Abstract
The role of core promoter elements in regulating transcription initiation is largely unknown for genes subject to complex regulation. Major histocompatibility complex class I genes are ubiquitously expressed and governed by tissue-specific and hormonal signals. Transcription initiates at multiple sites within the core promoter, which contains elements homologous to the canonical elements CCAAT, TATAA, Sp1 binding site (Sp1BS), and Initiator (Inr). To determine their functions, expression of class I transgenes with individually mutated elements was assessed. Surprisingly, all mutant promoters supported transcription. However, each mutated core promoter element had a distinct effect on expression: CAAT box mutations modulated constitutive expression in nonlymphoid tissues, whereas TATAA-like element mutations dysregulated transcription in lymphoid tissues. Inr mutations aberrantly elevated expression. Sp1BS element mutations resulted in variegated transgene expression. RNA polymerase II binding and histone H3K4me3 patterns correlated with transgene expression; H3K9me3 marks partially correlated. Whereas the wild-type, TATAA-like, and CAAT mutant promoters were activated by gamma interferon, the Sp1 and Inr mutants were repressed, implicating these elements in regulation of hormonal responses. These results lead to the surprising conclusion that no single element is required for promoter activity. Rather, each plays a distinct role in promoter activity, chromatin structure, tissue-specific expression, and extracellular signaling.
INTRODUCTION
Transcription of genes by RNA polymerase II (Pol II) is a highly regulated process that requires the integration of multiple signaling pathways at the extended promoter. Regulation is mediated by specific interactions of transcription factors with promoter DNA sequences, resulting in the assembly of the transcription machinery and onset of transcription (1–5). RNA Pol II promoters are divided conceptually into two domains: distal and core promoter regions. Although the diversity of transcription factor binding sites and the complexity of their organization in distal, upstream regulatory regions is well recognized (6), it is increasingly apparent that core promoter regions are also highly diverse (7–13). Core promoters, defined as the minimal length of DNA necessary to direct accurate transcription initiation by Pol II, can be grouped according to the presence of specific DNA sequence elements, such as CAAT and TATAA boxes (12, 14), Initiator (Inr) (9, 15–18), TFIIB response element (BRE), and downstream promoter element (DPE), or the presence of CpG islands (19, 20) or ATG deserts (21). Additionally, Sp1 binding sites (Sp1BS) often are located within the core promoter region. Several core promoter elements (e.g., TATAA box, Inr, BRE, DCE, and DPE) were shown to have a role in recruiting distinct general transcription factors (GTF) and integrating signals from distal regulatory elements (22–24). Until recently, the conventional view of core promoters was based largely on the characterization of promoters with simple TATAA or Inr cores; this view is being increasingly challenged by the characterization of complex promoters. Genome-wide analyses have shown that only a small fraction (5 to 10%) of promoters possess a canonical TATA box (25). Many promoters do not have any of the other previously identified core promoter elements (26, 27).
Promoters can also be distinguished on the basis of their transcription start site (TSS) use. Promoters associated with tissue-specific genes most commonly initiate transcription at a single site or a few tightly clustered ones. In contrast, promoters associated with ubiquitously expressed genes initiate transcription at multiple, dispersed start sites. Computational analyses have revealed a correlation between the presence in core promoters of either a TATAA box or Inr with single or multiple TSS, respectively. However, no in vivo functional analyses of core promoters with multiple TSS have been reported. Thus, it is not known whether any of the previously identified core promoter elements are functional in these core promoters.
The finding that core promoters are more complex than previously appreciated (28, 29) led us to examine the roles of individual core promoter elements in transcription of the major histocompatibility complex (MHC) gene. The MHC class I gene is an excellent model in which to characterize the functions of individual core promoter elements. The class I genes, whose products provide immune surveillance, are ubiquitously expressed but are subject to tissue-specific regulation. Expression of individual MHC class I genes varies by two orders of magnitude among different tissues: expression is highest in lymphoid tissues, lower in peripheral organs, and barely detectable in neuronal cells. In addition, MHC class I gene expression is modulated by cytokines and hormones that activate or repress transcription (30–32).
We reported previously that a transgene derived from the MHC class I gene, the PD1 gene, displayed the same tissue-specific patterns of expression and response to hormonal stimuli as endogenous mouse MHC class I genes (H-2K genes) (33, 34). Therefore, all of the DNA sequence elements necessary for regulated in vivo transcription are contained within the 1 kb of 5′ distal and core promoter elements. The extended promoter also contains two DPE that contribute to the overall levels of expression (22). Tissue-specific expression (TSE) is achieved through a promoter-distal complex regulatory element, located between bp −700 and −800, that modulates transcription through overlapping enhancer and silencer elements (35). Hormone/cytokine signaling is mediated by a series of promoter-proximal elements located between bp −68 and −500 (32, 36, 37).
Transcription initiates at multiple sites in the core promoter, where a prominent TSS defines the +1 position. The core promoter, located within an ATG desert, extends from bp −68 to +14. TSS use is regulated, with upstream sites preferentially targeted in basal TAF1/TFIID-dependent transcription and downstream sites preferentially used during activated, TAF1-independent transcription. Contained within the core promoter are sequences with homology to the canonical core promoter elements, CAAT, TATAA-like, Sp1BS, and Inr (33). These core promoter elements are highly conserved across the class I multigene family within a species and among mammalian species, consistent with important functional roles (38). For example, the CAAT and Inr elements are completely conserved, and mouse class I genes have a canonical TATAA element in the position of the PD1 TATAA-like element (38).
In the present study, we have assessed the in vivo functional roles of each of these core promoter sequence elements in the context of the native PD1 in transgenic mice. Surprisingly, none of the elements (CAAT, TATAA-like, Inr, or Sp1BS) is necessary for promoter function in vivo. However, these elements are functional: all four elements contribute to the absolute level of transcription but have distinct roles in regulating tissue-specific expression or modulating cytokine responses. Furthermore, analysis of the relationship between promoter activity and chromatin structure suggests that chromatin modifications generally reflect promoter activity. Taken together, these findings reveal novel functions for canonical core promoter elements in an MHC class I promoter with multiple TSS. They also provide evidence that core promoters with multiple TSS are flexible platforms that do not depend on a single element to support transcription initiation.
MATERIALS AND METHODS
Cell line and transfections.
The HeLa cell line (ATCC) was grown as previously described (22). Transfections were carried out using Fugene 6 (Life Technologies) according to the manufacturer's instructions. Levels of PD1 expression were assessed by flow cytometry using anti-SLA antibody (see Table 3 at DinahSingerLab.cancer.gov).
Mice.
C57BL/10 mice homozygous for the PD1 transgene were generated as described previously (39). The wild-type (WT) transgene contains a 1-kb regulatory region upstream of the TSS, the entire coding region, and 730 bp immediately following the polyadenylation site (40). The promoter mutations were made within the context of the DNA of this transgene (Fig. 1A). (Detailed cloning strategies can be found at DinahSingerLab.cancer.gov.) Another wild-type transgene, the PD1L transgene, has been described previously (30, 31, 34, 39). All studies were carried out in accordance with the National Institutes of Health Animal Care and Use committee (protocol EIB-076).
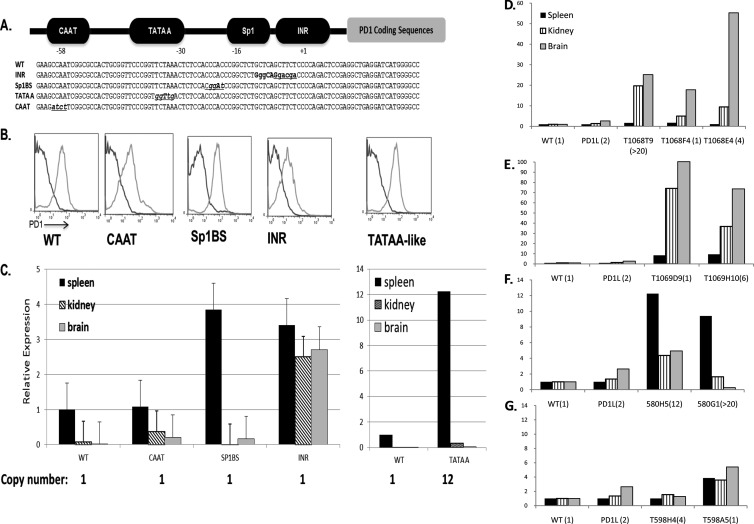
Fig 1.
