Abstract
Here we show that simian immunodeficiency virus (SIV) infection of rhesus macaques results in rapid upregulation of tetherin (BST-2 or CD317) on peripheral blood lymphocytes, including the CD4+ CCR5+ T cell targets of virus infection, with a peak of induction that coincides with peak alpha interferon (IFN-α) levels in plasma, and that tetherin remains above baseline levels throughout chronic infection. These observations are consistent with a role for tetherin in innate immunity to immunodeficiency virus infection.
TEXT
Mammals have evolved a number of cellular factors that interfere with specific steps of virus replication. One such factor, tetherin (BST-2 or CD317), is an interferon-inducible transmembrane protein that inhibits the release of enveloped viruses from infected cells (1, 2).
Tetherin has broad antiviral activity against diverse families of enveloped viruses (3–8), and many viruses have in turn acquired countermeasures to tetherin (1, 2, 4, 5, 9–11). The primate lentiviruses have evolved to use at least three different viral gene products to oppose tetherin. Whereas most simian immunodeficiency viruses (SIVs) use Nef to counteract the tetherin proteins of their nonhuman primate hosts (9, 10, 12), human immunodeficiency virus type 1 (HIV-1) Vpu and HIV-2 Env counteract human tetherin due to the absence of sequences in the cytoplasmic domain of the molecule required for recognition by Nef (13–15).
The antiviral activity of tetherin in vivo is dependent on immune activation and interferon-induced upregulation, as illustrated by a comparison of murine leukemia virus replication levels in wild-type versus tetherin-deficient mice (16). Recent studies have also shown that overexpression or cross-linking of tetherin can activate NF-κB, leading to the induction of proinflammatory cytokines (17, 18, 29). Furthermore, an earlier study identified tetherin as a ligand for ILT7, a receptor expressed by plasmacytoid dendritic cells (pDCs), and demonstrated that engagement of ILT7 inhibits interferon production by pDCs (19). Thus, in addition to impairing virus release from infected cells, tetherin may serve as innate sensor of virus infection and as a negative regulator of interferon responses.
In accordance with the induction of tetherin by type I interferons, an upregulation of tetherin mRNA transcription in CD4+ lymphocytes and a corresponding decrease in HIV-1 loads were observed in HIV-1/hepatitis C virus (HCV)-coinfected individuals treated with pegylated alpha interferon (IFN-α) (20). Homann et al. also demonstrated that tetherin expression is upregulated on the surfaces of peripheral blood mononuclear cells (PBMCs), including CD4+ lymphocytes, from HIV-1+ individuals (21). Tetherin expression is highest during acute infection, declines during chronic infection, and further decreases in patients receiving antiretroviral therapy (21). These observations are consistent with virus replication driving the interferon response and, in turn, tetherin induction.
In the present study, we performed a longitudinal analysis of tetherin expression in SIV-infected rhesus macaques. Changes in tetherin expression were monitored in peripheral blood for a group of six rhesus macaques following intrarectal challenge with SIVmac239. These animals constituted the control group of a vaccine study and were therefore naive to SIV at the time of challenge. At each time point, PBMCs were stained with a 9-color panel that included antibodies to CD3, CD4, CD8, CD20, CD28, CD95, CCR5, CCR7, and tetherin. Tetherin was detected with a fluorescein isothiocyanate (FITC)-conjugated monoclonal antibody (HM1.24), previously shown to bind to both human and rhesus macaque tetherin by flow cytometry and Western blot analysis (9). PBMCs were also stained in parallel with a similar panel that included an IgG2a isotype control in place of the antibody to tetherin to determine the level of nonspecific staining. The samples were then analyzed by polychromatic flow cytometry.
