Abstract
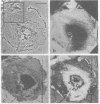
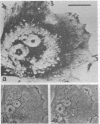
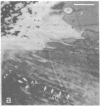
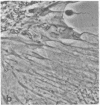
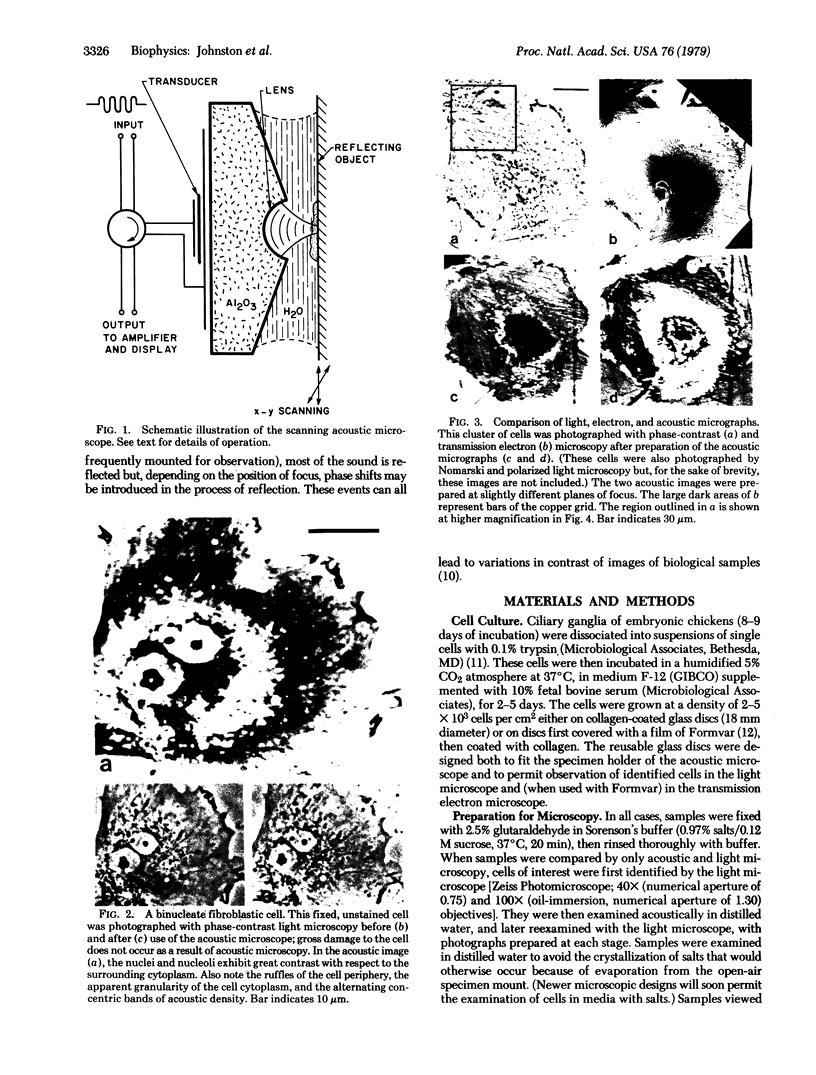
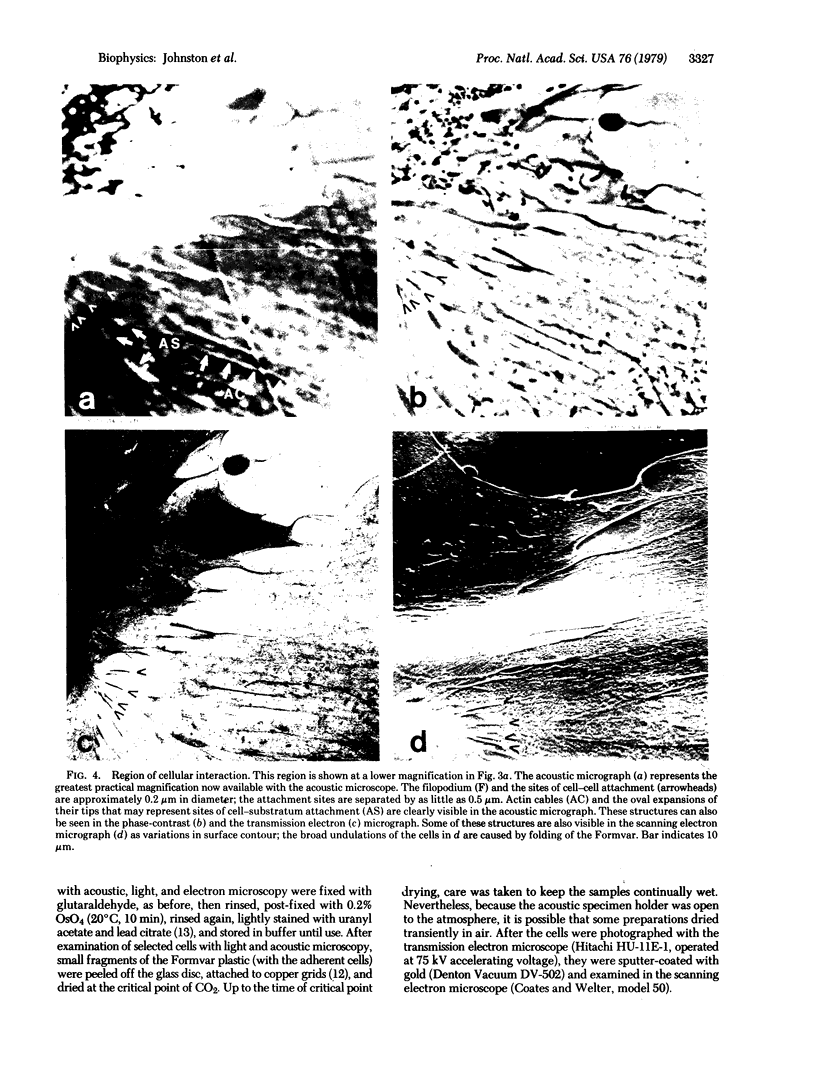
Recent advances now permit the use of scanning acoustic microscopy for the analysis of subcellular components. By sequential viewing of identified fixed cells with acoustic, light, and electron microscopy, we have established that the acoustic microscope can readily detect such features as nuclei and nucleoli, mitochondria, and actin cables. Under optimal conditions, images can even be obtained of filopodia, slender projections of the cell surface that are approximately 0.1-0.2 micron in diameter. Small objects separated by as little as 0.5-0.7 micron can successfully be resolved. Three aspects of the acoustic micrographs prepared in this preliminary survey seem especially prominent. These are, first, the extraordinary level of acoustic contrast that can differentiate the various cytoplasmic organelles, even in regions of very thin cytoplasm; second, the reversals in acoustic contrast that occur when altering the plane of focus; and third, the sensitivity of the acoustic response to overall cytoplasmic thickness. The acoustic microscope uses a novel source of contrast that is based on local mechanical properties. In addition, it can provide a degree of resolution that is comparable to that of the light microscope.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen R. D., David G. B., Nomarski G. The zeiss-Nomarski differential interference equipment for transmitted-light microscopy. Z Wiss Mikrosk. 1969 Nov;69(4):193–221. [PubMed] [Google Scholar]