Core promoter elements contribute to tissue-specific expression regulation. (A) Diagrammatic representation of the PD1 core promoter upstream of PD1 coding sequences and sequences of the wild-type (WT) core promoter and core promoter element mutations that were used in the study. (B) Representative fluorescence-activated cell sorter profiles of PBL from individual WT and mutant promoter transgenic mice from separate founder lines (light gray curves). Dark gray curves represent staining of negative-control C57/BL6 mice with the relevant antibody. Profiles are representative of the majority of mice of each of the transgenic lines. (C) Real-time PCR analysis of PD1 RNA levels in tissues of the different core promoter mutant transgenic mice relative to the WT. The CAAT mutant (T1068F4), Sp1BS mutant (T598A5), Inr mutant (T1069D9), and TATAA-like mutant (T580H5) are also shown. At least 3 mice from each founder line were analyzed individually (data are averages ± standard deviations [SD]). Transgene copy number for strains analyzed are listed. (D to G) PD1 RNA levels in spleen, kidney, and brain were determined in independently derived lines of CCAAT mutants (D), Inr mutants (E), TATAA-like mutants (F), and Sp1BS mutants (G) and compared to the WT and to published data from a second transgenic line, PD1L, that has a wild-type promoter (34). Numbers in parentheses next to the transgene name indicate transgene copy number. Note that in each tissue, the data are expressed relative to the wild-type level in that tissue.
Copy number.
Transgene gene copy number was determined by either Southern blotting or quantitative real-time PCR (qPCR) as described previously (41, 42).
IFN-γ treatment of transgenic mice.
Mice were injected intraperitoneally with 50 to 100 kU of mouse gamma interferon (IFN-γ; CalBiochem, Gibbstown, NJ) or an equal volume of saline. Tissues were harvested 24 h postinjection. Peripheral blood lymphocytes (PBL) obtained before and after treatment were analyzed by flow cytometry of cells stained with PT85 anti-SLA antibody to verify IFN-γ induction of MHC class I expression. Levels of PD1 and endogenous H2-K RNA were assessed by qPCR of RNA extracted from tissues. The experiment was performed 2 times with three mice analyzed independently. The following lines were used: WT, PD1; CAAT, T1068F4; TATAA-like, T580H5; Inr, T1069H10; and Sp1BS, T598A5.
RNA.
RNA extraction was performed on individual mice using an RNeasy kit (Qiagen) according to the manufacturer's instructions. Each sample was subjected to DNase treatment using a DNase kit (Ambion).
Northern blotting was conducted according to standard protocols. The membranes were probed with a 1-kb XbaI/SacI coding sequence fragment of the PD1 gene. 18S RNA probe was generated by cutting 18S plasmid with EcoRI.
Primer extension assays were performed as described previously (43). Each lane was loaded with 10 μg of RNA.
RT-PCR was performed using a high-capacity cDNA reverse transcription kit (Life Technologies) according to the manufacturer's instructions.
Real-time PCR was performed as described previously (30) using an ABI7900 device with SYBR PCR master mix (Life technologies). For tissue RNA expression, the calculations were conducted using a standard curve method and were compared to the level of 18S in the tissues. All results reported are averages from 3 independent experiments in at least 2 different lines of each strain to exclude possible insertion position effects. Endogenous MHC class I levels were measured the same way using published primers (44).
ChIP.
Spleens, kidneys, and brains of mice were harvested, and chromatin immunoprecipitations (ChIPs) were performed using the MAGNA tissue kit (Millipore) according to the manufacturer's instructions. Reactions were analyzed by qPCR using the primers described elsewhere for PD1 (30). Antibodies and primers that were used are detailed separately at DinahSingerLab.cancer.gov. Quantitative PCR was performed using an ABI7900 device with a SYBR green real-time PCR kit (Life technologies). Results were calculated using the ΔΔCT method of bound/input DNA compared to IgG antibody control. Input is DNA from the sheared chromatin fraction before immunoprecipitation. For each core promoter mutant, three separate ChIP experiments were performed for each of the lines analyzed, using pools of 3 spleens, 2 kidneys, or 1 brain. The strains used were the following: WT, PD1; CAAT, T1068T9 and T1068F4; TATAA-like, T580G1 and T580H5; Inr, T1069H10; and Sp1, T598A5.
Nucleosome occupancy.
Nuclei were prepared from spleen tissue, and nucleosome occupancy was determined as previously described (30).
RESULTS
Mutant core promoter transgenes are expressed in vivo.
Our earlier studies found that none of the core promoter mutations abrogated expression of a reporter gene in transient transfections of HeLa cells or transcription in vitro (33). This led us to ask whether the core promoter elements affected promoter activity in vivo. In order to assess their role in the context of the native MHC class I gene, PD1, we mutated the four elements (CAAT, TATAA-like, Sp1BS, and Inr) individually (Fig. 1A; also see data at DinahSingerLab.cancer.gov). Each construct consisted of 1 kb of 5′ upstream sequence, 3.4 kb of coding sequence, and 1.1 kb of 3′ downstream sequence. The wild-type PD1 genomic clone has been shown previously to direct normal patterns of expression in PD1L transgenic mice (39, 40). The mutants were first assessed for their expression in transient-transfection assays in HeLa cells. Consistent with the reporter assays, all of the core promoter element mutations directed cell surface expression of the PD1 gene at levels comparable to those of the wild type (see Fig. 1C at DinahSingerLab.cancer.gov).
To examine the role of these elements in vivo, we generated transgenic mice with either the WT promoter or mutant core promoters driving the PD1 gene. Multiple founders of each construct were derived and analyzed for transgene copy number and cell surface expression of the transgenic PD1 class I molecule (Fig. 1; also see Table 1 at DinahSingerLab.cancer.gov). Transgene copy numbers varied between 1 and >20 across all of the lines (Fig. 1; also see Table 2 at DinahSingerLab.cancer.gov). Founder mice with the WT promoter transgene expressed PD1 on their peripheral blood lymphocytes (PBL) (Fig. 1B, WT). Surprisingly, all of the core promoter mutants were capable of directing expression of PD1 transgenes (Fig. 1B). Among the Inr and TATAA-like mutants, virtually all of the founder mice expressed the transgene on their PBL; the large majority of the SP1BS and CAAT founder mice expressed the transgene (see Table 1 at DinahSingerLab.cancer.gov).
Integration sites varied among the founders, as evidenced by In situ hybridization of metaphase spreads (data not shown; also see Fig. 3 and Table 2 at DinahSingerLab.cancer.gov). Of note, in one of the TATAA-like mutant founder lines, the transgene integrated into the endogenous MHC locus H-2. Nevertheless, as will be discussed below, its pattern of expression differed from that of the H-2 class I gene but paralleled that of another TATAA-like mutant founder line with an integration site on a different chromosome. Similarly, one of the Sp1BS mutant transgenes integrated into the same locus as the wild-type transgene. However, the WT and Sp1BS transgenes displayed distinct patterns of expression (Fig. 1C and G). These findings indicate that expression is integration site independent.
Taken together, these results demonstrate that no single core promoter element is absolutely required for the activity of a MHC class I promoter.
Core promoter elements contribute to the regulation of tissue-specific expression.
The finding that the core promoter mutants were expressed in vivo raised the question of whether the core promoter elements play a role in either tissue-specific patterns of expression or responses to hormonal or cytokine stimuli. In order to determine whether any of the core promoter elements contribute to tissue-specific expression, we established two or more lines from founder mice of each promoter mutant and assessed the levels of RNA from different tissues of the transgenic mice both by real-time quantitative PCR to determine absolute levels and by Northern blotting to confirm the synthesis of full-length intact message. Three tissues representing the highest to lowest levels of endogenous MHC class I expression were examined: spleen, kidney, and brain.
