Abstract
Replication of plus-stranded RNA viruses is greatly affected by numerous host-encoded proteins that act as restriction factors. Cyclophilins, which are a large family of cellular prolyl isomerases, have been found to inhibit Tomato bushy stunt tombusvirus (TBSV) replication in a Saccharomyces cerevisiae model based on genome-wide screens and global proteomics approaches. In this report, we further characterize single-domain cyclophilins, including the mammalian cyclophilin A and plant Roc1 and Roc2, which are orthologs of the yeast Cpr1p cyclophilin, a known inhibitor of TBSV replication in yeast. We found that recombinant CypA, Roc1, and Roc2 strongly inhibited TBSV replication in a cell-free replication assay. Additional in vitro studies revealed that CypA, Roc1, and Roc2 cyclophilins bound to the viral replication proteins, and CypA and Roc1 also bound to the viral RNA. These interactions led to inhibition of viral RNA recruitment, the assembly of the viral replicase complex, and viral RNA synthesis. A catalytically inactive mutant of CypA was also able to inhibit TBSV replication in vitro due to binding to the replication proteins and the viral RNA. Overexpression of CypA and its mutant in yeast or plant leaves led to inhibition of tombusvirus replication, confirming that CypA is a restriction factor for TBSV. Overall, the current work has revealed a regulatory role for the cytosolic single-domain Cpr1-like cyclophilins in RNA virus replication.
INTRODUCTION
Genome-wide screens have led to the identification of many host factors that affect positive-strand RNA [(+)RNA] virus infections, including Tomato bushy stunt virus (TBSV), West Nile virus, Brome mosaic virus (BMV), hepatitis C virus (HCV), dengue virus, and Droshophila virus C in yeast or animal cells (1–9). Among the identified host factors, there are numerous inhibitory host proteins that serve as restriction factors for (+)RNA viruses. However, the functions of the majority of these host proteins during (+)RNA virus replication have not been fully revealed. The restriction factors likely interfere with the viral replication process, which takes place in membrane-bound viral replicase complexes (VRCs) in the cytoplasm of infected cells. The restriction factors potentially target the viral replication proteins, the viral RNA, or host-encoded proteins usurped by (+)RNA viruses to aid the replication process (10–19).
Recently, a major effort to dissect host restriction factors has been conducted with TBSV, which is a small (+)RNA virus that is used as a model virus to study virus replication, recombination, and virus-host interactions, based on a yeast (Saccharomyces cerevisiae) model host (20–25). Genome-wide screens of yeast genes and global proteomics approaches have led to the identification of over 500 host genes/proteins that affect TBSV replication or recombination or interact with the viral replication proteins or viral RNA (1, 3, 24–32). Interestingly, ∼25% of the identified factors seem to inhibit TBSV replication or recombination, suggesting that many host proteins might work as intrinsic restriction factors.
The tombusvirus VRC consists of two viral replication proteins (p33 and p92pol) and ∼10 host proteins (18, 30, 31, 33). The auxiliary p33 replication protein is an RNA chaperone involved in recruitment of TBSV (+)RNA to the site of replication, which is the cytosolic surface of peroxisomal membranes (34–37). The RdRp protein p92pol binds to the essential p33 replication protein for assembly of the functional VRC (22, 36, 38, 39). The recruited host proteins include heat shock protein 70 (Hsp70), eukaryotic elongation factor 1A (eEF1A), and the ESCRT (endosomal sorting complexes required for transport) family of host proteins, which are involved in the assembly of VRCs (10, 19, 33, 40–43). Other subverted host proteins in the VRC include glyceraldehyde-3-phosphate dehydrogenase (GAPDH), eEF1A, eEF1Bγ, and Ded1 DEAD box helicase, all of which have been shown to affect viral RNA synthesis (19, 40, 41, 43–48).
Among the cellular restriction factors that have been characterized is nucleolin (Nsr1p in yeast), an RNA-binding protein that interferes with the recruitment of the viral RNA for replication (49). Additional restriction factors are the WW domain proteins, such as Rsp5p, a Nedd4 family E3 ubiquitin ligase, that regulate the degradation of p92pol in yeast cells and inhibit the activity of VRCs in vitro (50, 51). Pkc1p kinase phosphorylates p33 close to the RNA-binding site and inhibits binding of p33 to the viral RNA, leading to suppression of TBSV replication (28).
An intriguing group of cellular proteins that inhibit TBSV replication in yeast is cyclophilins, such as the CypA-like single-domain Cpr1p and Cyp40-like multiple-domain Cpr7p cyclophilins and Ess1p parvulin, which decrease TBSV RNA accumulation in yeast (29, 52). Cyclophilins are a ubiquitous, highly conserved protein family with prolyl isomerase (PPIase) activity. Cyclophilins and the structurally unrelated FKB proteins (FK506-binding proteins) and parvulins include 13 and 29 prolyl isomerases in yeast and in plants, respectively (53, 54). PPIases catalyze cis-trans isomerization of the peptidyl-prolyl bonds (i.e., rotation of X-proline bonds from a trans to cis conformation) that alter the structure, function, or localization of the client proteins (53–55). The isomerization of the peptidyl-prolyl bonds is often needed for protein refolding after trafficking through cellular membranes (55). Cyclophilins are also involved in the assembly of multidomain proteins, muscle differentiation, detoxification of reactive oxygen species, and immune responses. Cyclophilins have been implicated in cancer, atherosclerosis, diabetes, and neurodegenerative diseases (54, 56, 57). Cyclophilin expression is induced by both biotic and abiotic stresses, including salt stress, heat and cold shock, wounding, and viral and fungal infections (53, 58).
We previously showed that the cytosolic Cyp40-like Cpr7p is a strong inhibitor of TBSV replication in yeast and in vitro (52). Cpr7p, through its TPR (tetratricopeptide repeats) domain and not the cyclophilin domain, binds to the RNA-binding domain of p33 replication protein and inhibits the p33/p92-driven recruitment of TBSV RNA for replication and decreases the efficiency of VRC assembly (52). We have demonstrated that the TPR domain of Cpr7p cyclophilin has antiviral activity, while its cyclophilin domain is not needed for viral restriction during RNA virus infections.
The best-characterized PPIase in eukaryotes is the cytosolic single-domain cyclophilin A (CypA in mammals) and the homologous Cpr1p in yeast. Unlike the Cyp40-like cyclophilins, CypA and Cpr1p lack the TPR domain. Therefore, in this study, we investigated the mechanism of inhibition of TBSV replication by these single-domain cyclophilins. We found that CypA and the orthologous Arabidopsis thaliana Roc1 and Roc2 cyclophilins inhibit TBSV replication in a cell-free assay. In addition, we found that CypA binds to the RNA-binding domain of p33 and, surprisingly, to the viral RNA. The binding of CypA to these viral components results in inhibition of viral (+)RNA recruitment in vitro and blocks VRC assembly. Overexpression of CypA or its PPIase-inactive mutant led to reduced TBSV RNA accumulation in yeast cells and plant leaves, suggesting that binding of CypA to the viral components and blocking their functions is the mechanism of inhibition by CypA.
MATERIALS AND METHODS
Yeast strains and expression plasmids.
Saccharomyces cerevisiae strain BY4741 (MATa his3Δ1 leu2Δ0 met15Δ0 ura3Δ0) was obtained from Open Biosystems (Huntsville, AL). Recombinant human glutathione S-transferase (GST)-tagged CypA and H126Q CypA mutant proteins were produced in Escherichia coli cells carrying pGEX-CypA and pGEX-H126Q CypA plasmids (a generous gift of Philippe Gallay) (59).
The GST- or maltose-binding protein (MBP)-tagged recombinant A. thaliana Roc1 and Roc2 cyclophilin proteins were produced in E. coli from plasmids pGEX-His-Roc1 or pGEX-His-Roc2 or pMAL-Roc1 or pMAL-Roc2 (pMalc-2X based). To make these constructs, either pPR-N-Roc1 or pPR-N-Roc2 plasmid (29) was used as a template in PCRs with primers 3576 (CGAAGATCTATGGCGTTCCCTAAGGTATAC) and 3492 (CCGCTCGAGCTAAGAGAGCTGACCACAATC) for Roc1 and 3577 (CGCGGATCCATGGCGAATCCTAAAGTCTTC) and 3578 (CCGCTCGAGTTATGAACTTGGGTTCTTGAG) for Roc2. The obtained PCR products were digested with BglII and XhoI for Roc1 or BamHI and XhoI for Roc2 and were inserted between the BamHI and XhoI sites in pGEX-His-Re (50) or BamHI and SalI in pMalc-2X.
Yeast overexpression plasmids pYC-His-CypA or pYC-His-Flag-CypA and pYC-His- H126Q (CypAmut) or pYC-His-Flag-H126Q were prepared as follows. Plasmid pGEX-CypA or pGEX-H126Q was used as a template in PCR with primers 5031 (CCAGGGATCCATGGTCAACCCCACCGTGTTC) and 5032 (CCAGCTCGAGTTATTCGAGTTGTCCACAGTCAGCAATGG). The obtained PCR products were digested with BamHI and XhoI and were inserted between BamHI and XhoI sites in pYC-His or pYC-HisFlag, respectively. Plasmids pPR-N-CypA and pPR-N-H126Q (CypAmut) were prepared as follows: the above PCR products were digested with BamHI and XhoI and were ligated into pPR-N-Re (50) between the BamHI and SalI sites.
The plant overexpression plasmids were constructed as described previously (60). Briefly, pGD-Roc1, pGD-Roc2, pGD-CypA, and pGD- H126Q (CypA/mut) were obtained by digesting the PCR products of Roc1, Roc2, CypA, and CypA-H126Q with BamHI and XhoI and were inserted between the BamHI and SalI sites of the pGD plasmid. Plasmids pGD-p19 and pGD-CNV have been described previously (60).
To study the effect of cyclophilin overexpression on tombusviral RNA replication, we transformed the yeast parental strain (BY4741) or the double mutant strain ΔCpr1/ts-Ess1 (29) with three plasmids: pYC-His-H126Q, pGBK-Cup-Flag-p33-Gal-DI72 (a gift from D Barajas), and pGAD-Cup-Flag-p92 (50). Transformed yeast were selected on SC-ULH− (Ura−/Leu−/His−) plates, then pregrown for 12 h in ULH− medium containing 2% glucose at 29°C. After centrifugation at 2,000 rpm for 3 min and washing the pellet with selective medium containing 2% galactose, yeast were grown for 24 h in SC-ULH− medium containing 2% galactose at 34°C (in the case of the ΔCpr1/ts-Ess1 yeast strain) or 23°C (in the case of the BY4741 strain). Then, yeast were grown for 8 h in SC-ULH− medium containing 2% galactose and 50 μmol CuSO4 at 23°C. Total RNA extraction from yeast cells and Northern blotting and Western blotting were done as previously described (22, 23).
Analysis of protein-protein interactions in a split-ubiquitin assay.