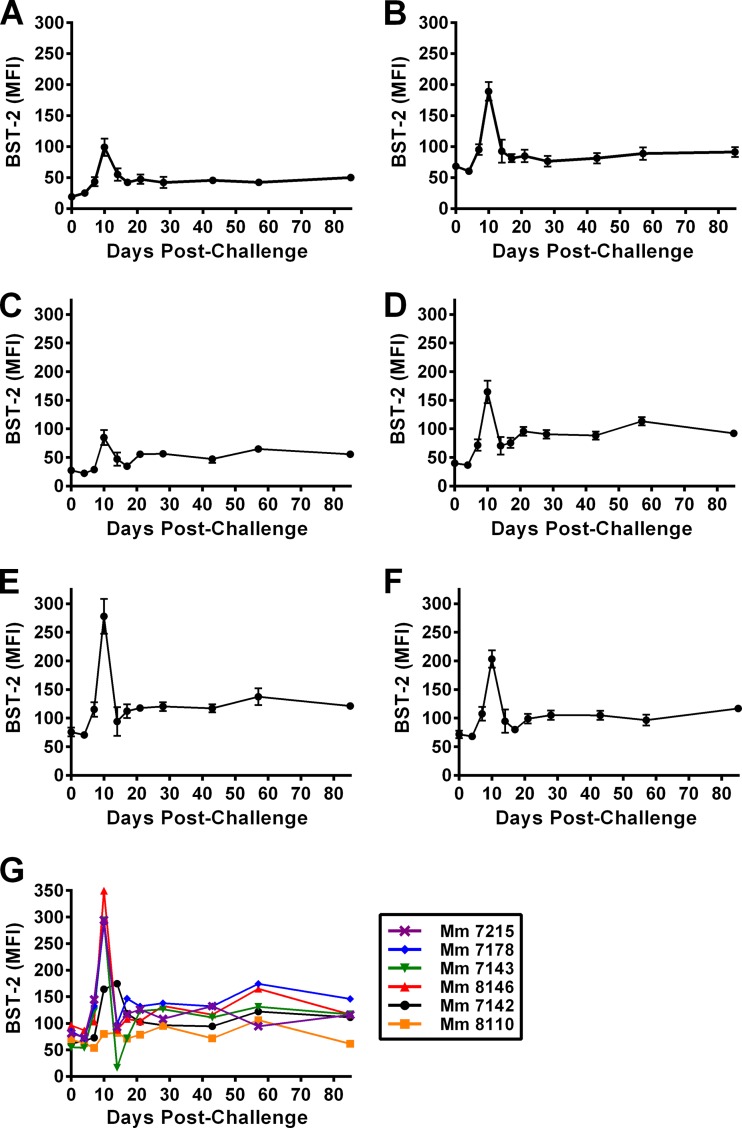
An increase in tetherin expression on CD20+ B cells, memory (CD95+) CD8+ T cells, naive CD4+ T cells (CD28moderate, CD95low), central memory CD4+ T cells (CD95high, CD28+, CCR7+, and CCR5−), transitional memory CD4+ T cells (CD95high, CD28+, CCR7−, and CCR5+), and effector memory CD4+ T cells (CD95+, CD28−, CCR7−, and CCR5dim) was detectable by day 7 postinfection (p.i.) and peaked by day 10 p.i. in four of the six animals (Fig. 1). One animal (Mm 7142) exhibited a delayed increase in tetherin upregulation, which peaked on day 14 p.i. Another animal (Mm 8110) did not show a detectable increase in tetherin expression at any time point after challenge (Fig. 1G). The lack of tetherin upregulation by Mm 8110 can be explained by the failure of infection to occur following mucosal SIV challenge of this animal (Fig. 2B), providing a useful, albeit unintended, internal control for these analyses. Comparison of the average changes in tetherin expression for the five infected animals revealed that the peak of tetherin upregulation occurred on day 10 p.i. in each of the lymphocyte subsets (Fig. 1). In each case, the extent of tetherin expression subsequently declined but remained above prechallenge levels during chronic infection (Fig. 1). Overall, these results reveal a significant increase in the surface expression of tetherin on multiple lymphocyte subsets, including CD4+ CCR5+ transitional memory T cells, which represent the principal targets of SIV infection.
Fig 1.
Tetherin is rapidly upregulated on multiple lymphocyte subsets in response to SIV infection. Naive rhesus macaques were challenged intrarectally with a single dose of pathogenic SIVmac239, PBMCs were isolated, and BST-2 expression was characterized by polychromatic flow cytometry at longitudinal time points. The mean fluorescence intensity (MFI) ± standard error of the mean (SEM) for BST-2 staining is shown for CD20+ B cells (A), memory CD8+ CD95+ T cells (B), naive CD4+ T cells (CD28moderate, CD95low) (C), central memory CD4+ T cells (CD95high, CD28+, CCR7+, and CCR5−) (D), transitional memory CD4+ T cells (CD95high, CD28+, CCR7−, and CCR5+) (E), and effector memory CD4+ T cells (CD95+, CD28−, CCR7−, and CCR5dim) (F). (G) The MFI of BST-2 staining is also shown for transitional memory CD4+ T cells of individual animals. In all cases, the MFI for the isotype control was subtracted to correct for variation in nonspecific staining.
