Mol. Physiol. 283:L1133–L1142 [DOI] [PubMed] [Google Scholar]
- 34.Gavard J. 2009. Breaking the VE-cadherin bonds. FEBS Lett. 583:1–6 [DOI] [PubMed] [Google Scholar]
- 35.Gavard J, Patel V, Gutkind JS. 2008. Angiopoietin-1 prevents VEGF-induced endothelial permeability by sequestering Src through mDia. Dev. Cell 14:25–36 [DOI] [PubMed] [Google Scholar]
- 36.Ogawa S, Shreeniwas R, Brett J, Clauss M, Furie M, Stern DM. 1990. The effect of hypoxia on capillary endothelial cell function: modulation of barrier and coagulant function. Br. J. Haematol. 75:517–524 [DOI] [PubMed] [Google Scholar]
- 37.Dejana E, Orsenigo F, Lampugnani MG. 2008. The role of adherens junctions and VE-cadherin in the control of vascular permeability. J. Cell Sci. 121:2115–2122 [DOI] [PubMed] [Google Scholar]
- 38.Dvorak HF. 2006. Discovery of vascular permeability factor (VPF). Exp. Cell Res. 312:522–526 [DOI] [PubMed] [Google Scholar]
- 39.Koster F, Foucar K, Hjelle B, Scott A, Chong YY, Larson R, McCabe M. 2001. Rapid presumptive diagnosis of hantavirus cardiopulmonary syndrome by peripheral blood smear review. Am. J. Clin. Pathol. 116:665–672 [DOI] [PubMed] [Google Scholar]
- 40.Gavrilovskaya I, Gorbunova E, Koster F, Mackow E. 2012. Elevated VEGF levels in pulmonary edema fluid and PBMCs from patients with acute hantavirus pulmonary syndrome. Adv. Virol. 2012:674360. 10.1155/2012/674360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gavrilovskaya I, Gorbunova E, Matthys V, Dalrymple N, Mackow E. 2012. The role of the endothelium in HPS pathogenesis and potential therapeutic approaches. Adv. Virol. 2012:467059. 10.1155/2012/467059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dvorak HF, Brown LF, Detmar M, Dvorak AM. 1995. Vascular permeability factor/vascular endothelial growth factor, microvascular hyperpermeability, and angiogenesis. Am. J. Pathol. 146:1029–1039 [PMC free article] [PubMed] [Google Scholar]
- 43.El-Hashemite N, Walker V, Zhang H, Kwiatkowski DJ. 2003. Loss of Tsc1 or Tsc2 induces vascular endothelial growth factor production through mammalian target of rapamycin. Cancer Res. 63:5173–5177 [PubMed] [Google Scholar]
- 44.Kim DD, Kleinman DM, Kanetaka T, Gerritsen ME, Nivaggioli T, Weber D, Duran WN. 2010. Rapamycin inhibits VEGF-induced microvascular hyperpermeability in vivo. Microcirculation 17:128–136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Land SC, Tee AR. 2007. Hypoxia-inducible factor 1alpha is regulated by the mammalian target of rapamycin (mTOR) via an mTOR signaling motif. J. Biol. Chem. 282:20534–20543 [DOI] [PubMed] [Google Scholar]
- 46.Xue Q, Nagy JA, Manseau EJ, Phung TL, Dvorak HF, Benjamin LE. 2009. Rapamycin inhibition of the Akt/mTOR pathway blocks select stages of VEGF-A164-driven angiogenesis, in part by blocking S6 kinase. Arterioscler. Thromb. Vasc. Biol. 29:1172–1178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gavrilovskaya IN, Gorbunova EE, Mackow ER. 2012. Andes virus infection of lymphatic endothelial cells causes giant cell and enhanced permeability responses that are rapamycin and vascular endothelial growth factor C sensitive. J. Virol. 86:8765–8772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gavrilovskaya IN, Gorbunova EE, Mackow ER. 2010. Pathogenic hantaviruses direct the adherence of quiescent platelets to infected endothelial cells. J. Virol. 84:4832–4839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gavrilovskaya IN, Gorbunova EE, Mackow NA, Mackow ER. 2008. Hantaviruses direct endothelial cell permeability by sensitizing cells to the vascular permeability factor VEGF, while angiopoietin 1 and sphingosine 1-phosphate inhibit hantavirus-directed permeability. J. Virol. 