The split-ubiquitin assay was performed based on methods that rely on the Dualmembrane kit 3 (Dualsystems). Analysis of p33 interactions with cyclophilins was done as described previously (46). Briefly, the obtained prey constructs pPR-N-CypA, pPR-N-CypA/mut, pPR-N-Roc1, and pPR-N-Roc2 (29), pPR-N-RE as a negative control, pPR-N-CPR1 (29) as a positive control, and the bait construct pGAD-BT2-N-His33 (50) were cotransformed into yeast strain NMY51/ADH-His92-Kan. Colonies were selected on TLUHA− (Trp−/Leu−/Ura−/His−/Ade−) synthetic minimal medium plates to test for p33-cyclophilin interactions.
Protein purification from E. coli.
Expression and purification of the recombinant MBP-tagged TBSV p33, p33 derivatives, and p92 replication proteins and MBP-tagged host proteins (Mal-Roc1 and Mal-Roc2) from E. coli were carried out as described earlier (61). Purification of GST-tagged CypA, H126Q, Roc1, and Roc2 was carried out by using glutathione resin as described previously (52). The eluted recombinant proteins were aliquoted for storage at −80°C. The concentrations of the purified recombinant proteins were measured via the Bio-Rad protein assay (62). Protein fractions used for the replication assays were at least 95% pure, as determined by SDS-PAGE.
Cyclophilin proteins purified from yeast.
Yeast strain BY4741, transformed with plasmid pYC-His-Flag-CypA and yeast strain expressing GST-CPR1 (a generous gift from Brenda Andrews) (63) were streaked on SC-U− plates and then were grown for 24 h in 50 ml SC-U− medium containing 2% galactose at 29°C. After dilution to 250 ml in SC-U− medium containing 2% galactose, yeast cells were grown for 18 h at 29°C. A 400-μg aliquot of the pelleted yeast was taken for affinity purification using anti-FLAG M2 agarose in the case of HisFlag-CypA or GST-binding resin in the case of GST-CPR1 as described previously (22, 46, 52).
In vitro pulldown assay.
MBP-binding columns were used to bind MBP-p33, MBP-p33 derivatives, or MBP-p92 (61). The sonicated extracts from E. coli cells containing the MBP-tagged viral proteins were added to the columns and incubated for 20 min at 4°C with mixing. After binding, the affinity columns were washed three times with cold column buffer prior to loading of purified GST (negative control), GST-tagged CypA, or GST-H126Q (100 μg or 200 μg) followed by incubation for 30 min at 4°C with mixing. The columns were washed three times with cold column buffer, followed by elution of the bound protein complexes with MBP elution buffer (column buffer containing 0.18% maltose). The presence of GST-tagged proteins in the eluate was analyzed by SDS-PAGE, followed by Coomassie blue staining or Western blotting with an anti-GST antibody. Fractions of the MBP-p33 and MBP-p92 proteins isolated with GST-tagged proteins were stored at −80°C and further used in cell-free extract (CFE)-based TBSV replication assays.
RNA probes used for studying RNA-protein interactions.
An in vitro T7 transcription reaction was used as described previously (61) to generate 32P-labeled or unlabeled full-length DI-72 (+) and (−)RNAs. Purification of transcripts for the CFE-based TBSV replication assays was done as described earlier (61). A UV spectrophotometer (Beckman) was used for the quantification of RNA transcripts.
Gel mobility shift assay (EMSA).
Electrophoretic mobility shift assay (EMSA) experiments were performed as described previously (35) with minor modifications. Briefly, the binding assay was done in the presence of 20 mM HEPES (pH 7.4), 50 mM NaCl, 10 mM MgCl2, 1 mM diethiothreitol, 1 mM EDTA, 5% glycerol, 6 U of RNasin, and 0.1 μg of tRNA in a 10-μl reaction volume. 32P-labeled RNA probes (0.1 pmol) were used together with 0.4 μg or 0.8 μg of recombinant proteins. The effect of CypA on binding of p33C to the 32P-labeled RNA (∼0.1 pmol) was tested using 0.02 μg to 0.6 μg of recombinant GST-CypA or GST-H126Q and 0.02 μg of p33C. Mixtures were incubated at room temperature for 15 min and loaded on 5% nondenaturing polyacrylamide gels as described previously (35).
Purification of tombusvirus replicase from yeast and in vitro replicase assay.
Purification of the tombusvirus replicase was done as published previously (22, 46). The in vitro RdRp activity assay was performed as published previously (22, 46) by using full-length DI-72 (−)RNA template obtained by T7 transcription in vitro. The effect of cyclophilin proteins was measured in the replicase assay with or without preincubation of the purified RdRp with GST-CypA, GST-H126Q, or GST for 15 min at room temperature. RNase I digestion to remove single-stranded 32P-labeled RNA was performed at 37°C for 30 min in 1× RNase I buffer containing 0.1 μl of RNase I (Promega).
TBSV in vitro replication assay in cell-free yeast extract.
CFE from strain BY4741 able to support in vitro TBSV replication was prepared as published earlier (43). Recombinant cyclophilin proteins were added in different amounts (as indicated in the figure legends) to the CFE-based replication assay mixtures as described previously (43).
Protein copurification with the tombusviral replicase.
Yeast strain BY4741 (200 mg of pelleted yeast) cotransformed with plasmid pYC-His-CypA (or pYC-His-H126Q), pGBK-Cup-Flag-p33-Gal-DI72, or pGAD-Cup-Flag-p92 was used for FLAG affinity purification of the membrane-bound tombusvirus replicase (Flag-p33 and Flag-p92) by using anti-Flag M2-agarose as published previously (33). Flag-p33 was detected with anti-Flag antibody (1/10,000 dilution) and alkaline phosphatase (AP)-conjugated anti-mouse antibody (1/10,000). His6-CypA or His6-H126Q proteins were detected with anti-His6 antibody from mouse (Amersham; 1/10,000 dilution) and AP-conjugated anti-mouse (1/10,000) followed by NBT-BCIP detection (33).
Northwestern assay.
Northwestern was done as described previously (61) with modifications. Briefly, equal amounts (∼1 μg) of recombinant proteins were run in an 12% SDS-PAGE and then transferred to polyvinylidene difluoride (PVDF) membranes. The membranes were renatured at room temperature in a renaturation buffer [10 mM Tris-Hcl [pH 7.5], 1 mM EDTA, 50 mM NaCl, 0.1% Triton X-100, and 1× Denhardt's reagent, 0.1 mg/ml ssDNA and 2 μg/ml yeast RNA (USB)] with three buffer changes for 30 min each. The membranes were probed with 32P-labeled DI-72 (-)RNA for 2 h, washed three times with the renaturation buffer, air dried, and analyzed with a phosphorimager.
Over-expression of cyclophilins in N. benthamiana.
Agrobacterium tumefaciens (strain C58C1) carrying separately pGD-Roc1, pGD-Roc2, pGD-CypA, pGD-H126Q (CypA/mut), pGD-p19 or pGD-CNV were prepared as published earlier (60). Young Nicotiana benthamiana leaves were infiltrated with Agrobacterium tumefaciens cultures carrying either empty pGD-35S plasmid as a negative control or one of the plasmids, pGD-Roc1, pGD-Roc2, pGD-CypA, or pGD-H126Q (CypA/mut). The leaves were also agroinfiltrated with pGD-p19 for suppressing gene silencing. On the next day, the same plants leaves were infiltrated with Agrobacterium cultures carrying pGD-CNV to initiate Cucumber necrosis virus (CNV) infection. Agrobacterium strains were used at the following optical densities at 600 nm (OD600s): 0.25 for pGD-p19, 0.75 for pGD-cyclophillins (or pGD-35S), and 0.2 for pGD-CNV. Samples for RNA extraction were taken from the agroinfiltrated leaves 72 h after CNV agroinfiltration. Isolation of RNA and Northern blot analysis was performed by using a previously published method (60).
RESULTS
Strong inhibition of TBSV replication by CypA and Arabidopsis Roc1 and Roc2 single-domain cyclophilins in a cell-free replication assay.
Although both Cpr1p and Cpr7p are members of the cyclophilin family (54), the mechanisms of inhibition of TBSV replication by these two proteins must be different. This is because the inhibitory function of Cpr7p depends on its unique TPR domain, while its PPIase (cyclophilin) domain has a lesser inhibitory effect (52). In fact, the sole TPR domain is a more potent inhibitor of TBSV replication than the full-length protein. In contrast, Cpr1p has only a single cyclophilin domain but lacks the TPR domain (54).
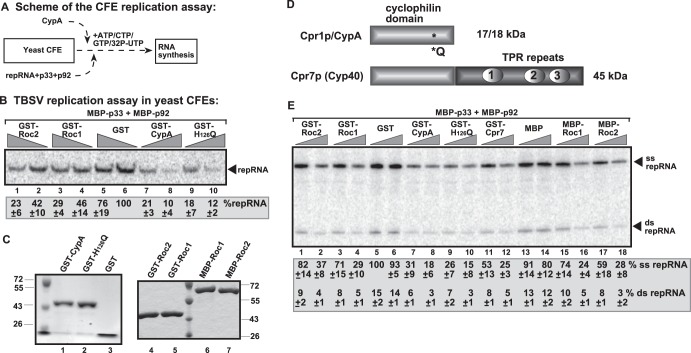
To dissect the mechanism of inhibition of TBSV replication by a cytosolic single-domain cyclophilin, we tested the yeast Cpr1p and the homologous human CypA protein. Cpr1p has been shown to inhibit TBSV replication in yeast. In addition, Cpr1p interacts with the p33 and p92 replication proteins (29). First, we used purified recombinant Cpr1p and the human CypA protein to measure inhibition of TBSV replication in a cell-free replication assay. This assay is based on a CFE prepared from yeast that can be programmed with purified recombinant p33 and p92 replication proteins and the viral (+)repRNA (replicon RNA) to support a single cycle of full replication in vitro (Fig. 1A) (43). Although, the recombinant yeast Cpr1p obtained from E. coli had no inhibitory effect (52), the recombinant CypA showed remarkably strong inhibition of TBSV replication in vitro (causing up to a 10-fold reduction) (Fig. 1B, lanes 7 and 8) on TBSV replication in the CFE-based assay. Interestingly, the purified recombinant Arabidopsis Roc1 (AtCyp18-3) and Roc2 (AtCyp19-3) proteins (Fig. 1C), which are cytosolic cyclophilins and orthologs of Cpr1p (53), also showed 3- to 5-fold inhibitory effects on TBSV replication in the CFE assay (Fig. 1B, lanes 1 to 4 versus lanes 5 and 6).
Fig 1.