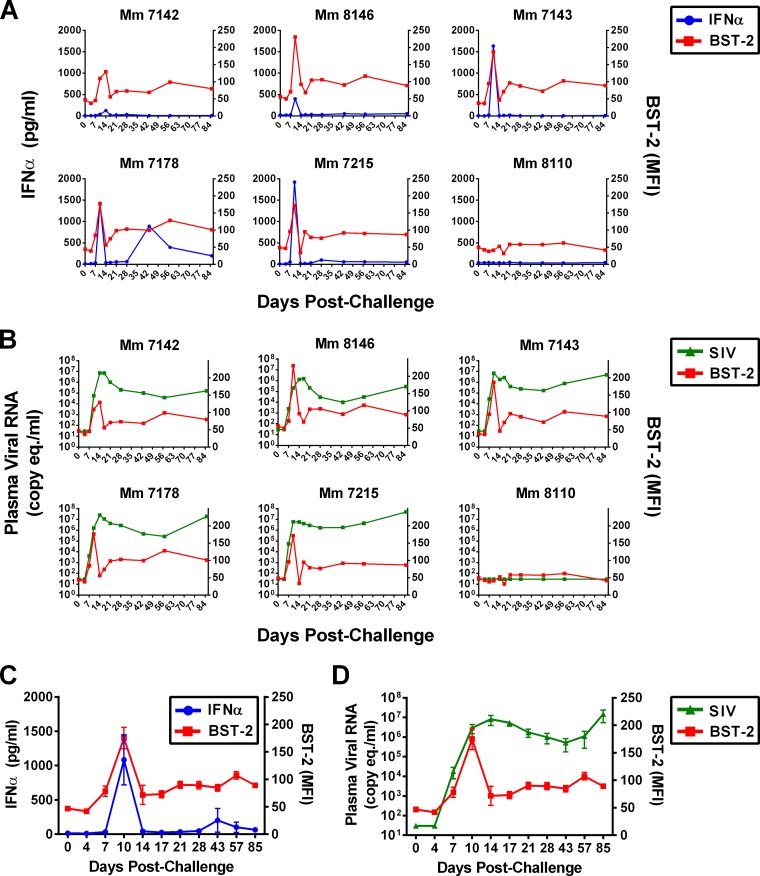
Fig 2.
The peak of IFN-α production coincides with the peak of tetherin induction but precedes peak viremia. (A) IFN-α levels in plasma (pg/ml) were measured using a macaque-specific enzyme-linked immunosorbent assay (ELISA) and are plotted relative to the MFI of BST-2 staining on CD4+ CD95+ T cells at each time point for individual animals. (B) Viral RNA loads in plasma (copy equvalent/ml) are shown at time points concurrent with the MFI of BST-2 staining on CD4+ CD95+ T cells for individual animals. The average MFIs for BST-2 staining on CD4+ CD95+ T cells relative to mean concentrations of IFN-α (C) and geometric mean viral RNA loads (D) in plasma are shown for the five SIV-infected animals. Error bars indicate SEMs at each time point.
Levels of IFN-α and viral RNA in plasma were measured at each of the time points after SIV challenge to determine the kinetics of tetherin induction with respect to the interferon response and virus replication. Tetherin upregulation on memory CD4+ CD95+ T cells closely coincided with IFN-α levels in plasma, consistent with the regulation of tetherin by type I interferons. Whereas no increase in tetherin or IFN-α was observed in the animal that did not become infected (Mm 8110), the peak of tetherin expression coincided precisely with the peak of IFN-α for each of the five SIV-infected animals (Fig. 2A). IFN-α levels declined by day 14 p.i. and, with the exception of a couple of time points for Mm 7178, were largely undetectable during chronic infection (Fig. 2A). Although a corresponding decrease in tetherin was also observed, tetherin expression on peripheral blood lymphocytes remained above baseline levels during chronic infection (Fig. 2A).
