Abstract
Effective strategies are needed to block mucosal transmission of human immunodeficiency virus type 1 (HIV-1). Here, we address a crucial question in HIV-1 pathogenesis: whether infected donor mononuclear cells or cell-free virus plays the more important role in initiating mucosal infection by HIV-1. This distinction is critical, as effective strategies for blocking cell-free and cell-associated virus transmission may be different. We describe a novel ex vivo model system that utilizes sealed human colonic mucosa explants and demonstrate in both the ex vivo model and in vivo using the rectal challenge model in rhesus monkeys that HIV-1-infected lymphocytes can transmit infection across the mucosa more efficiently than cell-free virus. These findings may have significant implications for our understanding of the pathogenesis of mucosal transmission of HIV-1 and for the development of strategies to prevent HIV-1 transmission.
INTRODUCTION
Novel microbicide and vaccine candidates for human immunodeficiency virus type 1 (HIV-1) are being evaluated preclinically for efficacy by assessing their ability to protect nonhuman primates against cell-free simian immunodeficiency virus (SIV) or simian-human immunodeficiency virus (SHIV) challenges. However, it remains unclear whether cell-associated virus (virus-infected donor mononuclear cells), cell-free virus, or both play the most important roles in initiating mucosal infection by HIV-1 (1–5). This distinction is critical, since effective strategies for blocking cell-free and cell-associated virus transmission may be very different (3, 6, 7). We sought to explore early events in mucosal transmission of HIV-1 and SIV by evaluating the relative efficiency of cell-associated and cell-free virus in initiating mucosal infection. To model these infection events, we developed a novel three-dimensional sealed human colonic mucosa explant system. We utilized this system in association with the SIV distal colon in vivo challenge model in rhesus macaques to evaluate the relative efficiency of initiating mucosal infection using cell-associated virus compared to that of initiating mucosal infection using cell-free virus in vivo.
MATERIALS AND METHODS
Viruses.
A replication-competent CC chemokine receptor type 5 (CCR5)-tropic HIV-1 strain expressing green fluorescence protein (GFP) [HIV-1(R5) NL4.3-BaL-GFP] (8) was utilized for human organ infections, and SIVmac251 (9) was utilized for rhesus monkey tissue infections.
TCID50 for cell-associated virus and cell-free virus.
The 50% tissue culture infective dose (TCID50) was determined as previously described (10). Briefly, 4 replicates each of cell-associated virus (starting with 200,000 cells/well) and cell-free virus (starting with concentrated virus) were added to the first column of a 96-well plate. Then, 5-fold dilutions were performed for a total of 9 serial dilutions (1:5 to 1:59). An additional column with no virus or cells added served as a negative control to measure the background. TZM-bl cells (NIH AIDS Research and Reference Reagent Program) expressing the reporter genes for firefly luciferase and β-galactosidase were added at a concentration of 5 × 104 cells per well in Dulbecco modified Eagle medium containing 10% fetal calf serum (FCS), 20 U/ml human interleukin-2 (IL-2; Hoffmann-La Roche, Nutley, NJ), and 20 μg/ml DEAE-dextran (Sigma-Aldrich, St. Louis, MO). Forty-eight hours after infection, cells were fixed and stained for β-galactosidase expression using X-Gal (5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside; β-galactosidase reporter gene staining kit, Sigma-Aldrich) as a substrate. A positive well was defined as one that contained 2 or more blue cells (10). The TCID50 was calculated using the Spearman-Kaerber formula (11).
Cell staining.
Peripheral blood mononuclear cells (PBMCs) or enriched CD4+ T lymphocytes were centrifuged for 7 min at 300 × g, and the supernatant was aspirated. Cells were incubated with 5 μM CellTracker Green (5-chloromethylfluorescein diacetate [CMFDA]; Life Technologies, Grand Island, NY) in RPMI 1640 (Corning, Manassas, VA) with 10% fetal bovine serum (FBS; Thermo Scientific, Logan, UT) and 1% penicillin-streptomycin (Life Technologies) (R10 medium) with 20 U/ml IL-2 for 40 min at 37°C. Alternatively, the cells were incubated in 0.45 μM CellTrace Far Red [1,3-dichloro-7-hydroxy-9,9-dimethyl-2(9H)-acridinone-succinimidyl-ester (DDAO-SE); Life Technologies] for 15 min at room temperature (RT). After washing, the stained PBMCs were incubated in fresh R10 containing 20 U/ml IL-2 at 37°C. Cells were washed in fresh R10 medium 3 times before adding them to the wells containing the sealed mucosal colon tissue.
Preparation and infection of sealed human explant.
Discarded human adult colon tissues were obtained after elective colectomy for nonresearch purposes, especially for colorectal cancer or intestinal blockage, within 2 h of surgery under institutional review board (Beth Israel Deaconess Medical Center) approval. Specimens from patients with inflammatory bowel disease were excluded. In all cases, samples for this study were taken adjacent to margins of resection in order to ensure the use of both macroscopically and microscopically normal tissues. Some of the materials were fixed in 4% paraformaldehyde and then embedded in paraffin for hematoxylin-eosin (H&E) staining to confirm a normal structure and tissue integrity. Nonfixed tissue samples were washed repeatedly and incubated for 15 min in R10 supplemented with 15 μg/ml gentamicin (Life Technologies), 15 μg/ml ciprofloxacin (Sigma-Aldrich), 120 U/ml nystatin (Sigma-Aldrich), and 25 μg/ml amphotericin B (Sigma-Aldrich). The muscularis propria side of the tissue was marked with black glitter particles (American Crafts, Orem, UT) to distinguish the mucosal side from the muscularis propria side during the sealing stage. The tissue was divided into circular pieces 4 mm in diameter with a biopsy punch and transferred to a 24-well plate with 0.5 ml of R10 supplemented with 20 U/ml IL-2, 5 μg/ml insulin (Sigma-Aldrich), 5 μg/ml transferrin (Sigma-Aldrich), 5 ng/ml selenium (Sigma-Aldrich), 2 ng/ml epidermal growth factor (Life Technologies), 15 μg/ml gentamicin, and 15 μg/ml ciprofloxacin. The biopsy specimens were sealed with a 3% solution of agarose (Lonza, Walkersville, MD) in double-distilled water. The mucosal side was positioned against a cold glass slide, and the opposite glitter-coated exposed side of the biopsy specimen was covered in gel. After rapid solidification of the gel, the biopsy specimens were transferred to a 24-well plate containing the culture medium described above with the luminal side facing upwards, allowing the unsealed mucosa to be exposed to free virus or cell-associated virus. Cell-free replication-competent HIV-1 (R5) NL4.3-BaL-GFP or CellTrace Far Red (DDAO-SE)-stained human CD4+ T lymphocytes infected with HIV-1 (R5) NL4.3-BaL-GFP were introduced on top of the sealed explants under shaking conditions at 37°C for 2 days. The sealed explant tissue specimens were then washed with R10 and maintained in culture for 3 additional days at 37°C before explant cell isolation.
MTT cell viability assay.
The 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyltetrazolium bromide (MTT) cell viability assay is based on the ability of mitochondrial dehydrogenase to convert MTT substrate (Sigma-Aldrich) into a blue formazan product. Tissue slices were incubated with the MTT substrate for 60 min at 37°C, followed by the addition of 500 μl ethanol to dissolve the colored crystal products. Samples were read using an enzyme-linked immunosorbent assay plate reader (Organon Teknika, Durham, NC) at a wavelength of 540 nm in reference to one of 650 nm (12). For protein determination, the same ethanol-extracted organ slices were dissolved with lysis buffer (200 μl double-distilled water, 0.1% Triton X-100), freeze-thawed twice, and sonicated, and the extract was clarified by centrifugation (3,000 × g for 10 min). The protein content of extracts was determined by a Bradford assay. Cell viability was determined after correction for the protein content of the extracts. The amount of color produced is proportional to the number of viable cells.
