Abstract
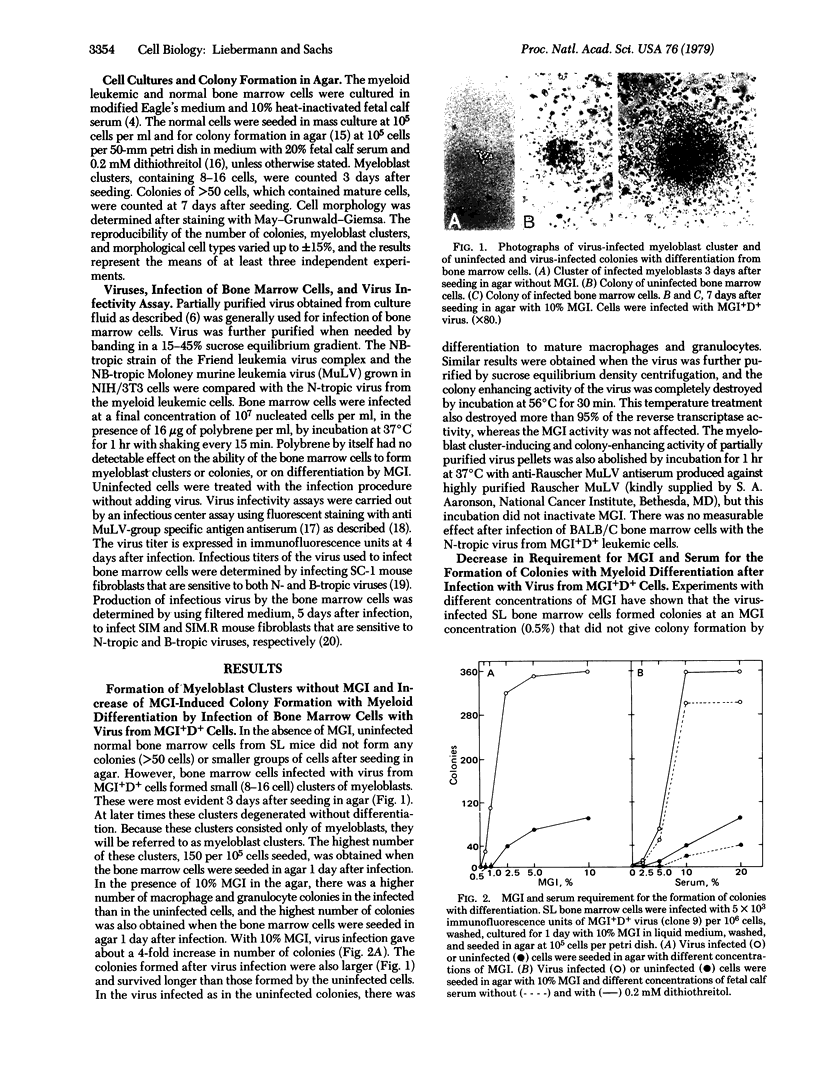
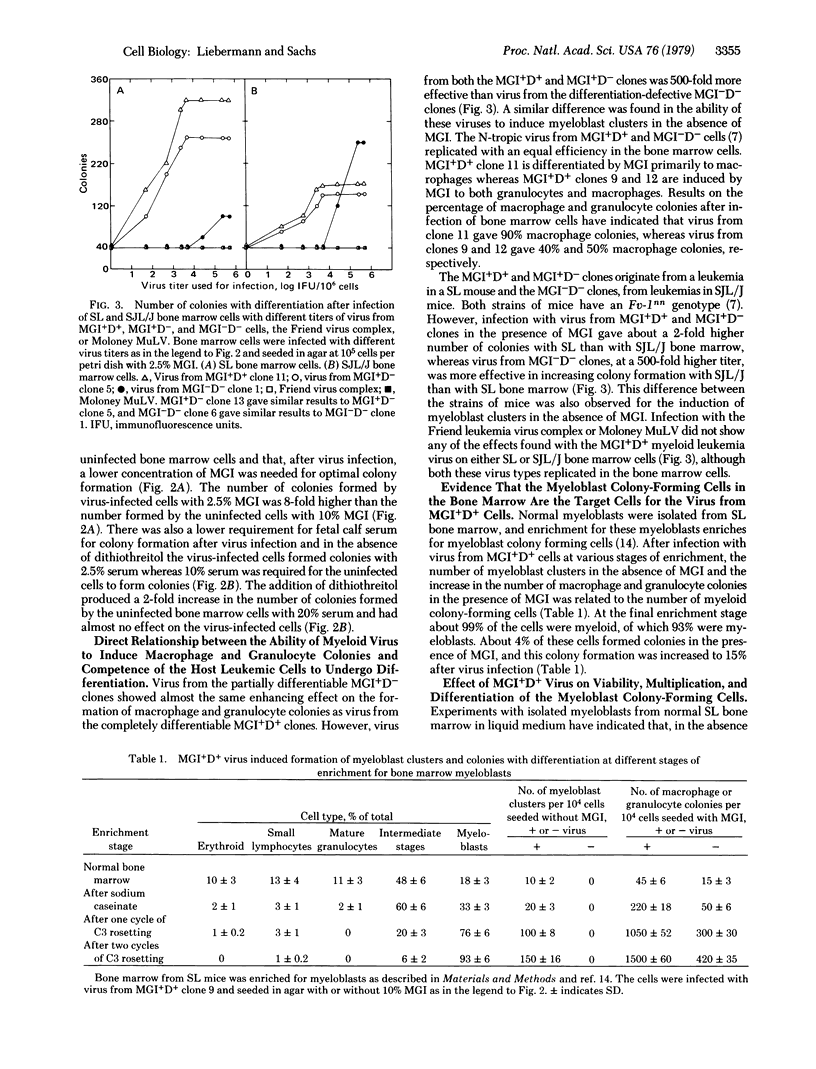
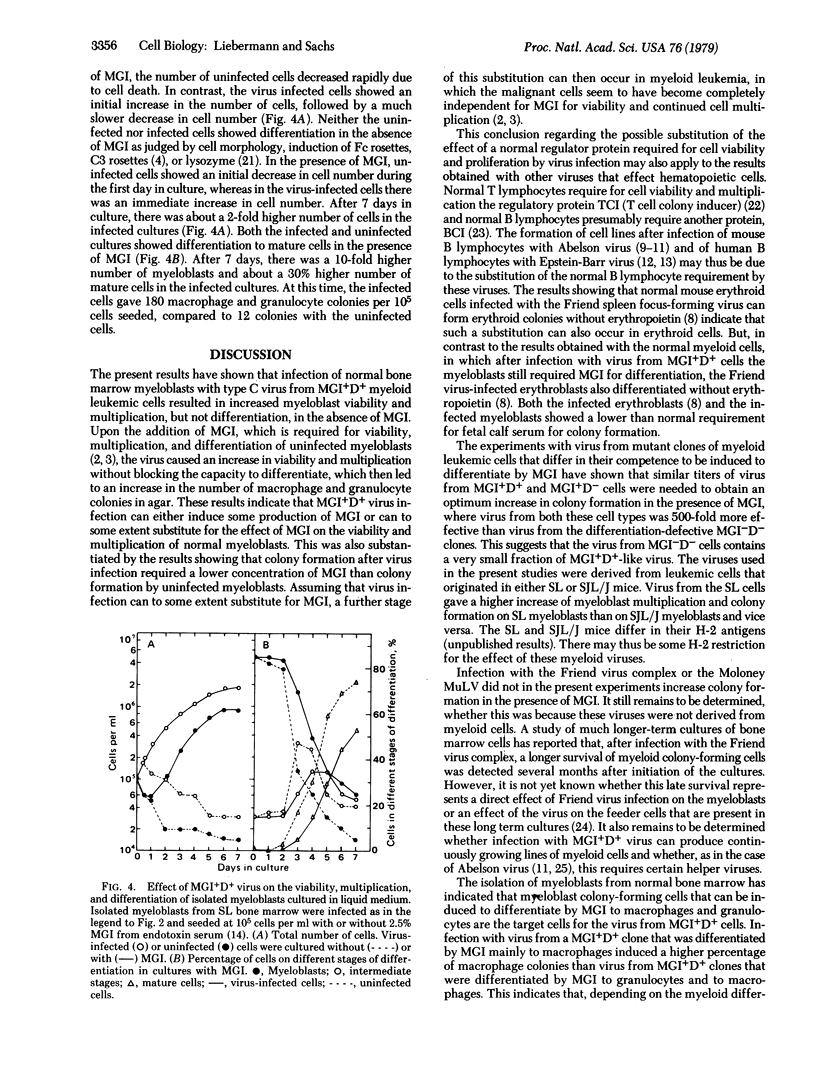
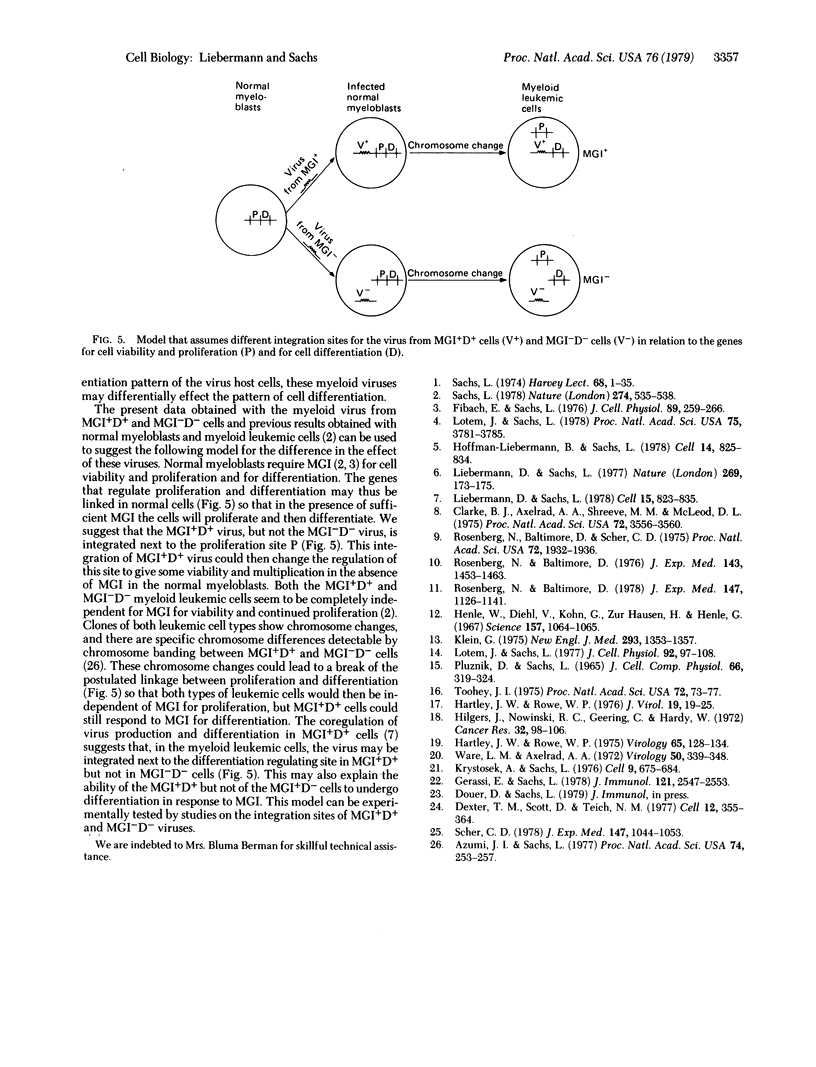
Clones of mouse myeloid leukemic cells that differ in their competence to be induced for normal cell differentiation by the protein inducer MGI produce type C virus. These viruses have been studied for their effect on the viability, multiplication, and differentiation of normal bone marrow cells either with or without the addition of MGI. Virus from leukemic clones that can differentiate normally to mature macrophages and granulocytes (MGI+D+ clones) induced some multiplication of myeloblasts in the bone marrow, but the cells did not differentiate without adding MGI. In the presence of MGI, this virus then induced an increased number of colonies whose cells differentiated to mature macrophages or granulocytes as in colonies of uninfected cells. Virus infection also resulted in a decrease in the amount of MGI and fetal calf serum that was required for colony formation. Virus from MGI+D+ clones, in the presence of MGI, was 500-fold more effective in increasing colony formation than virus from the differentiation-defective MGI-D- clones, although both types of virus replicated with equal efficiency in the normal bone marrow cells. No such increase was obtained after infection with the Friend leukemic virus complex or the Moloney murine leukemia virus. Infection with virus from a MGI+D+ clone that was differentiated by MGI mainly to macrophages induced a higher percentage of macrophage colonies than virus from MGI+D+ clones that were differentiated by MGI to granulocytes and macrophages. Studies with isolated myeloblast colony-forming