A similar analysis of tetherin upregulation with respect to virus replication revealed that tetherin expression peaked prior to peak viremia. Viral loads peaked on day 14 p.i. for four animals in which tetherin upregulation peaked on day 10 p.i. and on day 17 p.i. for one animal (Mm 7142) in which tetherin upregulation peaked on day 14 p.i. (Fig. 2B). Longitudinal changes in tetherin expression on CD4+ CD95+ T cells for the five SIV-infected animals with respect to mean IFN-α concentrations in plasma and geometric mean viral loads are summarized in Fig. 2C and D, respectively.
Despite the close temporal relationship between tetherin induction and IFN-α levels, the magnitude of tetherin upregulation did not correlate with the magnitude of IFN-α production. Although tetherin expression on memory CD4+ T cells was generally higher in animals with higher concentrations of IFN-α in plasma, this trend was not significant (Pearson correlation, P = 0.27). Likewise, we did not observe a correlation between viral loads and tetherin expression on memory CD4+ T cells (Pearson correlation, P = 0.45). However, there was a significant relationship between viral loads and IFN-α levels on day 10 p.i. (Pearson correlation, P = 0.02), reflecting a role for virus replication in driving the interferon response.
Coincident with peak viremia, tetherin expression sharply declined by day 14 postinfection. This decrease in tetherin expression was paralleled by a decrease in IFN-α production, which, with a couple of exceptions, remained below the limit of detection throughout chronic infection. These declines correspond well to the massive infection and depletion of 30 to 60% of memory CD4+ T cells that occurs 10 to 14 days after SIV infection (22) and to the well-documented loss of circulating pDCs during acute infection, as a result of cell death and/or pDC homing to lymphoid compartments (23, 24). Thus, decreases in IFN-α and tetherin levels during peak viremia are probably due, at least in part, to the loss of IFN-α production as a result of pDC dysregulation. Consequently, tetherin may contribute little to the resolution of acute viremia, which may explain why viral loads do not decline with the peak of tetherin induction.
In accordance with ongoing immune activation that is a hallmark of pathogenic SIV infection (25), tetherin expression remained above baseline levels during chronic infection. Although IFN-α concentrations in plasma were below the limit of detection at most time points, elevated levels of tetherin are consistent with the broad upregulation of interferon-stimulated genes previously observed during chronic SIV infection of rhesus macaques by transcriptional profiling (26). NF-κB signaling and the release of proinflammatory cytokines as results of tetherin cross-linking represent a possible positive-feedback mechanism contributing to the maintenance of elevated levels of tetherin expression during chronic infection (17, 18, 29). Ongoing upregulation of tetherin may also help to explain the selective pressure for nef-deleted SIV to acquire resistance to tetherin over months of persistent virus replication in animals (27, 28).
Based on the ability of tetherin to suppress IFN-α production by pDCs through interactions with ILT7 (19), it has been suggested that tetherin downmodulation in HIV- or SIV-infected cells may enhance immune activation by interfering with the dampening of the interferon response. However, given the general upregulation of tetherin observed on all lymphocyte subsets (and presumably other cell types) in response to SIV infection, the physiological relevance of tetherin downregulation as a contributing factor to immune activation is unclear. It seems unlikely that the disruption of tetherin-ILT7 interactions as a result of tetherin downmodulation in virus-infected cells, which represent only a fraction of the total lymphocyte population, would overcome negative signals to pDCs coming from ILT7 interactions with tetherin on the vast majority of uninfected cells.
In summary, we took advantage of the ability to control the timing of SIV infection in experimentally inoculated rhesus macaques to generate a high-resolution data set on the kinetics of tetherin upregulation with respect to virus replication and the type I interferon response. Similar longitudinal analyses in HIV-infected patients are not possible, particularly during acute infection, as the precise time of HIV-1 acquisition is rarely known and sample collection is much more limited. Our results reveal that tetherin is rapidly upregulated on multiple lymphocyte subsets, including the CD4+ CCR5+ T cell targets of virus infection, with a peak of induction that coincides with peak IFN-α levels in plasma, sharply declines coincident with peak viremia, and remains above baseline levels throughout the chronic phase of infection. Thus, tetherin is upregulated on the relevant targets of virus infection with kinetics consistent with a role in the earliest stages of the innate immune response to immunodeficiency virus infection.
ACKNOWLEDGMENTS
We thank Jackie Gillis and Michelle Connole in the Division of Immunology at the New England Primate Research Center for flow cytometry support. We also thank Chugai Pharmaceutical Co., Kanagawa, Japan, for providing the tetherin-specific monoclonal antibody HM1.24.
