Abstract
The NS1 protein of influenza A virus is known to downregulate apoptosis early in infection in order to support virus replication (O. P. Zhirnov, T. E. Konakova, T. Wolff, and H. D. Klenk, J. Virol. 76:1617–1625, 2002). In the present study, we analyzed the development of autophagy, another mechanism to protect cells from degradation that depends on NS1 expression. To this end, we compared autophagy in cells infected with wild-type (WT) influenza virus and virus lacking the NS1 gene (delNS1 virus). The results show that in WT-infected cells but not in delNS1 virus-infected cells, synthesis of the autophagy marker LC3-II, the lipidated form of microtubule light chain-associated protein LC3, is stimulated and that LC3-II accumulates in a perinuclear zone enriched with double-layered membrane vesicles characteristic of autophagosomes. Transfection experiments revealed that NS1 expressed alone was unable to upregulate autophagy, whereas hemagglutinin (HA) and M2 were. Proteolytic cleavage of HA increased autophagy. Taken together, these observations indicate that NS1 stimulates autophagy indirectly by upregulating the synthesis of HA and M2. Thus, it appears that NS1, besides downregulating apoptosis, is involved in upregulation of autophagy and that it supports the survival of infected cells by both mechanisms.
INTRODUCTION
Autophagy is a mechanism that allows cell survival under stress conditions, such as amino acid starvation, energy deprivation, and virus infection (1). It is a constitutive process that involves recycling of cytosolic proteins and organelles by lysosomal degradation and an immune response by virus protein degradation and extracellular presentation of degraded molecules to immune recognition (2, 3). Autophagy thereby provides nutrients for maintenance and repair of vital functions of the host, including immune defense. Autophagy is mediated by autophagosomes, specialized double-membrane vesicles originating from the Golgi apparatus, endoplasmic reticulum, and mitochondria, and it is regulated by the interaction of more than 30 autophagy-related cellular genes (Atgs) (4). Two stages can be discriminated in the autophagy process: an initial stage of autophagosome formation and a terminal effector stage (maturation) involving fusion of autophagosomes with lysosomes and degradation of the intra-autophagosomal content. Phosphatidylinositol-3-kinase class III (PI3K-III), beclin (Atg6), and LC3 (Atg8) are important factors involved in autophagy (5). Viruses have developed strategies either to counteract autophagy or to employ the autophagic pathway for their own benefit (1, 3).
Influenza A virus modulates autophagy (6–8) as well as apoptosis, another pathway regulating survival of infected cells (8). To allow host cell survival and to promote viral replication, apoptosis is downregulated early after infection by the interaction of the PI3K (class I)-AKT complex with the virus protein NS1 (9–12). As a consequence, rapid apoptosis develops in cells infected with influenza virus lacking the NS1 gene (13–15). However, when overproduced in transfected cells, NS1 induces apoptosis (16, 17). Autophagy, on the other hand, is regulated by the viral protein M2. M2 stimulates the initial stage of autophagosome formation but inhibits the terminal stage of autophagosome-lysosome fusion (6, 7).
There is evidence that autophagy and apoptosis are coupled pathways in influenza virus-infected cells, as well as in noninfected cells (18, 19). It was therefore of interest to find out whether NS1 is involved not only in apoptosis but also in the regulation of the autophagic process. To throw light on this issue, the development of autophagy was studied in cells infected with influenza virus lacking the NS1 gene (delNS1 virus) and wild-type (WT) virus and in cells expressing individual viral proteins. The data support the concept that NS1, in addition to its regulatory role in the antiapoptotic response, is involved in stimulation of autophagy and that this action is mediated by NS1-dependent amplification of M2 and hemagglutinin (HA) synthesis. It was also found that proteolytic cleavage of HA upregulates autophagy in infected cells.
MATERIALS AND METHODS
Cells and viruses.
Influenza A/PR/8/34 (H1N1) WT virus and its isogenic mutant delNS1 virus (13) were propagated in 7-day-old embryonated chicken eggs. Virus titers were measured by virus focus assay in Madin-Darby canine kidney cells (line MDCK-II; collection of the Institute of Virology, Marburg, Germany). MDCK and monkey kidney cells (line CV-1; European Collection of Cell Cultures [ECACC]) were passaged in Dulbecco's minimal essential medium (DMEM) containing 10% bovine fetal calf serum (FCS; Gibco-BRL, Germany). For infection, 2-day-old confluent cell monolayers were incubated with egg-grown influenza virus (1 to 3 PFU/cell) for 1 h at 37°C. After infection, cells were washed and incubated with DMEM without serum at 37°C for different periods of time and were then prepared for either microscopic examination, gel electrophoresis, or immunofluorescence analysis. Membrane-permeant lysosomal protease inhibitor E64d (Sigma-Aldrich) was dissolved in dimethyl sulfoxide, diluted to a final concentration of 10 μg/ml in complete DMEM, and added to mock- and virus-infected cell monolayers for 9.5 h.
Cell fractionation.
CV-1 and MDCK cells were infected with either WT or delNS1 viruses at a multiplicity of infection (MOI) of 1. At 9.5 h postinfection (p.i.), cells were disrupted by sonication in a Branson model 450 digital sonifier at power level 6 for 30 s, and cytoplasmic homogenates were prepared by centrifugation at 1,000 × g for 10 min at 4°C. Next, cytoplasmic homogenates were subdivided into 2 parts, and one aliquot was treated with 0.7% nonionic detergent NP-40. Soluble (S40) and membrane pellet (P40) fractions were obtained from the other aliquot by centrifugation at 105,000 × g (40,000 rpm) for 1 h (TLA-55 rotor, Optima MAX-XP ultracentrifuge). Fractions were analyzed by Western blot (WB) analysis using LC3-specific antibody and an enhanced chemiluminescent (ECL) procedure.
Cell transfection experiments.
The pCAGGS vector containing a combined chicken beta-actin and rabbit beta-globin hybrid promoter and the human cytomegalovirus immediate early promoter was used for expression of the NS1 protein. 293T cells were grown on 30-mm dishes to 60 to 90% confluence and transfected with 4.0 μg pCAGGS or pCAGGS carrying influenza virus A/WSN/33 (H1N1) genes (PB1, PB2, PA, NP, HA, NA, NEP, NS1, M1, M2) (20). Similar experiments were carried out with wild-type HA of A/FPV/Rostock/34 (H7N1) or with HA mutants in which the polybasic cleavage site had been converted into a monobasic cleavage site by deletion (H7-del) or alanine substitution (H7-aaa) (mutants 1 and 2, respectively, in reference 21). Cell transfection was performed with the Lipofectamine 2000 transfection reagent (7 μl; Qiagen, Germany) according to the manufacturer's protocol. DNA-Lipofectamine mixtures were prepared in Opti-MEM medium (Gibco-BRL, Germany). Transfected cells were incubated in DMEM containing 0.9% FCS and ampicillin-streptomycin for 27 and 40 h. This medium did not stimulate autophagy in cells. After incubation, cells were subjected to polypeptide analysis by polyacrylamide gel electrophoresis (PAGE).