As noted above, transgene copy numbers varied among the lines. Because it has been reported that levels of transgene expression are correlated with copy number, we first examined tissue expression patterns in those lines with a single copy of the transgene. The WT and the Inr, CAAT, and Sp1BS mutant constructs each generated a single-copy line; all transgenic lines derived from the TATAA-like mutant had multiple copies. To allow direct comparisons among the tissues, PCR results were normalized both to 18S RNA levels and to the level of PD1 RNA in the spleens of the WT PD1 transgenic mouse (Fig. 1C). The WT transgene was expressed with a pattern indistinguishable from that of a previously described PD1 transgene, the PD1L transgene, or the endogenous MHC class I genes (Fig. 1C). Importantly, each mutant promoter transgene expressed with a pattern distinct from that of the wild type (Fig. 1C). Furthermore, in at least one of the three tissues examined, each of the single-copy mutant lines expressed at a level higher than that of the wild type (Fig. 1C). Thus, mutation of the Inr promoter resulted in elevated transgene expression in spleen, kidney, and brain (Fig. 1C). In contrast, the level of CAAT mutant transgene expression in the spleen was indistinguishable from that of the wild type, but it was significantly higher in both kidney and brain (Fig. 1C). Expression of the single-copy Sp1BS mutant transgene was higher than that in the wild type in the spleen and brain (Fig. 1C). Although the TATAA mutant had multiple copies of the transgene, expression was markedly increased in spleen but only modestly in brain or kidney (Fig. 1C).
To determine whether the observed patterns of expression are integration site dependent, at least two independently derived transgenic lines, with at least 3 mice from each line, were analyzed separately for each promoter mutant (Fig. 1D to G; note that the expression of each tissue is normalized to the WT level of that tissue). Analysis of the remaining lines of transgenic mice revealed that the patterns observed for the individual single-copy line generally paralleled the overall patterns observed among multiple lines for a single mutant and differed from the WT (Fig. 1D to G).
Taken together, these results demonstrate that expression of this transgene is largely independent of copy number when assessed across multiple tissues. Copy number-independent expression has been observed previously for another MHC class I transgene, H-2Kb (45). Importantly, the data further indicate that the core promoter elements of the MHC class I gene can function as negative regulators of tissue-specific expression.
To derive a more accurate picture of the expression pattern for a single promoter element than could be generated from considering a single transgenic line, the expression data for a single promoter mutant were pooled (Fig. 2). Indeed, the pooled results largely paralleled those obtained with the single-copy transgenes. Thus, although all of the promoter element mutants were active in all three tissues examined, each mutant displayed a pattern of expression that differed from both the wild type and the other mutants. Mutation of the Inr element led to loss of tissue specificity and to significantly elevated expression in all three tissues examined relative to the wild type (Fig. 2B). Therefore, the Inr element of the MHC class I promoter contributes to both the magnitude and the tissue-specific patterns of expression. In contrast to the Inr mutant, the level of expression in the spleen of the construct mutated in the CAAT element was the same as that of the wild type but was significantly higher than that of the wild type in both kidney and brain (Fig. 2D). Thus, mutation of the CAAT element results in a loss of tissue specificity. Mutation of the Sp1 binding site did not markedly alter the tissue pattern of expression (Fig. 2C). However, the effect of mutating the Sp1BS element on expression levels was variegated among the transgenic founders. As will be discussed below, three of the founder lines did not express constitutively. Among the Sp1BS mutant transgenic lines that did express constitutively, they maintained tissue specificity but differed in their absolute levels of expression (see Fig. 2 at DinahSingerLab.cancer.gov). The level of expression was not directly correlated with copy number, since the single-copy transgene expressed at levels significantly higher than the WT. Mutating the TATAA-like element increased the absolute level of promoter activity, but the effect was seen primarily in the spleen and not in the other two tissues (Fig. 2E). This pattern of expression was observed in both strains of TATAA-like mutant promoter transgenes, where one transgene integrated into the mouse MHC locus on chromosome 17 and the other did not (see Fig. 2 and Table 2 at DinahSingerLab.cancer.gov). The same expression patterns were observed in Northern analysis for all of the mutants, indicating that the PCR was not detecting aberrant transcription (Fig. 2). Total PD1 protein levels, as assessed by Western blotting, in all of the mutants paralleled the RNA levels (data not shown).
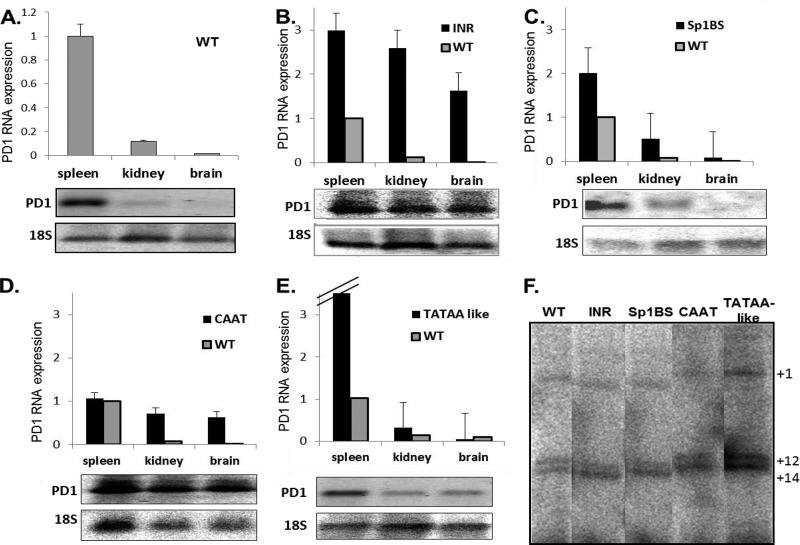
Fig 2.
Core promoter elements affect tissue-specific expression. The graphs represent qPCR averages of expressing strains for each mutant. The Northern blot analyses of PD1 RNA levels in tissues of the different core promoter mutant transgenic mice are representative. The WT (A), Inr mutants (T1069D9 and T1069H10) (B), Sp1BS mutants (T598A5 and T598H4) (C), CAAT mutants (T1068E4, T1068F4, and T1068T9) (D), and TATAA-like mutants (T580G1 and T580H5) (E) are shown. Experiments were repeated 3 times with at least 3 mice from each core promoter mutant transgenic line. (F) RNA primer extension analysis of in vivo TSS in spleen from WT and promoter mutant transgenic mice: Inr mutant (T1069D9), Sp1BS mutant (T598A5), CAAT mutant (T1068F4), and TATAA-like mutant (T580H5). The numbers on the right indicate base pairs relative to the TSS at +1. All of the samples were run on the same gel but were cropped for illustration purposes. This gel is representative of 3 experiments.
Thus, these core promoter elements are functional but in a distinctly different way than that of previously described canonical core promoter elements. It is striking that none of the core promoter element mutations resulted in levels of expression that were lower than that of the wild type, leading to the remarkable conclusion that these elements negatively regulate tissue-specific transcription.
Core promoter elements do not affect start site use.
We have shown both in vivo and in in vitro transcription assays that class I transcription initiates at a series of distinct transcription start sites (46). In order to assess whether the promoter activity of the mutants is due to novel start site selection in vivo, we performed primer extension assays on RNA extracted from spleens of mice transgenic for the various core promoter element mutations. Transcription in the WT transgenic mice initiated predominantly at bp +1, +12, and +14, as previously observed. The same start site use was observed in all of the promoter mutants, although some variation in the relative use of those start sites was noted (e.g., the preferential usage of bp +14 in the mutants) (Fig. 2F). Thus, mutation of the core promoter elements did not shift transcription initiation to an alternative promoter.
Promoter elements affect RNA Pol II occupancy along the MHC class I gene.
The finding that mutations in core promoter elements affect steady-state levels of class I RNA could reflect changes in the assembly of the transcription machinery, in transcription initiation, or even after transcription initiation (47). To distinguish among these possibilities, we examined RNA Pol II occupancy across the PD1 gene in tissues of mice with the different mutant transgenes. If the observed effects of the promoter mutations were transcriptional, the patterns of expression would be reflected in the extent of Pol II occupancy. In wild-type PD1 transgenic mice, the Pol II occupancy pattern paralleled RNA expression in the spleen, kidney, and brain (Fig. 3A). The highest Pol II levels were measured around the promoter in the spleen. Pol II occupancy also was consistently observed around bp −800, the site of the regulatory element that governs tissue-specific levels of expression (TSE), and around bp +4500, at exon 5. Consistent with their transcript levels (Fig. 2A), the levels of Pol II occupancy across the gene in the kidney and brain were low to undetectable, respectively.
