Abstract
αvβ3 integrin represents a novel sensing system which detects herpes simplex virus (HSV) and bacterial constituents. In cooperation with Toll-like receptor 2 (TLR2), it elicits an innate response that leads to activation of type I interferon (IFN), NF-κB, and a specific set of cytokines. We report that this defensive branch is functional in cells which represent experimental models of epithelial, including keratinocytic, and neuronal cells. These are the major targets of HSV in vivo. HSV entered the three cell lines via distinct routes. Hence, the defensive response was independent of the route of virus entry. Soluble gH/gL sufficed to elicit type I IFN and NF-κB activation and represents the viral pathogen-associated molecular pattern (PAMP) of this defense system.
TEXT
Pattern recognition receptors (PRRs) are responsible for sensing pathogens and for initiating signaling cascades which culminate in the innate response, whose hallmarks are the production of interferons (IFNs) and proinflammatory cytokines and the activation of the transcription factor NF-κB (1). Based on the nature of the pathogen-associated molecular pattern (PAMP) and the properties of the respective PRR, the innate response to herpesviruses can be divided into three main branches (for an in-depth review, see reference 2). Thus, the Toll-like receptors (TLRs) localized at the plasma membranes, e.g., TLR2, recognize proteic or lipidic PAMPs. The endosomal TLR3 and TLR7, as well as the cytoplasmic RIG-I (RNA helicase retinoic acid-inducible gene I), sense single- or double-stranded RNAs. The endosomal TLR9 and a number of cytosolic molecules, such as DAI (DNA-dependent activator of interferon-regulatory factor), IFI16 (gamma interferon-inducible protein 16), and AIM2 (absent in melanoma 2), etc., sense double-stranded DNA (2, 3).
Until recently, the prevalent paradigm has been that the IFN production occurs in response to activation of the cytosolic sensors, whereas the TLRs located at the plasma membrane elicit a predominantly inflammatory response (4–6). In particular, until recently, TLR2 was known to sense bacteria and was thought not to be involved in recognition of viruses. When a role for TLR2 against herpes simplex virus (HSV) and other herpesviruses was documented, TLR2 was found to be polarized toward an inflammatory response (7, 8). Kurt-Jones and coworkers (9) found that encephalitis caused by HSV was less severe in mice lacking TLR2, thus providing evidence for a role of TLR2 in the response to HSV and highlighting an inflammatory effect (10). In contrast with this view, Barbalat et al. (11) demonstrated that TLR2 was capable of inducing a type I IFN in response to viral ligands in inflammatory monocytes; the PAMPs remained elusive. Key to understanding the significance of the multiple, apparently redundant, innate defense branches against herpesviruses is the fact that they have been often demonstrated in highly specific cell types. For example, the TLR9 response was observed in plasmacytoid cells, the TLR9 and TLR2 responses were observed in dendritic cells, and the TLR2 response was observed in NK cells (12–14). Alternatively, the model HEK-293T cells have been employed in such studies (10). The roles of the various branches of the innate response to HSV in cell targets of the infection, e.g., epithelial and neuronal cells, have so far received little attention, apart from a number of studies focused on in vivo or ex vivo responses (9, 15–17).
Recently, we reported that αvβ3 integrin serves as a novel sensing system for HSV (18). It elicits the production of IFN-α and -β and a specific set of cytokines and receptors, interleukin-10 (IL-10), IL-2, and IL2R, as well as NF-κB activation (18). The HSV-induced NF-κB activation has long been known (19–22); however, its dependence on αvβ3 integrin was previously unknown. Evidence rested in loss-of-function experiments in 293T cells, in which β3 integrin was silenced, and in gain-of-function experiments in the myelocytic integrin-negative K562 cells, in which αvβ3 integrin was transgenically expressed. Two unprecedented features of this response were the cooperation between αvβ3 integrin and TLR2 and the ability of the αvβ3 integrin-TLR2 sensing system to elicit the IFN response. Thus, the TLR2-dependent response was greatly enhanced in the presence of αvβ3 integrin and, conversely, strongly inhibited when β3 integrin was silenced. In addition, αvβ3 integrin elicited a TLR2-independent, weaker response which targeted IRF3 and IRF7 through a cascade which involved SRC, SYK, and CARD9 (18). Because αvβ3 integrin is widely expressed, we proposed that what has been usually described as the TLR2-dependent response is indeed the response to the αvβ3 integrin-TLR2 sensing system (18). The αvβ3 integrin-TLR2 defensive branch is counteracted by the viral immediate early protein ICP0, since the highest IFN and NF-κB response was seen in cells infected with an ICP0 mutant. While this virus replicates very poorly in wild-type (WT) cells (generally positive for both αvβ3 integrin and TLR2), it replicated to the WT virus level in cells lacking TLR2 or αvβ3 integrin and to an even higher level in cells lacking both αvβ3 integrin and TLR2. ICP0 degrades MYD88, the adaptor downstream of TLR2 (23); this is likely the mechanism by which the HSV ICP0 mutant counteracts the αvβ3 integrin-TLR2 defense system.
To address the question of whether the αvβ3 integrin-dependent system is a bona fide activator of the anti-HSV innate response, we asked whether it plays a role in cells which represent experimental models of epithelial, including keratinocytic, and neuronal cells. These are the major targets of HSV infection in vivo. Because both αvβ3 integrin and TLR2 sense herpes simplex virus virions by means of the envelope glycoprotein complex gH/gL (18, 24), we further asked whether soluble gH/gL was sufficient to elicit this branch of the innate response. We report that silencing of β3 integrin in keratinocyte HaCaT cells, in HeLa cells, and in neuronal SK-N-SH cells decreased the production of type I IFN and IL-10, as well as NF-κB activation. Soluble gH/gL sufficed to elicit type I IFN and NF-κB activation and represents the viral PAMP of this defense system. In addition, we observed that HSV entered the three cell lines via distinct pathways. Hence, this defensive response is independent of the route of HSV entry.