Immune fluorescent staining.
Uninfected cells and cells infected with either delNS1 or WT virus were fixed with 4% paraformaldehyde (Merck, Germany) overnight at 4°C, permeabilized with 0.1% detergent NP-40 (Pierce) for 3 min, and stained sequentially with rabbit antibody specific to LC3 (Cell Signaling) and secondary anti-rabbit–tetramethyl rhodamine isocyanate (TRITC) conjugate (Jackson) dissolved in phosphate-buffered saline (PBS) containing 0.5% bovine serum albumin (BSA). Nuclei were stained with 0.1 mg/ml of DAPI (4′,6-diamidino-2-phenylindole) for 30 min in the dark. Stained cells were analyzed using a Leica TCS SP5 confocal microscope. Fluorescence was quantified by image processing using the histogram operation of the IrfanView program (version 4.36). The level of fluorescence of 20-by-20-pixel squares in the perinuclear zone was measured in the red channel, and these values were normalized to the level of DAPI nuclear staining, calculated as the level of fluorescence of pixels in the intranuclear area measured in the blue channel. The levels of fluorescence of 10 separate squares in the peri- and intranuclear zones of 3 separate cells were calculated for mock-, delNS1 virus-, and WT-infected samples.
PAGE.
Polypeptides were electrophoresed in 12% polyacrylamide gels using Tris-glycine-SDS buffer, followed by Western blot analysis. For gel staining with Coomassie brilliant blue R-350, the protocol of Pharmacia was applied. For electrophoresis, cellular polypeptides were treated in dissociation buffer (2% SDS, 0.04 M dithiothreitol, 0.02 M Tris-HCl, pH 6.8) for 10 min at 75°C, and quantitatively equivalent aliquots of each sample were loaded into the gel well.
WB analysis.
After SDS-PAGE, the polypeptides were transferred from the gel onto Protran nitrocellulose membranes (pore size, 0.45 μm; Schleicher & Schuell, Germany) by semidry electroblotting with Tris-glycine buffer (pH, ∼9.8) containing 15% ethanol and 0.03% SDS. To quantitatively normalize the samples in one set of the PAGE-WB analysis, the same amounts of protein were loaded into the wells of the gels. The membranes were washed with 150 mM PBS and incubated for 2 h at 20°C in 3% dried milk in PBS. After washing with PBS, the membranes were incubated overnight at 4°C in PBS containing 1% BSA and antisera specific for protein LC3 (NB600-1384; Novus), beclin (NB110-87318; Novus), actin (A5441; Sigma), kinase AKT and its phosphorylated form (AKT-Ser476-pho), S6k and its phosphorylated form (S6K-Ser240/244-pho), activated caspase 3a (Cell Signaling), and hemagglutinin of influenza viruses A/PR/8/34 (H1N1) and A/Aichi/68 (H3N2) (collection of the Institute of Virology, Marburg, Germany). Mouse monoclonal antibodies against influenza virus NP (clone A3; CDC, Atlanta, GA) and M1 (Serotec, Germany) proteins were used. After incubation with the first antibody, membranes were exposed to horseradish peroxidase (HRP)-conjugated secondary antispecies antibodies (Dako, Germany), followed by the visualization of positive bands by ECL on Agfa film. Protein bands were scanned, and amounts of protein were calculated using the TINA program, version 2.09 (Institute of Virology, Marburg, Germany).
Electron microscopy.
Uninfected and infected CV-1 and MDCK cells were pelleted by centrifugation at 1,000 × g for 7 min at 4°C. Cell pellets were fixed with 2% glutaraldehyde overnight at 5°C, postfixed with 1% OsO4 for 1 h, dehydrated with ethanol, and rinsed with propylenoxide for 30 min. Cell specimens were embedded in resin, sectioned, and observed under a transmission electron microscope (JEM-100SX; JEOL, Japan).
RESULTS
To compare autophagy levels in cells infected with WT and delNS1 virus, 4 major autophagic parameters were analyzed (22). First, the amounts of the lipidated form (LC3-II) of microtubule light chain-associated protein LC3 were studied by PAGE-WB. Second, nonlipidated LC3-I (19 kDa) and lipidated LC3-II (16 kDa) were analyzed in pellets and supernatants obtained after cell fractionation and centrifugation. Third, intracellular perinuclear condensation of LC3, a characteristic for autophagosome vesicle formation, was assayed by immune fluorescent staining using anti-LC3 antibody. Fourth, the accumulation of characteristic double-layered autophagic vesicles was studied by electron microscopy. These investigations were performed in epithelial cell lines of canine (MDCK), monkey (CV-1), and human (293T) origin.
In cells infected with WT virus, lipidated LC3-II began to accumulate at about 5 to 6 h p.i. and remained at high levels until 14 to 16 h p.i. (Fig. 1A). In contrast, formation of LC3-II was reduced throughout infection with delNS1 virus (Fig. 1A). Gel scan calculation showed that the formation of LC3-II observed at 10 to 14 h p.i. in WT virus- and delNS1 virus-infected cells exceeded the level observed in uninfected cells by 150 to 175% and 45 to 60%, respectively (Fig. 1B). Unlike LC3-II, expression of another autophagy regulator, beclin-1, was not upregulated at the early-middle stage (7 to 11 h p.i.) in WT and delNS1 virus infection; a moderate increase of beclin-1 expression was observed only at the late stage (14 to 16 h p.i.) in both infections (Fig. 1A). This finding probably means that expression of beclin-1 is upregulated only late in infection and asynchronously with LC3 expression. Incubation with the lysosomal protease inhibitor E64d did not influence accumulation of LC3-II in WT virus- and delNS1 virus-infected cells, implying that degradation of LC3-II played a negligible role in both delNS1 virus- and WT-infected cells (not shown). Thus, these data suggest that the lower accumulation of LC3-II in delNS1 virus-infected cells was not the result of increased degradation in comparison to that in WT-infected cells but mainly reflected weaker stimulation of autophagosome formation. Taken together, these data support the concept that the autophagic process is poorly stimulated in delNS1 virus-infected cells and that the final lysosomal fusion stage and LC3 degradation are also at low levels similar to those in uninfected cells.
Fig 1.