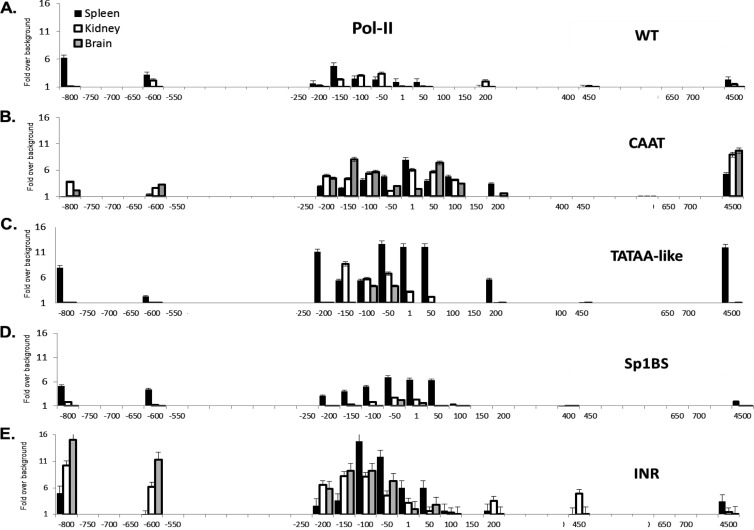
Fig 3.
Pol II occupancy correlates with activity of mutant core promoters. Pol II ChIP analysis of spleen, kidney, and brain from the WT and core promoter mutant strains. WT (A), CAAT (B), TATAA-like (C), Sp1BS (D), and Inr (E) core promoter mutant transgenic strains are shown. Note that the x axis denotes location relative to the TSS and is not to scale. ChIP was performed as described in Materials and Methods. Experiments were repeated three times with 3 mice from each core promoter mutant transgenic line. The strains that were used are detailed in Materials and Methods.
The patterns of Pol II occupancy across the transgenes of the mutant promoters also paralleled their patterns of RNA expression. The Sp1BS mutant transgene, which had the same tissue-specific patterns of expression as the wild type, also displayed the same overall patterns of Pol II promoter occupancy as the wild type in the different tissues but at a higher level in the spleen, consistent with its higher expression (Fig. 1C). Namely, Pol II occupancy clustered within the same region of the core promoter as in the wild type; another spike occurred around −800, where the TSE is located, and at exon 5 (Fig. 3D).
In the TATAA-like mutant, the overall pattern of Pol II occupancy paralleled that of the WT but was markedly higher across the transgene in the spleen and lower in kidney and brain (Fig. 3C), consistent with its higher expression. Surprisingly, binding of TATA binding protein (TBP) to the TATAA-like mutant promoter was higher than that in the WT (see Fig. 4A at DinahSingerLab.cancer.gov). Thus, although this element is highly conserved among all MHC class I genes in all species, it does not function as a canonical TATAA box. Rather, it functions as a tissue-specific regulatory element. (Since there are no other TATAA-like boxes within at least 500 bp downstream of the core promoter, the target sequence for TBP binding remains to be determined.) TBP binding to the endogenous H-2Kb, which has a canonical TATAA box, parallels that of the WT PD1 gene (see Fig. 4B at DinahSingerLab.cancer.gov). The function of the H-2Kb TATAA box remains to be determined.
Pol II occupancy on the CAAT mutant promoter in the spleen also was similar in magnitude to the wild type but appeared to be distributed more broadly (Fig. 3B). Again reflecting tissue patterns of expression, Pol II occupancies in the kidney and brain were the same in distribution and magnitude as the spleen (Fig. 3B). In contrast to the levels of Pol II around the core promoter, there was little Pol II occupancy on the TSE in the spleen. The accumulation of Pol II at the promoter in all three tissues, along with the loss of Pol II at the TSE, is consistent with the loss of tissue-specific expression caused by mutation of the CAAT element.
Finally, in transgenic mice with mutations of the Inr element, where tissue specificity is lost, Pol II occupancy is elevated and more broadly distributed across the core promoter than in the wild type (Fig. 3E). In contrast to the CAAT mutant, Pol II occupancy of the TSE was high in all three tissues and low at exon 5 (compare Fig. 3B to E). Thus, the mechanisms leading to loss of tissue-specific regulation in the CAAT and Inr mutants may be distinct. Taken together, these studies demonstrate that the core promoter elements play a role in establishing the magnitude of Pol II occupancy and transcription initiation.
Histone modifications associated with the MHC class I gene reflect the altered expression patterns of the mutant core promoter elements.
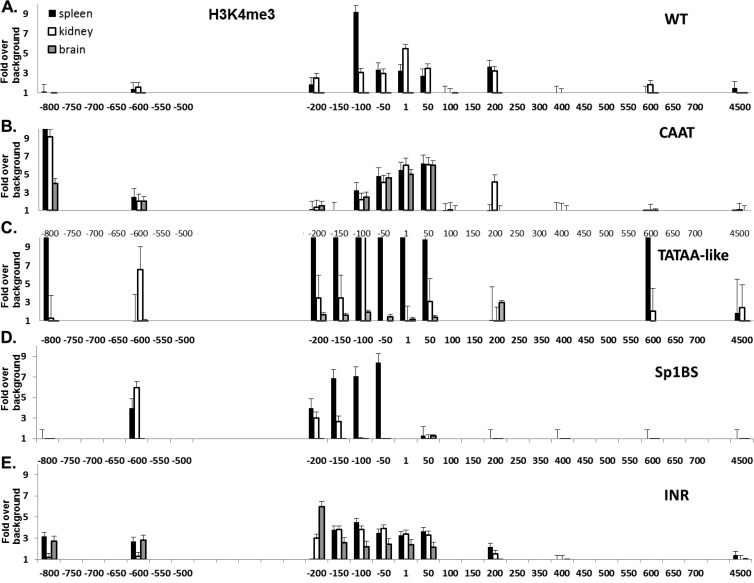
Different histone modifications are associated with genes that are either transcriptionally active or silent, leading us to ask whether there was a correlation between the expression patterns observed among the core promoter element mutants and their histone marks. We probed two distinct histone modifications: H3K4me3, a mark of active promoters, and H3K9me3, a mark of inactive transcription (48). The WT patterns of H3K4me3 levels around the core promoter are high in the spleen and kidney and undetectable in brain (Fig. 4A) (30). Among the core promoter element mutants, the patterns of H3K4me3 correlated closely with the patterns of tissue RNA expression and levels of Pol II occupancy. Thus, in the Sp1BS and TATAA-like mutant transgenics, H3K4me3 levels were high in those tissues where Pol II levels and expression were high but low in the other tissues (Fig. 4C and D). H3K4me3 levels were high in all three tissues of the CAAT and Inr mutants, where Pol II levels and expression were also high (Fig. 4B and E). Thus, there is a direct correlation between promoter activity and H3K4me3 chromatin modifications.
Fig 4.
Chromatin histone modification H3K4me3 correlates with activity of mutant core promoters. H3K4me3 ChIP analysis of spleen, kidney, and brain from the WT and core promoter mutant strains. ChIP was performed as described in Materials and Methods. WT (A), CAAT (B), TATAA-like (C), Sp1BS (D), and Inr (E) core promoter mutant transgenic strains are shown. Note that the x axis denotes location relative to the TSS and is not to scale. Experiments were repeated three times with 3 mice from each core promoter mutant transgenic line. The strains that were used are detailed in Materials and Methods.
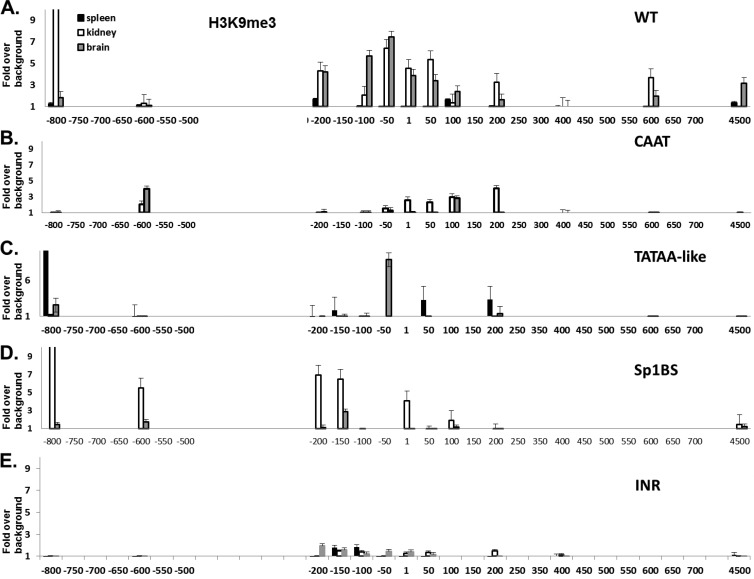
H3K9me3 marks, associated with silent genes, in the WT are inversely correlated with levels of transcription along the PD1 gene (Fig. 5A). In the CAAT and Inr promoter mutant transgenics, the H3K9me3 marks were low in all tissues (Fig. 5B and E), reflecting the aberrantly high PD1 expression in the tissues of those mice. Surprisingly, in the TATAA-like and Sp1BS core promoter mutants, the H3K9me3 patterns were not closely correlated with expression (Fig. 5C and D). In both of these mutants, H3K9me3 expression levels are low in the brain.