cells from the bone marrow have indicated that these are the target cells for the virus. Infections of these isolated myeloblasts with virus from MGI+D+ clones induced some multiplication without differentiation in the absence of MGI, and increased the viability and multiplication of the myeloblasts without inhibiting their ability to differentiate in the presence of MGI. The results, therefore, indicate that virus from MGI+D+ cells can increase the viability and multiplication of normal myeloblasts in the bone marrow without blocking the ability of these cells to be induced to differentiate by MGI, and that this effect was directly related to the competence of the leukemic host cells to be induced for normal differentiation. It is suggested that the difference between the effect of virus from MGI+D+ and MGI-D- cells may be due to a difference in their integration sites in relation to the genes that control cell viability, multiplication, and differentiation.
Full text
PDF
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Azumi J. I., Sachs L. Chromosome mapping of the genes that control differentiation and malignancy in myeloid leukemic cells. Proc Natl Acad Sci U S A. 1977 Jan;74(1):253–257. doi: 10.1073/pnas.74.1.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke B. J., Axelrad A. A., Shreeve M. M., McLeod D. L. Erythroid colony induction without erythropoietin by Friend leukemia virus in vitro. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3556–3560. doi: 10.1073/pnas.72.9.3556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dexter T. M., Scott D., Teich N. M. Infection of bone marrow cells in vitro with FLV: effects on stem cell proliferation, differentiation and leukemogenic capacity. Cell. 1977 Oct;12(2):355–364. doi: 10.1016/0092-8674(77)90111-8. [DOI] [PubMed] [Google Scholar]