82:5797–5806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gorbunova E, Gavrilovskaya IN, Mackow ER. 2010. Pathogenic hantaviruses Andes virus and Hantaan virus induce adherens junction disassembly by directing vascular endothelial cadherin internalization in human endothelial cells. J. Virol. 84:7405–7411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gorbunova EE, Gavrilovskaya IN, Mackow ER. 2013. Slit2-Robo4 receptor responses inhibit ANDV directed permeability of human lung microvascular endothelial cells. Antiviral Res. 99:108–112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gorbunova EE, Gavrilovskaya IN, Pepini T, Mackow ER. 2011. VEGFR2 and Src kinase inhibitors suppress Andes virus-induced endothelial cell permeability. J. Virol. 85:2296–2303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gavrilovskaya IN, Brown EJ, Ginsberg MH, Mackow ER. 1999. Cellular entry of hantaviruses which cause hemorrhagic fever with renal syndrome is mediated by beta3 integrins. J. Virol. 73:3951–3959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gavrilovskaya IN, Shepley M, Shaw R, Ginsberg MH, Mackow ER. 1998. Beta3 integrins mediate the cellular entry of hantaviruses that cause respiratory failure. Proc. Natl. Acad. Sci. U. S. A. 95:7074–7079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Raymond T, Gorbunova E, Gavrilovskaya IN, Mackow ER. 2005. Pathogenic hantaviruses bind plexin-semaphorin-integrin domains present at the apex of inactive, bent alphavbeta3 integrin conformers. Proc. Natl. Acad. Sci. U. S. A. 102:1163–1168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Borges E, Jan Y, Ruoslahti E. 2000. Platelet-derived growth factor receptor beta and vascular endothelial growth factor receptor 2 bind to the beta 3 integrin through its extracellular domain. J. Biol. Chem. 275:39867–39873 [DOI] [PubMed] [Google Scholar]
- 57.Byzova TV, Goldman CK, Pampori N, Thomas KA, Bett A, Shattil SJ, Plow EF. 2000. A mechanism for modulation of cellular responses to VEGF: activation of the integrins. Mol. Cell 6:851–860 [PubMed] [Google Scholar]
- 58.Hodivala-Dilke KM, McHugh KP, Tsakiris DA, Rayburn H, Crowley D, Ullman-Cullere M, Ross FP, Coller BS, Teitelbaum S, Hynes RO. 1999. Beta3-integrin-deficient mice are a model for Glanzmann thrombasthenia showing placental defects and reduced survival. J. Clin. Invest. 103:229–238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Reynolds LE, Wyder L, Lively JC, Taverna D, Robinson SD, Huang X, Sheppard D, Hynes RO, Hodivala-Dilke KM. 2002. Enhanced pathological angiogenesis in mice lacking beta3 integrin or beta3 and beta5 integrins. Nat. Med. 8:27–34 [DOI] [PubMed] [Google Scholar]
- 60.Robinson SD, Reynolds LE, Wyder L, Hicklin DJ, Hodivala-Dilke KM. 2004. Beta3-integrin regulates vascular endothelial growth factor-A-dependent permeability. Arterioscler. Thromb. Vasc. Biol. 24:2108–2114 [DOI] [PubMed] [Google Scholar]
- 61.Brugarolas J, Lei K, Hurley RL, Manning BD, Reiling JH, Hafen E, Witters LA, Ellisen LW, Kaelin WG., Jr 2004. Regulation of mTOR function in response to hypoxia by REDD1 and the TSC1/TSC2 tumor suppressor complex. Genes Dev. 18:2893–2904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Laplante M, Sabatini DM. 2012. mTOR signaling in growth control and disease. Cell 149:274–293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kim KS, Rajagopal V, Gonsalves C, Johnson C, Kalra VK. 2006. A novel role of hypoxia-inducible factor in cobalt chloride- and hypoxia-mediated expression of IL-8 chemokine in human endothelial cells. J. Immunol. 177:7211–7224 [DOI] [PubMed] [Google Scholar]