Fig 5.
Chromatin histone modification H3K9me3 correlates with activity of WT as well as with CAAT and Inr, but not with TATAA-like and Sp1BS, mutant core promoters. H3K9me3 ChIP analysis of spleen, kidney, and brain from the WT and core promoter mutant strains. ChIP was performed as described in Materials and Methods. WT (A), CAAT (B), TATAA-like (C), Sp1BS (D), and Inr (E) core promoter mutant transgenic strains are shown. Note that the x axis denotes location relative to the TSS and is not to scale. Experiments were repeated 3 times with 3 mice from each core promoter mutant transgenic line. The strains that were used are detailed in Materials and Methods.
These results indicate that the H3K4me3 activation marks associated with chromatin on the core promoter mutants are closely correlated with promoter activity, whereas the H3K9me3 marks are found in some cases but not others.
Sp1BS and Inr promoter mutants have aberrant responses to IFN-γ treatment.
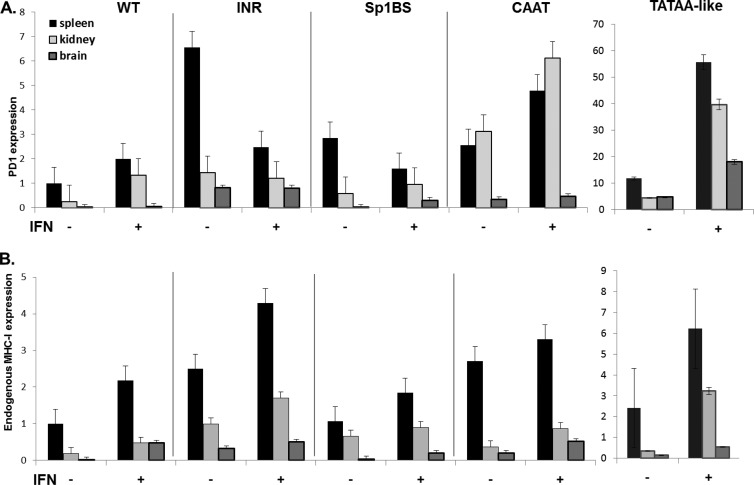
To further characterize the function of core promoter elements in vivo, we examined the responses of the different transgenic strains to extracellular signaling, as assessed by their gamma interferon (IFN-γ) responses (Fig. 6A). As internal controls for the IFN-γ, the levels of the endogenous MHC class I gene, H-2Kb, were monitored in the same mice (Fig. 6B). Transcription of both the WT PD1 transgene and endogenous MHC class I genes is activated by IFN-γ in all tissues (Fig. 6) (30). (It should be noted that the magnitude of the IFN-γ response in a given tissue varies among individual mice, both for endogenous and exogenous MHC class I genes, between about 1.5- and 5-fold.) As we have shown previously, the WT PD1 transgene response is indistinguishable from that of the endogenous H-2Kb genes (31). Interestingly, each of the mutant transgenes responded to IFN-γ treatment, as assessed by RNA levels, but responded differently. As expected, WT PD1 RNA levels were increased in all three tissues following IFN-γ treatment (Fig. 6A). Transgenes with mutations in either the TATAA-like or CAAT promoter element responded similarly to the wild type, with increased overall expression following IFN-γ treatment (Fig. 6A). Thus, although these two elements enable tissue-specific patterns of expression, neither is necessary for IFN-γ-activated transcription. Remarkably, and in striking contrast, PD1 RNA levels decreased markedly in response to IFN-γ in the spleens of mice transgenic for Inr and Sp1BS promoter element mutations (Fig. 6A). In these same tissues, the levels of endogenous H2-Kb RNA increased, demonstrating that the IFN-γ treatment was effective (Fig. 6B). Interferon treatment of a second Sp1BS transgenic line resulted in a similar decrease in splenic RNA expression (see Fig. 5 at DinahSingerLab.cancer.gov).
Fig 6.
Core promoter element mutants Sp1BS and Inr modulate the IFN-γ response. Transgenic mice were treated with IFN-γ as described in Materials and Methods. RNA from spleen, kidney, and brain was extracted and subjected to real-time PCR. (A) Real-time PCRs were performed using a specific exon 2 to 3 junction primer set of the PD1 gene. (B) Real-time PCRs using primers specific for the endogenous mouse MHC class I H2-Kb gene in the tissues of the same mice. All real-time PCRs are relative to an 18S standard. A summary of the P value calculations for the significance of IFN-γ induction is provided in Table 3 at DinahSingerLab.cancer.gov. The strains that were used are detailed in Materials and Methods.
These results lead to the surprising conclusion that whereas the CAAT and TATAA-like mutants are activated by IFN-γ, core promoters mutated in either Sp1BS or Inr element mediate an aberrant response to the extracellular signaling cascade initiated by IFN-γ.
Sp1 protein associates with cryptic binding sites in the promoter of the Sp1BS mutant.
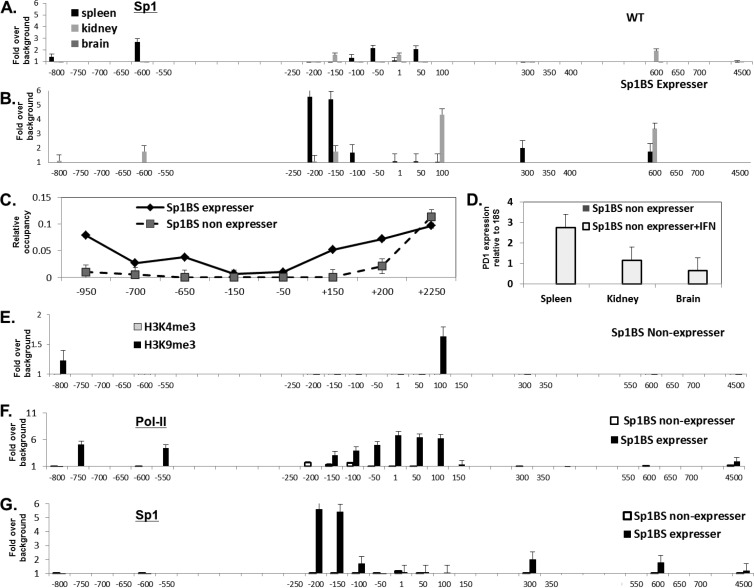
We have shown previously that the Sp1BS core promoter element contributes to the level of PD1 promoter activity in transient transfections of a reporter plasmid and that Sp1 protein binds to that site in vitro (33). Thus, it was surprising that mutation of the Sp1BS core promoter element did not abrogate promoter activity in the context of the full-length PD1 transgene in vivo. To determine if Sp1 protein itself plays a role in PD1 transcription in vivo, we assessed its association with the PD1 gene in tissues of the WT transgene and in the derivative Sp1BS core promoter element mutant transgenics. ChIP analysis of the WT PD1 gene localized Sp1 protein binding to the core promoter region in the spleen and kidney, with undetectable levels in the brain, paralleling the observed pattern of expression (Fig. 7A). Additional weak binding was observed in upstream and downstream regions. The Sp1 protein binding pattern was markedly different on the Sp1BS mutant transgene: no Sp1 protein binding was detected around the core promoter, but significant binding was observed upstream of the core promoter, as well as downstream in intron 1 in the spleen (Fig. 7B). Weaker binding of the same pattern was observed in kidney (Fig. 7B). Thus, the activity of the Sp1BS mutant promoter may be due to alternative sites of Sp1 protein binding that are enabled in the absence of the native core promoter site. Indeed, inspection of the PD1 sequence reveals a series of canonical Sp1 protein binding sites in the regions of detected binding (data not shown).