Cell-free TBSV replication assay supports an inhibitory role for CypA and two Arabidopsis single-domain cyclophilin orthologs. (A) Scheme of the CFE-based TBSV replication assay. (B) Denaturing PAGE analysis of the 32P-labeled TBSV repRNA products obtained in the CFE-based assay programmed with in vitro-transcribed TBSV DI-72 (+)repRNA and purified recombinant MBP-p33 and MBP-p92pol replication proteins of TBSV. Purified recombinant GST-tagged CypA, the GST-H126Q PPIase-deficient mutant, GST-Roc1, GST-Roc2, or GST (10 and 20 pmol) was added to CFE prepared from yeast strain BY4741. Each experiment was repeated three times. (C) SDS-PAGE analysis of the purified recombinant proteins used in the CFE-based assay. (D) Schematic representation of domains present in yeast Cpr1p (17 kDa), human CypA (18 kDa), and the yeast Cpr7p Cyp40-like cyclophilins. (E) Recombinant CypA inhibits the production of viral dsRNA and ssRNA in a CFE-based TBSV replication assay. Results from nondenaturing PAGE analysis of single- and double-stranded RNA products produced in the cell-free TBSV replication assay are shown. The assay included the purified recombinant GST-CypA, the GST-H126Q mutant, GST-Roc1, GST-Roc2, and GST-Cpr7p (7.5 and 15 pmol). Note that the dsRNA product represents the annealed (−)RNA and the (+)RNA, while the ssRNA products represent the newly made (+)RNA products. Samples were not heat treated (thus, both ssRNA and dsRNA products are present). The percentages of dsRNA and ssRNA in the samples are shown. Each experiment was repeated three times.
To test if minus- or plus-strand synthesis is inhibited by the above-mentioned cyclophilins (Fig. 1D), we measured the levels of double-stranded RNA (dsRNA), which correlates with minus-strand synthesis, and single-stranded RNA (ssRNA) (representing the newly made plus-strands) (38, 43) in the CFE assay. The recombinant CypA inhibited both dsRNA and ssRNA production to similar extents (Fig. 1E, lane 8 versus lanes 5 and 6), suggesting that RNA synthesis in general was inhibited by CypA through possible interference with the assembly of the VRC in vitro. Interestingly, the inhibition of TBSV RNA synthesis by CypA was as potent as that observed with the Cyp40-like multidomain Cpr7p protein (Fig. 1E, compare lanes 7 and 8 with lanes 11 and 12).
A similar picture can be drawn for the single-domain Arabidopsis Roc1 and Roc2 cyclophilins, both of which inhibited dsRNA and ssRNA production by ∼3-fold (Fig. 1E, lanes 2 and 4 versus lane 5). Tagging of Roc1 and Roc2 with GST or MBP did not make a major difference in their inhibitory potential (Fig. 1E, compare lanes 1 to 4 with lanes 15 to 18). Thus, we concluded that the closely related single-domain CypA and Roc1 and Roc2 cyclophilins inhibit RNA synthesis by the tombusvirus VRC in vitro.
The PPIase function of CypA is not required for inhibition of TBSV replication in a cell-free replication assay.
To test if the PPIase function of CypA is required for its inhibitory function, we used a purified recombinant CypA with a detrimental mutation (mutant H126Q) (Fig. 1C and D) (59). Mutant H126Q was as effective as the wild-type (wt) CypA in inhibition of TBSV replication in the CFE assay (Fig. 1B, lanes 9 and 10 versus lanes 7 and 8). Mutant H126Q was also able to reduce the production of dsRNA and ssRNA by up to 5-fold in vitro (Fig. 1E, lanes 9 and 10 versus lanes 7 and 8). Based on these data, we suggest that mutant H126Q is as potent an inhibitor as the wt CypA, indicating that the PPIase activity is not needed for the inhibitory effect of CypA on TBSV replication in vitro.
CypA inhibits VRC assembly in vitro.
Since the recombinant CypA was a potent inhibitor of TBSV replication in vitro, we used a two-step replication assay based on yeast CFE (43) to determine which steps of TBSV replication could be inhibited by CypA. In this assay, the first step includes VRC assembly on the endogenous membranes present in the CFE. The assay also contains the viral (+)repRNA, the recombinant p33 and p92 replication proteins obtained from E. coli, and rATP/rGTP (Fig. 2A, step 1). Under these conditions, the viral replication proteins recruit the (+)repRNA to the membrane, and the VRC becomes partially RNase and protease insensitive. The preassembled VRC, however, cannot initiate minus-strand synthesis yet, due to the absence of rCTP/rUTP (43). Then, during the second step, centrifugation and washing of the membranes removes all the proteins and molecules not bound to the membrane. Addition of rATP/rCTP/rGTP/32P-labeled rUTP to the membrane fraction of the CFE then initiates asymmetrical minus- and plus-strand RNA synthesis in vitro.
Fig 2.
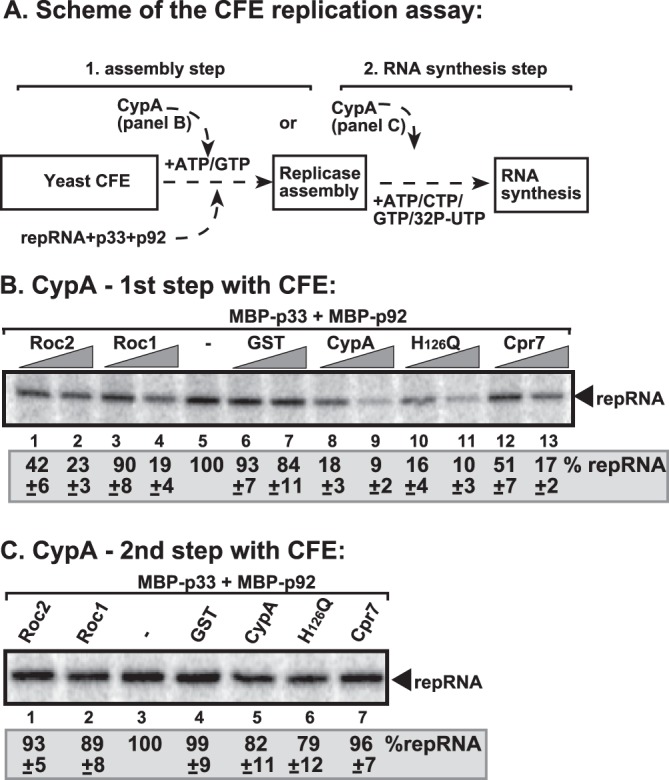
CypA inhibited assembly of the TBSV VRC in a stepwise CFE-based TBSV replication assay. (A) Scheme of the CFE-based TBSV replicase assembly and replication assays. Purified recombinant MBP-p33 and MBP-p92pol replication proteins of TBSV and in vitro-transcribed TBSV DI-72 (+)repRNA were added to CFE prepared from yeast strain BY4741 in step 1. The assay mixture either contained or lacked the purified recombinant GST-CypA or GST (7.5 and 15 pmol) during step 1. Note that the assay was performed in the presence of rATP/rGTP to facilitate TBSV VRC assembly, but not to support RNA synthesis in step 1. Centrifugation was used to collect the membrane fraction of the CFE after step 1, and after washing the membranes step 2 was performed in the presence of rATP/rCTP/rGTP and [32P]rUTP to support TBSV RNA replication. (B) Denaturing PAGE analysis of the 32P-labeled TBSV repRNA products obtained in the CFE-based assays when CypA and other cyclophilins were added during the first step. See further details in Fig. 1 and its legend. (C) Denaturing PAGE analysis of the 32P-labeled TBSV repRNA products obtained in the CFE-based assays when CypA and other cyclophilins were added in the second step. Three repeats of each experiment were performed. Note that all the cyclophilins used in panels B and C were GST tagged at the N terminus.
We found that addition of purified recombinant CypA during the first step (Fig. 2A) inhibited TBSV replication up to ∼90% (Fig. 2B, lanes 8 and 9 versus lanes 5 to 7), similar to the strong inhibitory effect of CypA during a standard CFE replication assay (Fig. 1A and B). This suggested that CypA strongly inhibits VRC assembly in vitro. Interestingly, the PPIase-inactive mutant H126Q was again as potent an inhibitor as the wt CypA (Fig. 2B, lanes 10 and 11), confirming that the PPIase function is not required to block TBSV replication. Importantly, CypA was as strong an inhibitor as Cpr7p (lanes 12 and 13), demonstrating that a single-domain cyclophilin can be as effective an inhibitor of TBSV replication as the TPR domain-containing Cpr7p. Roc1 and Roc2 also inhibited TBSV replication when added during the first step of the CFE assay (Fig. 2B, lanes 1 to 4 versus lanes 5 to 7), further demonstrating that these Arabidopsis cyclophilins have similar activities as CypA in inhibition of TBSV replication in vitro.
In contrast, addition of CypA, its mutant, or Roc1 and Roc2 exclusively during the second step of the CFE assay inhibited TBSV RNA replication by only 10 to 20% (Fig. 2C). These data are similar to those obtained with Cpr7p (Fig. 2C, lane 7) (52). We suggest that the lack of inhibition of viral RNA synthesis by cyclophilins after the VRC assembly step (i.e., during the second step of the CFE assay [Fig. 2A]) is due to the inaccessibility of the membrane-bound preassembled replicase by proteins (possibly due to physical hindrance and/or the membranous structure of the VRC).
To further test the effect of CypA on VRC assembly, we performed a second CFE assay in which the tombusvirus p33 and p92 replication proteins were expressed in yeast prior to CFE preparation. This approach allowed the membrane insertion of p33 and p92 and the formation of pre-VRCs in intact yeast cells in the absence of the viral RNA (38). Then, CFE was prepared and programmed with (+)repRNA in the presence or absence of purified recombinant CypA. We found that CypA or H126Q was able to efficiently inhibit TBSV ssRNA and dsRNA production in this assay (Fig. 3A, lanes 5 and 7). This suggested that CypA inhibits TBSV replication not through inhibiting the membrane association of the viral replication proteins or the preassembly of the protein components of the VRCs (both of which occurred in live yeast cells before CFE preparation in this assay), but instead is one of the subsequent steps, such as (+)RNA binding, activation of polymerase function of p92, or RNA synthesis.
Fig 3.