Whole-mount immunofluorescence.
Rhesus monkey colon biopsy specimens, about 2 mm3 in size, were sealed with 3% agar gel and exposed to cell-free virus or cell-associated virus in R10 medium with 20 U/ml IL-2 in a 24-well plate for 2 days. After viral exposure, the sealing gel was removed and the explants were washed 3 times with phosphate-buffered saline (PBS; Life Technologies). The explants were fixed with 2% paraformaldehyde in PBS for 15 min at RT, washed twice with PBS, and stored in 70% ethanol at 4°C overnight. The tissues were transferred into 50% methanol for 1 h at RT, washed twice with PBS, permeabilized with 1% Triton X-100 (Sigma-Aldrich) in PBS for 1 h, and blocked in CAS-Block solution (Life Technologies) for 2 h at RT (13). The tissues were incubated in a humid chamber with 4 μg/ml anti-p27 antibody derived from SIVmac251 (Advanced Biotechnologies Incorporated, Columbia, MD) in 0.5% Triton in CAS-Block solution (Life Technologies) at 4°C overnight. Tissues were washed 3 times with 0.1% Tween 20 (Sigma-Aldrich) in PBS and incubated overnight in a humid chamber at 4°C with a secondary antibody consisting of the Alexa Fluor 647 F(ab′)2 fragment of goat anti-mouse IgG (H+L; Life Technologies). Tissues were washed 3 times with PBS, fixed with 1% fresh paraformaldehyde in PBS for 15 min, stained with 1 μg/ml DAPI (4′,6-diamidino-2-phenylindole; Life Technologies) for 30 min, washed twice, and placed on a microscope slide with mounting buffer. All immunofluorescence images were captured with a Zeiss LSM510 upright confocal system (Carl Zeiss Microscopy, Thornwood, NY).
Explant cell isolation and identification of newly infected resident cells.
Cells were isolated from colon explants by incubating the biopsy specimen pieces in R10 medium supplemented with 0.5 mg/ml collagenase II (Sigma-Aldrich) for 25 min. Samples were then homogenized by 6 passages through a 10-ml syringe connected to a 15-gauge needle, followed by passage through a 70-μm-mesh-size nylon mesh cell strainer. Cells were washed using RPMI with 2% FCS. To identify new events of infection, cells were stained with stain from a LIVE/DEAD fixable yellow dead cell stain kit (Life Technologies) and CD4 peridinin chlorophyll protein-Cy5-5-specific (clone L200; Becton, Dickinson, Franklin Lakes, NJ), CD8 allophycocyanin-Cy7-specific (clone SK1; Becton, Dickinson), and CD3 Pacific Blue-specific (clone SP34.2; Becton, Dickinson) antibodies for 15 min and fixed with 1% formaldehyde. Samples were collected on an LSR II instrument (Becton, Dickinson) and analyzed using FlowJo software (version 9.3.1; Tree Star, Ashland, OR). Approximately 106 events were collected per sample. Dead cells were excluded from the analysis. CD3+ CD8− GFP-positive (GFP+) Far Red-negative (Far Red−) cells were defined to be newly infected.
Isolation and infection of human CD4+ T lymphocytes.
Total PBMCs were isolated from human blood by Ficoll-Hypaque gradient centrifugation and stimulated with 6.25 μg/ml concanavalin A (Sigma-Aldrich) and 20 U/ml IL-2. After 1 day of stimulation, CD4+ T cells were enriched from the PBMCs using a CD4+ T cell isolation kit II for negative CD4+ selection (Miltenyi Biotec, Auburn, CA) and a mass spectrometry column (Miltenyi Biotec). Purified CD4+ T cells were infected with HIV-1(R5) NL4.3-BaL-GFP at a multiplicity of infection (MOI) of 8 × 10−4. At 4 to 7 days postinfection, the cells were washed 3 times and stained with CellTrace Far Red (DDAO-SE).
Live/dead cell assays.
For experiments using live and dead cells, half of the uninfected CD4+ T lymphocytes, isolated using a CD4+ T cell isolation kit II for negative CD4+ selection, were fixed in 1% formaldehyde for half an hour and then washed 3 times with PBS before staining with CellTrace Far Red (DDAO-SE). The prefixed dead CD4+ T lymphocytes were mixed with live CD4+ T lymphocytes stained with CellTracker Green (CMFDA) at a live/dead cell ratio of 1:1 and resuspended in R10 before adding them atop the tissue explants.
In vivo cell-free and cell-associated virus infection.
Animal studies in nonhuman primates were conducted at the New England Primate Research Center, Southborough, MA, and at Bioqual, Rockville, MD, with approval by the Institutional Animal Care and Use Committees. The outbred adult rhesus macaques did not express the major histocompatibility complex class I alleles Mamu-A*01, Mamu-B*08, or Mamu-B*17. For in vivo challenges with cell-associated virus, PBMCs from an SIV-naive donor monkey were isolated by Ficoll gradient centrifugation, washed repeatedly, and then exposed to a low dose (MOI < 0.01) of SIVmac251. At 7 days postinfection, cells were washed 4 times with R10. A 1-ml inoculum of 5 × 105 infected PBMCs was administered atraumatically by the intrarectal route to sedated rhesus monkeys. The TCID50 of both free virus and infected rhesus PBMCs were determined on TZM-bl reporter cells. In addition, we confirmed by real-time PCR that the burden of SIV (up to 24,000 viral DNA copies/inoculum) was comparable to the burden of HIV-1 found in semen in vivo (up to 80,000 viral DNA copies/ml inoculum) (14–16). For intrarectal challenge with cell-free virus, the same SIVmac251 challenge stock was used. On the basis of the original TCID50 of the challenge stock, the viral stock was diluted with R10 to achieve the same average titer used for the cell-free challenges (1 ml of 92 TCID50/ml). Plasma SIV RNA levels were determined weekly after challenge by quantitative real-time PCR. Monkeys that developed viremia were not subjected to further weekly challenges.
Real-time PCR.
PBMCs from a naive monkey were exposed to SIVmac251 challenge stock at an MOI of <0.01. At 7 days postinfection, cells were washed 4 times with R10 and DNA was extracted using a Qiagen DNA microkit (Qiagen, Frederick, MD). All PCR assays were performed using TaqMan PCR master mix (Life Technologies) and analyzed with Stratagene MX4000 software, version 4.2 (Agilent, Santa Clara, CA). Run conditions were 95°C for 10 min, followed by 50 cycles of 95°C for 15 s and 60°C for 1.5 min. A quantitative real-time PCR for Gag was performed (17) by using 250 ng of DNA and 900 nM forward primer SIV-Gag-F (5′-GTCTGCGTCATCTGGTGCATTC-3′), 900 nM reverse primer SIV-Gag-R (5′-CACTAGGTGTCTCTGCACTATCTGTTTTG-3′), and 250 nM SIV Gag probe (5′-CTTCCTCAGTGTGTTTCACTTTCTCTTCTGCG-3′) linked to 6-carboxyfluorescein (FAM) and black hole quencher (BHQ) (Life Technologies). Relative cell numbers in each genomic DNA sample were determined as previously described (18). Briefly, the relative quantity of Gag was standardized to that of albumin in a known number of PBMCs, as determined by serial dilution. Forward primer AlbF (5′-TGCATGAGAAAACGCCAGTAA-3′), reverse primer AlbR (5′-ATGGTCGCCTGTTCACCAA-3′), and probe AlbP (5′-AGAAAGTCACCAAATGCTGCACGGAATC-3′) linked to FAM and BHQ (Life Technologies) were used.