- Fibach E., Sachs L. Control of normal differentiation of myeloid leukemic cells. XI. Induction of a specific requirement for cell viability and growth during the differentiation of myeloid leukemic cells. J Cell Physiol. 1976 Oct;89(2):259–266. doi: 10.1002/jcp.1040890209. [DOI] [PubMed] [Google Scholar]
- Gerassi E., Sachs L. Regulation of human T cell colonies by an inducing activity (TCI) produced by normal human and malignant mouse cells. J Immunol. 1978 Dec;121(6):2547–2553. [PubMed] [Google Scholar]
- Hartley J. W., Rowe W. P. Clonal cells lines from a feral mouse embryo which lack host-range restrictions for murine leukemia viruses. Virology. 1975 May;65(1):128–134. doi: 10.1016/0042-6822(75)90013-6. [DOI] [PubMed] [Google Scholar]
- Hartley J. W., Rowe W. P. Naturally occurring murine leukemia viruses in wild mice: characterization of a new "amphotropic" class. J Virol. 1976 Jul;19(1):19–25. doi: 10.1128/jvi.19.1.19-25.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henle W., Diehl V., Kohn G., Zur Hausen H., Henle G. Herpes-type virus and chromosome marker in normal leukocytes after growth with irradiated Burkitt cells. Science. 1967 Sep 1;157(3792):1064–1065. doi: 10.1126/science.157.3792.1064. [DOI] [PubMed] [Google Scholar]
- Hilgers J., Nowinski R. C., Geering G., Hardy W. Detection of avian and mammalian oncogenic RNA viruses (oncornaviruses) by immunofluorescence. Cancer Res. 1972 Jan;32(1):98–106. [PubMed] [Google Scholar]
- Hoffman-Liebermann B., Sachs L. Regulation of actin and other proteins in the differentiation of myeloid leukemic cells. Cell. 1978 Aug;14(4):825–834. doi: 10.1016/0092-8674(78)90338-0. [DOI] [PubMed] [Google Scholar]