- 64.Geimonen E, Neff S, Raymond T, Kocer SS, Gavrilovskaya IN, Mackow ER. 2002. Pathogenic and nonpathogenic hantaviruses differentially regulate endothelial cell responses. Proc. Natl. Acad. Sci. U. S. A. 99:13837–13842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pepini T, Gorbunova EE, Gavrilovskaya I, Mackow JE, Mackow ER. 2010. Andes virus regulation of cellular microRNAs contributes to hantavirus-induced endothelial cell permeability. J. Virol. 84:11929–11936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Farmer PJ, Bernier SG, Lepage A, Guillemette G, Regoli D, Sirois P. 2001. Permeability of endothelial monolayers to albumin is increased by bradykinin and inhibited by prostaglandins. Am. J. Physiol. Lung Cell. Mol. Physiol. 280:L732–L738 [DOI] [PubMed] [Google Scholar]
- 67.Forsythe JA, Jiang BH, Iyer NV, Agani F, Leung SW, Koos RD, Semenza GL. 1996. Activation of vascular endothelial growth factor gene transcription by hypoxia-inducible factor 1. Mol. Cell. Biol. 16:4604–4613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhou Q, Liu LZ, Fu B, Hu X, Shi X, Fang J, Jiang BH. 2007. Reactive oxygen species regulate insulin-induced VEGF and HIF-1alpha expression through the activation of p70S6K1 in human prostate cancer cells. Carcinogenesis 28:28–37 [DOI] [PubMed] [Google Scholar]
- 69.Wolff NC, Vega-Rubin-de-Celis S, Xie XJ, Castrillon DH, Kabbani W, Brugarolas J. 2011. Cell-type-dependent regulation of mTORC1 by REDD1 and the tumor suppressors TSC1/TSC2 and LKB1 in response to hypoxia. Mol. Cell. Biol. 31:1870–1884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Li Y, Inoki K, Yeung R, Guan KL. 2002. Regulation of TSC2 by 14-3-3 binding. J. Biol. Chem. 277:44593–44596 [DOI] [PubMed] [Google Scholar]
- 71.Ruvinsky I, Meyuhas O. 2006. Ribosomal protein S6 phosphorylation: from protein synthesis to cell size. Trends Biochem. Sci. 31:342–348 [DOI] [PubMed] [Google Scholar]
- 72.Mutlu GM, Sznajder JI. 2005. Mechanisms of pulmonary edema clearance. Am. J. Physiol. Lung Cell. Mol. Physiol. 289:L685–L695 [DOI] [PubMed] [Google Scholar]
- 73.Schraufnagel DE. 2010. Lung lymphatic anatomy and correlates. Pathophysiology 17:337–343 [DOI] [PubMed] [Google Scholar]
- 74.Schraufnagel DE, Agaram NP, Faruqui A, Jain S, Jain L, Ridge KM, Sznajder JI. 2003. Pulmonary lymphatics and edema accumulation after brief lung injury. Am. J. Physiol. Lung Cell. Mol. Physiol. 284:L891–L897 [DOI] [PubMed] [Google Scholar]
- 75.Taylor SL, Wahl-Jensen V, Copeland AM, Jahrling PB, Schmaljohn CS. 2013. Endothelial cell permeability during hantavirus infection involves factor XII-dependent increased activation of the kallikrein-kinin system. PLoS Pathog 9:e1003470. 10.1371/journal.ppat.1003470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Vaheri A, Strandin T, Hepojoki J, Sironen T, Henttonen H, Makela S, Mustonen J. 2013. Uncovering the mysteries of hantavirus infections. Nat. Rev. Microbiol. 11:539–550 [DOI] [PubMed] [Google Scholar]
- 77.Bahram F, Claesson-Welsh L. 2010. VEGF-mediated signal transduction in lymphatic endothelial cells. Pathophysiology 17:253–261 [DOI] [PubMed] [Google Scholar]
- 78.Birks JW, Klassen LW, Gurney CW. 1975. Hypoxia-induced thrombocytopenia in mice. J. Lab. Clin. Med. 86:230–238 [PubMed] [Google Scholar]
- 79.Gliki G, Abu-Ghazaleh R, Jezequel S, Wheeler-Jones C, Zachary I. 2001. Vascular endothelial growth factor-induced prostacyclin production is mediated by a protein kinase C (PKC)-dependent activation of extracellular signal-regulated protein kinases 1 and 2 involving PKC-delta and by mobilization of intracellular Ca2+. Biochem. J. 353:503–512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.He H, Venema VJ, Gu X, Venema RC, Marrero MB, Caldwell RB. 1999. Vascular endothelial growth factor signals endothelial cell production of nitric oxide and prostacyclin through flk-1/KDR activation of c-Src. J. Biol. Chem. 274:25130–25135 [DOI] [PubMed] [Google Scholar]