Silencing of β3 integrin in HaCaT, HeLa, and SK-N-SH cells.
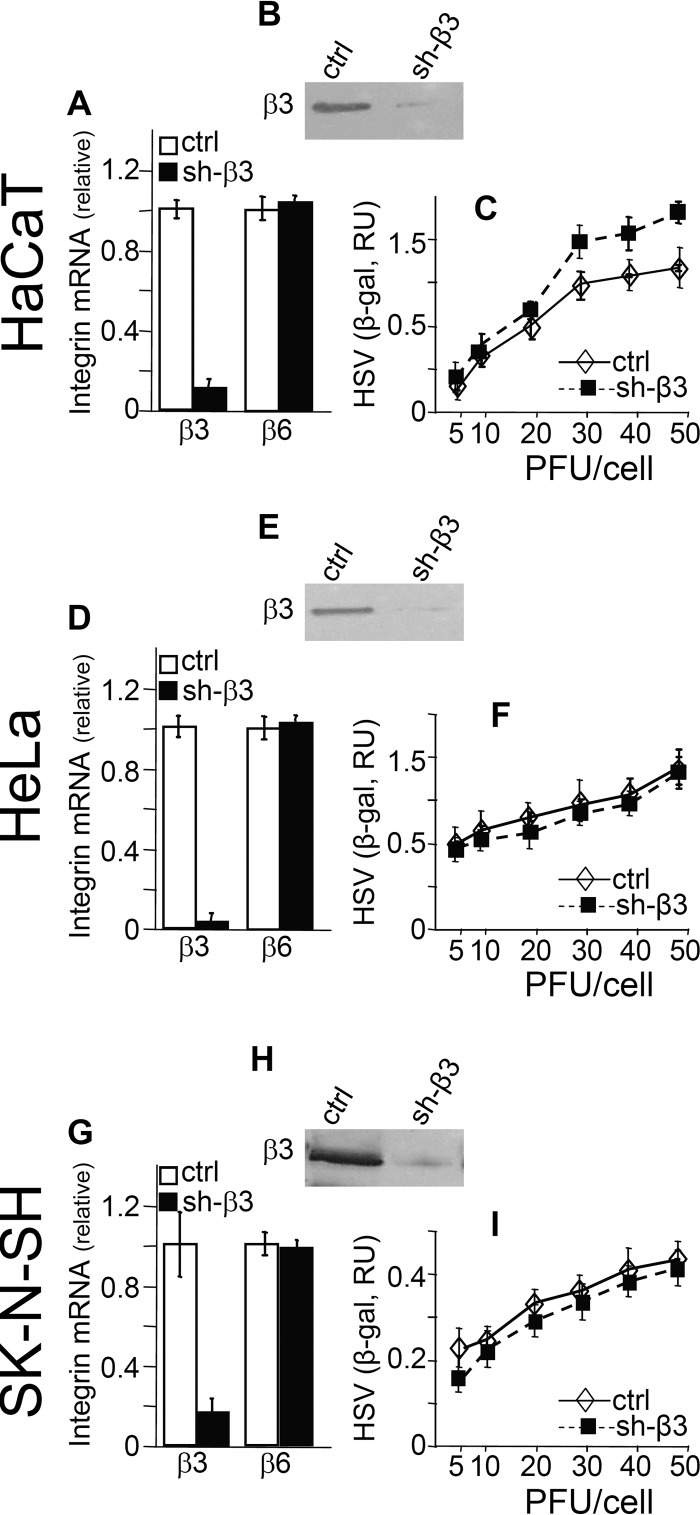
HaCaT, HeLa, and SK-N-SH cells express β3 integrin at similar levels, as measured by quantitative real-time PCR (qRT-PCR), with threshold cycle change (ΔCT) values of 13.56, 12.28, and 14.07, respectively. To determine the role of the αvβ3 integrin-dependent branch of innate immunity, we stably silenced β3 integrin by means of lentiviruses encoding short hairpin RNA to β3 (shRNA-β3) as described previously (18, 25). The silenced cells are herein called sh-β3 cells. Control cells were silenced by a control lentivirus (18). The extent of silencing was determined by qRT-PCR quantification of β3 integrin expression according to procedures described in detail elsewhere (18). Figure 1A, D, and G show that the extent of β3 integrin silencing in the three cell lines ranged between 80 and 95% at the mRNA level; it was confirmed at the protein level by immunoblotting (Fig. 1B, E, and H). The lack of off-target effects was assessed by measuring the effect of β3 integrin silencing on expression of β6 integrin, which was not modified (Fig. 1A, D, and G). Inasmuch as the innate response to HSV is influenced by the amount of virus which infects the cells, we ascertained that β3 integrin silencing did not grossly hamper HSV infection. To this end, β3 integrin-silenced and control cells were infected with increasing multiplicity of infection (MOI) of the recombinant HSV R8102, which carries a LacZ gene under the control of the α27 immediate early promoter (26). Considerable evidence indicates that the extent of β-galactosidase (β-gal) expression is proportional to the amount of virus which infects the cells (26, 27). Figure 1C, F, and I show that in β3 integrin-silenced cells, infection was not significantly inhibited in any cell type up to 20 PFU/cell, except for a modest increase in HaCaT cells.
Fig 1.