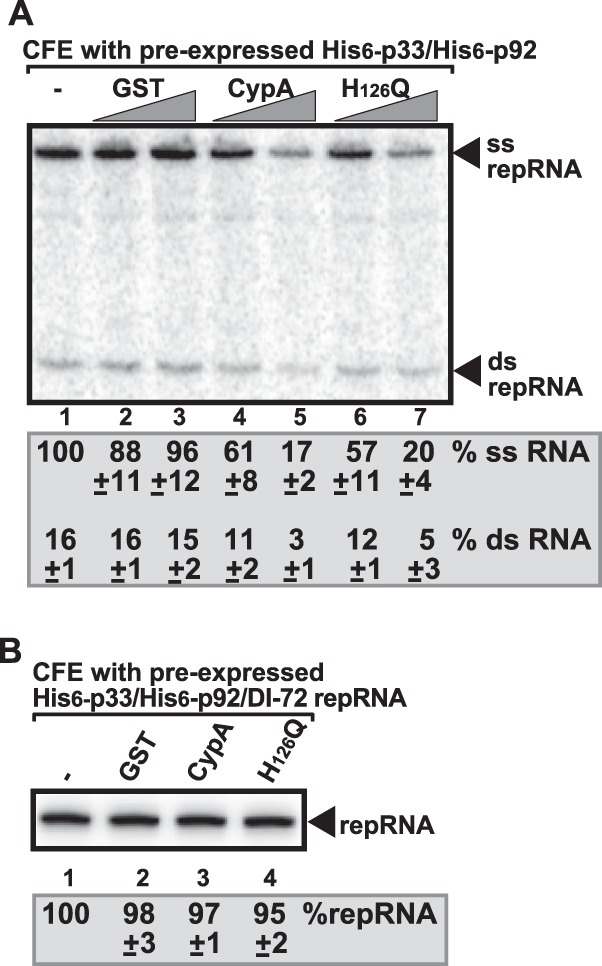
Recombinant CypA cannot inhibit the activity of the preassembled VRC in a CFE-based TBSV replication assay. (A) Nondenaturing PAGE analysis of single- and double-stranded RNA products produced in the cell-free TBSV replication assay. Note that the wt yeast expressed p33 and p92 replication proteins, allowing the preassembly of the VRC in living yeast cells prior to CFE preparation. The CFE was programmed with DI-72(+)repRNA only for the in vitro assay. The percentages of dsRNA and ssRNA in the samples are shown. Each experiment was repeated three times. (B) Denaturing PAGE analysis of repRNA products obtained in the cell-free TBSV replication assay. Note that the wt yeast expressed p33 and p92 replication proteins and the DI-72 repRNA; thus, completely assembled VRCs are formed in yeast prior to CFE preparation. Therefore, here we did not add viral components (p33/p92/repRNA) to the CFE prior to the in vitro assay. Note that all the cyclophilins used in panels A and B were GST tagged at the N terminus.
We also tested if the preassembled and active VRCs from yeast could be inhibited by addition of CypA in the CFE assay (Fig. 3B). These membrane-bound VRCs already contained the endogenous viral RNA (38). The added CypA or H126Q was unable to inhibit TBSV replication in this assay (Fig. 3B, lanes 3 and 4). This confirmed that the preassembled and active VRCs are not accessible to CypA.
CypA is associated with the viral replicase and binds to the RNA-binding region of the viral replication proteins.
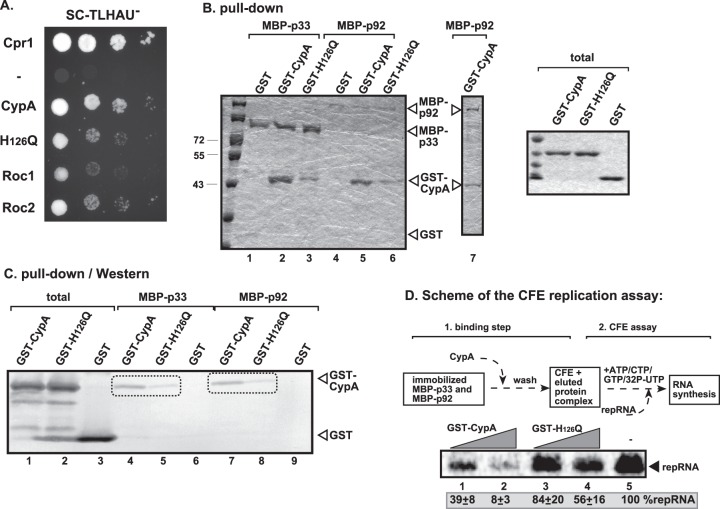
The inhibitory effect of a cellular protein on TBSV replication could be due to the effect of the given host protein on cellular factors coopted for viral replication, an effect on viral factors, or due to indirect effects on host metabolism. To identify the target of CypA, first we tested if CypA is present in the tombusviral VRCs. We isolated the membrane fractions from yeast containing the tombusviral VRCs, then used affinity purification of the p33 replication protein, the most abundant component of the VRC (33). We found that CypA copurified with the tombusvirus replicase from yeast (Fig. 4A and B, lanes 1 versus 2). The H126Q mutant was also copurified (Fig. 4A, lane 3), suggesting that PPIase activity is not needed for its recruitment.
Fig 4.
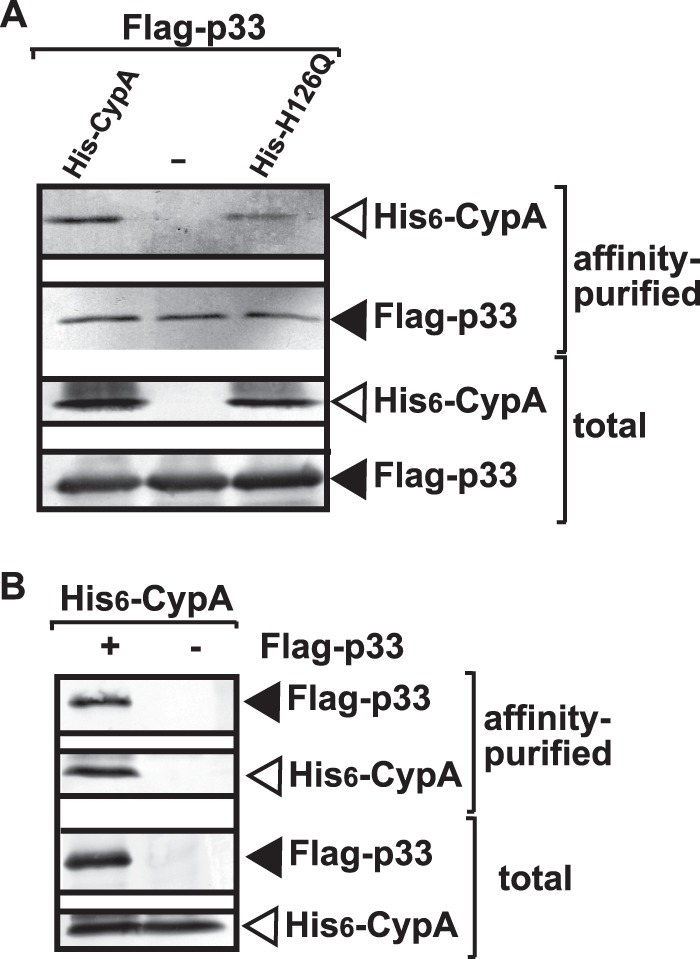
Copurification of CypA with the p33 replication protein from yeast. (A) The membrane-bound VRCs were collected by centrifugation, followed by solubilization and FLAG affinity purification from yeast coexpressing His6-CypA or His6-H126Q. (Top two panels) Western blot analysis of copurified His6-CypA or His6-H126Q with the FLAG-tagged p33 from yeast extracts performed using a FLAG affinity column with anti-His and anti-FLAG antibodies, respectively. (Bottom panels) Western blot of His6-CypA or His6-H126Q and the FLAG-tagged p33 in the total yeast extract performed with anti-His and anti-FLAG antibodies, respectively. (B) Negative control, showing the lack of nonspecific binding of His6-CypA to the FLAG affinity column. Details are provided in panel A and its legend.
To test if CypA interacts with the p33 replication protein, we performed a MYTH (split-ubiquitin) membrane-based yeast two-hybrid assay. As expected based on prior Cpr1p studies (29), CypA interacted with p33 (Fig. 5A). The interaction of Roc1 and Roc2 with p33 was also detected in the same assay (Fig. 5A). Interestingly, the interaction between H126Q and p33 was noticeably weaker than the binding of p33 to wt CypA (Fig. 5A). This was also confirmed in a pulldown experiment, which indicated ∼3-fold less-efficient binding between H126Q and either p33 or p92 than wt CypA (Fig. 5B, lanes 3 and 6 versus lanes 2 and 5; see also C, lanes 5 and 8 versus lanes 4 and 7). Based on these data, we propose that the sequence or the activity of the PPIase domain in CypA is critical for efficient binding to p33 and the overlapping p92 replication proteins.
Fig 5.
Interaction between CypA and p33 replication protein. (A) A split-ubiquitin MYTH assay was used to test binding between p33 and the shown full-length cyclophilin proteins. The bait p33 was coexpressed with the prey proteins in yeast. Cpr1 and the empty prey vector (NubG) were used as positive and negative controls, respectively. (B) Affinity binding (pulldown) assay to detect interaction between GST-CypA and the MBP-tagged viral replication proteins. MBP-p33 and MBP-p92 produced in E. coli were immobilized on amylose affinity columns. Then, GST-CypA expressed in E. coli was passed through the amylose affinity columns with immobilized MBP-tagged proteins. The affinity-bound proteins were eluted with maltose from the columns. The eluted proteins were analyzed by SDS-PAGE and Coomassie-staining. Lane 7 shows a sample where MBP-p92 could be better visualized than in lanes 4 to 6. The panel on the right shows the input purified recombinant proteins, based on Coomassie-stained SDS-PAGE. (C) The eluted proteins were also analyzed by Western blotting with anti-GST antibody to confirm the binding of GST-CypA to MBP-p92. Lanes 1 to 3 show the input purified recombinant proteins. (D) Inhibition of TBSV replication by a preformed GST-CypA:MBP-p33:MBP-p92 complex. As shown schematically on the top, we incubated the GST-CypA or GST-H126Q PPIase-deficient mutant (100 and 200 μg) with the immobilized MBP-tagged proteins to form the protein complex on the column. Then, the bound protein complexes were eluted with maltose from the columns. The eluted proteins were used together with (+)repRNA to program the yeast CFE as shown. The image shows the results of denaturing PAGE analysis of RNA products obtained in the cell-free TBSV replication assay.
To study if the interaction between CypA and p33/p92 is relevant for the inhibitory effect on TBSV replication, we affinity purified the p33/p92:CypA complex, followed by addition of the complex as a sole source of p33 and p92 to the CFE-based TBSV replication assay (Fig. 5D). Interestingly, the p33/p92:CypA complex supported TBSV replication poorly (at 8%), suggesting that binding of CypA to the replication proteins blocks their replication function. In contrast, the H126Q mutant was less effective in this function than the wt CypA (Fig. 5D, lanes 3 and 4 versus lane 5). This was likely due to the reduced binding of the H126Q mutant to p33/p92 complex in vitro.
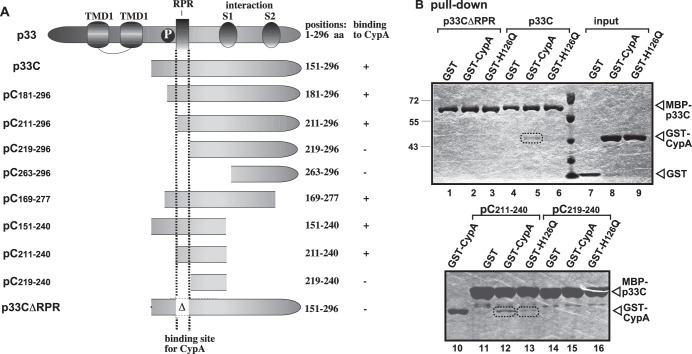
To map the CypA-binding site in the overlapping TBSV replication proteins, we used pulldown experiments with immobilized MBP-p33 and its truncation derivatives (Fig. 6A) and E. coli lysate containing GST-tagged CypA. These experiments revealed that CypA binds to the arginine-proline-rich (RPR) motif in p33 (Fig. 6A and B). Indeed, deletion of the RPR motif inhibited p33 binding to CypA (p33CΔRPR) (Fig. 6B, lane 2). The RPR motif represents the RNA-binding site in p33 and p92 replication proteins that is required for specific viral (+)RNA recruitment and replicase assembly (35, 61, 64).