Fig 7.
Sp1BS mutant transgene constitutive nonexpresser binds Sp1 anomalously and is induced to express by IFN-γ treatment. (A and B) ChIP analyses with anti-Sp1-specific antibody on tissues from WT (A) and Sp1BS expresser mice (T598A5) (B). (C) The relative nucleosome occupancy across the PD1 gene in the spleens of expresser and nonexpresser Sp1BS mutant transgenic mice (T598A5 and T598J5, respectively) was determined as described in Materials and Methods. (D) SP1BS nonexpresser (T598J5) mice were treated with IFN-γ or mock treated as described in Materials and Methods; the levels of PD1 RNA in spleen, kidney, and brain were measured by real-time quantitative PCR. (E) ChIP assays for H3K4me3 and H3K9me3 were performed on chromatin extracted from spleens of Sp1BS nonexpresser (T598J5) mice; compare to data for the Sp1BS expresser shown in in Fig. 4 and 5. (F) ChIP for Pol II was performed on chromatin extracted from spleens of Sp1BS expresser and nonexpresser (T598A5 and T598J5, respectively) mice. (G) ChIP for Sp1 antibody was performed on chromatin extracted from spleens of Sp1BS expresser and nonexpresser (T598A5 and T598J5, respectively) mice. ChIP was performed as described in Materials and Methods. In panels A to C and E to G, the x axis denotes location relative to the TSS and is not to scale.
All Sp1BS core promoter mutant transgenes are capable of supporting transcription.
As noted above, while 11 of the 14 founder mice with the Sp1BS core promoter element mutation constitutively expressed high levels of surface PD1 on their PBL, three did not. Indeed, no steady-state PD1 RNA could be detected in spleen, kidney, or brain of those mice by PCR, nor could any transcription initiation be detected by primer extension (Fig. 7D and data not shown). To determine whether the lack of detectable expression was due to chromatin inaccessibility of the DNA, nucleosome occupancy was assessed in chromatin from spleens of both Sp1BS mutant lines that constitutively expressed the transgene (termed expressers) and those that did not (nonexpressers). Unexpectedly, we found that spleens from both the expressers and nonexpressers have a nucleosome-free region around the core promoter with a pattern that parallels that observed for the WT promoter (Fig. 7C). Thus, the promoters of the Sp1BS mutant transgenic nonexpressers are in an open chromatin conformation and presumably are accessible to the transcription machinery. However, the chromatin modifications associated with the Sp1BS mutant transgenes reflect their expression status: H3K4me3 is associated with the chromatin of the expresser line (Fig. 4D) but not with the nonexpresser Sp1BS mutant transgenic lines (Fig. 7E). In contrast, the H3K9me3 mark associated with inactive promoters is found on chromatin from the nonexpresser transgenic line but not the expresser Sp1BS mutant transgenic lines (Fig. 7E, nonexpresser, and 5D, expresser).
To determine whether differences in Pol II occupancy are responsible for the differences between the constitutive nonexpresser and expresser Sp1BS mice, we next examined the binding of Pol II and Sp1 protein to the Sp1BS transgene of the nonexpresser line. Pol II binds around the promoter region of the nonexpresser, but the binding is much lower than that to the expresser (Fig. 7F). In addition, whereas the majority of Pol II binding is centered on the TSS in the line that expresses the transgene, in the expresser line Sp1 protein binding appears to be concentrated upstream of the TSS. Sp1 protein binding to the nonexpresser transgene was barely detectable (Fig. 7G). The causal relationships among Pol II and Sp1 binding and histone modifications are not known. Similarly, it is not known what distinguishes the few Sp1BS mutant transgenic mice that do not constitutively express from the majority that do.
Although the nonexpresser Sp1BS mutant promoters did not support detectable basal transcription, we asked whether transcription could be activated by IFN-γ. Remarkably, in all three tissues from the constitutive nonexpresser line, IFN-γ induced PD1 transcription to about the same extent and with the same pattern as the endogenous H-2Kb class I gene (Fig. 7D; also see Fig. 6 at DinahSingerLab.cancer.gov). Thus, the Sp1BS mutant promoter in transgenic lines that do not detectably express constitutively are not completely inactive, since the chromatin is open and normally responsive to treatment. Although effects of insertion site and copy number on expression cannot be definitively ruled out, these results do demonstrate that all of the transgenics with an Sp1BS core promoter element mutation are capable of expressing the transgene. The finding that IFN-γ induces PD1 expression in the constitutive nonexpresser Sp1BS mutant lines was particularly surprising in view of the result (Fig. 6A) that IFN-γ reduced expression of the transgene in spleens of the expresser Sp1BS mutant promoter transgenic line. The possible basis for this difference will be discussed below.
DISCUSSION
The core promoter is defined as the region surrounding the TSS where general transcription factors bind and assemble a transcription preinitiation complex. Implicit in this perspective is that the core promoter is a passive recipient of upstream signals, and its canonical elements serve only as docking sites and not as active regulatory elements. The studies we report here challenge that view for genes subject to complex regulatory mechanisms. We show that in the MHC class I gene, which is ubiquitously expressed but subject to both tissue-specific and dynamic regulation, none of the core promoter elements, which are homologous to canonical core promoter elements, is essential for promoter activity. However, they do actively participate in integrating upstream signals and in regulating transcription.
Transcription of the MHC class I gene begins at multiple sites between bp −18 and +14, with the major site defined as bp +1. By definition, the core promoter spans this segment. In the region extending from −58 to +14 are 4 elements with homologies to known core promoter elements: the CAAT box, the TATAA-like box, Sp1BS, and Inr. All of the promoters with mutations in individual core promoter elements within the context of the native gene in vivo were transcriptionally active. None was essential for promoter function.
Even more surprising, all of the mutations were capable of supporting constitutive expression at levels higher than the wild type. Furthermore, transcription initiated at the same start sites as the wild type. Indeed, all of these finding suggest that these MHC class I core promoter elements are negative regulators of transcription, a conclusion consistent with the known regulation of constitutive MHC class I expression. The PD1 extended promoter contains a complex regulatory element, located between bp −771 and −696, consisting of overlapping enhancer and silencer elements. Tissue-specific levels of transcription are controlled by the relative levels of silencer binding factors, which are inversely correlated with MHC class I RNA levels. In contrast, enhancer binding factors do not change markedly among different tissues (35). Thus, we speculate that mutation of core promoter elements relieves silencer function, resulting in increased transcription. Each class I core promoter element contributes in a unique way to establishing appropriate levels of tissue-specific basal and hormonal dynamic transcription. Together, they coordinate an orchestrated response to upstream signals: the CAAT box modulates expression in nonlymphoid tissues (kidney and brain), whereas the TATAA-like elements control transcription in lymphoid tissues (spleen). The Inr is a negative regulator of transcription in both lymphoid and nonlymphoid tissues.
The role of the Sp1BS element in regulating constitutive expression is less clear. Unlike the other core promoter element mutations, mutation of the Sp1BS element results in variegated expression among the different lines of transgenic mice. In those lines where it is expressed, the mutant Sp1BS transgene maintains the wild-type tissue-specific patterns of expression at levels equal to or higher than that of the wild type. In the constitutive nonexpresser line, the chromatin around the promoter is in an open conformation with low levels of bound Pol II. That these differences in expression are due to insertion site or copy number cannot be completely excluded. However, it is important to note that the highest level of expression was observed with the single-copy transgene, raising the possibility that the Sp1BS element functions as a general modulator of constitutive promoter activity.