This work was supported by Public Health Service grants AI098485, AI087498, and AI071306, National Institutes of Health grants RR000164 (TNPRC) and RR000168/OD011103, and, in part, federal funds from the National Cancer Institute, National Institutes of Health, under contract HHSN261200800001E.
The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government.
Footnotes
Published ahead of print 9 October 2013
REFERENCES
- 1.Van Damme N, Goff D, Katsura C, Jorgenson RL, Mitchell R, Johnson M, Stephens EB, Guatelli J. 2008. The interferon-induced protein BST-2/CD317 restricts release of virions from infected cells and is down-regulated from the cell surface by HIV-1 Vpu. Cell Host Microbe 3:245–252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Neil SJD, Zang T, Bieniasz PD. 2008. Tetherin inhibits retrovirus release and is antagonized by HIV-1 Vpu. Nature 451:425–430 [DOI] [PubMed] [Google Scholar]
- 3.Jouvenet N, Neil SJD, Zhadina M, Zang T, Kratovac Z, Lee Y, McNatt M, Hatziioannou T, Bieniasz PD. 2009. Broad-spectrum inhibition of retroviral and filoviral particle release by tetherin. J. Virol. 83:1837–1844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kaletsky RL, Francica JR, Agrawal-Gamse C, Bates P. 2009. Tetherin-mediated restriction of filovirus budding is antagonized by the Ebola glycoprotein. Proc. Natl. Acad. Sci. U. S. A. 106:2886–2891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mansouri M, Viswanathan K, Douglas JL, Hines J, Gustin J, Moses AV, Früh K. 2009. Molecular mechanism of BST2/tetherin downregulation by K5/MIR2 of Kaposi's sarcoma-associated herpesvirus. J. Virol. 83:9672–9681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sakuma T, Noda T, Urata S, Kawaoka Y, Yasuda J. 2009. Inhibition of Lassa and Marburg virus production by tetherin. J. Virol. 83:2382–2385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weidner JM, Jiang D, Pan X-B, Chang J, Block TM, Guo J-T. 2010. Interferon-induced cell membrane proteins, IFITM3 and tetherin, inhibit vesicular stomatitis virus infection via distinct mechanisms. J. Virol. 84:12646–12657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mangeat B, Cavagliotti L, Lehmann M, Gers-Huber G, Kaur I, Thomas Y, Kaiser L, Piguet V. 2012. Influenza virus partially counteracts restriction imposed by tetherin/BST-2. J. Biol. Chem. 287:22015–22029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jia B, Serra-Moreno R, Neidermyer W, Rahmberg A, Mackey J, Fofana Ben I, Johnson WE, Westmoreland S, Evans DT. 2009. Species-specific activity of SIV Nef and HIV-1 Vpu in overcoming restriction by tetherin/BST2. PLoS Pathog. 5:e1000429. 10.1371/journal.ppat.1000429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang F, Wilson SJ, Landford WC, Virgen B, Gregory D, Johnson MC, Munch J, Kirchhoff F, Bieniasz PD, Hatziioannou T. 2009. Nef proteins from simian immunodeficiency viruses are tetherin antagonists. Cell Host Microbe 6:54–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Le Tortorec A, Neil SJD. 2009. Antagonism to and intracellular sequestration of human tetherin by the human immunodeficiency virus type 2 envelope glycoprotein. J. Virol. 83:11966–11978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sauter D, Schindler M, Specht A, Landford WN, Münch J, Kim K-A, Votteler J, Schubert U, Bibollet-Ruche F, Keele BF, Takehisa J, Ogando Y, Ochsenbauer C, Kappes JC, Ayouba A, Peeters M, Learn GH, Shaw G, Sharp PM, Bieniasz P, Hahn BH, Hatziioannou T, Kirchhoff F. 2009. Tetherin-driven adaptation of Vpu and Nef function and the evolution of pandemic and nonpandemic HIV-1 strains. Cell Host Microbe 6:409–421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sauter D, Specht A, Kirchhoff F. 2010. Tetherin: holding on and letting go. Cell 141:392–398 [DOI] [PubMed] [Google Scholar]