Fig 6.
Map of the binding site of the TBSV p33 protein to CypA. (A) Schematic representation of viral p33 and its derivatives used in the binding assay (each MBP tagged at the N terminus). The various domains included the following: TMD, transmembrane domain; RPR, arginine-proline-rich RNA-binding domain; P, phosphorylated serine and threonine; S1 and S2, subdomains involved in the p33:p33/p92 interaction. The results of the in vitro binding experiments are summarized (+ or − labels are based on results of two repeat experiments). (B) Results of the pulldown assay to detect interactions between GST-CypA and the MBP-tagged viral p33 protein derivatives. See further details in Fig. 5B and its legend.
CypA binds to the viral RNA and inhibits recruitment of viral RNA for replication.
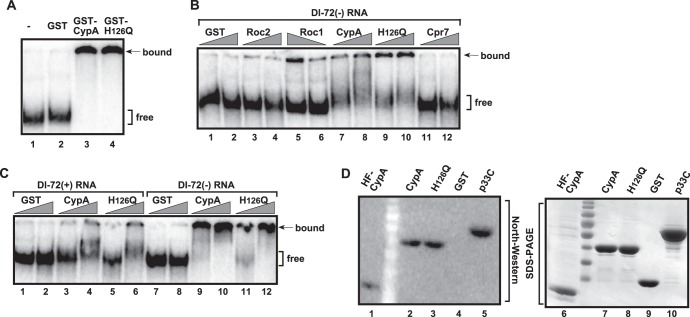
The observation that the H126Q mutant inhibited TBSV replication as efficiently as the wt CypA in the CFE assay (Fig. 1), yet the H126Q mutant, unlike wt CypA, bound inefficiently to the viral replication proteins (Fig. 5 and 6B), suggested that there must be an additional target for CypA and H126Q that affects the efficiency of TBSV inhibition. Therefore, we tested the binding activity of CypA to the viral RNA by using purified recombinant protein (Fig. 7A). The EMSA revealed that both CypA and the H126Q mutant bound to the viral (+)RNA efficiently, and even more to (−)RNA (Fig. 7A to C). Similarly, we also observed that the Arabidopsis Roc1 bound to the viral RNA (Fig. 7B, lane 5), albeit less efficiently than CypA. Viral (−)RNA binding by Arabidopsis Roc2 was undetectable (Fig. 7B, lane 4). In contrast, Cpr7p Cyp40-like cyclophilin did not bind to the viral RNA under the same conditions (Fig. 7B, lanes 11 and 12). We confirmed the viral RNA-binding ability of the E. coli-expressed CypA and H126Q mutant (Fig. 7D, lanes 2 and 3) and the yeast-expressed CypA (Fig. 7D, lane 1) in a Northwestern blotting assay.
Fig 7.
CypA binds to TBSV RNA. (A) In vitro EMSA binding assay with purified CypA and viral RNA template. The assay mixture contained 32P-labeled DI-72 (−)repRNA (∼0.1 pmol) plus 0.4 or 0.8 μg of purified recombinant CypA and H126Q mutant, as shown. The free or CypA-bound RNA was separated on nondenaturing 5% acrylamide gels. (B) EMSA binding assay with the GST-tagged Roc1, Roc2, Cpr7p, and Cpr1p, which were expressed in E. coli. The assay mixture contained 32P-labeled DI-72 (−)repRNA (∼0.1 pmol), plus 0.4 or 0.8 μg of purified recombinant cyclophilins, as shown. See further details in panel A and its legend. (C) Comparison of CypA or H126Q binding to either (+) or (−)-stranded RNA, based on an EMSA. See further details in panel A and its legend. (D, left image) Northwestern analysis of RNA binding by the purified recombinant CypA. The GST-tagged CypA, GST-H126Q mutant, and the p33C (the soluble C-terminal RNA-binding region only) replication protein were expressed in E. coli, while the His6-Flag-tagged HF-CypA was expressed in yeast. The probe was the 32P-labeled template DI-72 (−)RNA (∼50 pmol). (Right image) SDS-PAGE analysis of the affinity-purified recombinant proteins. The gel was stained with Coomassie blue. Each experiment was repeated at least two times. Note that all the cyclophilins used in panels A to D were GST tagged at the N terminus.
Viral RNA binding by CypA suggests that CypA might affect various functions of the viral RNA in cells. Therefore, we tested if CypA could affect the selective binding of p33 to the p33RE (p33 recognition element), which is required for replication, in an EMSA. As expected, p33 efficiently bound and shifted the (+)RNA carrying the p33RE (Fig. 8A, lanes 6 and 8) (35). However, addition of increasing amounts of recombinant CypA inhibited the formation of the p33:RNA complex in vitro (Fig. 8, lanes 2 and 3). Interestingly, the H126Q mutant also efficiently inhibited the formation of the p33:RNA complex in vitro (Fig. 8A, lanes 11 and 12). These data are consistent with the model that CypA can inhibit the selective recognition of the viral (+)RNA by the p33 replication protein, thus likely interfering with the recruitment of the viral (+)RNA for replication.
Fig 8.
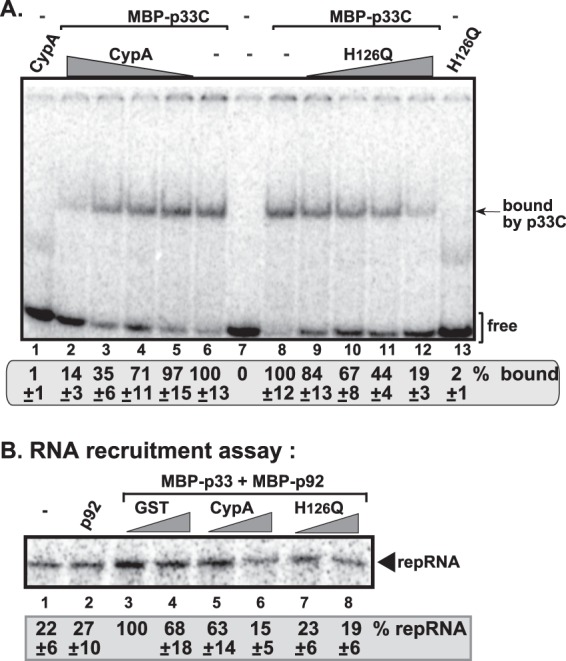
CypA inhibits the binding of p33 replication protein to the TBSV RNA and (+)RNA recruitment. (A) In vitro EMSA binding assay with purified MBP-p33C [an N-terminally truncated version of p33, which shows selective binding to the viral (+)RNA] in the presence of CypA or H126Q. The 32P-labeled RNA template was RII(+)-SL (∼0.1 pmol), which is the p33RE [part of RII(+)] and binds selectively to p33. The assay contained 0.02 μg of purified recombinant MBP-p33C, plus 0.02, 0.06, 0.2, or 0.6 μg of purified recombinant GST-CypA or GST- H126Q, as shown. The samples in lanes 1 and 13 contained 0.6 μg of purified recombinant GST-CypA or GST- H126Q in the absence of p33C. Purified recombinant GST did not affect the p33-RNA interaction (data not shown). See further details in Fig. 7 and its legend. (B) The viral (+)RNA recruitment assay was based on CFE. The 32P-labeled TBSV (+)repRNA template was added together with purified p33/p92 and CypA or H126Q to CFE prepared from yeast strain BY4741. The membrane association of the 32P-labeled DI-72 (+)repRNA template was measured by using denaturing PAGE gels. Note that the 32P-labeled TBSV (+)repRNA template inefficiently associated with the membrane, even in the absence of p33/p92 (lane 1), likely due to nonspecific binding to an RNA-binding host protein in the membrane. Note that all the cyclophilins used in panels A and B were GST tagged at the N terminus.
To test this model directly, we performed an in vitro viral (+)RNA recruitment assay based on yeast CFE with endogenous membranes (43) (Fig. 8B). We found the purified recombinant CypA and H126Q mutant strongly inhibited the recruitment of (+)repRNA by p33 and p92 replication proteins to the cellular membranes (Fig. 8B, lanes 5 and 6 and 7 and 8 versus lanes 3 and 4). These data strongly supported that CypA can inhibit (+)repRNA recruitment, which is critical for TBSV replication (35, 39, 65, 66). Moreover, the PPIase activity is not needed for this inhibitory function.
CypA inhibits de novo initiation by the tombusvirus replicase in vitro.
By binding to the viral RNA and to the replication proteins, CypA might also interfere with RNA synthesis. To test this, we performed an in vitro assay with affinity-purified tombusvirus replicase on an added minus-strand template in the presence or absence of recombinant CypA (Fig. 9A). The detergent-solubilized and affinity-purified tombusvirus replicase from yeast lacks endogenous RNA, which is removed during purification, and can only synthesize cRNA products on added TBSV templates (22, 39, 67). The purified replicase, unlike the membrane-bound replicase in the CFE-based assay, cannot perform a complete cycle of RNA synthesis (22, 39, 67). The purified tombusvirus replicase produces three types of products on the exogenous DI-72 RNA: a full-length de novo-initiated product (FL), internal initiation products (data not shown), and 3′-terminal extension product (3′TEX) (Fig. 9B, lane 1). Interestingly, the addition of CypA strongly inhibited the FL product (up to 5-fold reduction) (Fig. 9B, lanes 3 and 6). The H126Q mutant was also a strong inhibitor (Fig. 9B, lanes 4 and 5), suggesting that CypA inhibits de novo initiation, which is required for standard TBSV replication in infected cells.
Fig 9.
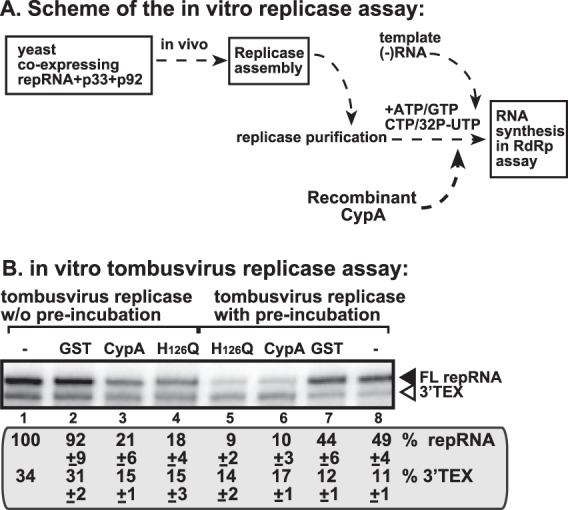
CypA inhibits de novo RNA synthesis by the affinity-purified tombusvirus replicase. (A) Scheme of the replicase preparation and the in vitro replication assay. Note that the original viral template RNA in the replicase from yeast was removed during replicase solubilization/purification. Therefore, an added (−)repRNA was tested for replicase activity. (B) Representative denaturing gel of 32P-labeled RNA products synthesized by the purified tombusvirus replicase in vitro in the presence of 0.5 μg of purified recombinant GST-CypA, GST- H126Q, or GST. The in vitro assays were programmed with DI-72 (−)repRNA, and the mixtures also contained ATP/CTP/GTP and [32P]UTP. All the components were added at the same time (lanes 1 to 4), or the replicase was preincubated with GST-CypA, GST- H126Q, or GST for ∼5 min prior to the assay (lanes 5 to 8). The level of cRNA synthesis producing FL (the full-length product, made via de novo initiation from the 3′-terminal promoter) is shown as percentage of FL product in the control sample. Note that this replicase preparation also synthesized 3′-terminal extension products (3′TEX). Each experiment was repeated three times. Note that all the cyclophilins used in panel B were GST tagged at the N terminus.