Statistical analysis.
All data are presented as the mean ± standard error of the mean (SEM), as noted below. Statistical differences between groups were assessed using a two-tailed Student t test. A P value of <0.05 was considered statistically significant.
RESULTS
Establishment of explant system.
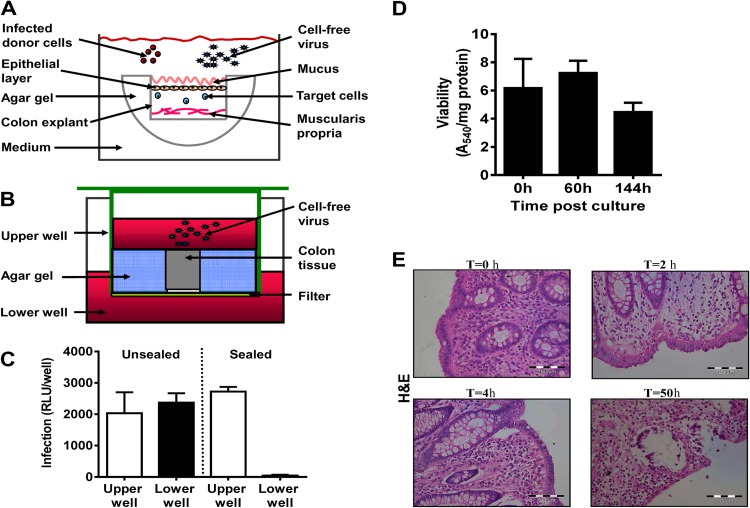
To model early events in HIV-1/SIV infection and to study viral acquisition across a mucosal barrier, distal colon tissue specimens from SIV-naive rhesus monkeys and uninfected humans were cut into 2- to 4-mm3 pieces. Colon explants were placed on a cold surface with the mucosal layer facing down, and agar gel was added to seal the basal and lateral surfaces (19) (Fig. 1A). When such a tissue specimen from a rhesus monkey was placed in a transwell and concentrated free SIVmac251 (1.7 × 105 TCID50/ml, as determined in vitro using TZM-bl reporter cells) was added on top of the sealed mucosal layer (Fig. 1B), the virus did not penetrate to the lower well (Fig. 1C), demonstrating that the gel sealant was successful in preventing viral penetration through the cut sides of the explant. Moreover, sealing the sides and the internal side of colon tissue pieces in agar gel prevented the penetration of 100% India Ink dye into the internal side of the organ (data not shown), confirming that the use of agar gel is a highly effective sealing method.
Fig 1.
Colon explant system. (A) Illustration of the explant sealing process. The sides and bottom of mucosal colon tissue pieces were sealed with 3% agar gel. The sealed tissue was placed in a well containing medium with the uncovered mucosal layer facing upward. Infected donor cells or cell-free virus was added to the medium to infect the sealed organ. (B) Validation of tissue explant sealing. Transwell plates were used to verify that free virus particles did not penetrate through the sides of the tissue explants. Sealed colon tissue was placed in the upper wells, and additional gel was used to seal the sides of the tissue blocks. Medium containing cell-free virus was added on top of the organ explants, while medium without virus was added to the lower wells. The wells were incubated at 37°C for 48 h. (C) To validate the sealing efficiency, replicating virus was quantified by collecting supernatants from the upper and lower wells, and virus titers were determined on TZM-bl reporter cells. An unsealed block exposed to cell-free virus in a transwell served as a control. Data reflect means ± SEMs (n = 3). RLU, relative light units. (D) Viability of sealed human colon tissues in organ culture. Human naive mucosal colon tissue specimens were cut into 4-mm circular pieces. Tissue pieces were sealed on the sides with agar gel and incubated for the indicated times. An MTT viability assay was carried out, and the results were corrected for the protein content of the human tissue as described in Materials and Methods. Data are means ± SEM (n = 5). (E) Histological sections (5 μm) were prepared from cultures of human colon tissues immediately following surgery and at times (T) of 2, 4, and 50 h after culture. The paraffin-embedded slides were stained with H&E.
Determination of viability.
To monitor the viability of the colon explants over time, an MTT assay was performed. The results demonstrated stable dehydrogenase enzyme-specific activity in the sealed human colon explants (Fig. 1D), indicating tissue viability during the first 6 days of organ culture. In order to determine the morphological integrity of the colonic tissue explants over time, we kept the sealed colon tissue pieces in culture, as described above, and took samples for paraffin section at several time points. The pathological analysis of the H&E staining clarified that at time zero the epithelial layer was morphologically fully intact (Fig. 1E). At 2 h postculture, it was possible to see subnuclear vacuolization; however, the epithelial layer remained intact. At 4 h, microscopic regions showed minimal separation between the epithelial layer and the basement membrane, but the continuity of the epithelial layer was preserved. At 50 h, there were widespread degenerative changes in the epithelial layer to a degree that would compromise the continuity of the epithelial layer (Fig. 1E). However, it should be noted that by using confocal microscopy, the first barrier, the mucin layer (built up mainly from mucin 2, a very stable glycoprotein), appeared to be preserved in the colon explant model during the time of the experiments and for up to 6 days (data not shown).
Relative infectivity of cell-associated and cell-free SIVmac251.
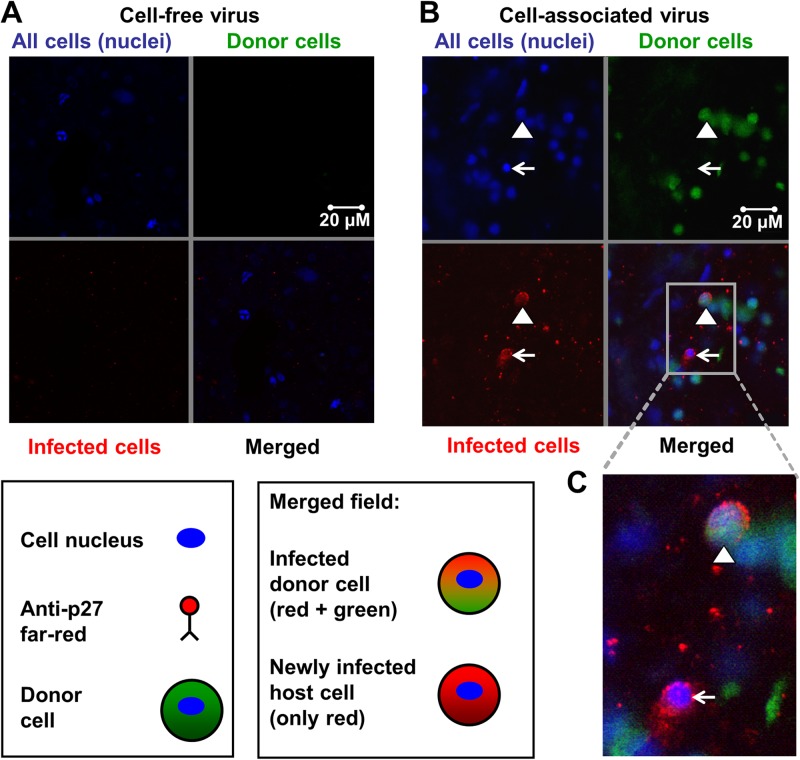
We then sought to evaluate the relative infectivity of cell-associated and cell-free SIVmac251 in the sealed colon explant model. PBMCs as a source of cell-associated virus and plasma as a source of cell-free virus were isolated from the blood of an SIVmac251-infected rhesus monkey with a plasma viral load of 9.1 × 107 SIV RNA copies/ml. Semen was not used as a source of virus because of the donor-to-donor variability in the number of white blood cells present in semen samples and the small volume accessible (20). The explants were sealed and placed into medium-containing wells. PBMCs were washed and stained with CellTracker Green to facilitate discrimination between these cells and resident cells in the explants. The TCID50 of both cell-free and cell-associated virus were determined in vitro using TZM-bl reporter cells. One milliliter of plasma (2.3 × 103 TCID50/ml) or R10 medium containing 106 stained PBMCs from the same infected monkey (1.8 × 102 TCID50/ml) was added to the mucosal surface of the colon tissue. Two days later, the tissues were washed, fixed, and stained using whole-mount immunofluorescence. While we did not observe infection initiated by cell-free virus (Fig. 2A, merged field), the donor PBMCs not only penetrated into the colon epithelial layer but also initiated new infection of resident target or host cells (Fig. 2B and C, merged fields).