Silencing of β3 integrin in HaCaT, HeLa, and SK-N-SH cells and infection with the HSV recombinant R8102 of β3 integrin-silenced and control cells. Stable silencing was performed by means of lentiviruses encoding shRNA to β3 integrin (18, 25). The β3 integrin-silenced cells are indicated as sh-β3. Control (ctrl) cells received a control lentivirus with no insert. (A, D, and G) The extent of β3 integrin silencing was determined by quantitative real-time PCR (qRT-PCR) by means of the real-time PCR primers inventoried in the TaqMan gene expression assay, for ITGB3 (Hs01001469_m1) or ITGB6 (Hs00168458_m1) and GADPH (Hs02758991_g1) (Applied Biosystems). qRT-PCR was performed as described previously (18), and relative changes in gene expression were determined by 2−ΔΔCT. The results were normalized relative to control cells, for which the values were set as 1. (B, E, and H) The silencing was confirmed at the protein level by immunoblotting with polyclonal antibody (PAb) 1932 (Chemicon) to the β3 integrin subunit. The lack of off-target effect of β3 integrin silencing was determined by measuring the expression of β6 integrin, which was not changed relative to that in control cells (A, D, and G). RU, relative units. (C, F, and I) Infection of HaCaT, HeLa, and SK-N-SH was not grossly altered following β3 integrin silencing. sh-β3 and ctrl cells in 96 wells were infected with the HSV recombinant R8102, which carries a LacZ gene under the α27 promoter (26), at the indicated increasing MOI (PFU/cell), and harvested at 8 h after infection. The extent of infection was determined as β-gal expression (26), by means of o-nitrophenyl-β-d-galactopyranoside as the substrate. Each point represents the average of triplicates ± standard deviation.
Silencing of β3 integrin decreases NF-κB response in HaCaT, HeLa, and SK-N-SH cells.
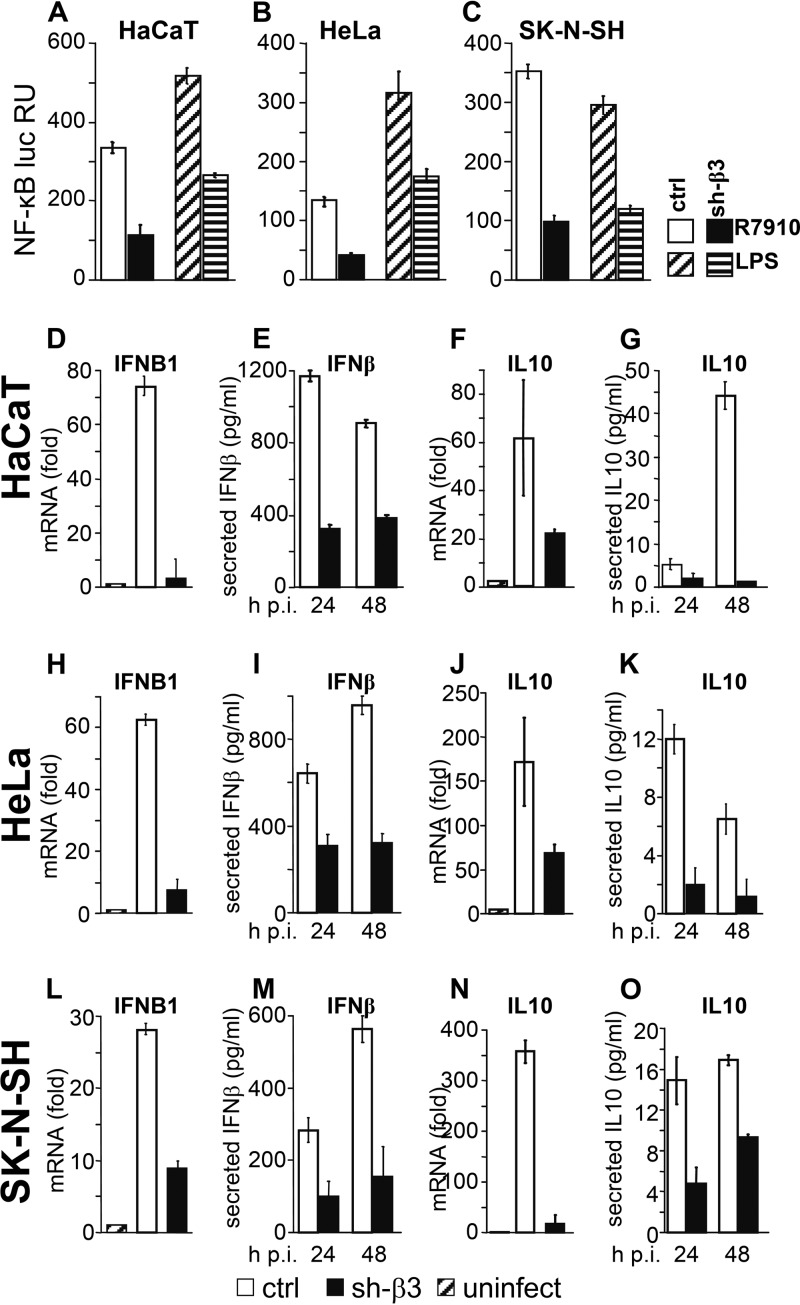
As a first measure of the effect of β3 integrin silencing on innate response, we determined the extent of NF-κB activation after infection with HSV. We made use of the HSV R7910 recombinant, which has a deletion within the gene encoding the immediate early protein ICP0 (28). ICP0 counteracts part of the host innate response (23, 29, 30), including the part mediated by αvβ3 integrin and TLR2 (18). To evaluate the response of the β3 integrin-silenced cells to bacterial components, cells were exposed to a commercial preparation of lipopolysaccharide (LPS) (L2630; Sigma-Aldrich, Milan, Italy) shown to induce a TLR2 response (18). Control and sh-β3 HaCaT, HeLa, and SK-N-SH cells were transfected with plasmids encoding luciferase (luc) under the NF-κB promoter (NF-κB–luc) and with Renilla luc, in a 130:1 ratio, and thereafter maintained for 3 days under preexhausted medium (18). They were then infected with R7910 (20 PFU/cell) for 6 h or exposed to LPS (100 ng/ml) for 4 h. Figure 2A to C show that silencing of β3 integrin resulted in a strong inhibition of NF-κB induction by either R7910 or LPS. Thus, both the virally and bacterially induced responses are hampered in the absence of β3 integrin. The finding that NF-κB induction was inhibited but not suppressed upon β3 integrin silencing is consistent with the notion that several innate sensing systems, including RIG-I as well as the gD interaction with herpesvirus entry mediator (HVEM), lead to NF-κB activation (2, 19, 31).