CypA inhibits tombusvirus replication in yeast.
To compare the activity of the yeast Cpr1p with that of the mammalian CypA, we overexpressed these proteins in wt yeast or in yeast that lacked CPR1 and carried a temperature-sensitive ESS1 parvulin (which complements CPR1 in TBSV replication in yeast) (29). Overexpression of CypA in wt yeast led to an ∼50% reduction in TBSV repRNA accumulation (Fig. 10A, lanes 1 to 4). The inhibitory effect of CypA overexpression was even more pronounced in cpr1Δ/ts-ess1 yeast, resulting in 25% TBSV replication only (Fig. 10B, lanes 1 to 4). Similar to Cpr1p (29), the overexpression of CypA did not affect the level of p33 replication protein in the two yeast strains tested, indicating that CypA is unlikely to destabilize the replication proteins. These data established that, similar to Cpr1p (29), CypA is a potent inhibitor of TBSV replication in yeast.
Fig 10.
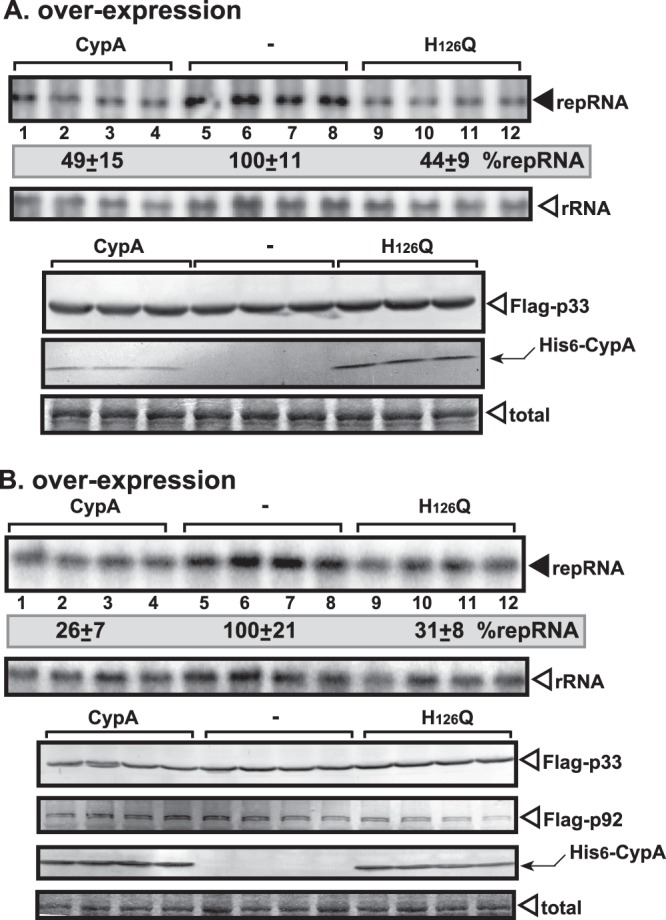
Overexpression of CypA or H126Q inhibits TBSV repRNA accumulation in yeast. The wt (A) or cpr1Δ/ts-ess1 (B) yeast strains were used for these experiments. (Top panel) Northern blot analysis of TBSV repRNA accumulation in yeast overproducing the His6-tagged CypA or H126Q. The repRNA levels were normalized based on rRNA loading. (Middle panel) Northern blot analysis showing the level of rRNA loading. (Bottom panels) Detection of Flag-tagged p33 by Western blotting using anti-Flag antibody. Detection of the overproduced His6-tagged CypA or H126Q by Western blotting with anti-His antibody in yeast is shown. The total protein level in each sample was analyzed by SDS-PAGE and Coomassie blue staining. Note that all of the cyclophilins expressed in yeast (panels A and B) were His6 tagged at the N terminus.
Interestingly, the H126Q mutant was as potent an inhibitor of TBSV replication as the wt CypA when overexpressed in both yeast strains (Fig. 10A and B, lanes 9 to 12). Thus, the PPIase activity of CypA is not required for its inhibitory effect on TBSV replication. This finding is in agreement with the CFE-based data.
Inhibition of tombusvirus accumulation in N. benthamiana plants expressing Roc1 and Roc2 cyclophilins.
To test if the single-domain cyclophilins can inhibit tombusvirus accumulation in plants, we separately overexpressed Roc1, Roc2, and human CypA in leaves of N. benthamiana. Tombusvirus RNA accumulation revealed a reduction of up to 12-fold in leaves overexpressing either Roc1 or Roc2 cyclophilins (Fig. 11A, lanes 13 to 20 versus lanes 9 to 12). Overexpression of Roc1 (Fig. 11B) or Roc2 (data not shown) did not have phenotypic effects on the N. benthamiana plants. The plants overexpressing Roc1 were protected from the lethal necrosis caused by tombusvirus infection (Fig. 11B). Similarly, expression of CypA or the H126Q mutant also led to close to a 10-fold reduction in tombusviral RNA (Fig. 11A) and greatly reduced necrosis (data not shown), confirming that the mammalian CypA protein is a potent inhibitor of tombusvirus replication in both plants and yeast.
Fig 11.
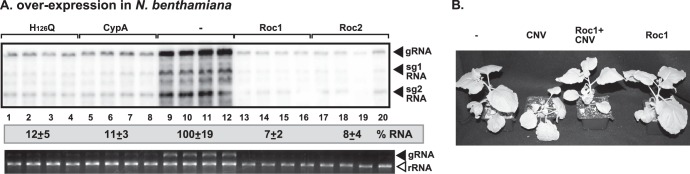
Overexpression of single-domain cyclophilins inhibits tombusvirus RNA accumulation in Nicotiana benthamiana. (A, top panel) Northern blot analysis of CNV (a very close relative of TBSV) RNA accumulation in plants overproducing Arabidopsis Roc1, Roc2, human CypA, or H126Q mutant. The repRNA levels were normalized based on rRNA loading. (Bottom panel) Ethidium bromide-stained agarose gel showing the level of rRNA loading. Note that N. benthamiana leaves transiently expressed the cyclophilins from the 35S promoter introduced via agroinfiltration. Samples were taken 3 days after agroinfiltration with CNV. (B) The phenotypes of plants overexpressing Roc1 and the symptoms induced by CNV infection. The picture was taken 10 days after agroinfiltration with CNV. Note that all the cyclophilins expressed in plants (panels A and B) were untagged.
DISCUSSION
The emerging picture from recent genome-wide screens and global proteomics approaches with tombusviruses indicates that many host proteins act as intrinsic restriction factors by inhibiting virus replication (28, 32, 44, 49). Among the host restriction factors are cyclophilins, which are a large family of peptidyl-prolyl cis-trans isomerases with protein chaperone-like function. Through isomerization of peptidyl-prolyl bonds, cyclophilins play important roles in protein folding, maturation, and conformational changes in client proteins and affect protein trafficking (54, 55). All cyclophilins share a 109-amino-acid-long cyclophilin-like domain that performs the PPIase activity, while additional unique sequences or domains in each member of the family are important for selection of protein substrates and for subcellular compartmentalization.
Previous works have identified six yeast PPIases, including four cyclophilins among the eight S. cerevisiae cyclophilins, which bind to the tombusvirus p33 replication protein (29). Evidence was obtained that the cytosolic Cpr1p and Cpr7p, the mitochondrial Cpr3p, as well as the parvulin Ess1p inhibited TBSV replication in yeast (29, 52). However, the Cyp40-like Cpr7p inhibited TBSV replication via its unique TPR domain, which is absent in Cpr1p and its orthologous mammalian CypA and Arabidopsis Roc1 and Roc2 cyclophilins (52). This suggests that the small Cpr1p and the homologous plant and human proteins with a single cyclophilin domain function using different mechanisms than the TPR domain in Cpr7p in restriction of TBSV replication.
Since the E. coli-expressed Cpr1p lacked inhibitory activity in the CFE-based TBSV replication assay (52), we decided to dissect the mechanism for this by using the orthologous mammalian CypA and the Arabidopsis Roc1 and Roc2 cyclophilins. Detailed mechanistic studies revealed that CypA inhibits TBSV replication by inhibiting the viral (+)RNA recruitment for replication and by blocking VRC assembly. CypA also inhibits the RNA synthesis steps if it gets recruited to the VRC during assembly. However, CypA does not seem to be able to enter the already-assembled VRC, suggesting that CypA cannot remodel the VRC once the membrane-bound VRC is formed. Altogether, these events lead to reduced RNA synthesis and potent inhibition of TBSV replication.
Comparison of the mechanisms of inhibition of TBSV replication by the TPR-containing Cpr7p (52) and the Cpr1-like CypA revealed several similarities as well as differences in their activities. The similarities included the inhibition of early steps of TBSV replication, including RNA recruitment and VRC assembly, efficient binding of both cyclophilins to the RPR sequence of p33/p92 involved in RNA binding, and the lack of requirement of PPIase activity for the inhibitory function. Also, neither protein can inhibit the function of the preassembled tombusvirus VRCs in vitro. The differences in the mechanisms between CypA and Cpr7p were also substantial and included the following findings: (i) the domains involved in inhibition is the TPR domain in the case of Cpr7p and the cyclophilin domain in the case of CypA; (ii) also, CypA binds to the viral RNA, which seems to be important, while Cpr7p-driven inhibition is through binding to p33/p92 replication proteins; (iii) CypA becomes recruited to the VRC, while this has not yet been shown for Cpr7p. Those mechanistic features of CypA, which are based on RNA binding, are actually similar to the those for cellular nucleolin, which is also a restriction factor that inhibits the recruitment of viral (+)RNA into replication through binding to viral (+)RNA (49). Thus, it seems that different cellular restriction factors target similar early steps during tombusvirus replication. These antiviral activities by these restriction factors might be possible, since the tombusvirus (+)RNA and replication proteins could be more exposed at the early stage, before VRC assembly, than during the later stages when the membrane-bound VRCs are tightly protected (38, 43).