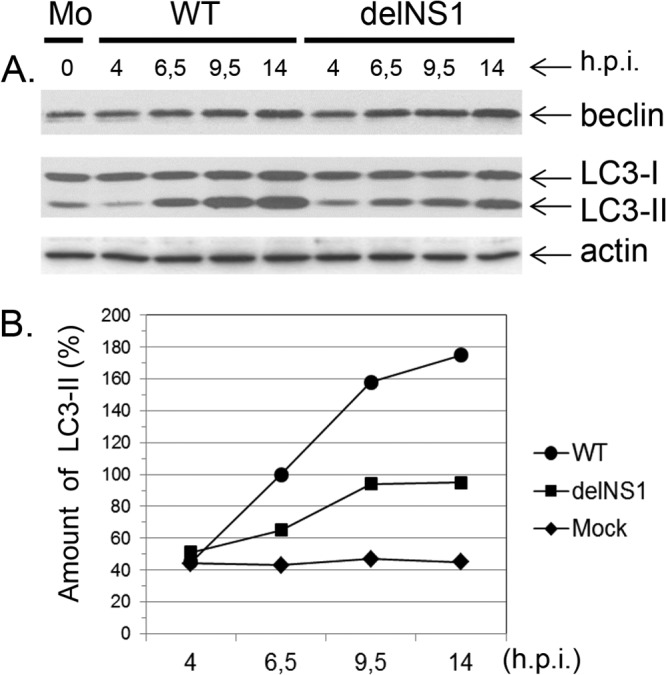
LC3-I and LC3-II profiles in CV-1 cells infected with WT and delNS1 viruses. (A) CV-1 cells were infected with either WT or delNS1 influenza A/PR/8/34 (H1N1) viruses at an MOI of 1. At 4, 6.5, 9.5, and 15 h p.i., equivalent amounts of cell homogenates were subjected to PAGE and WB analysis using antibodies specific for beclin-1, LC3, actin, and secondary species-specific HRP conjugates. Protein bands were visualized by exposure to Agfa film using the enhanced chemiluminescent West Dura reagent. Mo, mock-infected cells. (B) The amount of LC3-II relative to that of LC3-I (100%) was calculated by scanning analysis using the TINA program.
In order to confirm that accumulation of LC3-II was associated with lipid autophagosome vesicles, fractionation of cells for soluble protein and membrane pellet fractions was performed. After fractionation, LC3-I was found predominantly in the supernatant, whereas most of the LC3-II was present in the membrane pellet fractions, and the amounts of LC3-II were higher in the pellets of WT-infected cells than in those obtained from cells infected with delNS1 virus or from uninfected cells (Fig. 2, rows LC3-I and LC3-II). As expected, HA was detected only in membrane pellets and was fully absent in supernatants (Fig. 2, lanes HA0). Moreover, after detergent treatment, however, LC3-II and HA were found in the soluble fraction (not shown).
Fig 2.
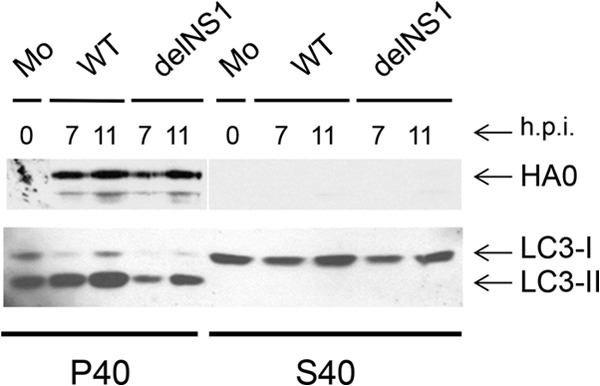
Subcellular distribution of LC3-I and LC3-II in cells infected with WT and delNS1 viruses. MDCK cells were infected with either WT or delNS1 influenza A/PR/8/34 (H1N1) viruses at an MOI of 1. At 7 and 11 h p.i., equal amounts of cells were disrupted by short sonication and cytoplasmic homogenates were prepared. Supernatant (S40) and membrane pellet (P40) fractions were obtained by centrifugation at 40,000 rpm (∼110,000 × g) for 1 h. Polypeptides were analyzed by PAGE-WB using LC3- and viral HA-specific antibodies and the ECL procedure. Equivalent aliquots of each subcellular fraction in relation to the volume of the initial cell sample were loaded into the wells.
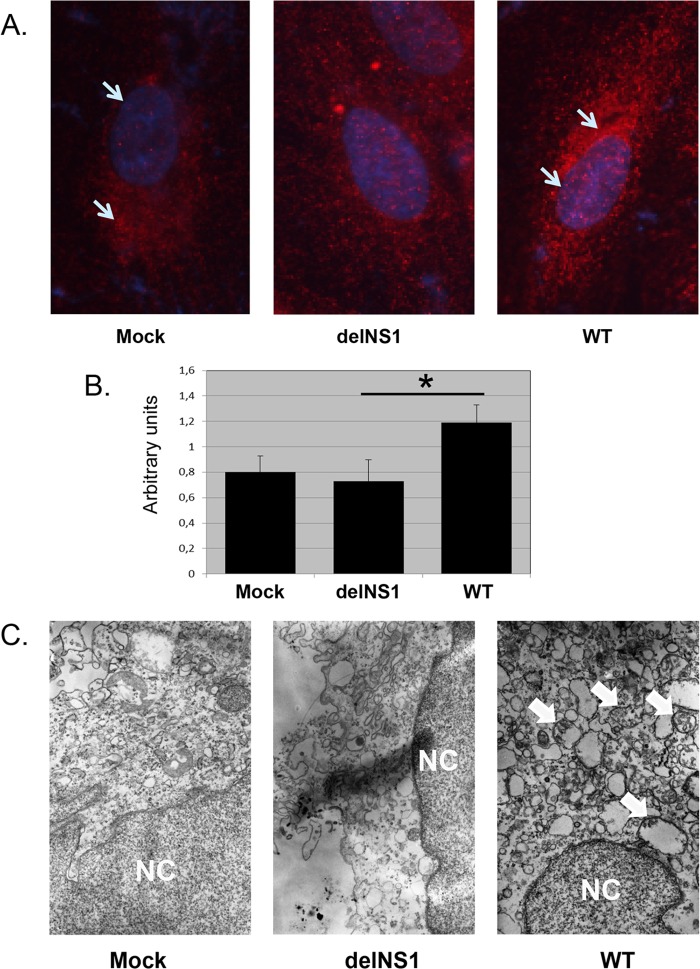
Analysis by immunofluorescence microscopy revealed low expression levels and diffuse cytoplasmic distribution of LC3-I/LC3-II in noninfected cells (Fig. 3A; mock) and in cells infected with delNS1virus (Fig. 3A, delNS1). In contrast, the intensity of LC3 staining was markedly increased in WT-infected cells, and about 30 to 40% of the cells in the monolayer displayed punctate-like accumulation concentrating preferentially in the perinuclear area, indicating stimulation of autophagosome formation (Fig. 3A, WT). Immune fluorescence staining also showed a higher concentration of LC3 in the perinuclear zone of WT-infected cells than in that of mock- and delNS1 virus-infected cells, whereas perinuclear LC3 accumulation was similar in mock- and delNS1 virus-infected cells (Fig. 3B). In agreement with these data, electron microscopic examination showed small amounts of autophagic double-layered vesicles in mock- and delNS1 virus-infected cells and significantly larger amounts in WT-infected cells (Fig. 3C; mock, delNS1, and WT).