Fig 2.
Innate response in β3 integrin-silenced or control HaCaT, HeLa, and SK-N-SH cells. (A to C). sh-β3 and control (ctrl) cells were transfected with a plasmid encoding luciferase (luc) under the NF-κB promoter and Renilla luc (Promega) and kept under preexhausted medium for 3 days. They were then infected with R7910 (20 PFU/cell) or exposed to lipopolysaccharide (LPS; 100 ng/ml) from a commercial source (L2630; Sigma-Aldrich, Milan, Italy) for 4 h. NF-κB activity, read by means of the Dual-Glo luciferase report assay system (Promega), was expressed as the NF-κB/Renilla luc ratio. Each value represents the average of triplicates ± standard deviation. (D to O) sh-β3 and ctrl cells were infected with R7910 (20 PFU/cell) and harvested 6 h after infection (h p.i.) for determination of IFNB1 and IL-10 mRNA levels by qRT-PCR. Alternatively, the infected cell media were harvested 24 or 48 h after infection for determination of secreted IFN-β and IL-10, by means of the VeriKine kit (Pestka Biomedical Laboratories [PBL] Interferon Source) or enzyme-linked immunosorbent assay (ELISA) kit (Thermo Scientific, Pierce) as detailed previously (18). Each column represents the average of triplicates ± standard deviation.
As outlined above, in addition to NF-κB activation, hallmarks of the β3 integrin/TLR2 response in 293T cells are the production of IFN-α and -β and of a characteristic set of cytokines, including IL-10. Therefore, we measured the effect of β3 integrin silencing on the expression and secretion of IFN-β and of IL-10. WT and sh-β3 integrin-silenced HaCaT, HeLa, and SK-N-SH cells were infected with R7910 and harvested at 6 h after infection. The infected cell media were harvested at 24 and 48 h after infection for cytokine determination (18). In all three cell lines, qRT-PCR values for the IFNB1 (Fig. 2D, H, and L) and IL-10 (Fig. 2F, J, and N) mRNA levels, relative to the glyceraldehyde-3-phosphate dehydrogenase (GADPH) mRNA level, strongly increased upon cell infection and there was a dramatic decrease in these levels for sh-β3 cells relative to control cells. This inhibition resulted in a dramatic decrease in secretion of IFN and IL-10 (Fig. 2E, I, and M and G, K, and O, respectively). Thus, the production of IFN-β and IL-10 in epithelial and neuronal cell lines in response to R7910 infection is dependent on β3 integrin.
Soluble gH/gL suffices to induce the β3 integrin-dependent NF-κB response.
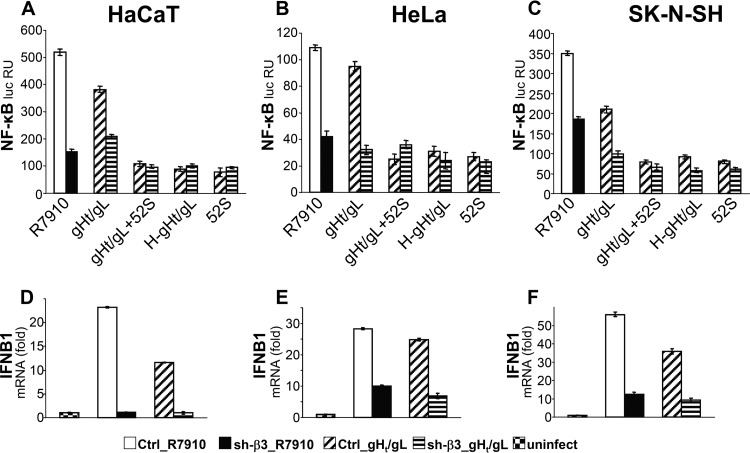
The viral ligands which elicit the TLR2-dependent IFN response remain generally elusive (11). Previously, we demonstrated that both αvβ3 integrin and TLR2 interact physically with gH/gL (18, 24). Thus, gH/gL cross-links the two receptors of this sensing system. Here, we asked whether a soluble form of gH/gL (32) suffices to induce the innate response in HaCaT, HeLa, and SK-N-SH cells. The control and β3 integrin silenced cells, transfected with NF-κB–luc and Renilla luc, were exposed to a truncated soluble form of gH/gL (gHt/gL) (32) (1.5 μM) for 4 h. This treatment was sufficient to elicit an NF-κB response, comparable to that elicited by R7910 (Fig. 3A to C). In the β3 integrin-silenced cells, the response was decreased by 40 to 50%. Soluble preparations of proteins may contain contaminants which elicit an innate response, among which are heat-stable bacterial components. We verified that the response elicited by soluble gHt/gL was authentic, either by preincubating gHt/gL with a neutralizing monoclonal antibody (MAb) to gH/gL, named 52S (33), or by heat denaturing gHt/gL. Both treatments reduced the NF-κB response to the background level (Fig. 3A to C). The IFN response elicited by gHt/gL was dependent on β3 integrin. Control and sh-β3 HaCaT, HeLa, and SK-N-SH cells were exposed to gHt/gL, or to R7910 as a control, for 6 h, and IFNB1 expression was quantified by qRT-PCR. The results in Fig. 3D to F show a dramatic decrease in IFNB1 expression in β3 integrin-silenced cells. Cumulatively, the experiments show that gHt/gL was sufficient to elicit an IFNB1 response dependent on β3 integrin in epithelial and neuronal cell lines.