- 14.Evans DT, Serra-Moreno R, Singh RK, Guatelli JC. 2010. BST-2/tetherin: a new component of the innate immune response to enveloped viruses. Trends Microbiol. 18:388–396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Serra-Moreno R, Evans DT. 2012. Adaptation of human and simian immunodeficiency viruses for resistance to tetherin/BST-2. Curr. HIV Res. 10:277–282 [DOI] [PubMed] [Google Scholar]
- 16.Liberatore RA, Bieniasz PD. 2011. Tetherin is a key effector of the antiretroviral activity of type I interferon in vitro and in vivo. Proc. Natl. Acad. Sci. U. S. A. 108:18097–18101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cocka LJ, Bates P. 2012. Identification of alternatively translated tetherin isoforms with differing antiviral and signaling activities. PLoS Pathog. 8:e1002931. 10.1371/journal.ppat.1002931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Galão RP, Le Tortorec A, Pickering S, Kueck T, Neil SJD. 2012. Innate sensing of HIV-1 assembly by Tetherin induces NFκB-dependent proinflammatory responses. Cell Host Microbe 12:633–644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cao W, Bover L, Cho M, Wen X, Hanabuchi S, Bao M, Rosen DB, Wang Y-H, Shaw JL, Du Q, Li C, Arai N, Yao Z, Lanier LL, Liu Y-J. 2009. Regulation of TLR7/9 responses in plasmacytoid dendritic cells by BST2 and ILT7 receptor interaction. J. Exp. Med. 206:1603–1614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pillai SK, Abdel-Mohsen M, Guatelli J, Skasko M, Monto A, Fujimoto K, Yukl S, Greene WC, Kovari H, Rauch A, Fellay J, Battegay M, Hirschel B, Witteck A, Bernasconi E, Ledergerber B, Gunthard HF, Wong JK, Günthard HF. 2012. Role of retroviral restriction factors in the interferon-α-mediated suppression of HIV-1 in vivo. Proc. Natl. Acad. Sci. U. S. A. 109:2–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Homann S, Smith D, Little S, Richman D, Guatelli J. 2011. Upregulation of BST-2/tetherin by HIV infection in vivo. J. Virol. 85:10659–10668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mattapallil JJ, Douek DC, Hill B, Nishimura Y, Martin M, Roederer M. 2005. Massive infection and loss of memory CD4+ T cells in multiple tissues during acute SIV infection. Nature 434:1093–1097 [DOI] [PubMed] [Google Scholar]
- 23.Brown KN, Wijewardana V, Liu X, Barratt-Boyes SM. 2009. Rapid influx and death of plasmacytoid dendritic cells in lymph nodes mediate depletion in acute simian immunodeficiency virus infection. PLoS Pathog. 5:e1000413. 10.1371/journal.ppat.1000413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reeves RK, Evans TI, Gillis J, Wong FE, Kang G, Li Q, Johnson RP. 2012. SIV infection induces accumulation of plasmacytoid dendritic cells in the gut mucosa. J. Infect. Dis. 206:1462–1468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chahroudi A, Bosinger SE, Vanderford TH, Paiardini M, Silvestri G. 2012. Natural SIV hosts: showing AIDS the door. Science 335:1188–1193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jacquelin B, Mayau V, Targat B, Liovat AS, Kunkel D, Petitjean G, Dillies M, Roques P, Butor C, Silvestri G, Giavedoni L, Lebon P, Barré-Sinoussi F, Benecke A, Müller-Trutwin M. 2009. Nonpathogenic SIV infection of African green monkeys induces a strong but rapidly controlled type I IFN response. J. Clin. Invest. 119:3544–3555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Alexander L, Illyinskii P, Lang S, Means R, Lifson J, Mansfield K, Desrosiers R. 2003. Determinants of increased replicative capacity of serially passaged simian immunodeficiency virus with nef deleted in rhesus monkeys. J. Virol. 77:6823–6835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Serra-Moreno R, Jia B, Breed M, Alvarez X, Evans DT. 2011. Compensatory changes in the cytoplasmic tail of gp41 confer resistance to tetherin/BST-2 in a pathogenic nef-deleted SIV. Cell Host Microbe 9:46–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tokarev A, Suarez M, Kwan W, Fitzpatrick K, Singh R, Guatelli J. 2013. Stimulation of NF-κB activity by the HIV restriction factor BST2. J. Virol. 87:2046–2057 [DOI] [PMC free article] [PubMed] [Google Scholar]