Based on the in vitro, yeast, and plant data obtained with wt and PPIase mutant CypA, we suggest that the RNA-binding function of CypA could also be critical for its viral restriction function. This is supported by the following observations: (i) the de novo-initiated FL product decreased in the presence of CypA (Fig. 9B); (ii) the H126Q mutant behaved similarly to the wt CypA in inhibiting tombusvirus replication in vitro, in yeast, and in plant, although the H126Q mutant bound to the viral replication proteins much less efficiently than the wt CypA; (iii) also, both wt CypA and the H126Q mutant inhibited the selective binding of p33 replication protein to the p33RE (Fig. 8). These observations could be explained by the ability of CypA to bind viral RNA that hinders the interaction of the viral RNA with the viral replication proteins. However, the possibility also remains that the affinity of CypA(H126Q) to TBSV replication proteins (Fig. 5) is sufficient to inhibit the RNA-binding activity of p33. Masking the RPR motif of p33 by CypA and even by CypA(H126Q) rather than their viral RNA-binding function is still a reasonable explanation for their inhibitory effects on RNA replication. Although refolding of the replication proteins by the PPIase activity of CypA, resulting in inhibition of their functions, cannot be excluded, the observation that the PPIase-inactive H126Q mutant is also an effective inhibitor of TBSV replication makes the importance of PPIase activity more difficult to demonstrate. We suggest that cyclophilins are effective inhibitors of TBSV replication most likely at the beginning of natural infections, when the amounts of viral proteins and the viral (+)RNA is still low in cells, and so the relative amount of cyclophilin is very high, resulting in a block of recruitment of the viral (+)RNA for replication and inhibition of the replicase function.
Although most of the mechanistic studies were performed with the mammalian CypA protein due to its high activity in vitro, we also obtained evidence that the Arabidopsis CypA-like Roc1 and Roc2 cyclophilins also have similar inhibitory functions in the CFE assay (Fig. 1). In addition, Roc1 and Roc2 also bound to both the viral replication proteins and the viral RNA (Fig. 7B), suggesting that these Arabidopsis cyclophilins play a comparable role to the CypA protein. Arabidopsis has 29 PPIases, including 7 cytosolic single-domain (CypA-like) cyclophilins (53), and so it is possible that additional plant members of this large family could have a restriction function during tombusvirus replication in plants.
Investigations with many viruses have indicated that cellular cyclophilins are either coopted to facilitate viral infections or are used by the host as intrinsic restriction factors (69). For example, similar to our findings with Cpr1p CypA-like proteins and Cyp40 homologs, cyclophilins have been shown to inhibit accumulation of several RNA viruses, including influenza A virus, West Nile virus, alfanodaviruses, and HIV-1 (5, 52, 69). Interestingly, HIV targets CypA via the retroviral Vif protein, which inhibits the incorporation of CypA into the viral particles (70). Overall, cyclophilins are potent inhibitors of several RNA viruses, and they might be part of the innate response of the host against some viruses. Since PPIases are conserved and ubiquitous proteins, their roles could be widespread against many viruses.
The picture is more complex, however, since several RNA viruses subvert cyclophilins to facilitate their replication. The list of these viruses includes flaviviruses and coronaviruses (5, 71). HCV usurps the cytosolic CypA to facilitate the assembly of the HCV VRC through influence on the cleavage of the NS5A-NS5B fusion protein and the folding of the NS5A and NS5B RdRp proteins (59, 72–74). CypA was shown to bind to the flavivirus NS5 replication protein and is a likely component of the flaviviral VRC (75).
Overall, cyclophilins seem to play important roles during RNA virus infections. The current work has revealed a new role for CypA-like proteins as intrinsic inhibitors of tombusvirus replication. This function for CypA is likely conserved from yeast to plants.
ACKNOWLEDGMENTS
We thank Pogany Judit for critical reading of the manuscript and for very helpful suggestions. We thank Philippe Gallay (Scripps Research Institute) and Brenda Andrews (University of Toronto) for yeast strains and plasmids.
This work was supported by the National Institutes of Health (NIAID; 1R21AI096323).
Footnotes
Published ahead of print 2 October 2013
REFERENCES
- 1.Panavas T, Serviene E, Brasher J, Nagy PD. 2005. Yeast genome-wide screen reveals dissimilar sets of host genes affecting replication of RNA viruses. Proc. Natl. Acad. Sci. U. S. A. 102:7326–7331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cherry S, Doukas T, Armknecht S, Whelan S, Wang H, Sarnow P, Perrimon N. 2005. Genome-wide RNAi screen reveals a specific sensitivity of IRES-containing RNA viruses to host translation inhibition. Genes Dev. 19:445–452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jiang Y, Serviene E, Gal J, Panavas T, Nagy PD. 2006. Identification of essential host factors affecting tombusvirus RNA replication based on the yeast Tet promoters Hughes Collection. J. Virol. 80:7394–7404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kushner DB, Lindenbach BD, Grdzelishvili VZ, Noueiry AO, Paul SM, Ahlquist P. 2003. Systematic, genome-wide identification of host genes affecting replication of a positive-strand RNA virus. Proc. Natl. Acad. Sci. U. S. A. 100:15764–15769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Krishnan MN, Ng A, Sukumaran B, Gilfoy FD, Uchil PD, Sultana H, Brass AL, Adametz R, Tsui M, Qian F, Montgomery RR, Lev S, Mason PW, Koski RA, Elledge SJ, Xavier RJ, Agaisse H, Fikrig E. 2008. RNA interference screen for human genes associated with West Nile virus infection. Nature 455:242–245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li Q, Brass AL, Ng A, Hu Z, Xavier RJ, Liang TJ, Elledge SJ. 2009. A genome-wide genetic screen for host factors required for hepatitis C virus propagation. Proc. Natl. Acad. Sci. U. S. A. 106:16410–16415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Randall G, Panis M, Cooper JD, Tellinghuisen TL, Sukhodolets KE, Pfeffer S, Landthaler M, Landgraf P, Kan S, Lindenbach BD, Chien M, Weir DB, Russo JJ, Ju J, Brownstein MJ, Sheridan R, Sander C, Zavolan M, Tuschl T, Rice CM. 2007. Cellular cofactors affecting hepatitis C virus infection and replication. Proc. Natl. Acad. Sci. U. S. A. 104:12884–12889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sessions OM, Barrows NJ, Souza-Neto JA, Robinson TJ, Hershey CL, Rodgers MA, Ramirez JL, Dimopoulos G, Yang PL, Pearson JL, Garcia-Blanco MA. 2009. Discovery of insect and human dengue virus host factors. Nature 458:1047–1050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tai AW, Benita Y, Peng LF, Kim SS, Sakamoto N, Xavier RJ, Chung RT. 2009. A functional genomic screen identifies cellular cofactors of hepatitis C virus replication. Cell Host Microbe 5:298–307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nagy PD. 2008. Yeast as a model host to explore plant virus-host interactions. Annu. Rev. Phytopathol. 46:217–242 [DOI] [PubMed] [Google Scholar]
- 11.Nagy PD, Pogany J. 2008. Multiple roles of viral replication proteins in plant RNA virus replication. Methods Mol. Biol. 451:55–68 [DOI] [PubMed] [Google Scholar]
- 12.Nagy PD, Wang RY, Pogany J, Hafren A, Makinen K. 2011. Emerging picture of host chaperone and cyclophilin roles in RNA virus replication. Virology 411:374–382 [DOI] [PubMed] [Google Scholar]
- 13.Li Z, Nagy PD. 2011. Diverse roles of host RNA binding proteins in RNA virus replication. RNA Biol. 8:305–315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.den Boon JA, Diaz A, Ahlquist P. 2010. Cytoplasmic viral replication complexes. Cell Host Microbe 8:77–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Salonen A, Ahola T, Kaariainen L. 2005. Viral RNA replication in association with cellular membranes. Curr. Top. Microbiol. Immunol. 285:139–173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miller S, Krijnse-Locker J. 2008. Modification of intracellular membrane structures for virus replication. Nat. Rev. Microbiol. 6:363–374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fernandez-Garcia MD, Mazzon M, Jacobs M, Amara A. 2009. Pathogenesis of flavivirus infections: using and abusing the host cell. Cell Host Microbe 5:318–328 [DOI] [PubMed] [Google Scholar]
- 18.Nagy PD, Pogany J. 2012. The dependence of viral RNA replication on co-opted host factors. Nat. Rev. Microbiol. 10:137–149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nagy PD, Barajas D, Pogany J. 2012. Host factors with regulatory roles in tombusvirus replication. Curr. Opin. Virol. 2:685–692 [DOI] [PubMed] [Google Scholar]
- 20.Nagy PD, Pogany J. 2006. Yeast as a model host to dissect functions of viral and host factors in tombusvirus replication. Virology 344:211–220 [DOI] [PubMed] [Google Scholar]
- 21.White KA, Nagy PD. 2004. Advances in the molecular biology of tombusviruses: gene expression, genome replication, and recombination. Prog. Nucleic Acid Res. Mol. Biol. 78:187–226 [DOI] [PubMed] [Google Scholar]
- 22.Panaviene Z, Panavas T, Serva S, Nagy PD. 2004. Purification of the cucumber necrosis virus replicase from yeast cells: role of coexpressed viral RNA in stimulation of replicase activity. J. Virol. 78:8254–8263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Panavas T, Nagy PD. 2003. Yeast as a model host to study replication and recombination of defective interfering RNA of Tomato bushy stunt virus. Virology 314:315–325 [DOI] [PubMed] [Google Scholar]
- 24.Nagy PD. 2011. The roles of host factors in tombusvirus RNA recombination. Adv. Virus Res. 81:63–84 [DOI] [PubMed] [Google Scholar]
- 25.Nagy PD, Pogany J. 2010. Global genomics and proteomics approaches to identify host factors as targets to induce resistance against Tomato bushy stunt virus. Adv. Virus Res. 76:123–177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Serviene E, Shapka N, Cheng CP, Panavas T, Phuangrat B, Baker J, Nagy PD. 2005. Genome-wide screen identifies host genes affecting viral RNA recombination. Proc. Natl. Acad. Sci. U. S. A. 102:10545–10550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Serviene E, Jiang Y, Cheng CP, Baker J, Nagy PD. 2006. Screening of the yeast yTHC collection identifies essential host factors affecting tombusvirus RNA recombination. J. Virol. 80:1231–1241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shah Nawaz Ul-Rehman M, Martinez-Ochoa N, Pascal H, Sasvari Z, Herbst C, Xu K, Baker J, Sharma M, Herbst A, Nagy PD. 2012. Proteome-wide overexpression of host proteins for identification of factors affecting tombusvirus RNA replication: an inhibitory role of protein kinase C. J. Virol. 86:9384–9395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mendu V, Chiu M, Barajas D, Li Z, Nagy PD. 2010. Cpr1 cyclophilin and Ess1 parvulin prolyl isomerases interact with the tombusvirus replication protein and inhibit viral replication in yeast model host. Virology 406:342–351 [DOI] [PubMed] [Google Scholar]