Fig 3.
A soluble form of HSV gH/gL (gHt/gL) suffices to induce NF-κB activation and IFNB1 production. (A to C) sh-β3 and control (Ctrl) cells, transfected with NF-κB–luc and Renilla luc, as described in the legend to Fig. 2, were exposed to gHt/gL (1.5 μM) for 4 h or infected with R7910 (20 PFU/cell). The production and characterization of gHt/gL were described previously (32). Where indicated, gHt/gL was preincubated for 1 h with MAb 52S (33) to gH/gL (gHt/gL + 52S) or heat inactivated for 15 min at 100°C (H-gHt/gL). Each column represents the average of triplicates ± standard deviation. (D to F) sh-β3 and control cells were exposed to gHt/gL (1.5 μM) for 6 h, infected with R7910 (20 PFU/cell), or left uninfected. IFNB1 mRNA levels were determined as detailed in the legend to Fig. 2, 6 h after infection. The mRNA level is expressed as fold increase in infected cells relative to that of uninfected cells (set as 1). Each column represents the average of triplicates ± standard deviation.
HSV enters HaCaT, HeLa, and SK-N-SH cells through different pathways.
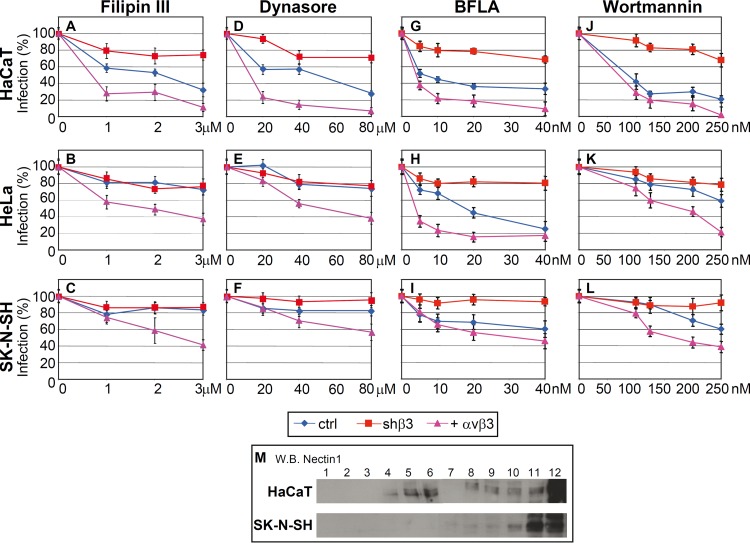
In addition to serving as a sensor and initiator of the innate response to HSV, αvβ3 integrin serves as a routing factor for HSV in 293T cells (34). It induces HSV to enter cells via a route dependent on lipid microdomains, dynamin 2, and acidic endosomes (34). This activity is exerted by αvβ3 integrin-mediated relocation of the gD receptor nectin1 to lipid microdomains (35). Here, we asked whether HSV enters HaCaT, HeLa, and SK-N-SH cells by the same route and whether it coincides with the one employed to enter 293T cells. We also assessed the dependence of the route of entry on αvβ3 integrin by comparing WT cells and cells in which β3 integrin was either silenced or overexpressed. The entry route was assessed by means of the following inhibitors. Filipin III is an inhibitor of lipid microdomains. Dynasore is an inhibitor of the dynamin 2 GTPase, which seals the invaginations and gives rise to endosomes. Bafilomycin A (BFLA) is an inhibitor of endosome acidification. Wortmannin is an inhibitor of phosphoinositide 3-kinase (PI3K). We have observed an overlap between sensitivity to BFLA and to wortmannin, implying that completion of the acidic endosome route of entry depends on PI3K activity.
Inhibition by filipin III differentiated the pathway of entry into HaCaT cells from that into HeLa and SK-N-SH cells. Figure 4A shows that infection of WT HaCaT cells was inhibited by this compound; hence, it occurs through lipid microdomains. In contrast, there was little effect on infection of HeLa cells (Fig. 4B) and an even smaller effect on SK-N-SH cells (Fig. 4C). The silencing of β3 integrin in HaCaT cells strongly reduced filipin III inhibition. Conversely, overexpression of αvβ3 integrin increased or conferred filipin III sensitivity; the latter phenomenon was seen also in HeLa and SK-N-SH cells. Thus, the dependence of HSV entry on lipid microdomains appeared to be a function of β3 integrin.
Fig 4.
(A to L) Effect of inhibitors on infection of R8102 into HaCaT, HeLa, and SK-N-SH cells. Cells were mock silenced (ctrl), had β3 integrin silenced (shβ3), or transiently overexpressed αvβ3 integrin (+ αvβ3). The stock solutions of filipin III (2.5 mM), dynasore (100 mM), bafilomycin A (BFLA) (160 mM), and wortmannin (2 M) (all from Sigma-Aldrich) in dimethyl sulfoxide were stored at −20°C. Cells were exposed to the indicated amounts of inhibitors for 1 h at 37°C and then infected with R8102 (3 PFU/cell) for 90 min in the presence of inhibitors (18). The viral inoculum was removed, and the cells were overlaid with medium containing inhibitors for 6 to 8 h. For filipin III and dynasore, cells were preincubated with the compounds at 37°C for 30 min and 60 min, respectively, and infected for 30 min (30 PFU/cell) in the same medium. Viral inoculum was removed; infected cells were overlaid without inhibitor and harvested 6 to 8 h after infection. Infection of R8102 was quantified by means of o-nitrophenyl-β-d-galactopyranoside; each point represents the average of triplicates ± standard deviation. (M) Flotation of membranes from HaCaT and SK-N-SH cells. Cells were solubilized in TNE buffer (10 mM Tris-HCl, 150 mM NaCl, 5 mM EDTA, 1% Triton X-100, 0.036 mg/ml each of the protease inhibitors Nα-p-tosyl-l-lysine-chloromethyl ketone [TLCK; Sigma-Aldrich] and N-tosyl-l-phenylalanine-chloromethyl ketone [TPCK; Sigma-Aldrich]) and layered at the bottom of a 5-35-42% sucrose gradient as detailed previously (39). After centrifugation at 34,000 rpm for 20 h at 4°C in a SW41 swing-out rotor, 12 fractions were collected from the top. Aliquots from each fraction were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Nectin-1 was detected by Western blotting (WB) with CK6 monoclonal antibody (Santa Cruz Biotechnologies) followed by peroxidase-conjugated anti-mouse antibody and enhanced-advance chemiluminescence (ECL-Advance kit; GE Healthcare). Numbers above the lanes indicate the numbers of the fractions. The part of the image relative to fractions 1 to 6 was exposed for 3 min; the part of the image relative to fractions 7 to 12 was exposed for 30 s.