Fig 3.
LC3 localization in CV-1 cells infected with WT and delNS1 viruses. CV-1 cells were infected with either WT or delNS1 influenza A/PR/8/34 (H1N1) virus at an MOI of 1. (A) Uninfected and infected cells were fixed at 9.5 h p.i. with 4% paraformaldehyde, permeabilized with 0.1% NP-40, and stained with rabbit monoclonal antibody specific to LC3 (Cell Signaling) and secondary affinity-purified anti-rabbit–TRITC conjugate (Jackson) (red). Nuclei were stained with DAPI (blue). Stained cells were analyzed under a confocal microscope. Magnifications, ×250. (B) Fluorescence-labeled LC3 was quantified by image processing analysis as described in Materials and Methods. The fluorescence of 10 separate 20-by-20 pixel squares in the perinuclear zones of 3 separate cells was measured and normalized by measuring nuclear fluorescence. Arbitrary units were calculated as the ratio of perinuclear red channel pixels/intranuclear blue channel pixels. *, significant difference (P < 0.01) between the values for delNS1 and WT viruses. (C) Uninfected and infected cells were pelleted at 1,000 × g for 5 min and processed for electron microscopy, as described in Materials and Methods. NC, nucleus. Bar, 0.5 μm.
The data shown here so far imply that the NS1 protein is involved in upregulation of autophagy in infected cells. It was now of interest to find out whether NS1 alone was sufficient to stimulate this process. To this end, 293T cells were transfected with plasmids expressing each protein (PB1, PA, PB2, NP, NEP, NS1, M2, M1, HA, NA) of influenza A/WSN/33 (H1N1) virus. Transfected cells were incubated for 27 and 40 h, and intracellular LC3-I/LC3-II profiles were identified by PAGE-WB. The majority of the viral proteins, including NS1, did not have a marked effect on LC3-II formation during 27-h (not shown) and 40-h (Fig. 4A) incubation periods. Also, NS1 proteins of A/PR/8/34 (H1N1) and A/Aichi/2/68 (H3N2) viruses did not stimulate autophagy (data not shown). Scanning analysis revealed that the LC3-I/LC3-II ratios (where the content of LC3-I plus that of LC3-II is referred as 100%) were higher in cells expressing M2 and HA (73% ± 6.7% and 31% ± 5.3%, respectively), whereas in all other cell samples, including mock-transfected cells, the ratios varied in the range of 19% ± 4.5%. An LC3-II increase in M2- and HA-expressing cells was also evident when LC3-II/actin ratios were calculated (not shown). The results obtained are in accordance with observations made by Gannagé and coauthors (6), indicating that M2 has the capacity, on the one hand, to initiate autophagosome formation and, on the other hand, to block the final maturation step of lysosome-autophagosome fusion. In addition, these data suggest that autophagy could be indirectly regulated by NS1, known to stimulate the synthesis of M2 and HA in infected cells (23–25).
Fig 4.
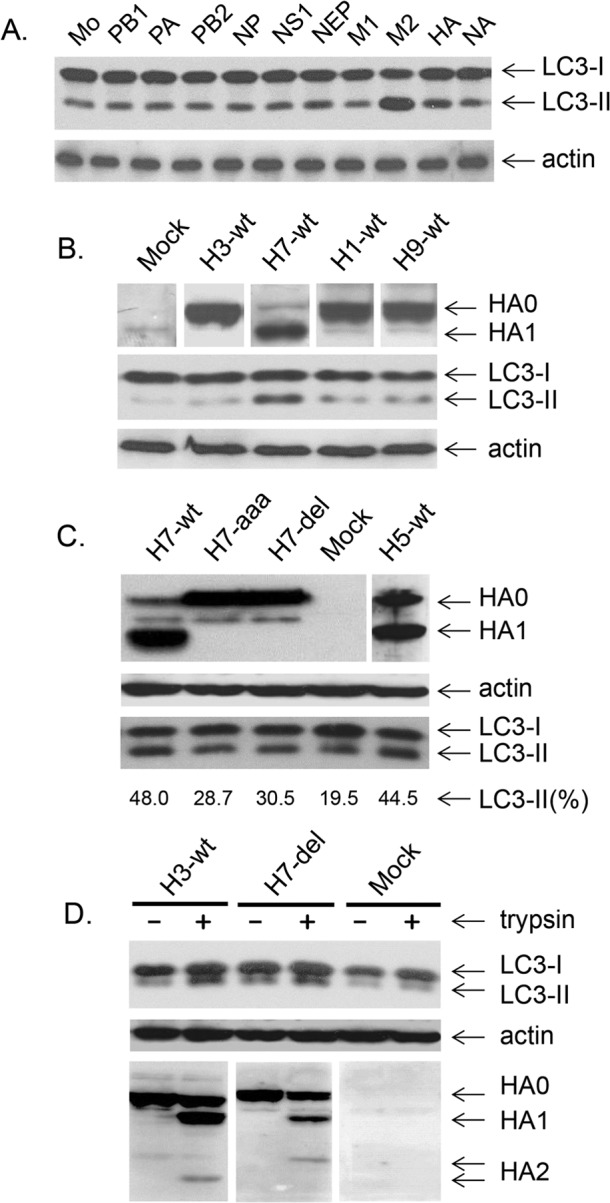
LC3-I/LC3-II profiles in 293T cells transfected with plasmids expressing individual virus proteins. (A and B) 293T cells transfected with pCAGGS vectors expressing protein PB1, PA, PB2, NP, NS1, NEP, M1, M2, HA, or NA of A/WSN/33 virus (A) or protein HA of wild-type viruses A/North Carolina/1918 (H1-wt), A/FPV/Rostock/34 (H7N1) (H7-wt), A/quail/Shantou/782/00 (H9N2) (H9-wt), and A/Aichi/68 (H3N2) (H3-wt) (B). At 40 h after transfection, intracellular LC3-I and LC3-II were detected by PAGE-WB analysis. Mock-transfected cells were also analyzed. Equal amounts of each cellular sample were loaded in each well of the gels. (C) H7 and H5 HAs containing polybasic cleavage sites of strains A/FPV/Rostock/34 (H7N1) (H7-wt), A/Kurgan/05/2001 (H5N1) (H5-wt), and HA mutants of strain A/FPV/Rostock/34 (H7N1) with monobasic cleavage sites (H7-del and H7-aaa) were expressed in 293T cells. After 40 h, equal aliquots of each sample of transfected cells were loaded in each well of the gels and analyzed by PAGE-WB for the expression of uncleaved HA0 and cleaved HA1, actin, LC3-I, and LC3-II. The amounts of LC3-II relative to those of LC3-I plus LC3-II (100%) were also determined by scanning of bands in the WB membrane image with the TINA program. (D) 293T cells were transfected with pCAGGS expressing HA, A/Aichi/68 (H3-wt), and the A/FPV/Rostock/34 mutant (H7-del) and incubated in DMEM containing 0.9% FCS. After 25 h, the medium was replaced with DMEM alone containing acetylated trypsin (T6763; Sigma) at a concentration of 3.5 μg/ml and cells were incubated for an additional 27 h. Cells were harvested, and equal amounts were loaded in each well of the gel and analyzed by PAGE-WB.