- Buckley I. K., Porter K. R. Electron microscopy of critical point dried whole cultured cells. J Microsc. 1975 Jul;104(2):107–120. doi: 10.1111/j.1365-2818.1975.tb04010.x. [DOI] [PubMed] [Google Scholar]
- Helfand S. L., Smith G. A., Wessells N. K. Survival and development in culture of dissociated parasympathetic neurons from ciliary ganglia. Dev Biol. 1976 Jun;50(2):541–547. doi: 10.1016/0012-1606(76)90174-3. [DOI] [PubMed] [Google Scholar]
- Lemons R. A., Quate C. F. Acoustic microscopy: biomedical applications. Science. 1975 May 30;188(4191):905–911. [PubMed] [Google Scholar]
- Ludueña M. A., Wessells N. K. Cell locomotion, nerve elongation, and microfilaments. Dev Biol. 1973 Feb;30(2):427–440. doi: 10.1016/0012-1606(73)90100-0. [DOI] [PubMed] [Google Scholar]
- Marmor M. F., Wickramasinghe H. K., Lemons R. A. Acoustic microscopy of the human retina and pigment epithelium. Invest Ophthalmol Vis Sci. 1977 Jul;16(7):660–666. [PubMed] [Google Scholar]
- Osborn M., Weber K. The display of microtubules in transformed cells. Cell. 1977 Nov;12(3):561–571. doi: 10.1016/0092-8674(77)90257-4. [DOI] [PubMed] [Google Scholar]
- Wessells N. K., Ludueña M. A., Letourneau P. C., Wrenn J. T., Spooner B. S. Thorotrast uptake and transit in embryonic glia, heart fibroblasts and neurons in vitro. Tissue Cell. 1974;6(4):757–776. doi: 10.1016/0040-8166(74)90014-7. [DOI] [PubMed] [Google Scholar]