The dynasore inhibition curves closely resembled those of filipin III. Specifically, HSV infection was dose-dependently inhibited by dynasore in HaCaT cells (Fig. 4D) but not in HeLa (Fig. 4E) and SK-N-SH (Fig. 4F) cells. Overexpression of αvβ3 integrin increased inhibition by dynasore in HaCaT cells and conferred some degree of inhibition in HeLa and SK-N-SH cells. The silencing of β3 integrin decreased dynasore inhibition in HaCaT cells.
BFLA inhibition curves differentiated the route of entry into HeLa cells from that into SK-N-SH cells. Infection of HeLa cells was impaired by this drug (Fig. 4H) (although with a curve somewhat different from that of HaCaT cells), whereas that of SK-N-SH (Fig. 4I) was only moderately affected. Of note, in all cells, β3 integrin silencing practically abolished inhibition by BFLA. αvβ3 integrin overexpression increased the extent of inhibition by BFLA in HaCaT and HeLa cells (Fig. 4G and H). Wortmannin inhibition curves were, at large, similar to those obtained with BFLA (Fig. 4J to L). Together, this series of experiments indicates that infection of HaCaT cells occurs via a route dependent on lipid microdomains, dynamin 2, and acidic endosomes. Infection of HeLa cells occurs via a route dependent on acidic endosomes but independent of lipid microdomains and dynamin 2. Infection of SK-N-SH cells was not sensitive to endosome acidification and was independent of lipid microdomains and dynamin 2. The requirement for a low-pH compartment in HSV infection of WT HaCaT and HeLA cells but not WT SK-N-SH cells is in agreement with previous data (36, 37). The finding that the inhibition by filipin III, as well as by the other inhibitors, is not complete in HaCaT cells suggests that not all the virion-cell interactions take place at the lipid microdomains. To address this question, we compared the distributions of nectin1 on the cell surface of HaCaT and SK-N-SH cells, by means of a cell membrane flotation experiment. By this approach, previously, we observed that when nectin1 localizes to lipid microdomains (exemplified by 293 cells overexpressing αvβ3 integrin), nectin1-containing membranes float to the middle-high fractions of the gradient. In contrast, when nectin1 is uniformly distributed on the cell surface, nectin1-containing membranes remain in the bottom fractions of the gradients. Figure 4M shows that in HaCaT cells, a portion of nectin1 was localized at the middle-high fractions of the gradient (lipid microdomains), whereas in SK-N-SH cells, nectin1 localized entirely at the bottom fractions (cell surface).
The conclusions to be drawn from this study are 3-fold.
(i) The arm of the innate response elicited by αvβ3 integrin functions in cells which represent experimental models of the in vivo targets of HSV, i.e., epithelial cells, including keratinocytes, and neuronal cells. This arm of the innate response leads to production of IFN-β and IL-10 and to NF-κB activation. In the past years, the prevailing view was that the IFN response was elicited upon activation of the cytosolic sensors, which recognize viral DNA or RNA. These arms of the innate response were detected mainly in specific cell types, e.g., dendritic, NK, plasmacytoid, or 293T model cells (2). In contrast with this view, the branch of the innate response described here is elicited by the αvβ3 integrin receptor located on the plasma membrane. Clearly, this response coexists with other branches of innate immunity and contributes to the overall type I IFN production elicited by HSV but is not the only one responsible for it. In agreement with this view, silencing of β3 integrin led to inhibition but not a complete abolishment of the NF-κB response (Fig. 2). Nonetheless, the role played by the β3 integrin-dependent arm of the innate response appears to be prominent in epithelial and neuronal cells, since the β3 integrin silencing inhibited IFN-β and IL-10 secretion and NF-κB activation from 60 to 80%, depending on the cells analyzed. The observation that the αvβ3 integrin-elicited signaling leads simultaneously to activation of type I IFN and of IL-10 suggests that this pathway is devoted mainly to establishing an antiviral state and to negatively controlling inflammation through IL-10.
(ii) A soluble form of gH/gL was sufficient to elicit the production of type I IFN and IL-10, as well as NF-κB activation. Thus, gH/gL represents the PAMP of the αvβ3 integrin-dependent innate response. Previously, we showed that gH/gL physically interacts with TLR2 (24) and that the αvβ3 integrin-dependent branch of the innate response in 293T cells involves the cooperation of αvβ3 integrin with TLR2 (18). Most likely, the response described here in HaCaT, HeLa, and SK-N-SH cells involves TLR2 in cooperation with αvβ3 integrin.