Fig 2.
SIVmac251-infected donor cells initiated host cell infections. One milliliter of plasma (2.4 × 103 TCID50/ml) or 1 × 106 PBMCs (18 TCID50/ml) from an SIV-infected monkey stained with CellTracker Green were added atop sealed colon explants obtained from SIV-naive rhesus macaque for 48 h. After viral exposure, organ explants were washed, fixed, and stained using whole-mount immunofluorescence. (A, B) DAPI stained cell nuclei blue (upper left quadrants), CellTracker Green stained donor cells green (upper right quadrants), and anti-p27 Far Red stained SIV p27 protein (SIV capsid) red (lower left quadrants). The lower right quadrants show the merged fields. For each of 2 independent experiments performed in triplicate, the entire tissue specimens (2 by 2 mm) were scanned by two independent investigators. (A) Representative confocal images of explants exposed to plasma containing cell-free SIVmac251. No infected cells were identified in the merged field after addition of free SIVmac251 atop sealed colon tissue. (B) Representative confocal images of explants exposed to PBMCs from an SIVmac251-infected monkey. The triangle points to an infected donor cell (green cytoplasm, red viral protein, blue nucleus), and the arrow points to a newly infected host cell (only red and blue staining). (C) High-powered view of the merged field.
Evaluation of migration into mucosal tissues.
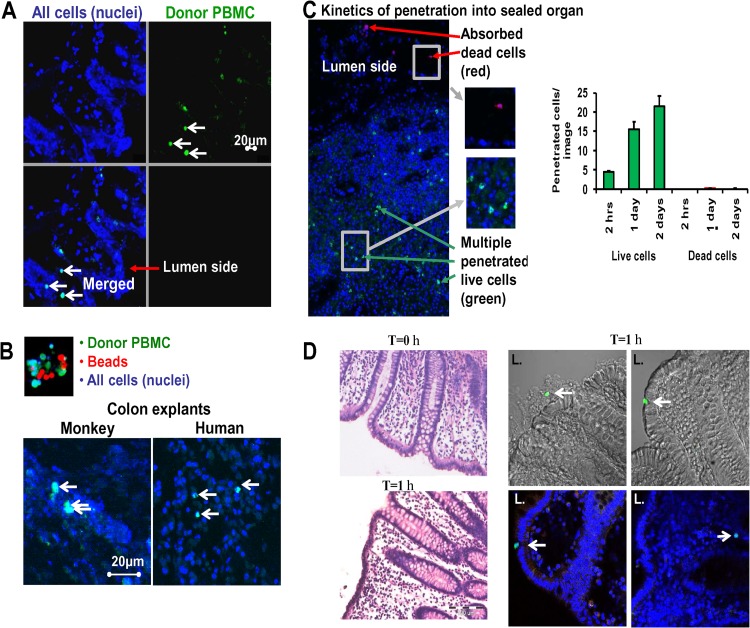
To examine whether allogeneic PBMCs can penetrate the mucin and migrate into the epithelial layer of the explant, CellTracker Green-stained PBMCs from SIV-naive monkeys were added to the exposed mucosal layer of sealed colon explants for 48 h. Next, we examined cryosection slides from the organs using fluorescence microscopy. The presence of green-stained donor PBMCs in the epithelial layer (Fig. 3A, merged field) confirmed the penetration of donor PBMCs into the explant tissue. To investigate whether the penetration of donor PBMCs into the epithelium is an active or passive process, a mixture of equal numbers of green-stained PBMCs and 6-μm red beads was added atop sealed colon tissues obtained from both rhesus monkeys and humans (Fig. 3B, top). Visualization by confocal microscopy revealed that while the inert red beads did not enter the colon mucosa, the green-stained donor PBMCs penetrated into monkey and human explant tissues (Fig. 3B, bottom), suggesting active cellular migration.
Fig 3.
Mucosal penetration by infected cells is an active process. Colon tissue from rhesus monkeys was sealed with the mucosal layer facing upward. Donor PBMCs (1 × 106 cells/well) from an SIVmac251-infected macaque were stained with CellTracker Green and incubated on top of the tissue for 48 h. Tissues were then washed and fixed with 1% paraformaldehyde. Tissue explants were assessed using fluorescence confocal microscopy and photographed at ×200 magnification. (A) Representative image of a cryosection from tissue exposed to donor PBMCs stained with CellTracker Green. (Upper left) All cell nuclei stained with DAPI (blue); (upper right) donor human PBMCs stained with CellTracker Green; (lower left) merged field. The white arrows point to donor PBMCs that migrated into the epithelial cell layer of the tissue. (B) (Top) A mix of red beads (diameter, 6 μm) and donor human PBMCs stained with CellTracker Green; (bottom) colonic explant tissue from a monkey and a human. PBMCs stained with CellTracker Green and red beads were added to these explants, and 48 h later the tissues were washed, fixed, and assessed by confocal microscopy for the presence of beads and donor cells in the cell layers below the mucosal. Arrows, donor PBMCs that migrated into the tissue. Penetration of red beads below the mucosal layer was not observed. The data shown are representative of 5 experiments performed in triplicate. Each of the 2- by 2-mm tissue specimens was scanned entirely by two independent investigators. (C) (Left) Live human donor CD4+ T lymphocytes cells stained with CellTracker Green and dead prefixed CD4+ T lymphocytes stained with CellTrace Far Red were added on top of sealed human colon explants. After 2, 24, and 48 h, the tissues were washed, fixed, and assessed by confocal microscopy from a side view for the presence of live (green) and dead (red) cells in the organ. In the representative side view taken 48 h after addition of cells, red arrows mark dead donor CD4+ T lymphocytes cells that absorbed to the luminal organ surface, while green arrows point to live CD4+ T lymphocytes cells that migrated into the colonic tissue. (C) (Right) Kinetics of human organ penetration by live and dead cells over time. Mucosal colon tissues 4 by 4 mm in area were exposed to live or dead CD4+ T lymphocytes cells at a live/dead cell ratio of 1:1. Tissues were scanned entirely, and pictures were taken (n = 6) and analyzed. Data represent those from 2 independent experiments. The graphs show means ± SEMs. (D) Histological sections (5 μm) were prepared from cultures of human colon tissues immediately following surgery and at 1 h after culture. (Left) The paraffin-embedded slides were stained with H&E. Live human donor CD4+ T lymphocytes stained green with CFSE were added on top of sealed fresh human colon explants. Following 1 h, the tissues were washed, fixed, and prepared for cryosection (30 μm). Confocal microscopy and Nomarski interference contrast optics were used to recognize and focus on the intact epithelial cells. (Right) A small number of CD4+ T cells stained green (arrows) were able to penetrate through the mucin layer, adsorb to the epithelial layer, and invade the entire surface of the mucosa. L., lumen side of the organ.