- Klein G. The Epstein-Barr virus and neoplasia. N Engl J Med. 1975 Dec 25;293(26):1353–1357. doi: 10.1056/NEJM197512252932607. [DOI] [PubMed] [Google Scholar]
- Krystosek A., Sachs L. Control of lysozyme induction in the differentiation of myeloid leukemic cells. Cell. 1976 Dec;9(4 Pt 2):675–684. doi: 10.1016/0092-8674(76)90131-8. [DOI] [PubMed] [Google Scholar]
- Liebermann D., Sachs L. Co-regulation of type C RNA virus production and cell differentiation in myeloid leukemic cells. Cell. 1978 Nov;15(3):823–835. doi: 10.1016/0092-8674(78)90267-2. [DOI] [PubMed] [Google Scholar]
- Liebermann D., Sachs L. Type C RNA virus production and cell competence for normal differentiation in myeloid leukaemic cells. Nature. 1977 Sep 8;269(5624):173–175. doi: 10.1038/269173a0. [DOI] [PubMed] [Google Scholar]
- Lotem J., Sachs L. Control of normal differentiation of myeloid leukemic cells. XII. Isolation of normal myeloid colony-forming cells from bone marrow and the sequence of differentiation to mature granulocytes in normal and D+ myeloid leukemic cells. J Cell Physiol. 1977 Jul;92(1):97–108. doi: 10.1002/jcp.1040920112. [DOI] [PubMed] [Google Scholar]
- Lotem J., Sachs L. In vivo induction of normal differentiation in myeloid leukemia cells. Proc Natl Acad Sci U S A. 1978 Aug;75(8):3781–3785. doi: 10.1073/pnas.75.8.3781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pluznik D. H., Sachs L. The cloning of normal "mast" cells in tissue culture. J Cell Physiol. 1965 Dec;66(3):319–324. doi: 10.1002/jcp.1030660309. [DOI] [PubMed] [Google Scholar]