(iii) In 293T cells, HSV is routed by αvβ3 integrin to a pathway of entry dependent on lipid microdomains, dynamin 2, and acidic endosomes. αvβ3 integrin itself in large part localized at the lipid microdomains (35). TLR2 partitions at or at the boundaries of lipid microdomains (38). The question then arose whether the αvβ3 integrin- and TLR2-dependent activation of the innate response took place only when virions entered cells through lipid microdomains. We observed that HSV entry into the three cell lines, HaCaT, HeLa, and SK-N-SH, occurred though different routes. Specifically, HSV entry into HaCaT cells was through a route dependent on lipid microdomains, dynamin 2, and acidic endosomes. HSV entry into HeLa cells was through a route independent of lipid microdomains and dynamin 2 but nonetheless through acidic endosomes. HSV entry into SK-N-SH cells was through a route independent of lipid microdomains, dynamin 2, and acidic endosomes. We conclude that elicitation of the type I IFN and NF-κB innate response takes place independently of the route followed by HSV to enter cells.
ACKNOWLEDGMENTS
We thank Juana Gerold for gift of lentiviruses encoding shRNA to β3 integrin and control lentiviruses.
This work was supported by the Italian Association for Cancer Research (AIRC), Milan, Italy, by the University of Bologna Ricerca Fondamentale Orientata (RFO), and by the Italian Ministry for University and Research (PRIN project).
Footnotes
Published ahead of print 9 October 2013
REFERENCES
- 1.Kawai T, Akira S. 2011. Toll-like receptors and their crosstalk with other innate receptors in infection and immunity. Immunity 34:637–650 [DOI] [PubMed] [Google Scholar]
- 2.Paludan SR, Bowie AG, Horan KA, Fitzgerald KA. 2011. Recognition of herpesviruses by the innate immune system. Nat. Rev. Immunol. 11:143–154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Conrady CD, Zheng M, Fitzgerald KA, Liu C, Carr DJ. 2012. Resistance to HSV-1 infection in the epithelium resides with the novel innate sensor, IFI-16. Mucosal Immunol. 5:173–183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Paladino P, Cummings DT, Noyce RS, Mossman KL. 2006. The IFN-independent response to virus particle entry provides a first line of antiviral defense that is independent of TLRs and retinoic acid-inducible gene I. J. Immunol. 177:8008–8016 [DOI] [PubMed] [Google Scholar]
- 5.Pham TH, Kwon KM, Kim YE, Kim KK, Ahn JH. 2013. DNA sensing-independent inhibition of herpes simplex virus 1 replication by DAI/ZBP1. J. Virol. 87:3076–3086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rasmussen SB, Jensen SB, Nielsen C, Quartin E, Kato H, Chen ZJ, Silverman RH, Akira S, Paludan SR. 2009. Herpes simplex virus infection is sensed by both Toll-like receptors and retinoic acid-inducible gene-like receptors, which synergize to induce type I interferon production. J. Gen. Virol. 90:74–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Compton T, Kurt-Jones EA, Boehme KW, Belko J, Latz E, Golenbock DT, Finberg RW. 2003. Human cytomegalovirus activates inflammatory cytokine responses via CD14 and Toll-like receptor 2. J. Virol. 77:4588–4596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang D, Shenk T. 2005. Human cytomegalovirus virion protein complex required for epithelial and endothelial cell tropism. Proc. Natl. Acad. Sci. U. S. A. 102:18153–18158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kurt-Jones EA, Chan M, Zhou S, Wang J, Reed G, Bronson R, Arnold MM, Knipe DM, Finberg RW. 2004. Herpes simplex virus 1 interaction with Toll-like receptor 2 contributes to lethal encephalitis. Proc. Natl. Acad. Sci. U. S. A. 101:1315–1320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu X, Fitzgerald K, Kurt-Jones E, Finberg R, Knipe DM. 2008. Herpesvirus tegument protein activates NF-kappaB signaling through the TRAF6 adaptor protein. Proc. Natl. Acad. Sci. U. S. A. 105:11335–11339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barbalat R, Lau L, Locksley RM, Barton GM. 2009. Toll-like receptor 2 on inflammatory monocytes induces type I interferon in response to viral but not bacterial ligands. Nat. Immunol. 10:1200–1207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lund J, Sato A, Akira S, Medzhitov R, Iwasaki A. 2003. Toll-like receptor 9-mediated recognition of herpes simplex virus-2 by plasmacytoid dendritic cells. J. Exp. Med. 198:513–520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sato A, Linehan MM, Iwasaki A. 2006. Dual recognition of herpes simplex viruses by TLR2 and TLR9 in dendritic cells. Proc. Natl. Acad. Sci. U. S. A. 103:17343–17348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Szomolanyi-Tsuda E, Liang X, Welsh RM, Kurt-Jones EA, Finberg RW. 2006. Role for TLR2 in NK cell-mediated control of murine cytomegalovirus in vivo. J. Virol. 80:4286–4291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bochud PY, Magaret AS, Koelle DM, Aderem A, Wald A. 2007. Polymorphisms in TLR2 are associated with increased viral shedding and lesional rate in patients with genital herpes simplex virus type 2 infection. J. Infect. Dis. 196:505–509 [DOI] [PubMed] [Google Scholar]
- 16.Jin H, Ma Y, Yan Z, Prabhakar BS, He B. 2012. Activation of NF-kappaB in CD8+ dendritic cells ex vivo by the gamma134.5 null mutant correlates with immunity against herpes simplex virus 1. J. Virol. 86:1059–1068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sørensen LN, Reinert LS, Malmgaard L, Bartholdy C, Thomsen AR, Paludan SR. 2008. TLR2 and TLR9 synergistically control herpes simplex virus infection in the brain. J. Immunol. 181:8604–8612 [DOI] [PubMed] [Google Scholar]