To determine whether the absence of inert beads in the colon mucosa was an artifact related to differential surface charges, we then compared the cellular migration of live versus dead CD4+ T cells into the allogeneic colonic epithelial layer. Isolated live CD4+ T cells stained with CellTracker Green and dead CD4+ T cells stained with CellTrace Far Red were added to the mucosal surface of sealed human colon explants (live/dead cell ratio, 1:1) and incubated. After 2, 24, and 48 h incubation, followed by repeated washes and fixation, the tissues were assessed by confocal microscopy for the presence of live and dead cells (Fig. 3C). While dead donor CD4+ T cells were detectable only at the luminal organ surface, live CD4+ T cells were observed throughout the mucosal epithelial layer with increasing numbers over time, indicative of active migration into the colonic tissue.
In order to determine the ability of CD4+ T cells to penetrate through the intact mucosal layer, we performed an experiment focusing on cell penetration in the first few hours (immediately after receiving the fresh organ). H&E staining indicated that the entire epithelial layer was intact before culturing (at 0 h). Most of the epithelial layer (∼98%) also remained intact at 1 h following the addition of the CD4+ T cells (Fig. 3D, left). Confocal microscopy and Nomarski optics allowed us to recognize and focus on the intact epithelial cells. We identified a small number of CD4+ T cells (Fig. 3D, right, stained green) that were able to penetrate the mucin layer, adsorb to the epithelial layer, and even invade the entire surface of the mucosa, demonstrating the ability of CD4+ T cells to penetrate even a fully intact epithelial layer.
Analysis of infectivity by HIV-1 in the explant model.
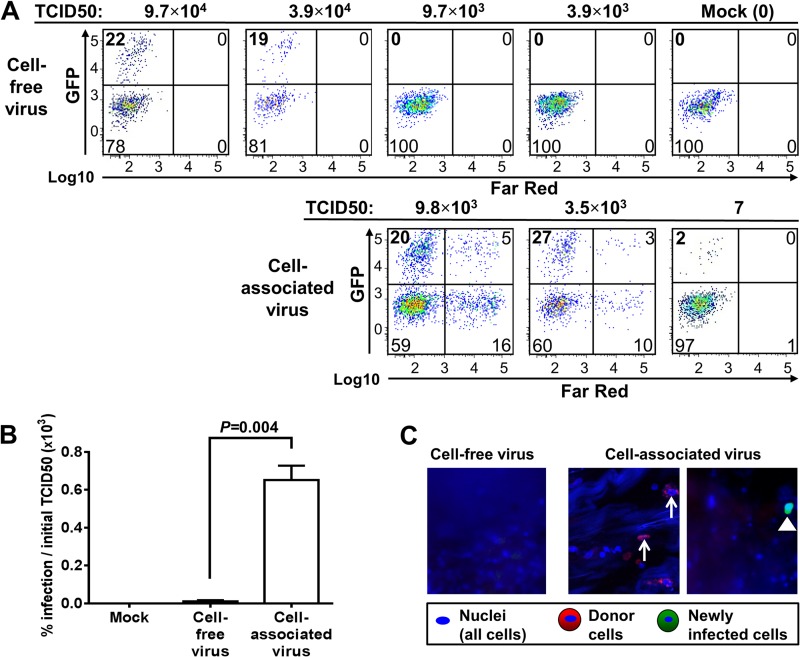
We evaluated the infectivity of cell-associated HIV-1 compared to that of cell-free virus in human colon tissues using a replication-competent CC chemokine receptor type 5 (CCR5)-tropic HIV-1 strain expressing green fluorescence protein (GFP) (NL4.3-BaL-GFP) (8). Uninfected human CD4+ T cells, which were enriched by negative selection, were exposed to HIV-1 expressing GFP in vitro. Five days later, the cells were washed and stained with stable CellTrace Far Red to differentiate donor cells and resident cells in the explant tissue. Cell-free HIV-1 expressing GFP or CD4+ T cells (stained with CellTrace Far Red) infected with HIV-1 expressing GFP were added on top of sealed fresh human colon explant specimens with decreasing TCID50 titers, as measured in vitro using TZM-bl reporter cells. Two days after the exposure to virus or infected cells, the tissue specimens were washed and the medium was replaced. The explant tissues were then incubated for an additional 3 days and, after digestion, subjected to flow cytometric analysis. To include T cells that had downregulated the expression of the CD4 molecule during HIV infection, the percentage of infected CD4+ T cells was determined by gating on live CD3+ CD8− T cells. Quantification of newly infected CD4+ T lymphocytes resident in the colon specimens (Fig. 4A, upper left quadrant of each histogram) indicated that the introduction of cell-associated virus was responsible for a high level of infection (27% newly infected resident CD4+ T cells were generated in the explant tissue by the introduction of 3.5 × 103 TCID50/well). To initiate a comparable infection using cell-free HIV-1, an approximately 10 times higher dose was required (19% newly infected resident CD4+ T cells were generated in the explant tissue by the introduction of 3.9 × 104 TCID50/well). Moreover, a TCID50/well of greater than 9.7 × 103 was required to initiate infection using cell-free HIV-1, while, strikingly, cell-associated HIV-1 initiated infection with as little virus as 7 TCID50/well. Our results indicate that the newly infected cells in the colon tissue after exposure to cell-associated as well as cell-free HIV-1 expressing GFP are primarily CD4+ T cells (>90%) (data not shown).
Fig 4.
Efficiency of cell-free and cell-associated virus in mucosal transmission of HIV-1. (A to C) The mucosal surface of normal human colonic tissue explants was exposed to cell-free replication-competent HIV-1 (R5) NL4.3-BaL-GFP or CellTrace Far Red-stained human CD4+ T lymphocytes infected with HIV-1 (R5) NL4.3-BaL-GFP and cultured for 5 days. The TCID50 was determined using TZM-bl reporter cells. The entire assay took 9 to 12 days. First, the donor CD4+ T lymphocytes cells were incubated with the virus for 4 to 7 days to generate cell-associated virus. Then, free virus or cell-associated virus was added on top of the fresh colon tissue for 2 days. Next, the organ tissue was washed and incubated for 3 additional days. (A) Identification of newly infected host cells in colon tissue explants exposed to different amounts of cell-free virus (top) or cell-associated virus (bottom). Isolated colonic cells were analyzed by flow cytometry. (Upper left quadrants) Newly infected CD4+ T lymphocytes (CD3+ CD8− GFP+ Far Red−) in colon explants as a percentage of all counted CD4+ T lymphocytes; (lower left quadrants) uninfected CD4+ T lymphocytes in colon tissue; (upper and lower right quadrants) CellTrace Far Red-stained donor CD4+ T lymphocytes that were attached to or that had penetrated into the explants. (B) Comparison of infection rates in 3 human colon explant tissue specimens using cell-free or cell-associated virus. The percentages of newly infected CD4+ T cells in the explants compared to the TCID50 used to initiate infection showed that cell-associated virus was significantly more efficient in initiating infection than cell-free HIV-1 (R5) NL4.3-BaL-GFP (P = 0.004, t test). (C) Confocal images of colon explants exposed to cell-free HIV-1 (R5) NL4.3-BaL-GFP (2.0 × 104 TCID50/ml) or CD4+ T lymphocyte-associated HIV-1 (R5) NL4.3-BaL-GFP (4.3 × 103 TCID50/ml). Fluorescence confocal microscopy showed no infection 5 days after exposure to cell-free virus. The triangle points to representative donor cells that entered the organ explant; arrows point to newly infected host cells (GFP+ Far Red−). Magnifications, ×200.