Interestingly, the uncleaved HAs of A/WSN/33 virus and of other viruses containing a single arginine at the cleavage site, such as A/North Carolina/1918 (H1N1), A/FPV/Rostock/34 (H7N1), A/quail/Shantou/782/00 (H9N2), and A/Aichi/68 (H3N2), were found to stimulate LC3-II only moderately (Fig. 4A, lane HA, and B). However, high levels of LC3-II were observed after expression of the cleaved HAs of subtypes H5 and H7 carrying multibasic cleavage sites (Fig. 4C, lanes H7-wt and H5-wt). Similarly, autophagy was only slightly increased by the uncleaved HA of the H7 mutants containing monobasic cleavage sites (Fig. 4C, lanes H7-aaa and H7-del) but was significantly higher after trypsin treatment, as was also the case with H3 HA (Fig. 4D). These observations clearly show that HA cleavage stimulates the upregulation of autophagy.
To demonstrate the regulatory effects of NS1 on the formation of other viral proteins, the synthesis of NP, HA, M1, and M2 in cells infected with WT and delNS1 viruses was analyzed. Infected cells were labeled at 6.5 h p.i. with [35S]methionine and cysteine for 2 h, and polypeptides were analyzed by PAGE, followed by autoradiography. As shown in Fig. 5A, (i) synthesis of the HA, M1, and M2 proteins was 3 to 5 times lower in delNS1 virus-infected cells than in WT-infected cells, whereas NP levels were similar, and (ii) M2 was synthesized as a minor component in WT-infected cells and close to the background level in delNS1 virus-infected cells. Similar results were obtained when protein synthesis was quantified on Western blots (Fig. 5B). These observations confirm that the markedly higher expression of M2 and HA in WT- than in delNS1 virus-infected cells correlates with the levels of autophagic markers.
Fig 5.
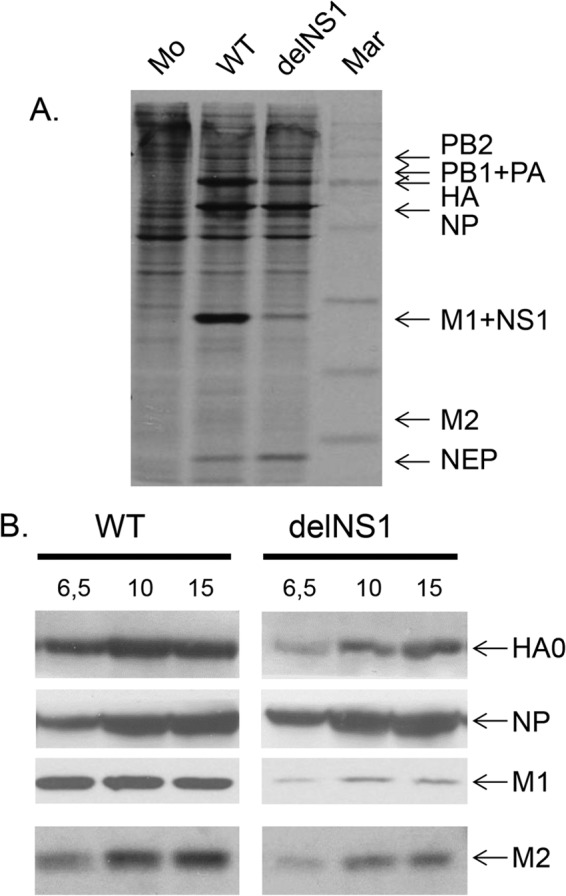
Early and late proteins in cells infected with WT and delNS1 influenza viruses. CV-1 cells were infected with either WT or delNS1 influenza A/PR/8/34 (H1N1) viruses at an MOI of 1. (A) At 6.5 h p.i., cells were incubated in medium containing a mixture of [35S]methionine and cysteine for 1.5 h and cellular polypeptides were analyzed by PAGE and autoradiography. Mar, (C-14 molecular size markers: 14, 20, 30, 45, 66, 97, and 220 kDa; Amersham). (B) Cell homogenates prepared at 6.5, 10, and 15 h p.i. were processed by PAGE-WB. Polypeptides were detected with antibodies specific for HA, NP, M1, and M2 and for HRP conjugates using ECL, and positive bands were calculated by scanning with the TINA program.
We then compared the development of autophagy and apoptosis in MDCK cells depending on the time after infection with WT and delNS1 viruses (Fig. 6). Downregulation of the phosphorylated form of protein kinase AKT (AKT-phospho473) and upregulation of activated caspase 3a and the apoptotic form of NP (aNP) were analyzed as markers of apoptosis (9, 26, 27). LC3-II and the phosphorylated form of the S6k protein (S6k-pho) were monitored in parallel as markers for autophagy (28). Whereas caspase 3a and aNP appeared earlier and more intensively after delNS1 virus infection, AKT-pho was markedly induced only in WT-infected cells, indicating that apoptosis rapidly developed in delNS1 virus-infected cells and was delayed in WT-infected cells. As indicated by the formation of LC3-II and similar to the data obtained in CV-1 cells (Fig. 1), autophagy was pronounced in WT-infected MDCK cells and reduced in delNS1 virus-infected ones (Fig. 6A). S6k-pho, as an indicator for mTOR activation (28), was detected at similar levels in mock-, WT-, and delNS1 virus-infected MDCK (Fig. 6B) and CV-1 (not shown) cells. The phosphorylated form of the effector protein kinase mTOR, known to negatively regulate autophagy, was also not induced in these cells (data not shown). The moderate downregulation of S6k-pho seen in delNS1 virus- and WT-infected cells at 12 to 14 h p.i. compared to the level of regulation at 9 h p.i. (Fig. 6B) correlates with autophagy stimulation developing late in infection (Fig. 1 and 6A). These observations indicate downregulation of autophagy and rapid development of apoptosis in delNS1 virus-infected cells, in contrast to the highly stimulated autophagy and delayed apoptosis observed in WT-infected cells.
Fig 6.