- 18.Gianni T, Leoni V, Chesnokova LS, Hutt-Fletcher LM, Campadelli-Fiume G. 2012. alphavbeta3-integrin is a major sensor and activator of innate immunity to herpes simplex virus-1. Proc. Natl. Acad. Sci. U. S. A. 109:19792–19797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Amici C, Rossi A, Costanzo A, Ciafre S, Marinari B, Balsamo M, Levrero M, Santoro MG. 2006. Herpes simplex virus disrupts NF-kappaB regulation by blocking its recruitment on the IkappaBalpha promoter and directing the factor on viral genes. J. Biol. Chem. 281:7110–7117 [DOI] [PubMed] [Google Scholar]
- 20.Gregory D, Hargett D, Holmes D, Money E, Bachenheimer SL. 2004. Efficient replication by herpes simplex virus type 1 involves activation of the IkappaB kinase-IkappaB-p65 pathway. J. Virol. 78:13582–13590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Santoro MG, Rossi A, Amici C. 2003. NF-kappaB and virus infection: who controls whom. EMBO J. 22:2552–2560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Taddeo B, Zhang W, Lakeman F, Roizman B. 2004. Cells lacking NF-kappaB or in which NF-kappaB is not activated vary with respect to ability to sustain herpes simplex virus 1 replication and are not susceptible to apoptosis induced by a replication-incompetent mutant virus. J. Virol. 78:11615–11621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.van Lint AL, Murawski MR, Goodbody RE, Severa M, Fitzgerald KA, Finberg RW, Knipe DM, Kurt-Jones EA. 2010. Herpes simplex virus immediate-early ICP0 protein inhibits Toll-like receptor 2-dependent inflammatory responses and NF-kappaB signaling. J. Virol. 84:10802–10811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Leoni V, Gianni T, Salvioli S, Campadelli-Fiume G. 2012. Herpes simplex virus glycoproteins gH/gL and gB bind Toll-like receptor 2, and soluble gH/gL is sufficient to activate NF-kappaB. J. Virol. 86:6555–6562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gerold G, Ajaj KA, Bienert M, Laws HJ, Zychlinsky A, de Diego JL. 2008. A Toll-like receptor 2-integrin beta3 complex senses bacterial lipopeptides via vitronectin. Nat. Immunol. 9:761–768 [DOI] [PubMed] [Google Scholar]
- 26.Cocchi F, Menotti L, Mirandola P, Lopez M, Campadelli-Fiume G. 1998. The ectodomain of a novel member of the immunoglobulin subfamily related to the poliovirus receptor has the attributes of a bona fide receptor for herpes simplex virus types 1 and 2 in human cells. J. Virol. 72:9992–10002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Geraghty RJ, Krummenacher C, Cohen GH, Eisenberg RJ, Spear PG. 1998. Entry of alphaherpesviruses mediated by poliovirus receptor-related protein 1 and poliovirus receptor. Science 280:1618–1620 [DOI] [PubMed] [Google Scholar]
- 28.Kawaguchi Y, Bruni R, Roizman B. 1997. Interaction of herpes simplex virus 1 alpha regulatory protein ICP0 with elongation factor 1delta: ICP0 affects translational machinery. J. Virol. 71:1019–1024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Boutell C, Everett RD. 2013. Regulation of alphaherpesvirus infections by the ICP0 family of proteins. J. Gen. Virol. 94:465–481 [DOI] [PubMed] [Google Scholar]
- 30.Roizman B. 2011. The checkpoints of viral gene expression in productive and latent infection: the role of the HDAC/CoREST/LSD1/REST repressor complex. J. Virol. 85:7474–7482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Randall RE, Goodbourn S. 2008. Interferons and viruses: an interplay between induction, signalling, antiviral responses and virus countermeasures. J. Gen. Virol. 89:1–47 [DOI] [PubMed] [Google Scholar]
- 32.Gianni T, Cerretani A, Dubois R, Salvioli S, Blystone SS, Rey F, Campadelli-Fiume G. 2010. Herpes simplex virus glycoproteins H/L bind to cells independently of {alpha}V{beta}3 integrin and inhibit virus entry, and their constitutive expression restricts infection. J. Virol. 84:4013–4025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Showalter SD, Zweig M, Hampar B. 1981. Monoclonal antibodies to herpes simplex virus type 1 proteins, including the immediate-early protein ICP 4. Infect. Immun. 34:684–692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gianni T, Gatta V, Campadelli-Fiume G. 2010. {alpha}V{beta}3-integrin routes herpes simplex virus to an entry pathway dependent on cholesterol-rich lipid rafts and dynamin2. Proc. Natl. Acad. Sci. U. S. A. 107:22260–22265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gianni T, Campadelli-Fiume G. 2012. alphaVbeta3-integrin relocalizes nectin1 and routes herpes simplex virus to lipid rafts. J. Virol. 86:2850–2855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nicola AV, Hou J, Major EO, Straus SE. 2005. Herpes simplex virus type 1 enters human epidermal keratinocytes, but not neurons, via a pH-dependent endocytic pathway. J. Virol. 79:7609–7616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nicola AV, McEvoy AM, Straus SE. 2003. Roles for endocytosis and low pH in herpes simplex virus entry into HeLa and Chinese hamster ovary cells. J. Virol. 77:5324–5332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Triantafilou M, Manukyan M, Mackie A, Morath S, Hartung T, Heine H, Triantafilou K. 2004. Lipoteichoic acid and toll-like receptor 2 internalization and targeting to the Golgi are lipid raft-dependent. J. Biol. Chem. 279:40882–40889 [DOI] [PubMed] [Google Scholar]
- 39.Gianni T, Campadelli-Fiume G, Menotti L. 2004. Entry of herpes simplex virus mediated by chimeric forms of nectin1 retargeted to endosomes or to lipid rafts occurs through acidic endosomes. J. Virol. 78:12268–12276 [DOI] [PMC free article] [PubMed] [Google Scholar]