To further evaluate the relative infectivity of cell-associated and cell-free virus, we performed three independent experiments using explanted human colon tissue from different patients who had undergone colonic resection. The percentages of infected CD4+ T lymphocytes, normalized by the number of infectious units used to initiate infection, suggested that cell-associated virus induced infection more efficiently than cell-free virus (Fig. 4B; P = 0.004). Using confocal microscopy, infection by free virus was not detected (Fig. 4C, left). However, we identified that donor CD4+ T lymphocytes penetrated into the mucosa of human colonic tissue (Fig. 4C, middle, red cells). We also identified cells expressing only GFP without Far Red staining, representing newly infected cells (Fig. 4C, right). Combined, these data provide additional evidence of initiation of infection by cell-associated virus.
In vivo evaluation of infectivity by cell-free or cell-associated SIVmac251.
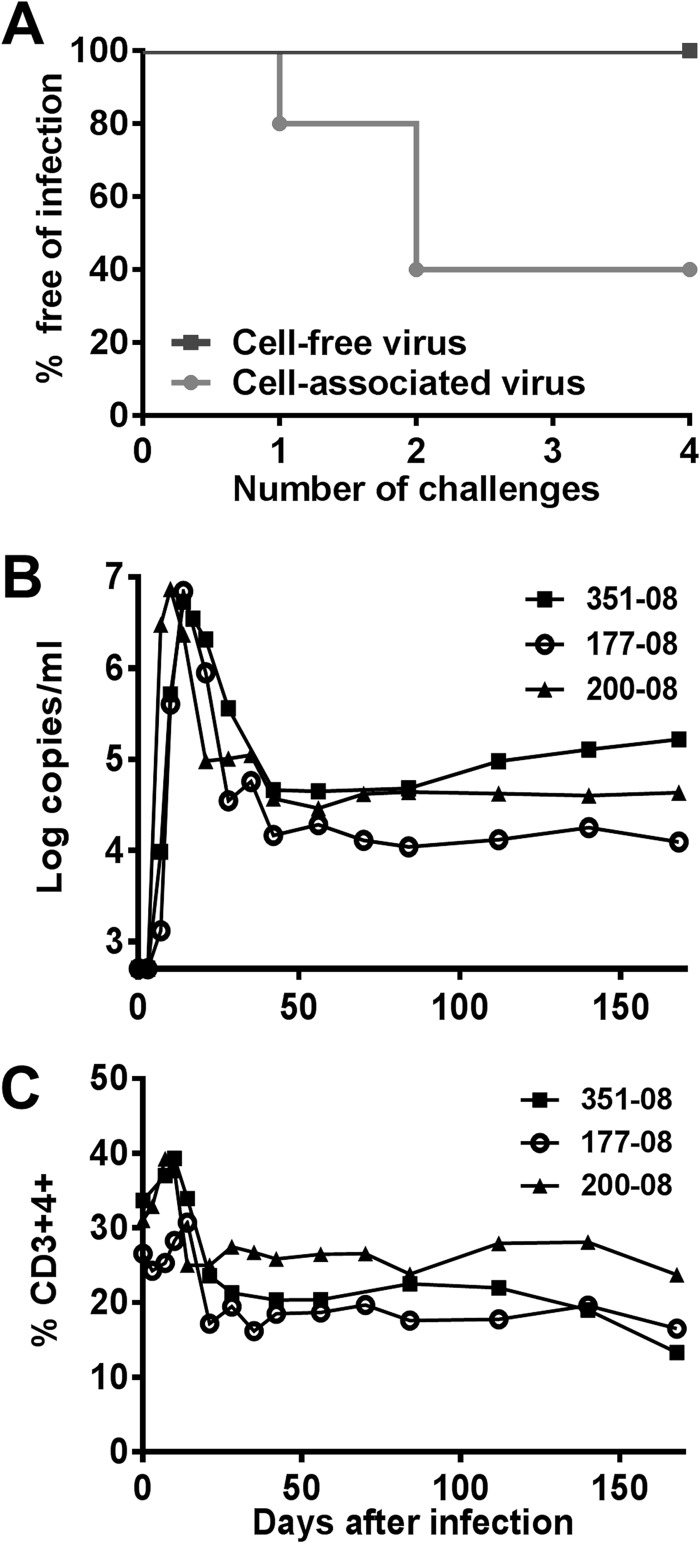
To validate these results in vivo, we next carried out a nonhuman primate study to compare the efficiency of cell-associated and cell-free SIVmac251 in initiating mucosal infections in rhesus monkeys. First, we measured the TCID50 of both the free virus stock and the infected PBMCs using TZM-bl reporter cells. Low doses of virus (92 TCID50/ml) were administered by the intrarectal route to 5 monkeys as cell-associated virus and to 4 monkeys as cell-free virus in 4 successive challenges (Fig. 5A). While this low dose of cell-free virus did not initiate an infection, cell-associated virus successfully initiated infection in 3 of 5 monkeys following only 2 challenges with the same dose of virus. The monkeys that became infected after intrarectal challenge with cell-associated virus exhibited a typical acute SIV infection with a peak viremia ranging from 5.3 × 106 to 7.3 × 106 viral RNA copies/ml at 10 to 14 days after challenge, followed by a loss of CD4+ T lymphocytes (Fig. 5B and C).
Fig 5.
Efficiency of cell-free and cell-associated virus in mucosal transmission of SIV in vivo. (A) Low dose of cell-associated but not cell-free SIVmac251-initiated infection of rhesus macaques by the intrarectal route. While none of 4 macaques became infected following 4 successive intrarectal challenges with 1 ml of cell-free SIVmac251 (92 TCID50/ml), 3 of 5 monkeys developed viremia after challenges with equivalent TCID50 of cell-associated SIVmac251. (B) Viral load (number of viral RNA copies/ml) measured over time in 3 infected monkeys following challenge with cell-associated SIV. (C) Percentages of CD4+ T lymphocytes evaluated over time in the blood of the infected monkeys.
DISCUSSION
The findings in our colonic explant model suggest that cell-associated HIV-1 (virus-infected lymphocytes) may be able to transmit infection across the rectal mucosa more efficiently than cell-free virus. This increased efficiency could be a consequence of the ability of CD4+ T lymphocytes to adsorb to the mucosal layer and, while attached to the mucosa, continually produce virus that can initiate infection (Fig. 4A). In contrast, the cell-free virus is either washed out of the lumen, trapped by the viscoelastic mucosal layer present at the colonic mucosa (21, 22), or blocked by the epithelial layer (23, 24). However, CD4+ T cells can actively migrate through mucosal tissue (14, 25, 26) (Fig. 2B, 3, and 4C), allowing infected cells to potentially transmit the virus to resident CD4+ T cells either via cell-to-cell contact or by release of virus below the mucosal barrier. Although we observed that over 90% of infected cells in the colon are CD4+ T cells (data not shown), we cannot rule out a role for macrophages and dendritic cells in late viral spreading.
It should be noted that the first barrier—the mucin layer (built up mainly from mucin 2, a very stable glycoprotein)—seems to be preserved in the colon explant model during the time of the experiments, and indeed, the barrier formed by the mucin layer prevented any penetration of the control dead cells and beads (Fig. 3B to D).