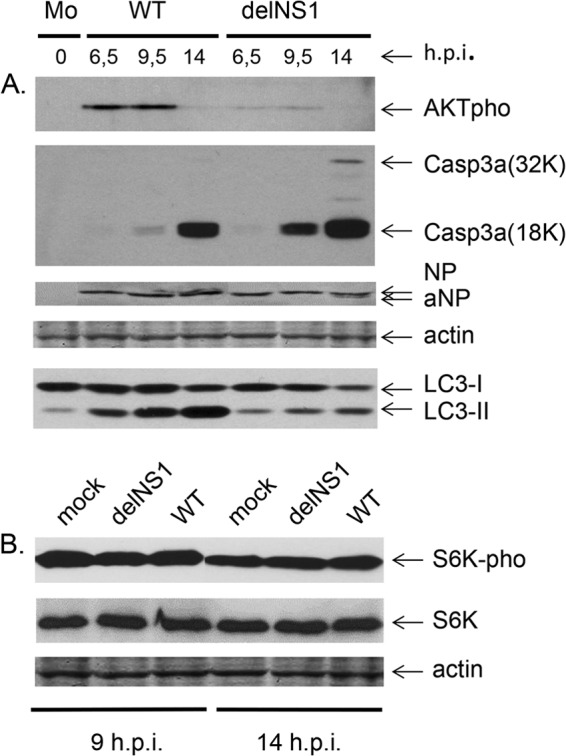
Apoptotic and autophagic markers in cells infected with WT and delNS1 influenza A/PR/8/34 viruses. (A) MDCK cells were infected with WT and delNS1 viruses at an MOI of 1. At 6.5, 9.5, and 14 h p.i., cellular polypeptides were analyzed by PAGE-WB using antibodies specific for AKTpho, 32K and 18K units of activated caspase 3 (Casp3a 32K, Casp3a 18K), actin, and viral NP and for secondary host species-specific HRP conjugates. (B) S6k/S6k-pho profiles were identified by PAGE-WB using rabbit anti-S6k and -SK6-pho antibodies and anti-rabbit antibody–HRP conjugate. Positive bands were detected by the ECL procedure using the West Dura luminescent reagent.
DISCUSSION
We have previously shown that replication of influenza virus provokes a two-phase response in infected cells: an antiapoptotic response at the early-middle stage and an apoptotic response at the late stage of infection (9). The NS1 protein was found to play a crucial role in the antiapoptotic pathway through activation of the protein kinase complex PI3K (class I)-AKT (9–12). Accordingly, delNS1 virus induced accelerated apoptosis in infected cells (13–15). In the present study, we uncovered a new regulatory loop which links NS1, HA, and M2 with autophagy in infected cells.
The available data suggest that influenza virus affects host autophagy by several mechanisms. On one side, viral protein M2 was suggested to interact with host beclin-1 to initiate autophagy by formation of autophagosomes. However, only a moderate increase of beclin-1 expression was observed at the late stage in both delNS1 virus- and WT-infected cells. This finding probably means that beclin-1 is involved in the upregulation of autophagy in influenza virus-infected cells late in infection and asynchronously with LC3 upregulation. Notably, this observation does not exclude the possibility that beclin-1 may modulate influenza virus pathogenesis by complexing with Bcl2 and Atg14 or by its posttranslational modifications, such as caspase cleavage, phosphorylation, or ubiquitination (29). On the other side, the M2 protein appeared to promote by an unknown mechanism the blockade of the final stage of autophagy, the fusion of autophagosomes with lysosomes (6, 7). Here we show that NS1 is also involved in upregulation of autophagy. NS1 most probably acts indirectly by stimulating the synthesis of M2 and HA, which in turn initiate autophagosome biogenesis. Accordingly, in delNS1 virus-infected cells, high LC3-I and low LC3-II levels, along with diffuse LC3 deposition, were observed throughout infection, indicating reduced autophagosome generation.
Taken together, these observations suggest the following scenario (summarized in Fig. 7). Early in infection, NS1, NP, PB1, PA, and PB2 are formed, whereas M2, M1, HA, and NA appear late (30). At the middle of infection, NS1 stimulates autophagosome formation by upregulation of M2 and HA synthesis, and it interferes with apoptosis through PI3K (class I)-AKT signaling. Both mechanisms promote survival of cells infected with WT virus. In delNS1 virus-infected cells, both prosurvival mechanisms are reduced due to the lack of NS1 and low expression of its regulatory targets, M2 and HA, which promotes accelerated apoptosis (13–15). In general, downregulation of autophagy and rapid development of apoptosis occurred in delNS1 virus-infected cells, in contrast to the highly stimulated autophagy and delayed apoptosis observed in WT-infected cells.
Fig 7.
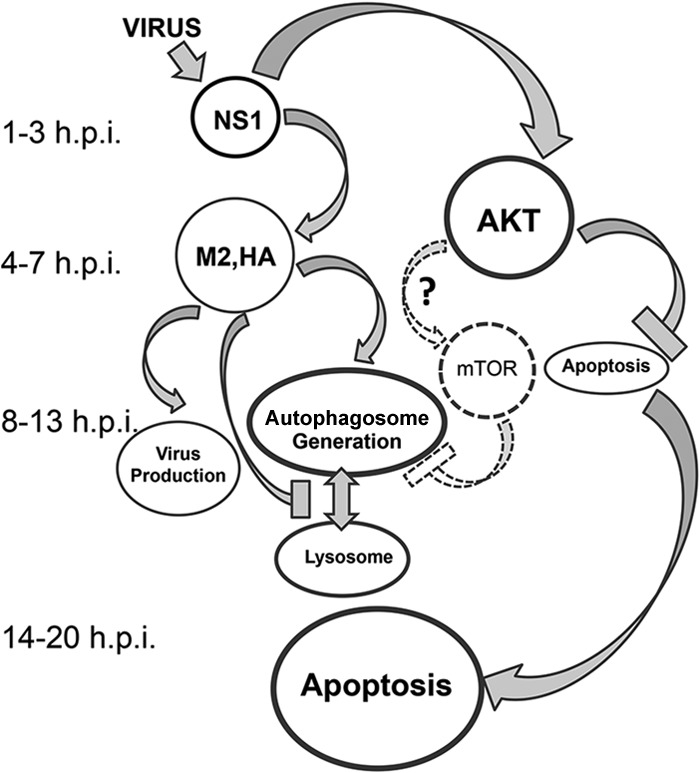
NS1-dependent regulatory links between autophagy and apoptosis in influenza virus-infected cells. NS1, synthesized as early as 1 h p.i., regulates autophagy and apoptosis by different mechanisms. First, NS1 initiates host protein kinase AKT, also known as protein kinase B (PKB), activation through binding to the p85beta subunit of PI3K class I (11, 12), resulting in the downregulation of apoptosis (9, 10). Second, NS1 amplifies synthesis of the late virus proteins HA, NA, M1, M2, and NEP. Amplification of M2 and HA stimulates formation of autophagosomes and suppresses their fusion with lysosomes (6). Thus, autophagy develops in influenza virus-infected cells after inhibition of apoptosis (19). The AKT-mTOR loop, a negative regulator of autophagosome generation (25), is not stimulated in cells infected with influenza H1N1 viruses and might be downregulated only in certain cell types infected with H5N1 virus (27). Late in virus replication (>13 h p.i.), AKT activation ceases and apoptosis develops when virus production has already declined (9). The exact mechanisms of apoptosis in influenza virus-infected cells remain to be discovered. Many influenza virus proteins, including NS1, NA, PB1-F2, and M1, possess proapoptotic abilities, and their overaccumulation late in infection can lead to apoptosis. An approximate time scale as times (hours) postinfection is shown on the left. Gray and dashed arrows and gray and dashed borders and hammer-like lines show up- and downregulatory actions, respectively.