- 30.Li Z, Pogany J, Panavas T, Xu K, Esposito AM, Kinzy TG, Nagy PD. 2009. Translation elongation factor 1A is a component of the tombusvirus replicase complex and affects the stability of the p33 replication co-factor. Virology 385:245–260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li Z, Barajas D, Panavas T, Herbst DA, Nagy PD. 2008. Cdc34p Ubiquitin-conjugating enzyme is a component of the tombusvirus replicase complex and ubiquitinates p33 replication protein. J. Virol. 82:6911–6926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shah Nawaz ul-Rehman M, Reddisiva Prasanth K, Baker J, Nagy PD. 2013. Yeast screens for host factors in positive-strand RNA virus replication based on a library of temperature-sensitive mutants. Methods 59:207–216 [DOI] [PubMed] [Google Scholar]
- 33.Serva S, Nagy PD. 2006. Proteomics analysis of the tombusvirus replicase: Hsp70 molecular chaperone is associated with the replicase and enhances viral RNA replication. J. Virol. 80:2162–2169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jonczyk M, Pathak KB, Sharma M, Nagy PD. 2007. Exploiting alternative subcellular location for replication: tombusvirus replication switches to the endoplasmic reticulum in the absence of peroxisomes. Virology 362:320–330 [DOI] [PubMed] [Google Scholar]
- 35.Pogany J, White KA, Nagy PD. 2005. Specific binding of tombusvirus replication protein p33 to an internal replication element in the viral RNA is essential for replication. J. Virol. 79:4859–4869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Panavas T, Hawkins CM, Panaviene Z, Nagy PD. 2005. The role of the p33:p33/p92 interaction domain in RNA replication and intracellular localization of p33 and p92 proteins of Cucumber necrosis tombusvirus. Virology 338:81–95 [DOI] [PubMed] [Google Scholar]
- 37.Stork J, Kovalev N, Sasvari Z, Nagy PD. 2011. RNA chaperone activity of the tombusviral p33 replication protein facilitates initiation of RNA synthesis by the viral RdRp in vitro. Virology 409:338–347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pogany J, Nagy PD. 2008. Authentic replication and recombination of Tomato bushy stunt virus RNA in a cell-free extract from yeast. J. Virol. 82:5967–5980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Panaviene Z, Panavas T, Nagy PD. 2005. Role of an internal and two 3′-terminal RNA elements in assembly of tombusvirus replicase. J. Virol. 79:10608–10618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang RY, Nagy PD. 2008. Tomato bushy stunt virus co-opts the RNA-binding function of a host metabolic enzyme for viral genomic RNA synthesis. Cell Host Microbe 3:178–187 [DOI] [PubMed] [Google Scholar]
- 41.Li Z, Pogany J, Tupman S, Esposito AM, Kinzy TG, Nagy PD. 2010. Translation elongation factor 1A facilitates the assembly of the tombusvirus replicase and stimulates minus-strand synthesis. PLoS Pathog. 6(11):e1001175. 10.1371/journal.ppat.1001175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Barajas D, Jiang Y, Nagy PD. 2009. A unique role for the host ESCRT proteins in replication of Tomato bushy stunt virus. PLoS Pathog. 5(12):e1000705. 10.1371/journal.ppat.1000705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pogany J, Stork J, Li Z, Nagy PD. 2008. In vitro assembly of the Tomato bushy stunt virus replicase requires the host heat shock protein 70. Proc. Natl. Acad. Sci. U. S. A. 105:19956–19961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Huang TS, Nagy PD. 2011. Direct inhibition of tombusvirus plus-strand RNA synthesis by a dominant-negative mutant of a host metabolic enzyme, GAPDH, in yeast and plants. J. Virol. 86:9090–9102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang RY, Stork J, Nagy PD. 2009. A key role for heat shock protein 70 in the localization and insertion of tombusvirus replication proteins to intracellular membranes. J. Virol. 83:3276–3287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kovalev N, Pogany J, Nagy PD. 2012. A co-opted DEAD-box RNA helicase enhances tombusvirus plus-strand synthesis. PLoS Pathog. 8(2):e1002537. 10.1371/journal.ppat.1002537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kovalev N, Barajas D, Nagy PD. 2012. Similar roles for yeast Dbp2 and Arabidopsis RH20 DEAD-box RNA helicases to Ded1 helicase in tombusvirus plus-strand synthesis. Virology 432:470–484 [DOI] [PubMed] [Google Scholar]
- 48.Sasvari Z, Izotova L, Kinzy TG, Nagy PD. 2011. Synergistic roles of eukaryotic translation elongation factors 1Bγ and 1A in stimulation of tombusvirus minus-strand synthesis. PLoS Pathog. 7(12):e1002438. 10.1371/journal.ppat.1002438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jiang Y, Li Z, Nagy PD. 2010. Nucleolin/Nsr1p binds to the 3′ noncoding region of the tombusvirus RNA and inhibits replication. Virology 396:10–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Barajas D, Li Z, Nagy PD. 2009. The Nedd4-type Rsp5p ubiquitin ligase inhibits tombusvirus replication by regulating degradation of the p92 replication protein and decreasing the activity of the tombusvirus replicase. J. Virol. 83:11751–11764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Qin J, Barajas D, Nagy PD. 2012. An inhibitory function of WW domain-containing host proteins in RNA virus replication. Virology 426:106–119 [DOI] [PubMed] [Google Scholar]
- 52.Lin JY, Mendu V, Pogany J, Qin J, Nagy PD. 2012. The TPR domain in the host Cyp40-like cyclophilin binds to the viral replication protein and inhibits the assembly of the tombusviral replicase. PLoS Pathog. 8(2):e1002491. 10.1371/journal.ppat.1002491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Romano PG, Horton P, Gray JE. 2004. The Arabidopsis cyclophilin gene family. Plant Physiol. 134:1268–1282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang P, Heitman J. 2005. The cyclophilins. Genome Biol. 6:226. 10.1186/gb-2005-6-7-226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Arevalo-Rodriguez M, Wu X, Hanes SD, Heitman J. 2004. Prolyl isomerases in yeast. Front. Biosci. 9:2420–2446 [DOI] [PubMed] [Google Scholar]
- 56.Lee J, Kim SS. 2010. Current implications of cyclophilins in human cancers. J. Exp. Clin. Cancer Res. 29:97. 10.1186/1756-9966-29-97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Galat A, Bua J. 2010. Molecular aspects of cyclophilins mediating therapeutic actions of their ligands. Cell. Mol. Life Sci. 67:3467–3488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kumari S, Roy S, Singh P, Singla-Pareek SL, Pareek A. 2012. Cyclophilins: proteins in search of function. Plant Signal Behav. 8:e22734. 10.4161/psb.22734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chatterji U, Bobardt M, Selvarajah S, Yang F, Tang H, Sakamoto N, Vuagniaux G, Parkinson T, Gallay P. 2009. The isomerase active site of cyclophilin A is critical for hepatitis C virus replication. J. Biol. Chem. 284:16998–17005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cheng CP, Jaag HM, Jonczyk M, Serviene E, Nagy PD. 2007. Expression of the Arabidopsis Xrn4p 5′-3′ exoribonuclease facilitates degradation of tombusvirus RNA and promotes rapid emergence of viral variants in plants. Virology 368:238–248 [DOI] [PubMed] [Google Scholar]
- 61.Rajendran KS, Nagy PD. 2003. Characterization of the RNA-binding domains in the replicase proteins of Tomato bushy stunt virus. J. Virol. 77:9244–9258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rajendran KS, Pogany J, Nagy PD. 2002. Comparison of turnip crinkle virus RNA-dependent RNA polymerase preparations expressed in Escherichia coli or derived from infected plants. J. Virol. 76:1707–1717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sopko R, Huang D, Preston N, Chua G, Papp B, Kafadar K, Snyder M, Oliver SG, Cyert M, Hughes TR, Boone C, Andrews B. 2006. Mapping pathways and phenotypes by systematic gene overexpression. Mol. Cell 21:319–330 [DOI] [PubMed] [Google Scholar]
- 64.Pathak KB, Jiang Z, Ochanine V, Sharma M, Pogany J, Nagy PD. 2013. Characterization of dominant-negative and temperature-sensitive mutants of tombusvirus replication proteins affecting replicase assembly. Virology 437:48–61 [DOI] [PubMed] [Google Scholar]
- 65.Pathak KB, Pogany J, Xu K, White KA, Nagy PD. 2012. Defining the roles of cis-acting RNA elements in tombusvirus replicase assembly in vitro. J. Virol. 86:156–171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Monkewich S, Lin HX, Fabian MR, Xu W, Na H, Ray D, Chernysheva OA, Nagy PD, White KA. 2005. The p92 polymerase coding region contains an internal RNA element required at an early step in tombusvirus genome replication. J. Virol. 79:4848–4858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Nagy PD, Pogany J. 2000. Partial purification and characterization of Cucumber necrosis virus and Tomato bushy stunt virus RNA-dependent RNA polymerases: similarities and differences in template usage between tombusvirus and carmovirus RNA-dependent RNA polymerases. Virology 276:279–288 [DOI] [PubMed] [Google Scholar]
- 68.Frausto SD, Lee E, Tang H. 2013. Cyclophilins as modulators of viral replication. Viruses 5:1684–1701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Strebel K, Luban J, Jeang KT. 2009. Human cellular restriction factors that target HIV-1 replication. BMC Med. 7:48. 10.1186/1741-7015-7-48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Takeuchi H, Buckler-White A, Goila-Gaur R, Miyagi E, Khan MA, Opi S, Kao S, Sokolskaja E, Pertel T, Luban J, Strebel K. 2007. Vif counteracts a cyclophilin A-imposed inhibition of simian immunodeficiency viruses in human cells. J. Virol. 81:8080–8090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.de Wilde AH, Li Y, van der Meer Y, Vuagniaux G, Lysek R, Fang Y, Snijder EJ, van Hemert MJ. 2013. Cyclophilin inhibitors block arterivirus replication by interfering with viral RNA synthesis. J. Virol. 87:1454–1464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kaul A, Stauffer S, Berger C, Pertel T, Schmitt J, Kallis S, Zayas M, Lohmann V, Luban J, Bartenschlager R. 2009. Essential role of cyclophilin A for hepatitis C virus replication and virus production and possible link to polyprotein cleavage kinetics. PLoS Pathog. 5(8):e1000546. 10.1371/journal.ppat.1000546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yang F, Robotham JM, Grise H, Frausto S, Madan V, Zayas M, Bartenschlager R, Robinson M, Greenstein AE, Nag A, Logan TM, Bienkiewicz E, Tang H. 2010. A major determinant of cyclophilin dependence and cyclosporine susceptibility of hepatitis C virus identified by a genetic approach. PLoS Pathog. 6(9):e1001118. 10.1371/journal.ppat.1001118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhou D, Mei Q, Li J, He H. 2012. Cyclophilin A and viral infections. Biochem. Biophys. Res. Commun. 424:647–650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Qing M, Yang F, Zhang B, Zou G, Robida JM, Yuan Z, Tang H, Shi PY. 2009. Cyclosporine inhibits flavivirus replication through blocking the interaction between host cyclophilins and viral NS5 protein. Antimicrob. Agents Chemother. 53:3226–3235 [DOI] [PMC free article] [PubMed] [Google Scholar]