The highly efficient infection by cell-associated HIV-1 in the colon explant system (cell-associated HIV-1 infection with as little virus as 7 TCID50/well; Fig. 4A) was supported by our results obtained using a rectal SIV challenge in rhesus macaques. Despite the high variance of biologic substrate in semen samples from HIV-1-infected patients, the inoculum of 50,000 PBMCs that was used to challenge the monkeys in vivo with a viral burden of up to 2.4 × 104 DNA copies/inoculum appears to simulate exposure to sexually transmitted HIV-1. The median numbers of leukocytes in semen have been shown to be 1 × 105 cells/ml of semen, and the cell-associated HIV-1 burden has been shown to be up to 80,000 DNA copies/ml seminal inoculum in HIV-1-infected males (14, 15, 27, 28). In contrast, previous rhesus macaque challenge models with cell-free SIV used very high viral doses (106 to 107 viral RNA copies/inoculum) (9, 29, 30) in order to initiate infection. Such a high cell-free viral burden has been reported in only 3 of 89 HIV-1-infected patients (31) and is much higher than the typical cell-free viral burden in the semen of infected individuals (maximum, 4 × 105 viral RNA copies/ml) (20). The superior infectivity of cell-associated HIV-1/SIV versus cell-free HIV-1/SIV in a mucosal infection through the colon (ex vivo and in vivo) may not be limited to colon infection, as recent publications indicate that cell-associated SIV can also initiate efficient infection through the intact vaginal mucosa in an in vivo macaque monkey model (14). The findings of early studies in the 1990s (32) that used highly concentrated cell-free virus (an amount 105 times higher than that received by the intravenous route) but that did not elicit infection by intravaginal challenge with limited numbers (105) of PBMCs from SIV-infected monkeys may have contributed to the abandonment of studies investigating the role of cell-associated virus in viral acquisition. While the vast majority of challenge studies have been performed with cell-free virus, some researchers have emphasized the potential importance of cell-associated HIV (3, 5, 16). On the basis of our results, future challenge studies should include both cell-free and cell-associated virus.
Our findings may have significant implications for the understanding of the pathogenesis of mucosal transmission of HIV-1 and for the development of strategies to prevent HIV-1 transmission.
In order to gain a better comprehension regarding the exact mechanism of cell-associated virus infectivity, further studies should use in vivo models and perhaps tissues from newly infected patients to determine the relative infectivity of invading cells compared to that of cells that remain attached to the lumen side. Topical microbicides that inactivate free virus but do not block infected cells may not protect against mucosal acquisition of virus. Also, viral antigens may be presented differently to the immune system when expressed on the surface of infected cells or as cell-free virus (7). This has implications for the roles of mucosal CD8+ T cells and antibody-dependent cell-mediated cytotoxicity, which may be capable of targeting infected cells and confer sterile protection from infection. In addition, the different modes of infection used by cell-associated HIV-1 (e.g., virological synapses, nanotubes, filopodia) (33–35) enable close contact between donor cells and target cells and can change the inhibition of antiretroviral drugs and neutralizing antibodies (6, 36). All of these considerations may play an important role in vaccine immunogen design.
ACKNOWLEDGMENTS
We sincerely thank Ashley Ladd for her technical support, Evan Cale for his revision of this paper, Dan Barouch for providing SIVmac251, Dana Gabuzda for supplying HIV-1(R5) NL4.3-BaL-GFP, the NIAID Vaccine Research Center for the kind gift of rhesus macaque PBMCs, Eli Pikarsky for his generous help with the pathology analysis and his good advice, and Zippora Shlomai for helping with the penetration assays.
This work was supported by Harvard Medical School Center for AIDS Research grant AI060354.
D.K.-G. designed and validated the explant system and performed the whole-mount immunofluorescence and the mucosal penetration assays. D.K.-G., M.A.L., S.L.H., and G.Z. designed, performed, and analyzed the fluorescence cell sorting assays for the infected human explants. D.K.-G., Y.Z., and C.A. performed the confocal assays. R.M.N. examined, prepared, and supplied the human colon tissues. D.K.-G. and W.W.Y. designed and conducted the PCR assays. D.K.-G., R.B.G., J.O., G.Z., and Y.Z. conducted integrity assays. D.K.-G., B.K.-S., S.L.H., M.A., and N.L.L. designed the in vivo study. B.K.-S. performed the in vivo data analysis. D.K.-G., B.K.-S., S.L.H., R.B.G., C.A., R.M.N., M.A., G.Z., and N.L.L. contributed to writing.
We declare no competing financial interests.
Footnotes
Published ahead of print 9 October 2013
This article is dedicated to Norman L. Letvin, a great scientist and dedicated mentor.
REFERENCES
- 1.Anderson DJ, Yunis EJ. 1983. “Trojan horse” leukocytes in AIDS. N. Engl. J. Med. 309:984–985 [PubMed] [Google Scholar]
- 2.Kashuba AD, Dyer JR, Kramer LM, Raasch RH, Eron JJ, Cohen MS. 1999. Antiretroviral-drug concentrations in semen: implications for sexual transmission of human immunodeficiency virus type 1. Antimicrob. Agents Chemother. 43:1817–1826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Virgin HW, Walker BD. 2010. Immunology and the elusive AIDS vaccine. Nature 464:224–231 [DOI] [PubMed] [Google Scholar]
- 4.Vernazza PL. 2005. HIV in semen: still more to be learned. AIDS Res. Ther. 2:11. 10.1186/1742-6405-2-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Letvin NL. 2008. Immunology of HIV infection, p 1204–1232 In Paul WE. (ed), Fundamental immunology, 6th ed. Wolter Kluwer/Lippincott, Williams & Wilkins, Philadelphia, PA [Google Scholar]
- 6.Abela IA, Berlinger L, Schanz M, Reynell L, Gunthard HF, Rusert P, Trkola A. 2012. Cell-cell transmission enables HIV-1 to evade inhibition by potent CD4bs directed antibodies. PLoS Pathog. 8:e1002634. 10.1371/journal.ppat.1002634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ferrari G, Pollara J, Kozink D, Harms T, Drinker M, Freel S, Moody MA, Alam SM, Tomaras GD, Ochsenbauer C, Kappes JC, Shaw GM, Hoxie JA, Robinson JE, Haynes BF. 2011. An HIV-1 gp120 envelope human monoclonal antibody that recognizes a C1 conformational epitope mediates potent antibody-dependent cellular cytotoxicity (ADCC) activity and defines a common ADCC epitope in human HIV-1 serum. J. Virol. 85:7029–7036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ancuta P, Autissier P, Wurcel A, Zaman T, Stone D, Gabuzda D. 2006. CD16+ monocyte-derived macrophages activate resting T cells for HIV infection by producing CCR3 and CCR4 ligands. J. Immunol. 176:5760–5771 [DOI] [PubMed] [Google Scholar]