AKT is known to be a negative regulator of autophagosome generation, acting through the activation of mTOR (28), and this function may not be in line with the proautophagic role of NS1. However, this discrepancy seems to be illusive, since we found that the AKT-mTOR regulatory loop fails to be upregulated in influenza virus-infected cells. S6k protein, which is phosphorylated by activated mTOR (28), was present in the phosphorylated form only at low levels in both WT- and delNS1 virus-infected MDCK and CV-1 cells, indicating a level of mTOR in infected cells close to the background level. Thus, it seems likely that the AKT-mTOR pathway loop has a negligible influence on the generation of autophagosomes in H1N1-infected cells, irrespective of whether or not NS1 is expressed. However, the AKT-mTOR pathway may be involved in the downregulation of autophagy in cells infected with H5N1 virus (31, 32). Moreover, HAs of highly pathogenic viruses H5 and H7, unlike those of low-pathogenic viruses of subtypes H1, H3, H9, etc., were found here to possess a strong capacity for autophagy stimulation due to their membrane-associated furin-dependent cleavage within cells. HAs with monobasic cleavage sites were also found here to increase their autophagy stimulation properties after external cleavage with trypsin. This mechanism may play a pathogenic role in influenza virus infection in the respiratory system, where such HAs are cleaved by respiratory transmembrane proteases (for a review, see reference 33). These observations suggest that regulation of autophagy varies depending on the virus strain and host cell metabolic peculiarities.
It is well-known that about 50% of carcinomas and their cell cultures have dysregulated autophagy pathways (34), and this fact determined our selection of host cell types for investigation. Here we studied CV-1, MDCK, and carcinoma 293T cell lines, which were characterized by normal constitutive levels of autophagy with a significant prevalence of LC3-I over that of LC3-II (Fig. 1B, 4A to D, and 6A). In contrast, cultures of other carcinoma-derived cells, such as lung Calu-3 and intestinal Caco-2 cells, were earlier found to display extremely high endogenous levels of autophagy stimulation and a remarkable predominance (more than 80%) of LC3-II over that of LC3-I (35). This abnormal carcinoma-dependent upregulation of autophagy in these cells prompted us to exclude them from the current investigation. To study autophagy in the natural respiratory tract under conditions of influenza virus infection, in vivo experiments with bronchial epithelium in infected mice are now in progress.
Many viruses, including influenza virus, are considered agents suitable for tumor therapy (36). This therapeutic capacity of the influenza delNS1 virus has been successfully explored with selected types of human cancers, such as Ras-activated (37), interferon-deficient (38), and protease-secreting (39) tumors. The observation described here, that delNS1 virus downregulates autophagy and stimulates apoptosis, throws light on some of the mechanisms underlying its oncolytic potential.
ACKNOWLEDGMENTS
We thank Olga Dolnik from the Institute of Virology (Marburg, Germany) and Anatoly Manykin and Elena Gushina from the D. I. Ivanovsky Institute of Virology (Moscow, Russia) for assistance in microscopic and electron microscopic assays. Mikhail Matrosovich is acknowledged for providing the DNA vector carrying the HA of influenza virus A/North Carolina/1918. Olga and Veronica Zhirnova are acknowledged for help with the manuscript.
This work is supported by the Deutsche Forschungsgemeinschaft (SFB 593; Germany) and research grant 13-04-01827 of the Russian Foundation of Basic Research.
We declare no conflict of interest.
Footnotes
Published ahead of print 11 September 2013
REFERENCES
- 1.Dumit VI, Dengiel J. 2012. Autophagosomal protein dynamics and influenza virus infection. Front. Immunol. 3:43. 10.3389/fimmu.2012.00043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Law AH, Lee DC, Yuen KY, Peiris M, Lau AS. 2010. Cellular response to influenza virus infection: a potential role for autophagy in CXCL10 and interferon-alpha induction. Cell. Mol. Immunol. 7:263–270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Deretic V, Levine B. 2009. Autophagy, immunity, and microbial adaptations. Cell Host Microbe 5:527–549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tanida I, Ueno T, Kominami E. 2008. LC3 and autophagy. Methods Mol. Biol. 445:77–88 [DOI] [PubMed] [Google Scholar]
- 5.Münz C. 2011. Beclin-1 targeting for viral immune escape. Viruses 3:1166–1178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gannagé M, Dormann D, Albrecht R, Dengjel J, Torossi T, Rämer PC, Lee M, Strowig T, Arrey F, Conenello G, Pypaert M, Andersen J, García-Sastre A, Münz C. 2009. Matrix protein 2 of influenza A virus blocks autophagosome fusion with lysosomes. Cell Host Microbe 6:367–380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhou Z, Jiang X, Liu D, Fan Z, Hu X, Yan J, Wang M, Gao GF. 2009. Autophagy is involved in influenza A virus replication. Autophagy 5:321–328 [DOI] [PubMed] [Google Scholar]
- 8.Comber JD, Robinson TM, Siciliano NA, Snook AE, Eisenlohr LC. 2011. Functional macroautophagy induction by influenza A virus without a contribution to major histocompatibility complex class II-restricted presentation. J. Virol. 85:6453–6463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhirnov OP, Klenk HD. 2007. Control of apoptosis in influenza virus-infected cells by up-regulation of Akt and p53 signaling. Apoptosis 12:1419–1432 [DOI] [PubMed] [Google Scholar]
- 10.Ehrhardt C, Wolff T, Pleschka S, Planz O, Beermann W, Bode JG, Schmolke M, Ludwig S. 2007. Influenza A virus NS1 protein activates the PI3K/Akt pathway to mediate antiapoptotic signaling responses. J. Virol. 81:3058–3067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hale BG, Jackson D, Chen YH, Lamb RA, Randall RE. 2006. Influenza A virus NS1 protein binds p85beta and activates phosphatidylinositol-3-kinase signaling. Proc. Natl. Acad. Sci. U. S. A. 103:14194–14199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shin YK, Li Y, Liu Q, Anderson DH, Babiuk LA, Zhou Y. 2007. SH3 binding motif 1 in influenza A virus NS1 protein is essential for PI3K/Akt signaling pathway activation. J. Virol. 81:12730–12739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhirnov OP, Konakova TE, Wolff T, Klenk HD. 2002. NS1 protein of influenza A virus down-regulates apoptosis. J. Virol. 76:1617–1625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lu X, Masic A, Li Y, Shin Y, Liu Q, Zhou Y. 2010. The PI3K/Akt pathway inhibits influenza A virus-induced Bax-mediated apoptosis by negatively regulating the JNK pathway via ASK1. J. Gen. Virol. 91(Pt 6):1439–1449 [DOI] [PubMed] [Google Scholar]