- Rosenberg N., Baltimore D. A quantitative assay for transformation of bone marrow cells by Abelson murine leukemia virus. J Exp Med. 1976 Jun 1;143(6):1453–1463. doi: 10.1084/jem.143.6.1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg N., Baltimore D., Scher C. D. In vitro transformation of lymphoid cells by Abelson murine leukemia virus. Proc Natl Acad Sci U S A. 1975 May;72(5):1932–1936. doi: 10.1073/pnas.72.5.1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg N., Baltimore D. The effect of helper virus on Abelson virus-induced transformation of lymphoid cells. J Exp Med. 1978 Apr 1;147(4):1126–1141. doi: 10.1084/jem.147.4.1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachs L. Control of normal cell differentiation and the phenotypic reversion of malignancy in myeloid leukaemia. Nature. 1978 Aug 10;274(5671):535–539. doi: 10.1038/274535a0. [DOI] [PubMed] [Google Scholar]
- Sachs L. Regulation of membrane changes, differentiation, and malignancy in carcinogenesis. Harvey Lect. 1974;68:1–35. [PubMed] [Google Scholar]
- Scher C. D. Effect of pseudotype on Abelson virus and Kirsten sarcoma virus-induced leukemia. J Exp Med. 1978 Apr 1;147(4):1044–1053. doi: 10.1084/jem.147.4.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toohey J. I. Sulfhydryl dependence in primary explant hematopoietic cells. Inhibition of growth in vitro with vitamin B12 compounds. Proc Natl Acad Sci U S A. 1975 Jan;72(1):73–77. doi: 10.1073/pnas.72.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ware L. M., Axelrad A. A. Inherited resistance to N- and B-tropic murine leukemia viruses in vitro: evidence that congenic mouse strains SIM and SIM.R differ at the Fv-1 locus. Virology. 1972 Nov;50(2):339–348. doi: 10.1016/0042-6822(72)90385-6. [DOI] [PubMed] [Google Scholar]