- 9.Liu J, Keele BF, Li H, Keating S, Norris PJ, Carville A, Mansfield KG, Tomaras GD, Haynes BF, Kolodkin-Gal D, Letvin NL, Hahn BH, Shaw GM, Barouch DH. 2010. Low-dose mucosal simian immunodeficiency virus infection restricts early replication kinetics and transmitted virus variants in rhesus monkeys. J. Virol. 84:10406–10412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vacharaksa A, Asrani AC, Gebhard KH, Fasching CE, Giacaman RA, Janoff EN, Ross KF, Herzberg MC. 2008. Oral keratinocytes support non-replicative infection and transfer of harbored HIV-1 to permissive cells. Retrovirology 5:66. 10.1186/1742-4690-5-66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Finney DJ. 1978. Statistical method in biological assay, 3rd ed, vol 3 Charles Griffin & Co. Ltd, London, United Kingdom [Google Scholar]
- 12.Kolodkin-Gal D, Zamir G, Edden Y, Pikarsky E, Pikarsky A, Haim H, Haviv YS, Panet A. 2008. Herpes simplex virus type 1 preferentially targets human colon carcinoma: role of extracellular matrix. J. Virol. 82:999–1010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Massler A, Kolodkin-Gal D, Meir K, Khalaileh A, Falk H, Izhar U, Shufaro Y, Panet A. 2011. Infant lungs are preferentially infected by adenovirus and herpes simplex virus type 1 vectors: role of the tissue mesenchymal cells. J. Gene Med. 13:101–113 [DOI] [PubMed] [Google Scholar]
- 14.Salle B, Brochard P, Bourry O, Mannioui A, Andrieu T, Prevot S, Dejucq-Rainsford N, Dereuddre-Bosquet N, Le Grand R. 2010. Infection of macaques after vaginal exposure to cell-associated simian immunodeficiency virus. J. Infect. Dis. 202:337–344 [DOI] [PubMed] [Google Scholar]
- 15.Politch JA, Mayer KH, Anderson DJ. 2009. Depletion of CD4+ T cells in semen during HIV infection and their restoration following antiretroviral therapy. J. Acquir. Immune Defic. Syndr. 50:283–289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Anderson DJ, Politch JA, Nadolski AM, Blaskewicz CD, Pudney J, Mayer KH. 2010. Targeting Trojan horse leukocytes for HIV prevention. AIDS 24:163–187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cline AN, Bess JW, Piatak M, Jr, Lifson JD. 2005. Highly sensitive SIV plasma viral load assay: practical considerations, realistic performance expectations, and application to reverse engineering of vaccines for AIDS. J. Med. Primatol. 34:303–312 [DOI] [PubMed] [Google Scholar]
- 18.Nishimura Y, Sadjadpour R, Mattapallil JJ, Igarashi T, Lee W, Buckler-White A, Roederer M, Chun TW, Martin MA. 2009. High frequencies of resting CD4+ T cells containing integrated viral DNA are found in rhesus macaques during acute lentivirus infections. Proc. Natl. Acad. Sci. U. S. A. 106:8015–8020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Collins KB, Patterson BK, Naus GJ, Landers DV, Gupta P. 2000. Development of an in vitro organ culture model to study transmission of HIV-1 in the female genital tract. Nat. Med. 6:475–479 [DOI] [PubMed] [Google Scholar]
- 20.Mayer KH, Boswell S, Goldstein R, Lo W, Xu C, Tucker L, DePasquale MP, D'Aquila R, Anderson DJ. 1999. Persistence of human immunodeficiency virus in semen after adding indinavir to combination antiretroviral therapy. Clin. Infect. Dis. 28:1252–1259 [DOI] [PubMed] [Google Scholar]
- 21.Mahalingam A, Jay JI, Langheinrich K, Shukair S, McRaven MD, Rohan LC, Herold BC, Hope TJ, Kiser PF. 2011. Inhibition of the transport of HIV in vitro using a pH-responsive synthetic mucin-like polymer system. Biomaterials 32:8343–8355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Habte HH, de Beer C, Lotz ZE, Roux P, Mall AS. 2010. Anti-HIV-1 activity of salivary MUC5B and MUC7 mucins from HIV patients with different CD4 counts. Virol. J. 7:269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gali Y, Arien KK, Praet M, Van den Bergh R, Temmerman M, Delezay O, Vanham G. 2010. Development of an in vitro dual-chamber model of the female genital tract as a screening tool for epithelial toxicity. J. Virol. Methods 165:186–197 [DOI] [PubMed] [Google Scholar]
- 24.Lawrence P, Portran D, Terrasse R, Palle S, Olivier T, Fantini J, Bourlet T, Pozzetto B, Delezay O. 2012. Selective transmigration of monocyte-associated HIV-1 across a human cervical monolayer and its modulation by seminal plasma. AIDS 26:785–796 [DOI] [PubMed] [Google Scholar]
- 25.Khanna KV, Whaley KJ, Zeitlin L, Moench TR, Mehrazar K, Cone RA, Liao Z, Hildreth JE, Hoen TE, Shultz L, Markham RB. 2002. Vaginal transmission of cell-associated HIV-1 in the mouse is blocked by a topical, membrane-modifying agent. J. Clin. Invest. 109:205–211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ibata B, Parr EL, King NJ, Parr MB. 1997. Migration of foreign lymphocytes from the mouse vagina into the cervicovaginal mucosa and to the iliac lymph nodes. Biol. Reprod. 56:537–543 [DOI] [PubMed] [Google Scholar]
- 27.Jung A, Maier R, Vartanian JP, Bocharov G, Jung V, Fischer U, Meese E, Wain-Hobson S, Meyerhans A. 2002. Recombination: multiply infected spleen cells in HIV patients. Nature 418:144. [DOI] [PubMed] [Google Scholar]
- 28.Wolff H, Anderson DJ. 1988. Immunohistologic characterization and quantitation of leukocyte subpopulations in human semen. Fertil. Steril. 49:497–504 [PubMed] [Google Scholar]
- 29.Keele BF, Li H, Learn GH, Hraber P, Giorgi EE, Grayson T, Sun C, Chen Y, Yeh WW, Letvin NL, Mascola JR, Nabel GJ, Haynes BF, Bhattacharya T, Perelson AS, Korber BT, Hahn BH, Shaw GM. 2009. Low-dose rectal inoculation of rhesus macaques by SIVsmE660 or SIVmac251 recapitulates human mucosal infection by HIV-1. J. Exp. Med. 206:1117–1134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Barouch DH, Liu J, Li H, Maxfield LF, Abbink P, Lynch DM, Iampietro MJ, SanMiguel A, Seaman MS, Ferrari G, Forthal DN, Ourmanov I, Hirsch VM, Carville A, Mansfield KG, Stablein D, Pau MG, Schuitemaker H, Sadoff JC, Billings EA, Rao M, Robb ML, Kim JH, Marovich MA, Goudsmit J, Michael NL. 2012. Vaccine protection against acquisition of neutralization-resistant SIV challenges in rhesus monkeys. Nature 482:89–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stekler J, Sycks BJ, Holte S, Maenza J, Stevens CE, Dragavon J, Collier AC, Coombs RW. 2008. HIV dynamics in seminal plasma during primary HIV infection. AIDS Res. Hum. Retroviruses 24:1269–1274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sodora DL, Gettie A, Miller CJ, Marx PA. 1998. Vaginal transmission of SIV: assessing infectivity and hormonal influences in macaques inoculated with cell-free and cell-associated viral stocks. AIDS Res. Hum. Retroviruses 14(Suppl 1):S119–S123 [PubMed] [Google Scholar]
- 33.Nobile C, Rudnicka D, Hasan M, Aulner N, Porrot F, Machu C, Renaud O, Prevost MC, Hivroz C, Schwartz O, Sol-Foulon N. 2010. HIV-1 Nef inhibits ruffles, induces filopodia, and modulates migration of infected lymphocytes. J. Virol. 84:2282–2293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sowinski S, Jolly C, Berninghausen O, Purbhoo MA, Chauveau A, Kohler K, Oddos S, Eissmann P, Brodsky FM, Hopkins C, Onfelt B, Sattentau Q, Davis DM. 2008. Membrane nanotubes physically connect T cells over long distances presenting a novel route for HIV-1 transmission. Nat. Cell Biol. 10:211–219 [DOI] [PubMed] [Google Scholar]
- 35.Sattentau Q. 2008. Avoiding the void: cell-to-cell spread of human viruses. Nat. Rev. Microbiol. 6:815–826 [DOI] [PubMed] [Google Scholar]
- 36.Sigal A, Kim JT, Balazs AB, Dekel E, Mayo A, Milo R, Baltimore D. 2011. Cell-to-cell spread of HIV permits ongoing replication despite antiretroviral therapy. Nature 477:95–98 [DOI] [PubMed] [Google Scholar]