- 15.Jackson D, Killip MJ, Galloway CS, Russell RJ, Randall RE. 2010. Loss of function of the influenza A virus NS1 protein promotes apoptosis but this is not due to a failure to activate phosphatidylinositol 3-kinase (PI3K). Virology 396:94–105 [DOI] [PubMed] [Google Scholar]
- 16.Schultz-Cherry S, Dybdahl-Sissoko N, Neumann G, Kawaoka Y, Hinshaw VS. 2001. Influenza virus NS1 protein induces apoptosis in cultured cells. J. Virol. 75:7875–7881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lam WY, Tang JW, Yeung AC, Chiu LC, Sung JJ, Chan PK. 2008. Avian influenza virus A/HK/483/97(H5N1) NS1 protein induces apoptosis in human airway epithelial cells. J. Virol. 82:2741–2751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kourtis N, Tavernarakis N. 2009. Autophagy and cell death in model organisms. Cell Death Differ. 16:21–30 [DOI] [PubMed] [Google Scholar]
- 19.McLean JE, Datan E, Matassov D, Zakeri ZF. 2009. Lack of Bax prevents influenza A virus-induced apoptosis and causes diminished viral replication. J. Virol. 83:8233–8246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Neumann G, Watanabe T, Ito H, Watanabe S, Goto H, Gao P, Hughes M, Perez DR, Donis R, Hoffmann E, Hobom G, Kawaoka Y. 1999. Generation of influenza A viruses entirely from cloned cDNAs. Proc. Natl. Acad. Sci. U. S. A. 96:9345–9350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wagner R, Gabriel G, Schlesner M, Alex N, Herwig A, Werner O, Klenk HD. 2013. Protease activation mutants elicit protective immunity against highly pathogenic avian influenza viruses of subtype H7 in chickens and mice. Emerg. Microbes Infect. 2:e7. 10.1038/emi2013.7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mizushima N, Yoshimori T, Levine B. 2010. Methods in mammalian autophagy research. Cell 140:313–326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hatada E, Hasegawa M, Shimizu K, Hatanaka M, Fukuda R. 1990. Analysis of influenza A virus temperature-sensitive mutants with mutations in RNA segment 8. J. Gen. Virol. 71(Pt 6):1283–1292 [DOI] [PubMed] [Google Scholar]
- 24.Ludwig S, Vogel U, Scholtissek C. 1995. Amino acid replacements leading to temperature-sensitive defects of the NS1 protein of influenza A virus. Arch. Virol. 140:945–950 [DOI] [PubMed] [Google Scholar]
- 25.Salvatore M, Basler CF, Parisien JP, Horvath CM, Bourmakina S, Zheng H, Muster T, Palese P, García-Sastre A. 2002. Effects of influenza A virus NS1 protein on protein expression: the NS1 protein enhances translation and is not required for shutoff of host protein synthesis. J. Virol. 76:1206–1212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhirnov OP, Konakova TE, Garten W, Klenk H. 1999. Caspase-dependent N-terminal cleavage of influenza virus nucleocapsid protein in infected cells. J. Virol. 73:10158–10163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhirnov OP, Klenk HD. 2009. Alterations in caspase cleavage motifs of NP and M2 proteins attenuate virulence of a highly pathogenic avian influenza virus. Virology 394:57–63 [DOI] [PubMed] [Google Scholar]
- 28.Liang C. 2010. Negative regulation of autophagy. Cell Death Differ. 17:1807–1815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kang R, Zeh HJ, Lotze MT, Tang D. 2011. The beclin 1 network regulates autophagy and apoptosis. Cell Death Differ. 18:571–580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Skehel JJ, Hay AJ. 1978. Influenza virus transcription. J. Gen. Virol. 39:1–8 [DOI] [PubMed] [Google Scholar]
- 31.Sun Y, Li C, Shu Y, Ju X, Zou Z, Wang H, Rao S, Guo F, Liu H, Nan W, Zhao Y, Yan Y, Tang J, Zhao C, Yang P, Liu K, Wang S, Lu H, Li X, Tan L, Gao R, Song J, Gao X, Tian X, Qin Y, Xu KF, Li D, Jin N, Jiang C. 2012. Inhibition of autophagy ameliorates acute lung injury caused by avian influenza A H5N1 infection. Sci. Signal. 5:ra16. 10.1126/scisignal.2001931 [DOI] [PubMed] [Google Scholar]
- 32.Ma J, Sun Q, Mi R, Zhang H. 2011. Avian influenza A virus H5N1 causes autophagy-mediated cell death through suppression of mTOR signaling. J. Genet. Genomics 38:533–537 [DOI] [PubMed] [Google Scholar]
- 33.Zhirnov OP, Klenk HD, Wright PF. 2011. Aprotinin and similar protease inhibitors as drugs against influenza. Antiviral Res. 92:27–36 [DOI] [PubMed] [Google Scholar]
- 34.Liu EY, Ryan KM. 2012. Autophagy and cancer—issues we need to digest. J. Cell Sci. 125(Pt 10):2349–2358 [DOI] [PubMed] [Google Scholar]
- 35.Zhirnov OP, Vorobjeva IV, Saphonova EA, Malyshev NA, Schwalm F, Klenk HD. 2013. Pathogenic effect of pandemic influenza virus H1N1 under replication in cultures of human cells. Voprosi Virusol. N. 4:20–28 (In Russian.) [PubMed] [Google Scholar]
- 36.Guo ZS, Thorne SH, Bartlett DL. 2008. Oncolytic virotherapy: molecular targets in tumor-selective replication and carrier cell-mediated delivery of oncolytic viruses. Biochim. Biophys. Acta 1785:217–231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bergmann M, Romirer I, Sachet M, Fleischhacker R, García-Sastre A, Palese P, Wolff K, Pehamberger H, Jakesz R, Muster T. 2001. A genetically engineered influenza A virus with ras-dependent oncolytic properties. Cancer Res. 61:8188–8193 [PubMed] [Google Scholar]
- 38.Muster T, Rajtarova J, Sachet M, Unger H, Fleischhacker R, Romirer I, Grassauer A, Url A, García-Sastre A, Wolff K, Pehamberger H, Bergmann M. 2004. Interferon resistance promotes oncolysis by influenza virus NS1-deletion mutants. Int. J. Cancer 110:15–21 [DOI] [PubMed] [Google Scholar]
- 39.Sturlan S, Stremitzer S, Bauman S, Sachet M, Wolschek M, Ruthsatz T, Egorov A, Bergmann M. 2010. Endogenous expression of proteases in colon cancer cells facilitate influenza A viruses mediated oncolysis. Cancer Biol. Ther. 10:592–599 [DOI] [PubMed] [Google Scholar]