- 81.Kaner RJ, Ladetto JV, Singh R, Fukuda N, Matthay MA, Crystal RG. 2000. Lung overexpression of the vascular endothelial growth factor gene induces pulmonary edema. Am. J. Respir. Cell Mol. Biol. 22:657–664 [DOI] [PubMed] [Google Scholar]
- 82.Liesmaa I, Leskinen HK, Kokkonen JO, Ruskoaho H, Kovanen PT, Lindstedt KA. 2009. Hypoxia-induced expression of bradykinin type-2 receptors in endothelial cells triggers NO production, cell migration, and angiogenesis. J. Cell. Physiol. 221:359–366 [DOI] [PubMed] [Google Scholar]
- 83.Miura S, Matsuo Y, Saku K. 2003. Transactivation of KDR/Flk-1 by the B2 receptor induces tube formation in human coronary endothelial cells. Hypertension 41:1118–1123 [DOI] [PubMed] [Google Scholar]
- 84.Suarez S, Ballmer-Hofer K. 2001. VEGF transiently disrupts gap junctional communication in endothelial cells. J. Cell Sci. 114:1229–1235 [DOI] [PubMed] [Google Scholar]
- 85.Thuringer D, Maulon L, Frelin C. 2002. Rapid transactivation of the vascular endothelial growth factor receptor KDR/Flk-1 by the bradykinin B2 receptor contributes to endothelial nitric-oxide synthase activation in cardiac capillary endothelial cells. J. Biol. Chem. 277:2028–2032 [DOI] [PubMed] [Google Scholar]
- 86.Mackow ER, Gavrilovskaya IN. 2009. Hantavirus regulation of endothelial cell functions. Thromb. Haemost. 102:1030–1041 [DOI] [PubMed] [Google Scholar]
- 87.Goldsmith CS, Elliott LH, Peters CJ, Zaki SR. 1995. Ultrastructural characteristics of Sin Nombre virus, causative agent of hantavirus pulmonary syndrome. Arch. Virol. 140:2107–2122 [DOI] [PubMed] [Google Scholar]
- 88.Alitalo K. 2011. The lymphatic vasculature in disease. Nat. Med. 17:1371–1380 [DOI] [PubMed] [Google Scholar]
- 89.Cam H, Houghton PJ. 2011. Regulation of mammalian target of rapamycin complex 1 (mTORC1) by hypoxia: causes and consequences. Target Oncol. 6:95–102 [DOI] [PubMed] [Google Scholar]
- 90.Humar R, Kiefer FN, Berns H, Resink TJ, Battegay EJ. 2002. Hypoxia enhances vascular cell proliferation and angiogenesis in vitro via rapamycin (mTOR)-dependent signaling. FASEB J. 16:771–780 [DOI] [PubMed] [Google Scholar]
- 91.Ikeda H, Shiojima I, Oka T, Yoshida M, Maemura K, Walsh K, Igarashi T, Komuro I. 2011. Increased Akt-mTOR signaling in lung epithelium is associated with respiratory distress syndrome in mice. Mol. Cell. Biol. 31:1054–1065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Brugarolas J, Kaelin WG., Jr 2004. Dysregulation of HIF and VEGF is a unifying feature of the familial hamartoma syndromes. Cancer Cell 6:7–10 [DOI] [PubMed] [Google Scholar]
- 93.Bett JS, Ibrahim AF, Garg AK, Kelly V, Pedrioli P, Rocha S, Hay RT. 2013. The P-body component USP52/PAN2 is a novel regulator of HIF1A mRNA stability. Biochem. J. 451:185–194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kulshreshtha R, Davuluri RV, Calin GA, Ivan M. 2008. A microRNA component of the hypoxic response. Cell Death Differ. 15:667–671 [DOI] [PubMed] [Google Scholar]
- 95.Mir MA, Duran WA, Hjelle BL, Ye C, Panganiban AT. 2008. Storage of cellular 5′ mRNA caps in P bodies for viral cap-snatching. Proc. Natl. Acad. Sci. U. S. A. 105:19294–19299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Winter J, Jung S, Keller S, Gregory RI, Diederichs S. 2009. Many roads to maturity: microRNA biogenesis pathways and their regulation. Nat. Cell Biol. 11:228–234 [DOI] [PubMed] [Google Scholar]