Although all four core promoter elements regulate constitutive, tissue-specific patterns of expression, only two, the CAAT and TATAA-like promoters, parallel the wild-type MHC class I gene response to interferon with increased transcription in spleen, kidney, and brain. In contrast, in vivo interferon treatment results in aberrant responses by the Inr and Sp1BS mutant promoters. In the Inr mutant, transcription in the kidney and brain is unaffected by interferon treatment, whereas it is repressed in spleen. The response of the Sp1BS mutant is tissue specific: interferon represses transcription in the spleen but induces it in brain and kidney. The mechanisms underlying the anomalous responses of the Sp1BS and Inr mutants are not known. It is possible that the responses reflect the distinct regulatory pathways utilized for basal and activated transcription of the class I gene. In the absence of interferon, transcription depends primarily on TFIID-mediated transcription complexes and upstream start sites, while interferon induces the coactivator CIITA, which bypasses the requirement for TFIID and targets downstream start sites (33). It is tempting to speculate that interferon treatment bypasses the roles of the Inr and Sp1BS elements in favor of remaining wild-type promoter elements. If those elements are intrinsically less active, the net result of interferon-induced bypassing of the Inr or Sp1BS element combined with activation of the other elements would be a reduction in overall expression. Consistent with this interpretation is the finding that among the Sp1BS mutant transgenic lines with undetectable basal class I expression, interferon treatment induced expression in the tissues of the Sp1BS transgenic mice. This suggests that whereas the Sp1BS element contributes to normal basal transcription, interferon targets other core promoter elements. Future experiments will assess this model.
The patterns of expression among the core promoter mutant transgenic mice were independent of transgene copy. Among the CCAT, Inr, and Sp1BS core promoter mutants, single-copy transgene lines were derived. In each, the transgene was expressed and displayed aberrant tissue expression relative to the WT, with at least one tissue having expression that was higher than that of the WT. Furthermore, lines with multiple copies displayed the same general pattern of expression as the cognate single-copy mutant. Although it is generally thought that transgene expression is copy number dependent, similar copy number-independent expression has been reported for an MHC class I Kb transgene (45). What governs whether there is an effect of copy number on promoter activity remains to be determined. It is noteworthy that in lines with multiple copies, all were integrated in tandem at a single site (see Fig. 3 at DinahSingerLab.cancer.gov). Whether this contributes to the copy number independence also remains to be seen.
Taken together, our findings lead to a model in which each of the core promoter elements responds to upstream signaling by extracellular pathways, tissue-specific pathways, or both. Transcription then initiates within the core promoter at multiple TSS, whose relative usage is determined by the integrated functions of the targeted core promoter element(s) (see Fig. 7 at DinahSingerLab.cancer.gov).
The MHC class I core promoter elements not only contribute to transcriptional regulation but also perform the conventional functions of docking sites for the assembly of the transcription initiation machinery. Thus, the activity of each of the mutant core promoters is a direct reflection of abundance of Pol II recruited to the promoter in each tissue. They also serve to establish the patterns of histone methylation. In all of the core promoter mutants, histone H3K4 trimethylation is directly correlated with the level of promoter activity of the mutants, whereas H3K9 methylation is inversely correlated. This finding suggests that epigenetic modifications can reflect intrinsic promoter activity rather than regulate it.
In conclusion, the present studies have revealed the complex functional architecture of the MHC class I core promoter, which has the 2-fold function of (i) recruiting general transcription factors to the transcription preinitiation complex and (ii) regulating both tissue-specific and extracellular signaling responses, presumably through binding of various transcription factors and looping with upstream enhancer and silencer elements. Unlike promoters that initiate at a single TSS and depend on specific core promoter elements for their function, the class I core promoter is best described as a flexible platform in which multiple elements contribute to regulating transcription initiation. No single element is required for the recruitment of either general transcription factors or Pol II. In support of this view of the core promoter, in vitro assays demonstrated TFIID binding efficiently both to a canonical TATA/Inr promoter (termed the super core promoter) and to variants mutated in the TATA or Inr elements (49). It remains to be determined whether core promoters associated with other genes that are subject to complex regulation and initiate at multiple TSS are similarly complex. Finally, the present findings also leave open the question of what identifies a core promoter within the context of the genome.
ACKNOWLEDGMENTS
We thank David Grindler, Sara Williford, and Alison Vollmer for their help in the initial analysis of the transgenic lines and Dara Wangsa for her help with the metaphase spread experiments. We also thank Kevin Howcroft, Brian Lewis, and Ranjan Sen and members of the laboratory for helpful discussions and critical review of the manuscript.
We declare that we have no conflict of interest.
This research was supported by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research.
Footnotes
Published ahead of print 9 September 2013
REFERENCES
- 1.Berk AJ. 1999. Activation of RNA polymerase II transcription. Curr. Opin. Cell Biol. 11:330–335 [DOI] [PubMed] [Google Scholar]
- 2.Roeder RG. 1996. The role of general initiation factors in transcription by RNA polymerase II. Trends Biochem. Sci. 21:327–335 [PubMed] [Google Scholar]
- 3.Chen JL, Attardi LD, Verrijzer CP, Yokomori K, Tjian R. 1994. Assembly of recombinant TFIID reveals differential coactivator requirements for distinct transcriptional activators. Cell 79:93–105 [DOI] [PubMed] [Google Scholar]
- 4.Gill G. 2001. Regulation of the initiation of eukaryotic transcription. Essays Biochem. 37:33–43 [DOI] [PubMed] [Google Scholar]
- 5.Kadonaga JT. 2004. Regulation of RNA polymerase II transcription by sequence-specific DNA binding factors. Cell 116:247–257 [DOI] [PubMed] [Google Scholar]
- 6.Struhl K. A paradigm for precision. Science 293:1054. [DOI] [PubMed] [Google Scholar]
- 7.Burke TW, Kadonaga JT. 1997. The downstream core promoter element, DPE, is conserved from Drosophila to humans and is recognized by TAFII60 of Drosophila. Genes Dev. 11:3020–3031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lagrange T, Kapanidis AN, Tang H, Reinberg D, Ebright RH. 1998. New core promoter element in RNA polymerase II-dependent transcription: sequence-specific DNA binding by transcription factor IIB. Genes Dev. 12:34–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Smale ST, Jain A, Kaufmann J, Emami KH, Lo K, Garraway IP. 1998. The initiator element: a paradigm for core promoter heterogeneity within metazoan protein-coding genes. Cold Spring Harb. Symp. Quant. Biol. 63:21–31 [DOI] [PubMed] [Google Scholar]
- 10.Kutach AK, Kadonaga JT. 2000. The downstream promoter element DPE appears to be as widely used as the TATA box in Drosophila core promoters. Mol. Cell Biol. 20:4754–4764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Smale ST. 2001. Core promoters: active contributors to combinatorial gene regulation. Genes Dev. 15:2503–2508 [DOI] [PubMed] [Google Scholar]
- 12.Butler JE, Kadonaga JT. 2002. The RNA polymerase II core promoter: a key component in the regulation of gene expression. Genes Dev. 16:2583–2592 [DOI] [PubMed] [Google Scholar]
- 13.Willy PJ, Kobayashi R, Kadonaga JT. 2000. A basal transcription factor that activates or represses transcription. Science 290:982–985 [DOI] [PubMed] [Google Scholar]
- 14.Singer VL, Wobbe CR, Struhl K. 1990. A wide variety of DNA sequences can functionally replace a yeast TATA element for transcriptional activation. Genes Dev. 4:636–645 [DOI] [PubMed] [Google Scholar]
- 15.Smale ST, Baltimore D. 1989. The “initiator” as a transcription control element. Cell 57:103–113 [DOI] [PubMed] [Google Scholar]