- 97.McNulty S, Flint M, Nichol ST, Spiropoulou CF. 2013. Host mTORC1 signaling regulates Andes virus replication. J. Virol. 87:912–922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Shrivastava-Ranjan P, Rollin PE, Spiropoulou CF. 2010. Andes virus disrupts the endothelial cell barrier by induction of vascular endothelial growth factor and downregulation of VE-cadherin. J. Virol. 84:11227–11234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Knox AJ, Corbett L, Stocks J, Holland E, Zhu YM, Pang L. 2001. Human airway smooth muscle cells secrete vascular endothelial growth factor: up-regulation by bradykinin via a protein kinase C and prostanoid-dependent mechanism. FASEB J. 15:2480–2488 [DOI] [PubMed] [Google Scholar]
- 100.Irigoyen M, Anso E, Martinez E, Garayoa M, Martinez-Irujo JJ, Rouzaut A. 2007. Hypoxia alters the adhesive properties of lymphatic endothelial cells. A transcriptional and functional study. Biochim. Biophys. Acta 1773:880–890 [DOI] [PubMed] [Google Scholar]
- 101.Hagendoorn J, Padera TP, Kashiwagi S, Isaka N, Noda F, Lin MI, Huang PL, Sessa WC, Fukumura D, Jain RK. 2004. Endothelial nitric oxide synthase regulates microlymphatic flow via collecting lymphatics. Circ. Res. 95:204–209 [DOI] [PubMed] [Google Scholar]
- 102.Miao RQ, Fontana J, Fulton D, Lin MI, Harrison KD, Sessa WC. 2008. Dominant-negative Hsp90 reduces VEGF-stimulated nitric oxide release and migration in endothelial cells. Arterioscler. Thromb. Vasc. Biol. 28:105–111 [DOI] [PubMed] [Google Scholar]
- 103.Sundstrom JB, McMullan LK, Spiropoulou CF, Hooper WC, Ansari AA, Peters CJ, Rollin PE. 2001. Hantavirus infection induces the expression of RANTES and IP-10 without causing increased permeability in human lung microvascular endothelial cells. J. Virol. 75:6070–6085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Li X, Kimura H, Hirota K, Kasuno K, Torii K, Okada T, Kurooka H, Yokota Y, Yoshida H. 2005. Synergistic effect of hypoxia and TNF-alpha on production of PAI-1 in human proximal renal tubular cells. Kidney Int. 68:569–583 [DOI] [PubMed] [Google Scholar]
- 105.Hayasaka D, Maeda K, Ennis FA, Terajima M. 2007. Increased permeability of human endothelial cell line EA.hy926 induced by hantavirus-specific cytotoxic T lymphocytes. Virus Res. 123:120–127 [DOI] [PubMed] [Google Scholar]
- 106.Mori M, Rothman AL, Kurane I, Montoya JM, Nolte KB, Norman JE, Waite DC, Koster FT, Ennis FA. 1999. High levels of cytokine-producing cells in the lung tissues of patients with fatal hantavirus pulmonary syndrome. J. Infect. Dis. 179:295–302 [DOI] [PubMed] [Google Scholar]
- 107.Morita I, Kanayasu T, Murota S. 1984. Kallikrein stimulates prostacyclin production in bovine vascular endothelial cells. Biochim. Biophys. Acta 792:304–309 [PubMed] [Google Scholar]
- 108.Willems C, van Aken WG. 1979. Production of prostacyclin by vascular endothelial cells. Haemostasis 8:266–273 [DOI] [PubMed] [Google Scholar]
- 109.Bozza FA, Shah AM, Weyrich AS, Zimmerman GA. 2009. Amicus or adversary: platelets in lung biology, acute injury, and inflammation. Am. J. Respir. Cell Mol. Biol. 40:123–134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Coller BS, Shattil SJ. 2008. The GPIIb/IIIa (integrin alphaIIbbeta3) odyssey: a technology-driven saga of a receptor with twists, turns, and even a bend. Blood 112:3011–3025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Alff PJ, Gavrilovskaya IN, Gorbunova E, Endriss K, Chong Y, Geimonen E, Sen N, Reich NC, Mackow ER. 2006. The pathogenic NY-1 hantavirus G1 cytoplasmic tail inhibits RIG-I- and TBK-1-directed interferon responses. J. Virol. 80:9676–9686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Yanagihara R, Silverman DJ. 1990. Experimental infection of human vascular endothelial cells by pathogenic and nonpathogenic hantaviruses. Arch. Virol. 111:281–286 [DOI] [PubMed] [Google Scholar]