- 16.Zenzie-Gregory B, Khachi A, Garraway IP, Smale ST. 1993. Mechanism of initiator-mediated transcription: evidence for a functional interaction between the TATA-binding protein and DNA in the absence of a specific recognition sequence. Mol. Cell Biol. 13:3841–3849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kaufmann J, Smale ST. 1994. Direct recognition of initiator elements by a component of the transcription factor IID complex. Genes Dev. 8:821–829 [DOI] [PubMed] [Google Scholar]
- 18.Lo K, Smale ST. 1996. Generality of a functional initiator consensus sequence. Gene 182:13–22 [DOI] [PubMed] [Google Scholar]
- 19.Du X, Han L, Guo AY, Zhao Z. 2012. Features of methylation and gene expression in the promoter-associated CpG islands using human methylome data. Comp. Funct. Genomics 2012:598987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Takeshima H, Yamashita S, Shimazu T, Ushijima T. 2011. Effects of genome architecture and epigenetic factors on susceptibility of promoter CpG islands to aberrant DNA methylation induction. Genomics 98:182–188 [DOI] [PubMed] [Google Scholar]
- 21.Lee MP, Howcroft K, Kotekar A, Yang HH, Buetow KH, Singer DS. 2005. ATG deserts define a novel core promoter subclass. Genome Res. 15:1189–1197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee N, Iyer SS, Mu J, Weissman JD, Ohali A, Howcroft TK, Lewis BA, Singer DS. 2010. Three novel downstream promoter elements regulate MHC class I promoter activity in mammalian cells. PLoS One 5:e15278. 10.1371/journal.pone.0015278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee DH, Gershenzon N, Gupta M, Ioshikhes IP, Reinberg D, Lewis BA. 2005. Functional characterization of core promoter elements: the downstream core element is recognized by TAF1. Mol. Cell. Biol. 25:9674–9686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lewis BA, Sims RJ, III, Lane WS, Reinberg D. 2005. Functional characterization of core promoter elements: DPE-specific transcription requires the protein kinase CK2 and the PC4 coactivator. Mol. Cell 18:471–481 [DOI] [PubMed] [Google Scholar]
- 25.Kim TH, Barrera LO, Zheng M, Qu C, Singer MA, Richmond TA, Wu Y, Green RD, Ren B. 2005. A high-resolution map of active promoters in the human genome. Nature 436:876–880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang MQ, Elnitski LL. 2008. Diversity of core promoter elements comprising human bidirectional promoters. BMC Genomics 9(Suppl 2):S3. 10.1186/1471-2164-9-S2-S3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yokoyama KD, Thorne JL, Wray GA. 2011. Coordinated genome-wide modifications within proximal promoter cis-regulatory elements during vertebrate evolution. Genome Biol. Evol. 3:66–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gerstein MB, Kundaje A, Hariharan M, Landt SG, Yan KK, Cheng C, Mu XJ, Khurana E, Rozowsky J, Alexander R, Min R, Alves P, Abyzov A, Addleman N, Bhardwaj N, Boyle AP, Cayting P, Charos A, Chen DZ, Cheng Y, Clarke D, Eastman C, Euskirchen G, Frietze S, Fu Y, Gertz J, Grubert F, Harmanci A, Jain P, Kasowski M, Lacroute P, Leng J, Lian J, Monahan H, O'Geen H, Ouyang Z, Partridge EC, Patacsil D, Pauli F, Raha D, Ramirez L, Reddy TE, Reed B, Shi M, Slifer T, Wang J, Wu L, Yang X, Yip KY, Zilberman-Schapira G, Batzoglou S, Sidow A, Farnham PJ, Myers RM, Weissman SM, Snyder M. 2012. Architecture of the human regulatory network derived from ENCODE data. Nature 489:91–100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sanyal A, Lajoie BR, Jain G, Dekker J. 2012. The long-range interaction landscape of gene promoters. Nature 489:109–113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kotekar AS, Weissman JD, Gegonne A, Cohen H, Singer DS. 2008. Histone modifications, but not nucleosomal positioning, correlate with major histocompatibility complex class I promoter activity in different tissues in vivo. Mol. Cell. Biol. 28:7323–7336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ehrlich R, Sharrow SO, Maguire JE, Singer DS. 1989. Expression of a class I MHC transgene: effects of in vivo alpha/beta-interferon treatment. Immunogenetics 30:18–26 [DOI] [PubMed] [Google Scholar]
- 32.Giuliani C, Saji M, Bucci I, Fiore G, Liberatore M, Singer DS, Monaco F, Kohn LD, Napolitano G. 2006. Transcriptional regulation of major histocompatibility complex class I gene by insulin and IGF-I in FRTL-5 thyroid cells. J. Endocrinol. 189:605–615 [DOI] [PubMed] [Google Scholar]
- 33.Howcroft TK, Raval A, Weissman JD, Gegonne A, Singer DS. 2003. Distinct transcriptional pathways regulate basal and activated major histocompatibility complex class I expression. Mol. Cell. Biol. 23:3377–3391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Maguire JE, Ehrlich R, Frels WI, Singer DS. 1990. Regulation of expression of a class I major histocompatibility complex transgene. J. Reprod. Fertil. Suppl. 41:59–62 [PubMed] [Google Scholar]
- 35.Weissman JD, Singer DS. 1991. A complex regulatory DNA element associated with a major histocompatibility complex class I gene consists of both a silencer and an enhancer. Mol. Cell. Biol. 11:4217–4227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lefebvre S, Berrih-Aknin S, Adrian F, Moreau P, Poea S, Gourand L, Dausset J, Carosella ED, Paul P. 2001. A specific interferon (IFN)-stimulated response element of the distal HLA-G promoter binds IFN-regulatory factor 1 and mediates enhancement of this nonclassical class I gene by IFN-beta. J. Biol. Chem. 276:6133–6139 [DOI] [PubMed] [Google Scholar]
- 37.Kirshner S, Palmer L, Bodor J, Saji M, Kohn LD, Singer DS. 2000. Major histocompatibility class I gene transcription in thyrocytes: a series of interacting regulatory DNA sequence elements mediate thyrotropin/cyclic adenosine 3′,5′-monophosphate repression. Mol. Endocrinol. 14:82–98 [DOI] [PubMed] [Google Scholar]
- 38.Howcroft T, Singer D. 2003. Expression of nonclassical MHC class Ib genes: comparison of regulatory elements. Immunol. Res. 27:1–30 [DOI] [PubMed] [Google Scholar]
- 39.Frels WI, Bluestone JA, Hodes RJ, Capecchi MR, Singer DS. 1985. Expression of a microinjected porcine class I major histocompatibility complex gene in transgenic mice. Science 228:577–580 [DOI] [PubMed] [Google Scholar]
- 40.Cohen H, Parekh P, Sercan Z, Kotekar A, Weissman JD, Singer DS. 2009. In vivo expression of MHC class I genes depends on the presence of a downstream barrier element. PLoS One 4:e6748. 10.1371/journal.pone.0006748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ballester M, Castello A, Ibanez E, Sanchez A, Folch JM. 2004. Real-time quantitative PCR-based system for determining transgene copy number in transgenic animals. Biotechniques 37:610–613 [DOI] [PubMed] [Google Scholar]
- 42.Joshi M, Keith Pittman H, Haisch C, Verbanac K. 2008. Real-time PCR to determine transgene copy number and to quantitate the biolocalization of adoptively transferred cells from EGFP-transgenic mice. Biotechniques 45:247–258 [DOI] [PubMed] [Google Scholar]
- 43.Weissman JD, Brown JA, Howcroft TK, Hwang J, Chawla A, Roche PA, Schiltz L, Nakatani Y, Singer DS. 1998. HIV-1 tat binds TAFII250 and represses TAFII250-dependent transcription of major histocompatibility class I genes. Proc. Natl. Acad. Sci. U. S. A. 95:11601–11606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Landel CP, Stabley DL, Bundesen LQ. 1997. PCR identification of class I major histocompatibility complex genes transcribed in mouse blastocyst and placenta. J. Reprod. Immunol. 33:31–43 [DOI] [PubMed] [Google Scholar]
- 45.Hatina J, Frangoulis B, Gregorova S, Plichtova R, Pla M, Forejt J. 1999. Lymphoid specificity of a copy number-related expression of the H2-Kb transgene. Mol. Immunol. 36:73–80 [DOI] [PubMed] [Google Scholar]
- 46.Howcroft TK, Weissman JD, Gegonne A, Singer DS. 2005. A T lymphocyte-specific transcription complex containing RUNX1 activates MHC class I expression. J. Immunol. 174:2106–2115 [DOI] [PubMed] [Google Scholar]
- 47.Bregman A, Avraham-Kelbert M, Barkai O, Duek L, Guterman A, Choder M. 2011. Promoter elements regulate cytoplasmic mRNA decay. Cell 147:1473–1483 [DOI] [PubMed] [Google Scholar]
- 48.Barski A, Cuddapah S, Cui K, Roh TY, Schones DE, Wang Z, Wei G, Chepelev I, Zhao K. 2007. High-resolution profiling of histone methylations in the human genome. Cell 129:823–837 [DOI] [PubMed] [Google Scholar]
- 49.Cianfrocco MA, Kassavetis GA, Grob P, Fang J, Juven-Gershon T, Kadonaga JT, Nogales E. 2013. Human TFIID binds to core promoter DNA in a reorganized structural state. Cell 152:120–131 [DOI] [PMC free article] [PubMed] [Google Scholar]